Seed Fatty Acids Modify Oviposition of Tenebrio molitor (Coleoptera: Tenebrionidae)
Abstract
:1. Introduction
2. Results
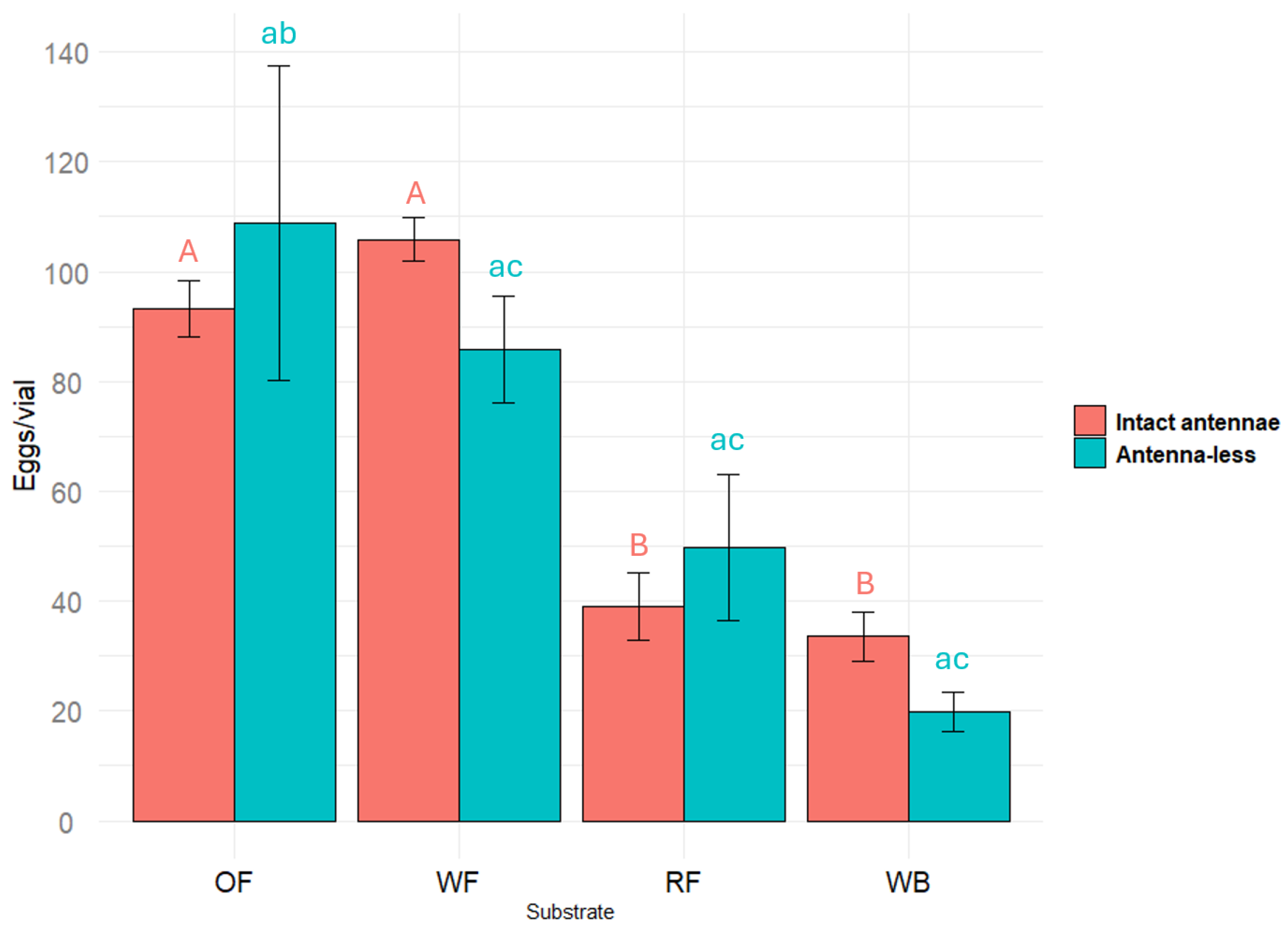
2.1. Oviposition on Different Substrates
2.2. The Substrate VOCs and Oviposition
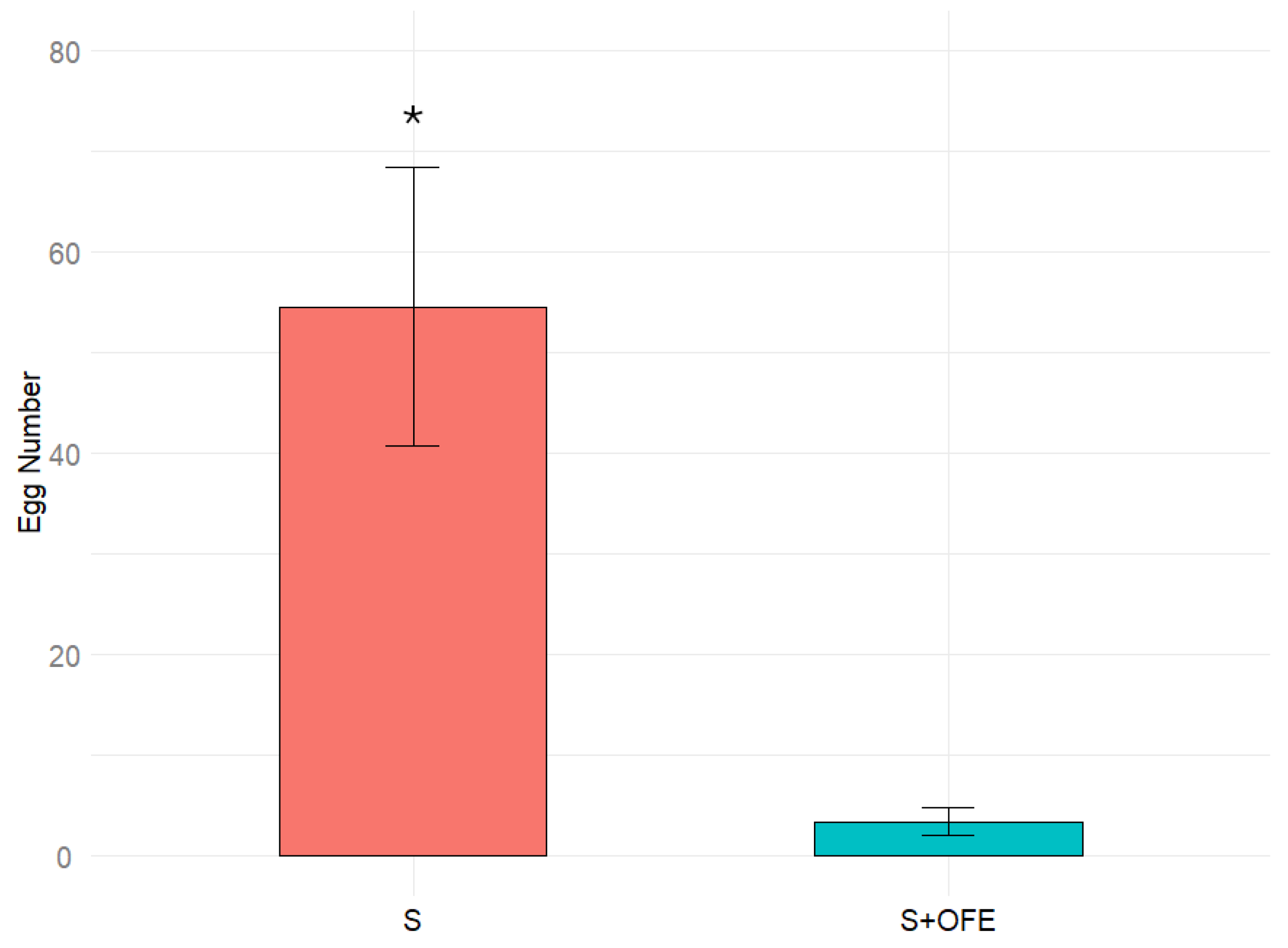
2.3. Oviposition on Sand
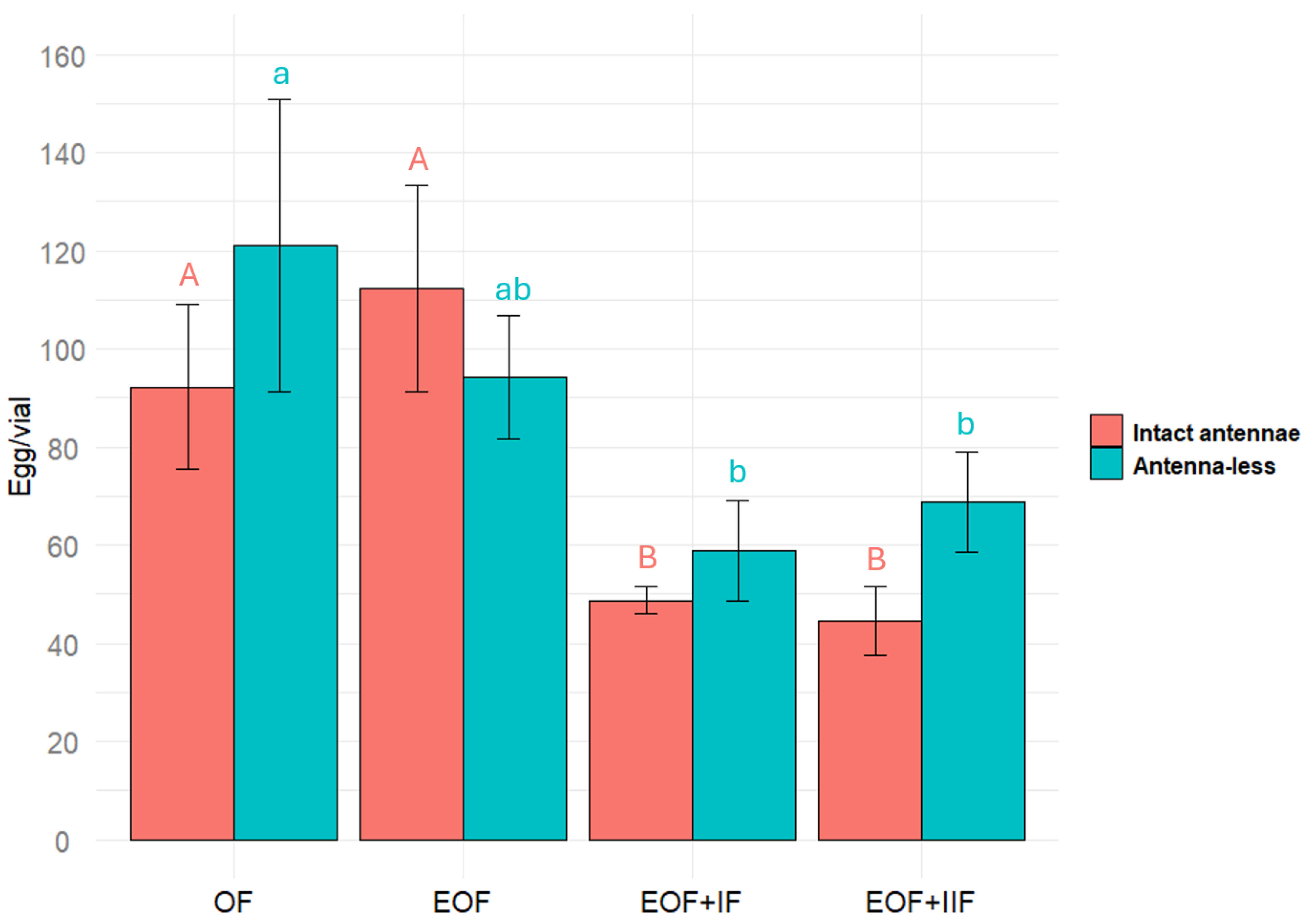
2.4. The Effect of Oat Extract
2.5. The Effect of Oat Extract Fractions
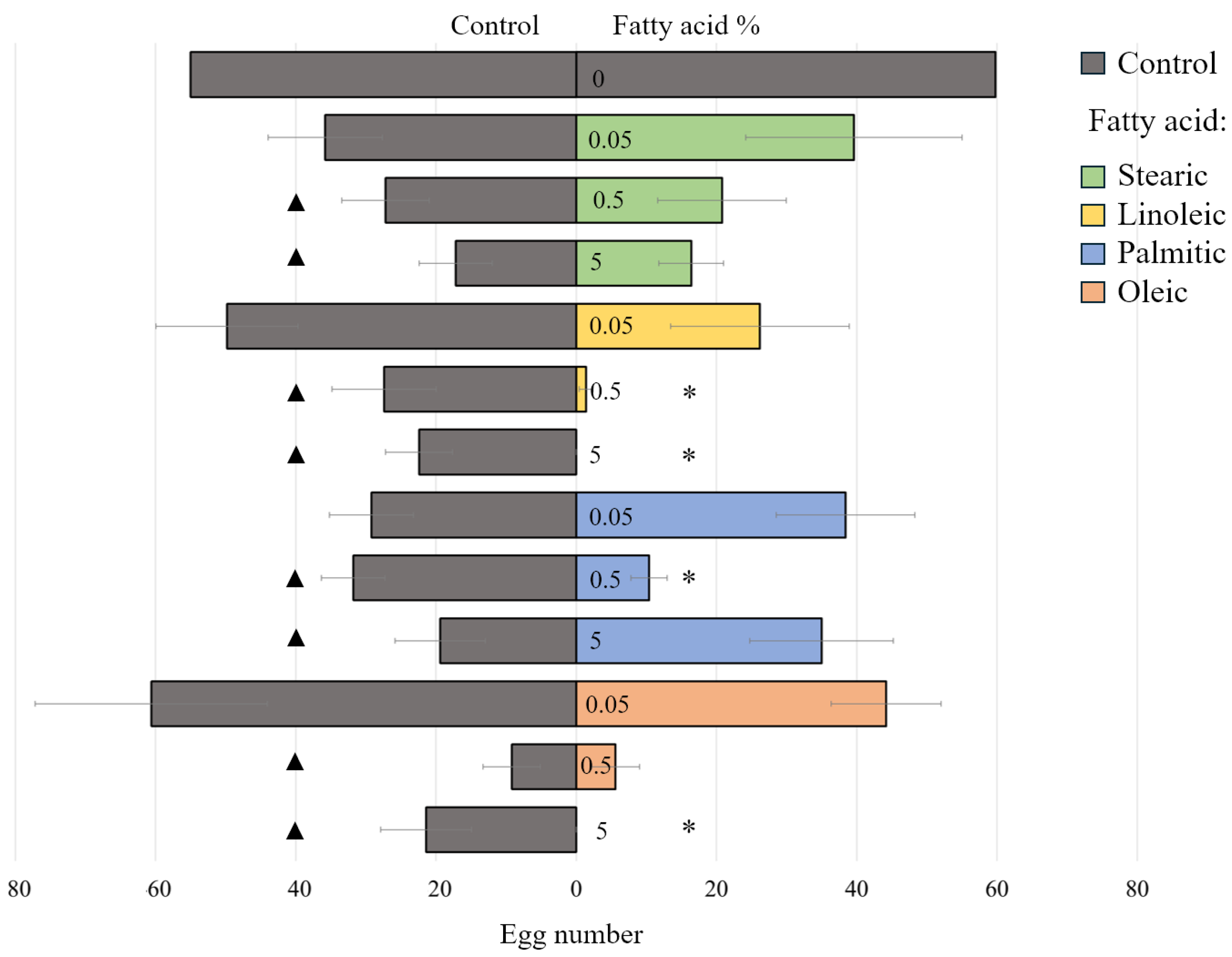
2.6. The Effect of Fatty Acids
3. Discussion
4. Materials and Methods
4.1. Insects
4.2. Oviposition Substrates
4.3. Fatty Acids
4.4. Oviposition Test
4.4.1. Difference Between Substrates
4.4.2. Sand Substrate
4.4.3. Oat Extract
4.4.4. Oat Extract Fractions
4.4.5. Fatty Acid Analysis
4.5. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johnson, S.N.; Rasmann, S. Root-Feeding Insects and Their Interactions with Organisms in the Rhizosphere. Annu. Rev. Entomol. 2015, 60, 517–535. [Google Scholar] [CrossRef] [PubMed]
- Linabury, M.C.; Turley, N.E.; Brudvig, L.A. Insects Remove More Seeds than Mammals in First-Year Prairie Restorations. Restor. Ecol. 2019, 27, 1300–1306. [Google Scholar] [CrossRef]
- Fleck, M.D.; Costa, E.C.; Boscardin, J.; Silva, J.M.D. Damage Caused by Seed-Feeding Insects in Senna corymbosa: A New Host Plant for the Subfamily Bruchinae. Floresta Ambiente 2020, 28, e20200008. [Google Scholar] [CrossRef]
- Karban, R.; Lowenberg, G. Feeding by Seed Bugs and Weevils Enhances Germination of Wild Gossypium Species. Oecologia 1992, 92, 196–200. [Google Scholar] [CrossRef]
- de Andrade, E.K.V.; Rodrigues, R.; Bard, G.D.C.V.; da Silva Pereira, L.; Baptista, K.E.V.; Cavalcanti, T.F.M.; Oliveira, A.E.A.; Souza, T.A.M.; Gomes, V.M. Identification, Biochemical Characterization and Biological Role of Defense Proteins from Common Bean Genotypes Seeds in Response to Callosobruchus maculatus Infestation. J. Stored Prod. Res. 2020, 87, 101580. [Google Scholar] [CrossRef]
- Khan, A.; Gumbs, F.A.; Persad, A. Pesticidal Bioactivity of Ackee (Blighia sapida Koenig) Against Three Stored-Product Insect Pests. Trop. Agric. 2002, 79, 217–223. [Google Scholar]
- Ramos-Elorduy, J.; González, E.A.; Hernández, A.R.; Pino, J.M. Use of Tenebrio molitor (Coleoptera: Tenebrionidae) to Recycle Organic Wastes and as Feed for Broiler Chickens. J. Econ. Entomol. 2002, 95, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Kouřimská, L.; Sabolová, M.; Horčička, P.; Rys, S.; Božik, M. Lipid Content, Fatty Acid Profile, and Nutritional Value of New Oat Cultivars. J. Cereal Sci. 2018, 84, 44–48. [Google Scholar] [CrossRef]
- Deruytter, D.; Coudron, C.L.; Teerlinck, S. Influence of Crate Size, Oviposition Time, Number of Adults and Cannibalism on the Reproduction of Tenebrio molitor. J. Insects Food Feed 2019, 5, 247–255. [Google Scholar] [CrossRef]
- Gerber, G.H.; Sabourin, D.U. Oviposition Site Selection in Tenebrio molitor (Coleoptera: Tenebrionidae). Can. Entomol. 1984, 116, 27–39. [Google Scholar] [CrossRef]
- Tasin, M.; Lucchi, A.; Ioriatti, C.; Mraihi, M.; De Cristofaro, A.; Boger, Z.; Anfora, G. Oviposition Response of the Moth Lobesia botrana to Sensory Cues from a Host Plant. Chem. Senses 2011, 36, 633–639. [Google Scholar] [CrossRef]
- Lei, Q.; Xu, L.; Tang, K.Y.; Yu, J.L.; Chen, X.F.; Wu, S.X.; Wang, J.J.; Jiang, H.B. An Antenna-Enriched Chemosensory Protein Plays Important Roles in the Perception of Host Plant Volatiles in Bactrocera dorsalis (Diptera: Tephritidae). J. Agric. Food Chem. 2024, 72, 2888–2897. [Google Scholar] [CrossRef]
- Fandino, R.A.; Haverkamp, A.; Bisch-Knaden, S.; Zhang, J.; Bucks, S.; Nguyen, T.A.T.; Schröder, K.; Werckenthin, A.; Rybak, J.; Stengl, M.; et al. Mutagenesis of Odorant Coreceptor Orco Fully Disrupts Foraging but Not Oviposition Behaviors in the Hawkmoth Manduca sexta. Proc. Natl. Acad. Sci. USA 2019, 116, 15677–15685. [Google Scholar] [CrossRef] [PubMed]
- Riddick, E.W. Benefits and Limitations of Factitious Prey and Artificial Diets on Life Parameters of Predatory Beetles, Bugs, and Lacewings: A Mini-Review. BioControl 2009, 54, 325–339. [Google Scholar] [CrossRef]
- Sun, Y.X.; Hao, Y.N.; Riddick, E.W.; Liu, T.X. Factitious Prey and Artificial Diets for Predatory Lady Beetles: Current Situation, Obstacles, and Approaches for Improvement: A Review. Biocontrol Sci. Technol. 2017, 27, 601–619. [Google Scholar] [CrossRef]
- Riddick, E.W.; Wu, Z.; Eller, F.J.; Berhow, M.A. Potential of 2,4-Dihydroxybenzoic Acid as an Oviposition Stimulant for Mass-Reared Ladybird Beetles. J. Insect Sci. 2019, 19, 9. [Google Scholar] [CrossRef] [PubMed]
- Sterna, V.; Zute, S.; Brunava, L.; Vicupe, Z. Lipid Composition of Oat Grain Grown in Latvia. In Proceedings of the 9th Baltic Conference on Food Science and Technology “FOODBALT”, Jelgava, Latvia, 8–9 May 2014. [Google Scholar]
- Leonova, S.; Shelenga, T.; Hamberg, M.; Konarev, A.V.; Loskutov, I.; Carlsson, A.S. Analysis of Oil Composition in Cultivars and Wild Species of Oat (Avena sp.). J. Agric. Food Chem. 2008, 56, 7983–7991. [Google Scholar] [CrossRef]
- Sahasrabudhe, M.R. Lipid Composition of Oats (Avena sativa L.). J. Am. Oil Chem. Soc. 1979, 56, 80–84. [Google Scholar] [CrossRef]
- Lidon, F.C.; Daccak, D.; Scotti-Campos, P.; Silva, M.M.; Bagulho, A.S.; Pais, I.; Galhano, C.; Ramalho, J.C.; Moreira, J.; Pessoa, M.F.; et al. An Integrated Chemical and Technological Approach for Assessing Portuguese Wheat Flours Quality and Lengthening Bread Shelf-Life. Emir. J. Food Agric. 2019, 31, 884–894. [Google Scholar] [CrossRef]
- Chew, S.C. Cold-Pressed Rapeseed (Brassica napus) Oil: Chemistry and Functionality. Food Res. Int. 2020, 131, 108997. [Google Scholar] [CrossRef]
- Waheed, A.; Ali, Z.; Akhter, M.; Mahmood, K. Lipid Classes and Fatty Acids Comparison of Oscar and Dunkeld Variety of Canola Seeds. Asian J. Chem. 2013, 25, 4279. [Google Scholar] [CrossRef]
- Apprich, S.; Tirpanalan, Ö.; Hell, J.; Reisinger, M.; Böhmdorfer, S.; Siebenhandl-Ehn, S.; Novalin, S.; Kneifel, W. Wheat Bran-Based Biorefinery 2: Valorization of Products. LWT-Food Sci. Technol. 2014, 56, 222–231. [Google Scholar] [CrossRef]
- Jung, G.W.; Uddin, M.S.; Kwon, K.T.; Chun, B.S. Comparison of Supercritical and Near-Critical Carbon Dioxide Extraction of Carotenoid-Enriched Wheat Bran Oil. Afr. J. Biotechnol. 2010, 9, 7702–7709. [Google Scholar] [CrossRef]
- Roh, G.H.; Meier, L.; Shrestha, B.; Hesler, S.P.; Zhu, J.J.; Kendra, P.E.; Loeb, G.M.; Tay, J.W.; Cha, D.H. A 2-Component Blend of Coconut Oil-Derived Fatty Acids as an Oviposition Deterrent against Drosophila suzukii (Drosophilidae: Diptera). J. Econ. Entomol. 2023, 116, 1671–1678. [Google Scholar] [CrossRef]
- Klüber, P.; Arous, E.; Jerschow, J.; Fraatz, M.; Bakonyi, D.; Rühl, M.; Zorn, H. Fatty Acids Derived from Oviposition Systems Guide Female Black Soldier Flies (Hermetia illucens) Toward Egg Deposition Sites. Insect Sci. 2024, 31, 1231–1248. [Google Scholar] [CrossRef]
- Li, G.; Ishikawa, Y. Leaf Epicuticular Wax Chemicals of the Japanese Knotweed Fallopia japonica as Oviposition Stimulants for Ostrinia latipennis. J. Chem. Ecol. 2006, 32, 595–604. [Google Scholar] [CrossRef]
- Parr, M.J.; Tran, B.; Simmonds, M.; Kite, G.; Credland, P. Influence of Some Fatty Acids on Oviposition by the Bruchid Beetle, Callosobruchus maculatus. J. Chem. Ecol. 1998, 24, 1577–1593. [Google Scholar] [CrossRef]
- Addesso, K.M.; Alborn, H.T.; Bruton, R.R.; McAuslane, H.J. A Multicomponent Marking Pheromone Produced by the Pepper Weevil, Anthonomus eugenii (Coleoptera: Curculionidae). Chemoecology 2021, 31, 247–258. [Google Scholar] [CrossRef]
- Roh, G.H.; Kendra, P.E.; Zhu, J.J.; Roda, A.; Loeb, G.M.; Tay, J.W.; Cha, D.H. Coconut Oil-Derived Five-Component Synthetic Oviposition Deterrent for Oriental Fruit Fly, Bactrocera dorsalis. Pest Manag. Sci. 2023, 79, 3852–3859. [Google Scholar] [CrossRef]
- Movva, V.; Zhu, J.; Roda, A.; Kendra, P.; Yang, X.; Cloonan, K.; Tay, J.W.; Cha, D.H. Deterrence and Behavioral Mode of Coconut Oil-Derived Free Fatty Acids on Zeugodacus cucurbitae Oviposition. Insect Sci. 2024. ahead of print. [Google Scholar] [CrossRef]

| Fatty Acids % | |||||
|---|---|---|---|---|---|
| Substrate | Fat Amount % | Oleic | Palmitic | Linoleic | Stearic |
| Oat [8,17,18,19] | 4–10 | 34.80 | 18.90 | 38.30 | 2.10 |
| Wheat [20] | 2–4 | 11.80 | 21.10 | 58.30 | 3.10 |
| Rapeseed [21,22] | 40–45 | 62.25 | 4.70 | 17.19 | 1.80 |
| Wheat bran [23,24] | 3–4 | 14.60 | 17.70 | 54.60 | 2.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bumbulytė, G.; Auškalnis, A.; Būda, V. Seed Fatty Acids Modify Oviposition of Tenebrio molitor (Coleoptera: Tenebrionidae). Plants 2025, 14, 848. https://doi.org/10.3390/plants14060848
Bumbulytė G, Auškalnis A, Būda V. Seed Fatty Acids Modify Oviposition of Tenebrio molitor (Coleoptera: Tenebrionidae). Plants. 2025; 14(6):848. https://doi.org/10.3390/plants14060848
Chicago/Turabian StyleBumbulytė, Gabrielė, Arijus Auškalnis, and Vincas Būda. 2025. "Seed Fatty Acids Modify Oviposition of Tenebrio molitor (Coleoptera: Tenebrionidae)" Plants 14, no. 6: 848. https://doi.org/10.3390/plants14060848
APA StyleBumbulytė, G., Auškalnis, A., & Būda, V. (2025). Seed Fatty Acids Modify Oviposition of Tenebrio molitor (Coleoptera: Tenebrionidae). Plants, 14(6), 848. https://doi.org/10.3390/plants14060848






