Do Faster-Growing Holoparasitic Plant Species Exhibit Broader Niches and Wider Global Distributions?
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Set Up
2.2. Growth Rate Data and Experimental Analysis
2.3. Global Occurrence Data
2.4. Niche Breadth Data
2.5. Global Distribution Area Data of Species
2.6. Statistical Analysis Methods
3. Results
3.1. Parasitism Experiment Results
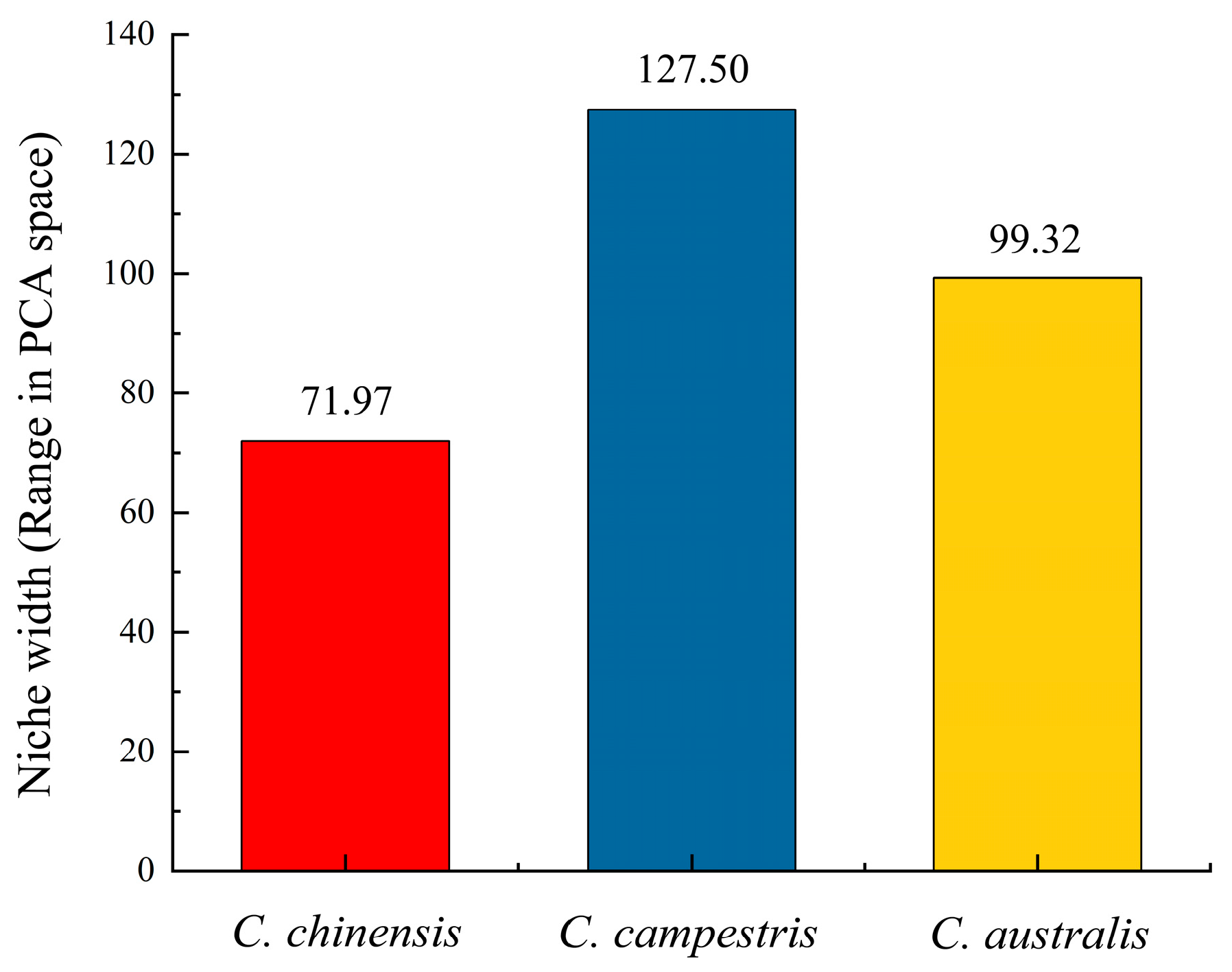
3.2. Niche Breadth and Global Distribution Area
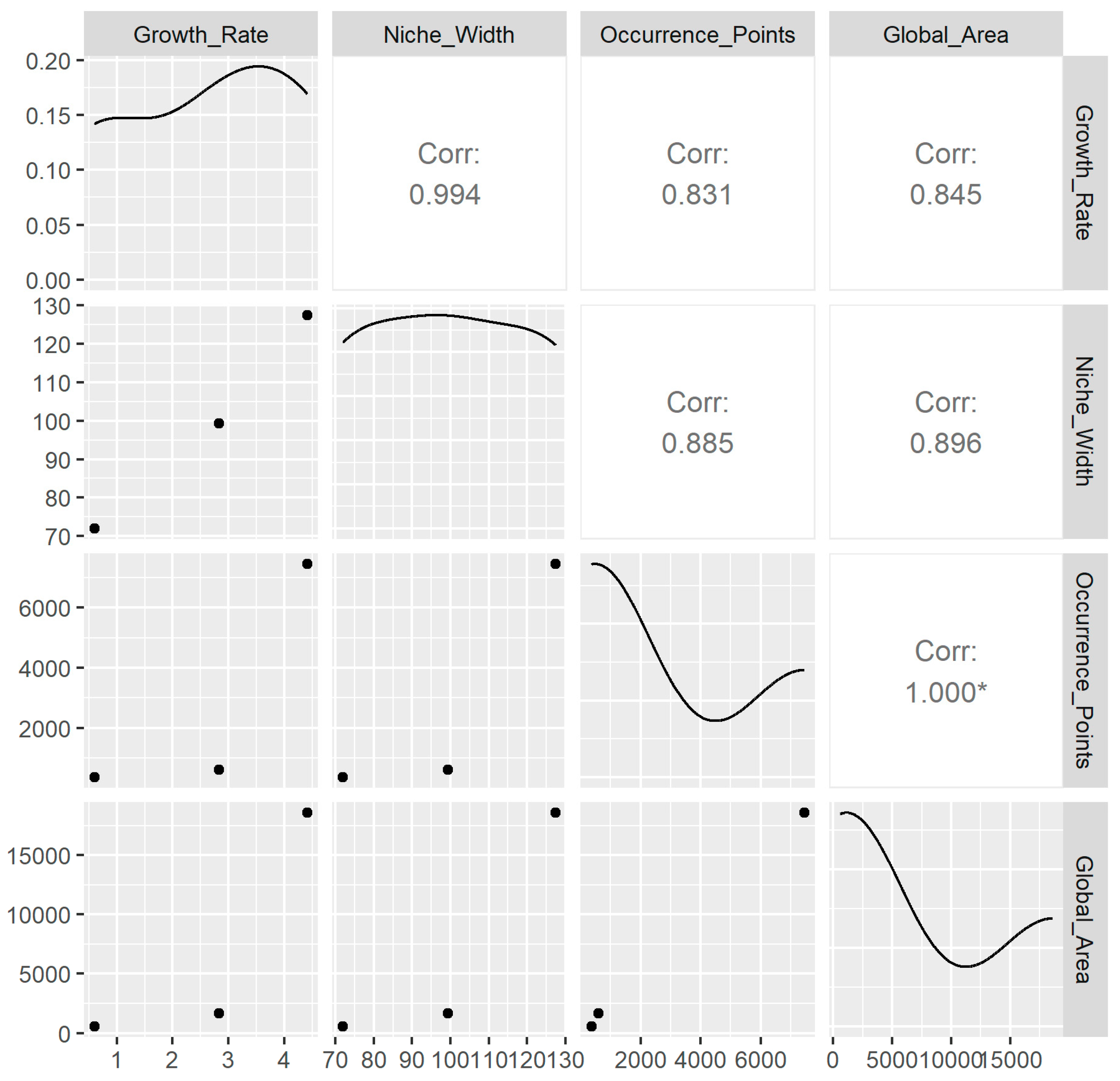
3.3. The Relationships Between the Variables
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilson, R.J.; Thomas, C.D.; Fox, R.; Roy, D.B.; Kunin, W.E. Spatial patterns in species distributions reveal biodiversity change. Nature 2004, 432, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Biancolini, D.; Pacifici, M.; Falaschi, M.; Bellard, C.; Blackburn, T.M.; Ficetola, G.F.; Rondinini, C. Global Distribution of Alien Mammals Under Climate Change. Glob. Change Biol. 2024, 30, e17560. [Google Scholar] [CrossRef]
- Poulin, R.; Morand, S. Parasite Biodiversity; Smithsonian Books: Washington, DC, USA, 2004. [Google Scholar]
- Hechinger, R.F.; Lafferty, K.D.; Dobson, A.P.; Brown, J.H.; Kuris, A.M. A Common Scaling Rule for Abundance, Energetics, and Production of Parasitic and Free-Living Species. Science 2011, 333, 445–448. [Google Scholar] [CrossRef]
- Price, P.W. General concepts on the evolutionary biology of parasites. Evolution 1977, 31, 405. [Google Scholar] [CrossRef] [PubMed]
- Nickrent, D.L.; Duff, R.J.; Colwell, A.E.; Wolfe, A.D.; Young, N.D.; Steiner, K.E.; de Pamphilis, C.W. Molecular Phylo-genetic and Evolutionary Studies of Parasitic Plants. In Molecular Sytematics of Plants II-DNA Sequencing; Soltis, D.E., Soltis, P.S., Doyle, J.J., Eds.; Kluwer Academic Publisher: Boston, MA, USA, 1998; pp. 211–241. [Google Scholar]
- Zhang, G.; Li, Q.; Sun, S. Diversity and distribution of parasitic angiosperms in China. Ecol. Evol. 2018, 8, 4378–4386. [Google Scholar] [CrossRef]
- Poulin, R.; Krasnov, B.R.; Mouillot, D.; Thieltges, D.W. The comparative ecology and biogeography of parasites. Philos. Trans. R. Soc. Lond. 2011, 366, 2379–2390. [Google Scholar] [CrossRef]
- Yuncker, T.G. The genus Cuscuta. Memoirs of the Torrey Botanical Club 1932, 18, 113–331. [Google Scholar]
- Stefanović, S.; Kuzmina, M.; Costea, M. Delimitation of major lineages within Cuscuta subgenus Grammica (dodders; Convolvulaceae) using plastid and nuclear DNA sequences. Am. J. Bot. 2007, 94, 568–589. [Google Scholar] [CrossRef]
- Lanini, W.T.; Kogan, M. Biology and management of Cuscuta in crops. Int. J. Agric. Nat. Resour. 2005, 32, 127–141. [Google Scholar] [CrossRef]
- Baráth, K. Effect of species environment on host preference of Cuscuta campestris. Plant Ecol. 2021, 222, 1023–1032. [Google Scholar] [CrossRef]
- Ren, Z.; Zagortchev, L.; Ma, J.; Yan, M.; Li, J. Predicting the potential distribution of the parasitic Cuscuta chinensis under global warming. BMC Ecol. 2020, 20, 1–14. [Google Scholar] [CrossRef]
- Yuan, Y.-G.; Yuan, Y.-G.; Gao, F.-L.; Gao, F.-L.; Yu, F.-H.; Yu, F.-H.; Li, J.-M.; Li, J.-M.; Li, M.-H.; Li, M.-H. Resource availability and parasitism intensity influence the response of soybean to the parasitic plant Cuscuta australis. Front. Plant Sci. 2023, 14, 1177154. [Google Scholar] [CrossRef]
- Masanga, J.; Mwangi, B.N.; Kibet, W.; Sagero, P.; Wamalwa, M.; Oduor, R.; Ngugi, M.; Alakonya, A.; Ojola, P.; Bellis, E.S.; et al. Physiological and ecological warnings that dodders pose an exigent threat to farmlands in Eastern Africa. Plant Physiol. 2021, 185, 1457–1467. [Google Scholar] [CrossRef]
- Erdogan, P. Parasitic Plants in Agriculture and Management; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Yang, B.-F.; Du, L.-S.; Li, J.-M. Effects of Cuscuta australis parasitism on the growth, reproduction and defense of Solidago canadensis. Chin. J. Appl. Ecol. 2015, 26, 3309–3314. [Google Scholar]
- Mills, P.B.; Hossie, T.J.; Murray, D.L. Niche determinants in a salamander complex: Does hybridism or reproductive parasitism explain patterns of distribution? Ecosphere 2020, 11, e03265. [Google Scholar] [CrossRef]
- Thuiller, W.; Albert, C.; Araujo, M.B.; Berry, P.M.; Cabeza, M.; Guisan, A.; Hickler, T.; Midgley, G.F.; Paterson, J.; Schurr, F.M.; et al. Predicting global change impacts on plant species’ distributions: Future challenges. Perspect. Plant Ecol. Evol. Syst. 2008, 9, 137–152. [Google Scholar] [CrossRef]
- Yan, X.L.; Wang, Z.H.; Ma, J.S. The Checklist of the Naturalized Plants in China; Shanghai Scientific and Technical Publishers: Shanghai, China, 2019. [Google Scholar]
- Hao, Q.; Ma, J.-S. Invasive alien plants in China: An update. Plant Divers. 2022, 45, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Costea, M.; Spence, I.; Stefanović, S.. Systematics of Cuscuta chinensis species complex (subgenus Grammica, Convolvu-laceae): Evidence for long-distance dispersal and one new species. Org. Divers. Evol. 2011, 11, 373–386. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Satistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 8 October 2024).
- Dixon, P. VEGAN, a package of R functions for community ecology. J. Veg. Sci. 2003, 14, 927–930. [Google Scholar] [CrossRef]
- Kambach, S.; Lenoir, J.; Decocq, G.; Welk, E.; Seidler, G.; Dullinger, S.; Gégout, J.; Guisan, A.; Pauli, H.; Svenning, J.; et al. Of niches and distributions: Range size increases with niche breadth both globally and regionally but regional estimates poorly relate to global estimates. Ecography 2018, 42, 467–477. [Google Scholar] [CrossRef]
- Cai, Q.; Welk, E.; Ji, C.; Fang, W.; Sabatini, F.M.; Zhu, J.; Zhu, J.; Tang, Z.; Attorre, F.; Campos, J.A.; et al. The relationship between niche breadth and range size of beech (Fagus) species worldwide. J. Biogeogr. 2021, 48, 1240–1253. [Google Scholar] [CrossRef]
- Liu, M.; Li, X.; Yang, L.; Chen, K.; Shama, Z.; Jiang, X.; Yang, J.; Zhao, G.; Huang, Y. Prediction of the Potential Distribution and Conservation Strategies of the Endangered Plant Tapiscia sinensis. Forests 2024, 15, 1677. [Google Scholar] [CrossRef]
- Environmental Systems Research Institute. ArcGIS 10.2; Environmental Systems Research Institute: Redlands, CA, USA, 2013; Available online: https://www.esri.com (accessed on 5 November 2024).
- Vijayvargiya, A. One-way analysis of variance. J. Valid. Technol. 2009, 15, 62–63. [Google Scholar]
- Ahmed, I.; Jena, A.K. Using non-parametric Kruskal-Wallis H test for assessing mean differences in the opinion of environmental sustainability. Int. J. Geogr. Geol. Environ. 2023, 5, 74–80. [Google Scholar] [CrossRef]
- Gerald, B. A brief review of independent, dependent and one sample t-test. Int. J. Appl. Math. Theor. Phys. 2018, 4, 50–54. [Google Scholar] [CrossRef]
- Carlson, C.J.; Dallas, T.A.; Alexander, L.W.; Phelan, A.L.; Phillips, A.J. What would it take to describe the global diversity of parasites? Proc. R. Soc. B 2020, 287, 20201841. [Google Scholar] [CrossRef]
- Pappalardo, P.; Morales-Castilla, I.; Park, A.W.; Huang, S.; Schmidt, J.P.; Stephens, P.R. Comparing methods for mapping global parasite diversity. Glob. Ecol. Biogeogr. 2019, 29, 182–193. [Google Scholar] [CrossRef]
- Hautier, Y.; Hector, A.; Vojtech, E.; Purves, D.; Turnbull, L.A. Modelling the growth of parasitic plants. J. Ecol. 2010, 98, 857–866. [Google Scholar] [CrossRef]
- Li, J.; Jin, Z.; Song, W. Do Native Parasitic Plants Cause More Damage to Exotic Invasive Hosts Than Native Non-Invasive Hosts? An Implication for Biocontrol. PLoS ONE 2012, 7, e34577. [Google Scholar] [CrossRef][Green Version]
- Prider, J.; Watling, J.; Facelli, J.M. Impacts of a native parasitic plant on an introduced and a native host species: Implications for the control of an invasive weed. Ann. Bot. 2009, 103, 107–115. [Google Scholar] [CrossRef]
- Hoberg, E.P.; Brooks, D.R. A macroevolutionary mosaic: Episodic host-switching, geographical colonization and di-versification in complex host-parasite systems. J. Biogeogr. 2008, 35, 1533–1550. [Google Scholar] [CrossRef]
- Cebrián-Camisón, S.; la Puente, J.M.-D.; Ruiz-López, M.J.; Figuerola, J. Do specialist and generalist parasites differ in their prevalence and intensity of infection? A test of the niche breadth and trade-off hypotheses. Int. J. Parasitol. 2024, 55, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Sexton, J.P.; Montiel, J.; Shay, J.E.; Stephens, M.R.; Slatyer, R.A. Evolution of ecological niche breadth. Annu. Rev. Ecol. Evol. Syst. 2017, 48, 183–206. [Google Scholar] [CrossRef]
- Slatyer, R.A.; Hirst, M.; Sexton, J.P. Niche breadth predicts geographical range size: A general ecological pattern. Ecol. Lett. 2013, 16, 1104–1114. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.H. On the Relationship between Abundance and Distribution of Species. Am. Nat. 1984, 124, 255–279. [Google Scholar] [CrossRef]

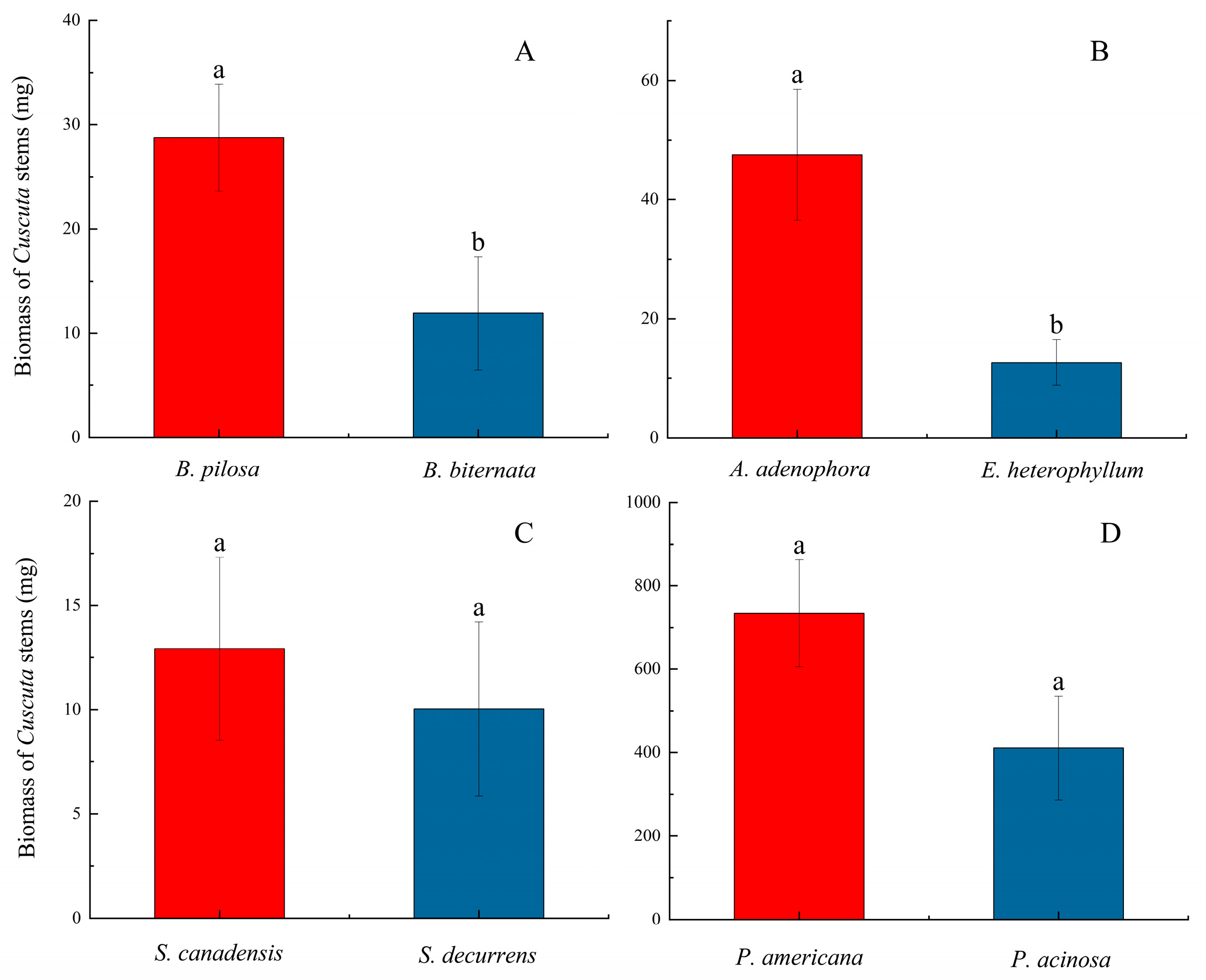
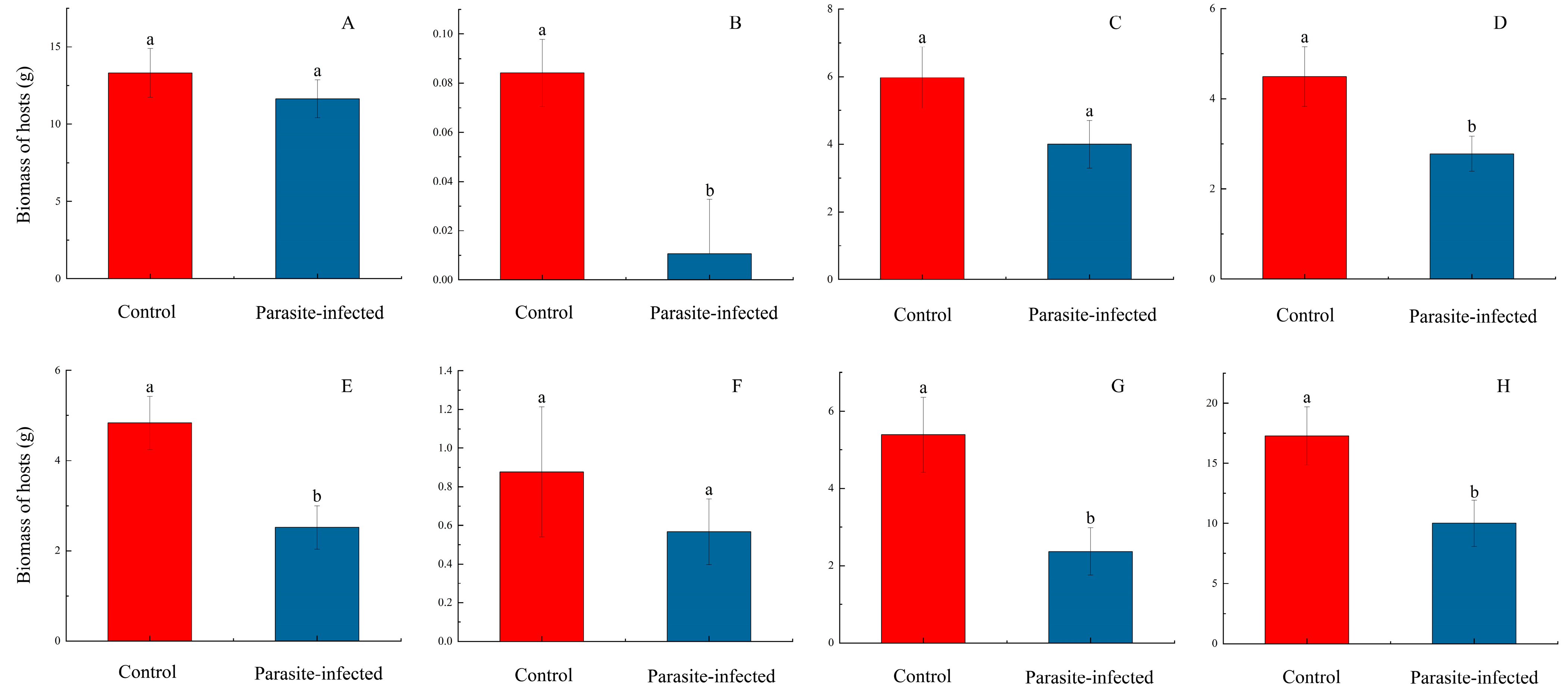
| Family | Native Host | Non-Native American Host |
|---|---|---|
| Asteraceae | Bidens biternata (Lour.) Merr. et Sherff | Bidens pilosa L. |
| Asteraceae | Eupatorium heterophyllum DC. | Ageratina adenophora (Sprengel) R. M. King & H. Robinson |
| Asteraceae | Solidago decurrens Lour. | Solidago canadensis L. |
| Phytolaccaceae | Phytolacca acinosa Roxb. | Phytolacca americana |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Hu, J. Do Faster-Growing Holoparasitic Plant Species Exhibit Broader Niches and Wider Global Distributions? Plants 2025, 14, 831. https://doi.org/10.3390/plants14060831
Zhang Q, Hu J. Do Faster-Growing Holoparasitic Plant Species Exhibit Broader Niches and Wider Global Distributions? Plants. 2025; 14(6):831. https://doi.org/10.3390/plants14060831
Chicago/Turabian StyleZhang, Quanzhong, and Jinming Hu. 2025. "Do Faster-Growing Holoparasitic Plant Species Exhibit Broader Niches and Wider Global Distributions?" Plants 14, no. 6: 831. https://doi.org/10.3390/plants14060831
APA StyleZhang, Q., & Hu, J. (2025). Do Faster-Growing Holoparasitic Plant Species Exhibit Broader Niches and Wider Global Distributions? Plants, 14(6), 831. https://doi.org/10.3390/plants14060831






