Effect of Schinus areira L. Essential Oil on Attraction, Reproductive Behavior, and Survival of Ceratitis capitata Wiedemann
Abstract
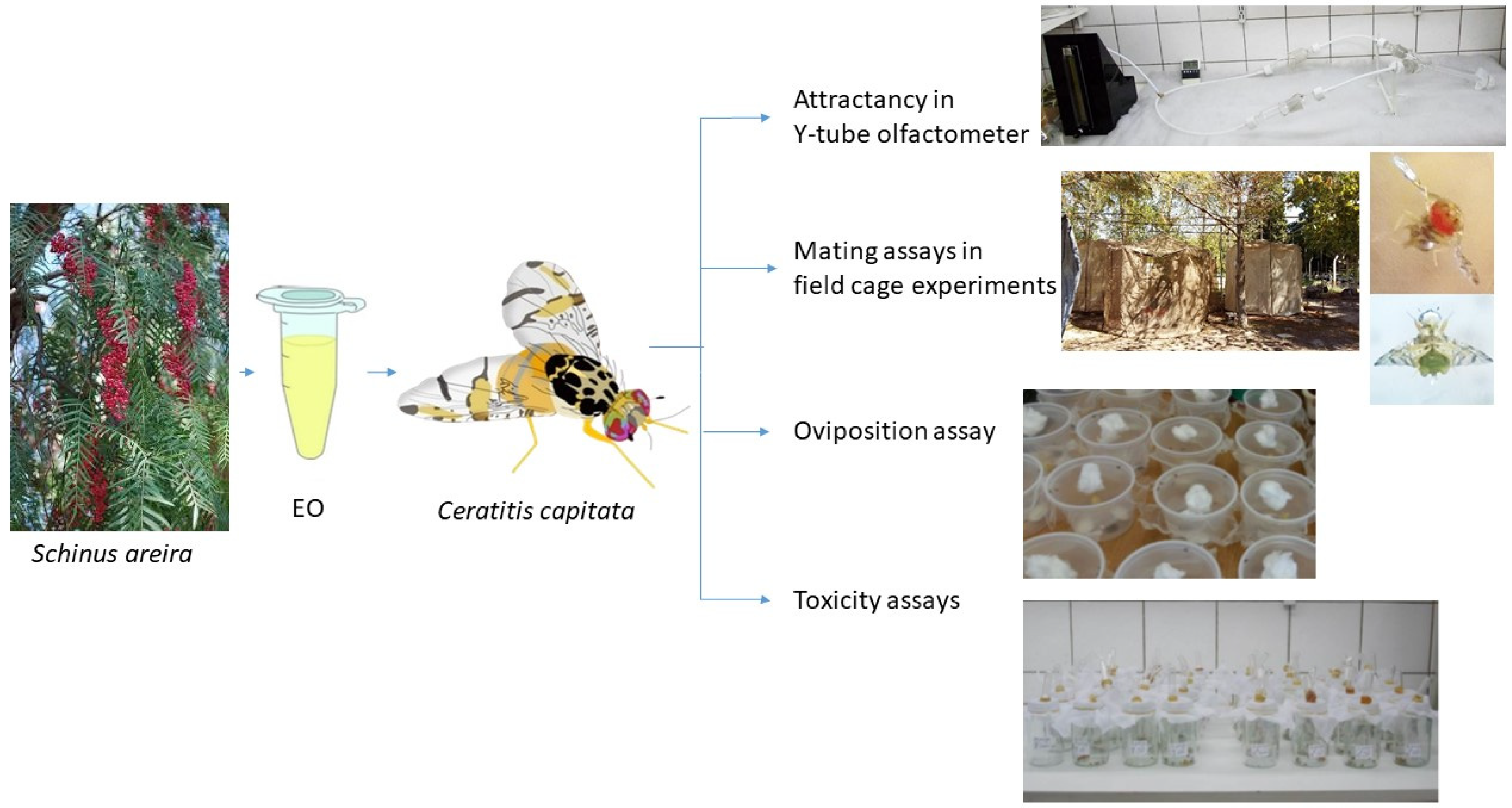
1. Introduction
2. Results
2.1. Composition of the Essential Oil of Schinus areira
2.2. Behavioral Responses
2.3. Mating
2.3.1. Mating Success
2.3.2. Latency to Mate
2.3.3. Copula Duration
2.3.4. Mating Location
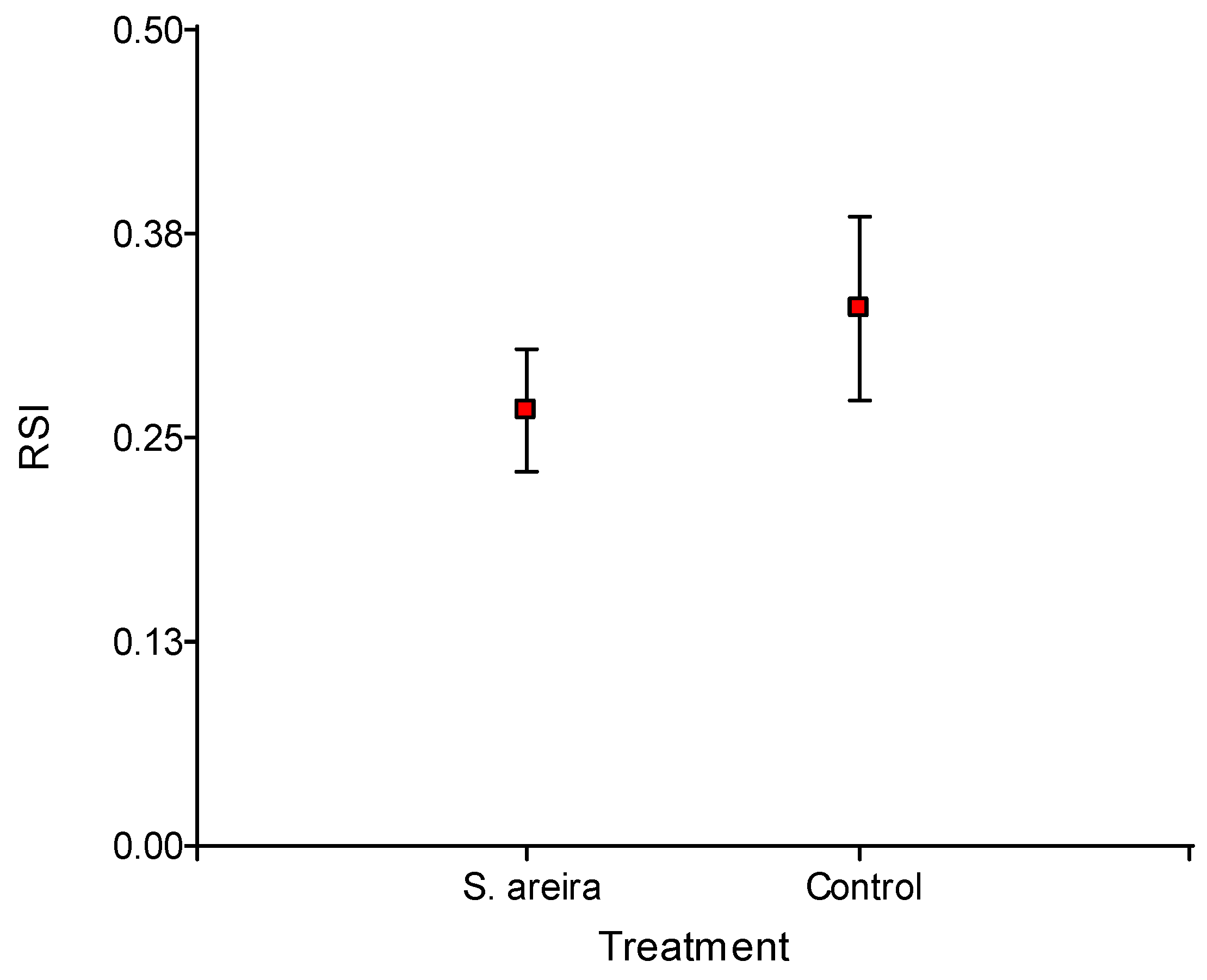
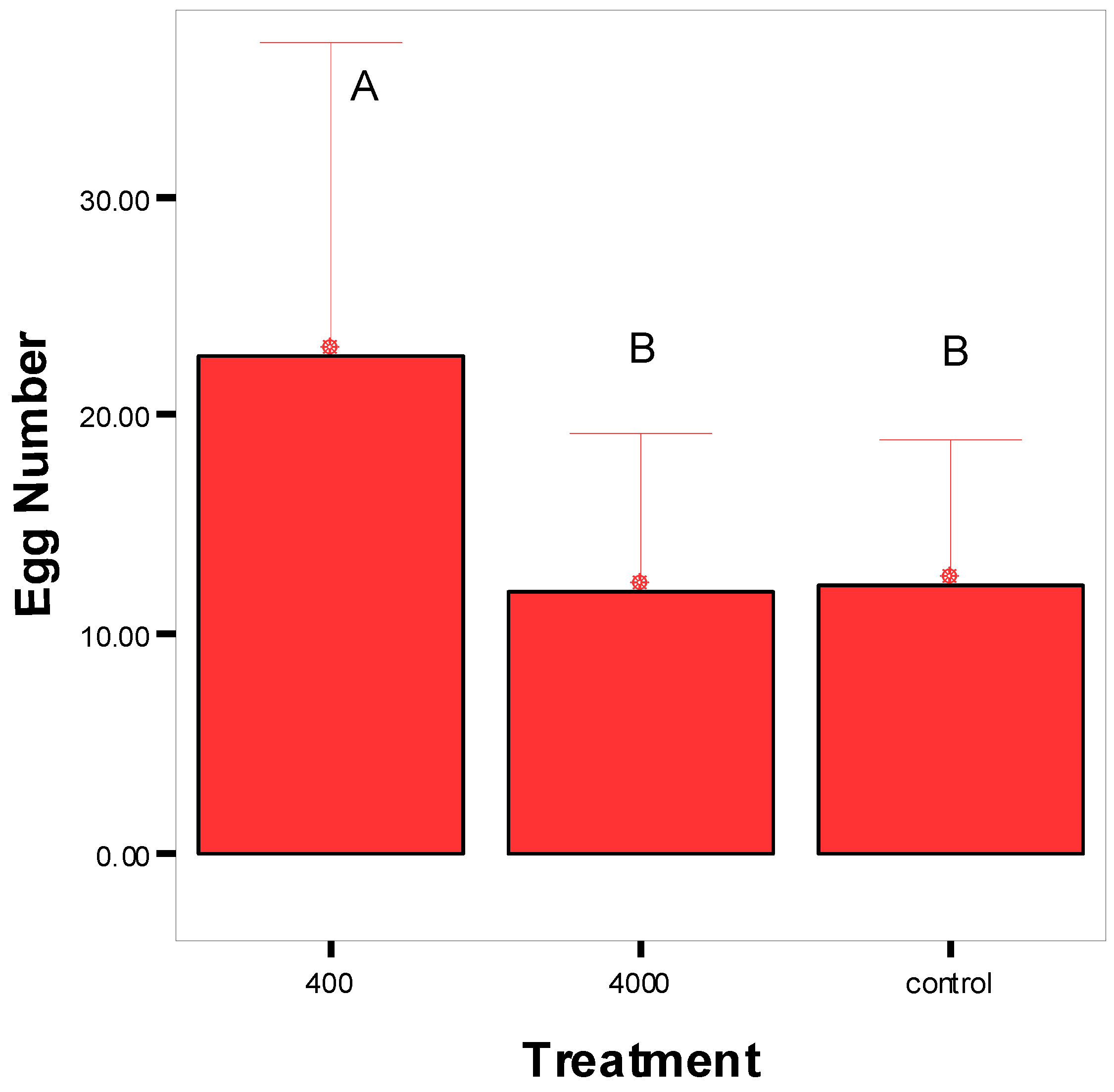
2.4. Oviposition on Superficially Treated Grapes
2.5. Survival Response
3. Materials and Methods
3.1. Insects
3.2. EO Production and Analysis
3.3. Behavioral Responses
3.4. Mating Assay of Sterile Males
3.5. Oviposition on Superficially Treated Grapes
3.6. Survival Response
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EOs | Essential oils |
| RSI | Ratio sterility index |
| SIT | Sterile insect technique |
| IPM | Integrated pest management |
| GC–MS | Gas chromatography–mass spectrometry |
| tsl | Thermo-sensitive lethal |
Appendix A
| RI | Compound | Area (%) |
|---|---|---|
| 839 | 2,4-Dimethyl-1-heptene | tr |
| 927 | Tricyclene | 1.2 |
| 939 | α-Pinene | 5.6 |
| 956 | Camphene | 9.2 |
| 977 | Sabinene | 0.9 |
| 984 | β-Pinene | 3.7 |
| 992 | β-Myrcene | 4.4 |
| 1014 | α-Phellandrene | 18.8 |
| 1031 | p-Cymene | 4.8 |
| 1037 | Limonene | 9.6 |
| 1039 | β-Phellandrene | 7.3 |
| 1063 | ɣ-Terpinene | tr |
| 1090 | Terpinolene | 0.1 |
| 1108 | Nonanal | 0.2 |
| 1132 | 43 (99.9); 93 (48.8); 71 (43.3); 69 (42.0); 94 (38.1); 95 (36.0); 111 (35.8); 79 (35.2); 139 (34.7); 55 (34.4). | tr |
| 1151 | 43 (99.9); 55 (75.0); 41 (72.4); 92 (68.8); 91 (63.7); 69 (61.1); 93 (51.4); 81 (48.6); 83 (46.4); 79 (42.1) | tr |
| 1183 | 95 (99.9); 110 (36.2); 41 (24.2); 109 (23.7); 81 (21.2); 67 (18.5); 39 (12.6); 55 (11.5); 108 (11.2); 53 (93) | tr |
| 1189 | 1-Terpinen-4-ol | 0.3 |
| 1197 | Cryptone | tr |
| 1203 | α-Terpineol | tr |
| 1213 | cis-Sabinol | tr |
| 1254 | 43 (99.9); 97 (58.3); 107 (42.8); 41 (31.8); 55 (23.0); 39 (16.7); 79 (15.5); 69 (14.3); 109 (14.2); 53 (13.9) | 0.4 |
| 1290 | Bornyl acetate | 1.9 |
| 1338 | δ-Elemene | 0.1 |
| 1353 | α-Cubebene | tr |
| 1383 | α-Copaene | 0.1 |
| 1396 | β-Elemene | 0.9 |
| 1417 | α-Gurjunene | 0.6 |
| 1432 | β-Caryophyllene | 1.9 |
| 1450 | Aromadendrene | tr |
| 1459 | (cis)-Muurola-3,5-diene | 0.1 |
| 1468 | α-Humulene | 0.6 |
| 1473 | (cis)-Muurola-4(14),5-diene | 0.3 |
| 1481 | cadina-1(6),4-diene(trans) | 0.1 |
| 1484 | γ-Muurolene | 0.4 |
| 1493 | (trans)-Muurola-4(14),5-diene | 1.4 |
| 1502 | Viridiflorene | 0.2 |
| 1507 | Bicyclogermacrene | 3.7 |
| 1524 | γ-Cadinene | 0.8 |
| 1528 | δ-Amorphene | 4.4 |
| 1532 | (trans)Calamenene | 0.7 |
| 1543 | (trans)Cadina-1(2),4-diene | 0.2 |
| 1547 | α-Cadinene | 0.2 |
| 1552 | α-Calacorene | tr |
| 1559 | Elemol | 2.2 |
| 1586 | Ledol | 0.3 |
| 1590 | Germacrene D-4-ol | 1.1 |
| 1592 | Spathulenol | 0.9 |
| 1617 | Viridiflorol | 0.5 |
| 1622 | 97 (99.9); 41 (97.3); 81 (79.6); 55 (77.7); 79 (75.1); 69 (73.6); 107 (63.1); 67 (54.4); 93 (53.5); 177 (46.7). | 2.5 |
| 1647 | Eudesmol (10-epi-gamma) | 0.5 |
| 1661 | Cadinol (epi-alpha) | 0.5 |
| 1664 | Muurolol (epi-alpha) | 1.3 |
| 1671 | α-Cadinol | 1.2 |
| 1674 | α-Eudesmol | 0.8 |
| 1715 | 84 (99.9); 41 (66); 81 (65.7); 55 (50.8); 83 (44); 109 (43.2); 67 (41.1); 69 (39.9); 121 (29.9); 93 (29.2). | 0.6 |
References
- Liu, H.; Zhang, C.; Hou, B.-H.; Ou-Yang, G.-C.; Ma, J. Interspecific Competition Between Ceratitis capitata and Two Bactrocera spp. (Diptera: Tephritidae) Evaluated via Adult Behavioral Interference Under Laboratory Conditions. J. Econ. Entomol. 2017, 110, 1145–1155. [Google Scholar] [CrossRef] [PubMed]
- Guillén, D.; Sánchez, R. Expansion of the National Fruit Fly Control Programme in Argentina. In Area-Wide Control of Insect Pests; Vreysen, M.J.B., Robinson, A.S., Hendrichs, J., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 653–660. ISBN 978-1-4020-6058-8. [Google Scholar]
- Liquido, N.J.; McQuate, G.T.; Suiter, K.A.; Norrbom, A.L.; Yee, W.L.; Chang, C.L. Compendium of Fruit Fly Host Plant Information. In Area-Wide Management of Fruit Fly Pests; CRC Press: Boca Raton, FL, USA, 2019; pp. 363–368. ISBN 978-0-429-35573-8. [Google Scholar]
- Broughton, S.; De Lima, C.P.F. Field Evaluation of Female Attractants for Monitoring Ceratitis capitata (Diptera: Tephritidae) Under a Range of Climatic Conditions and Population Levels in Western Australia. J. Econ. Entomol. 2002, 95, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Piñero, J.C.; Souder, S.K.; Smith, T.R.; Fox, A.J.; Vargas, R.I. Ammonium Acetate Enhances the Attractiveness of a Variety of Protein-Based Baits to Female Ceratitis capitata (Diptera: Tephritidae). J. Econ. Entomol. 2015, 108, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Catalá-Oltra, M.; Llácer, E.; Dembilio, O.; Pla, I.; Urbaneja, A.; Pérez-Hedo, M. Remating in Ceratitis capitata Sterile Males: Implications in Sterile Insect Technique Programmes. J. Appl. Entomol. 2021, 145, 958–965. [Google Scholar] [CrossRef]
- Hendrichs, J.; Robinson, A.S.; Cayol, J.P.; Enkerlin, W. Medfly Areawide Sterile Insect Technique Programmes for Prevention, Suppression or Eradication: The Importance of Mating Behavior Studies. Fla. Entomol. 2002, 85, 1–13. [Google Scholar] [CrossRef]
- Magaña, C.; Hernández-Crespo, P.; Ortego, F.; Castañera, P. Resistance to Malathion in Field Populations of Ceratitis capitata. J. Econ. Entomol. 2007, 100, 1836–1843. [Google Scholar] [CrossRef]
- Raga, A.; Sato, M.E. Effect of Spinosad Bait against Ceratitis capitata (Wied.) and Anastrepha fraterculus (Wied.) (Diptera: Tephritidae) in Laboratory. Neotrop. Entomol. 2005, 34, 815–822. [Google Scholar] [CrossRef]
- Boulahia Kheder, S.; Trabelsi, I.; Aouadi, N. From Chemicals to IPM Against the Mediterranean Fruit Fly Ceratitis capitata (Diptera, Tephritidae). In Integrated Pest Management and Pest Control—Current and Future Tactics; Soloneski, S., Ed.; InTechOpen: Rijeka, Croatia, 2012; ISBN 978-953-51-0050-8. [Google Scholar]
- Regnault-Roger, C.; Vincent, C.; Arnason, J.T. Essential Oils in Insect Control: Low-Risk Products in a High-Stakes World. Annu. Rev. Entomol. 2012, 57, 405–424. [Google Scholar] [CrossRef]
- Klassen, W.; Vreysen, M.J.B. Area-Wide Integrated Pest Management and the Sterile Insect Technique. In Sterile Insect Technique –Principles and Practice in Area-Wide Integrated Pest Management; CRC Press: Boca Raton, FL, USA, 2021; pp. 75–112. [Google Scholar]
- Katsoyannos, B.I.; Kouloussis, N.A.; Papadopoulos, N.T. Response of Ceratitis capitata to Citrus Chemicals under Semi-natural Conditions. Entomol. Exp. Appl. 1997, 82, 181–188. [Google Scholar] [CrossRef]
- Shelly, T.E.; Pahio, E. Relative Attractiveness of Enriched Ginger Root Oil and Trimedlure to Male Mediterranean Fruit Flies (Diptera: Tephritidae). Fla. Entomol. 2002, 85, 545–551. [Google Scholar] [CrossRef]
- Shelly, T.E.; Mcinnis, D.O.; Pahio, E.; Edu, J. Aromatherapy in the Mediterranean Fruit Fly (Diptera: Tephritidae): Sterile Males Exposed to Ginger Root Oil in Prerelease Storage Boxes Display Increased Mating Competitiveness in Field-Cage Trials. J. Econ. Entomol. 2004, 97, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Shelly, T.E. Aromatherapy and Medfly SIT. Fruit Flies of Economic Importance: From Basic to Applied Knowledge. In Proceedings of the 7th International Symposium on Fruit Flies of Economic Importance, Salvador, Brazil, 10–15 September 2006; pp. 59–69. [Google Scholar]
- Shelly, T.E.; Epsky, N.D. Exposure to Tea Tree Oil Enhances the Mating Success of Male Mediterranean Fruit Flies (Diptera: Tephritidae). Fla. Entomol 2015, 98, 1127–1133. [Google Scholar] [CrossRef][Green Version]
- Papachristos, D.P.; Kimbaris, A.C.; Papadopoulos, N.T.; Polissiou, M.G. Toxicity of Citrus Essential Oils against Ceratitis capitata (Diptera: Tephritidae) Larvae. Ann. Appl. Biol. 2009, 155, 381–389. [Google Scholar] [CrossRef]
- Alves, T.J.S.; Murcia, A.; Wanumen, A.C.; Wanderley-Teixeira, V.; Teixeira, Á.A.C.; Ortiz, A.; Medina, P. Composition and Toxicity of a Mixture of Essential Oils Against Mediterranean Fruit Fly, Ceratitis capitata (Wiedemann) (Diptera: Tephritidae). J. Econ. Entomol. 2019, 112, 164–172. [Google Scholar] [CrossRef]
- Benelli, G.; Flamini, G.; Canale, A.; Cioni, P.L.; Conti, B. Toxicity of Some Essential Oil Formulations against the Mediterranean Fruit Fly Ceratitis capitata (Wiedemann) (Diptera Tephritidae). Crop Prot. 2012, 42, 223–229. [Google Scholar] [CrossRef]
- López, S.B.; López, M.L.; Aragón, L.M.; Tereschuk, M.L.; Slanis, A.C.; Feresin, G.E.; Zygadlo, J.A.; Tapia, A.A. Composition and Anti-Insect Activity of Essential Oils from Tagetes L. Species (Asteraceae, Helenieae) on Ceratitis capitata Wiedemann and Triatoma infestans Klug. J. Agric. Food Chem. 2011, 59, 5286–5292. [Google Scholar] [CrossRef]
- Miguel, M.G.; Almeida, M.L.; Gonçalves, M.A.; Figueiredo, A.C.; Barroso, J.G.; Pedro, L.M. Toxic Effects of Three Essential Oils on Ceratitis capitata. J. Essent. Oil-Bear. Plants 2010, 13, 191–199. [Google Scholar] [CrossRef]
- Murray, A.P.; Murray, M.G. Phytochemistry, Traditional Uses and Bioactivity of the Medicinal Plant Schinus areira L. (Anacardiaceae): A Review. Nat. Prod. J. 2017, 7, 97–103. [Google Scholar] [CrossRef]
- Descamps, L.R.; Chopa, C.S.; Ferrero, A.A. Activity of Schinus areira (Anacardiaceae) Essential Oils against the Grain Storage Pest Tribolium castaneum. Nat. Prod. Commun. 2011, 6, 887–891. [Google Scholar] [CrossRef]
- Tapia Mattar, V.; Borioni, J.L.; Hollmann, A.; Rodriguez, S.A. Insecticidal Activity of the Essential Oil of Schinus areira against Rhipibruchus picturatus (F.) (Coleoptera: Bruchinae), and Its Inhibitory Effects on Acetylcholinesterase. Pestic. Biochem. Physiol. 2022, 185, 105134. [Google Scholar] [CrossRef]
- Machado, C.D.; Raman, V.; Rehman, J.U.; Maia, B.H.L.N.S.; Meneghetti, E.K.; Almeida, V.P.; Silva, R.Z.; Farago, P.V.; Khan, I.A.; Budel, J.M. Schinus Molle: Anatomy of Leaves and Stems, Chemical Composition and Insecticidal Activities of Volatile Oil against Bed Bug (Cimex Lectularius). Rev. Bras. Farmacogn. 2018, 29, 1–10. [Google Scholar] [CrossRef]
- Abdel-Sattar, E.; Zaitoun, A.A.; Farag, M.A.; Gayed, S.H.E.; Harraz, F.M.H. Chemical Composition, Insecticidal and Insect Repellent Activity of Schinus molle L. Leaf and Fruit Essential Oils against Trogoderma granarium and Tribolium castaneum. Nat. Prod. Res. 2010, 24, 226–235. [Google Scholar] [CrossRef]
- Benzi, V.; Stefanazzi, N.; Ferrero, A.A. Biological Activity of Essential Oils from Leaves and Fruits of Pepper Tree (Schinus molle L.) to Control Rice Weevil (Sitophilus oryzae L.). Chilean J. Agric. Res. 2009, 69, 154–159. [Google Scholar] [CrossRef]
- Bigliani, M.C.; Rossetti, V.; Grondona, E.; Presti, S.L.; Paglini, P.M.; Rivero, V.; Zunino, M.P.; Ponce, A.A. Chemical Compositions and Properties of Schinus areira L. Essential Oil on Airway Inflammation and Cardiovascular System of Mice and Rabbits. Food Chem. Toxicol. 2012, 50, 2282–2288. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectorscopy, 4th ed.; Allured Pub. Corp.: Carol Stream, IL, USA, 2007; ISBN 978-1-932633-21-4. [Google Scholar]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; Gonzalez, L.; Tablada, M.; Robledo, C.W. InfoStat, Version 2017; Centro de Transferencia InfoStat, FCA, Universidad Nacional de Córdoba: Córdoba, Argentina, 2017. [Google Scholar]
- FAO; IAEA; USDA. Manual de Control de Calidad del Producto en la Cría Masiva y Liberación de Moscas de la Fruta Estériles; 7.0 (2019); IAEA: Vienna, Austria, 2022. [Google Scholar]
- Barud, F.J.; Gómez, M.P.; Ruiz, M.J.; Bachmann, G.E.; Goane, L.; Segura, D.F.; Lara, N.; Murúa, F.; Asfennato, A.; Gómez, E.; et al. Sexual Competitiveness of Sterile Ceratitis capitata Males Exposed to Essential Oils from Non-host Plant Species Native to Argentina. Entomol. Exp. Appl. 2023, 171, 146–155. [Google Scholar] [CrossRef]
- Jofré Barud, F.; López, S.; Tapia, A.; Feresin, G.E.; López, M.L. Attractant, Sexual Competitiveness Enhancing and Toxic Activities of the Essential Oils from Baccharis spartioides and Schinus polygama on Ceratitis capitata Wiedemann. Ind. Crops Prod. 2014, 62, 299–304. [Google Scholar] [CrossRef]
- IBM Corp. IBM SPSS Statistics for Windows 2015; IBM Corp.: Armonk, NY, USA, 2015. [Google Scholar]
- Kurdelas, R.R.; López, S.; Lima, B.; Feresin, G.E.; Zygadlo, J.; Zacchino, S.; López, M.L.; Tapia, A.; Freile, M.L. Chemical Composition, Anti-Insect and Antimicrobial Activity of Baccharis Darwinii Essential Oil from Argentina, Patagonia. Ind. Crops Prod. 2012, 40, 261–267. [Google Scholar] [CrossRef]
- Sampietro, D.A.; Belizan, M.M.E.; Baptista, Z.P.T.; Vattuone, M.A.; Catalán, C.A.N. Essential Oils from Schinus Species of Northwest Argentina: Composition and Antifungal Activity. Nat. Prod. Commun. 2014, 9, 1019–1022. [Google Scholar] [CrossRef]
- Cutro, A.C.; Castelli, M.V.; López, S.N.; Rosales, M.A.; Hollmann, A.; Rodriguez, S.A. Chemical Composition of Schinus areira Essential Oil and Antimicrobial Action against Staphylococcus aureus. Nat. Prod. Res. 2019, 35, 2931–2936. [Google Scholar] [CrossRef]
- Scalerandi, E.; Flores, G.A.; Palacio, M.; Defagó, M.T.; Carpinella, M.C.; Valladares, G.; Bertoni, A.; Palacios, S.M. Understanding Synergistic Toxicity of Terpenes as Insecticides: Contribution of Metabolic Detoxification in Musca domestica. Front. Plant Sci. 2018, 9, 1579. [Google Scholar] [CrossRef]
- De Sena Filho, J.G.; Soares De Almeida, A.; Pinto-Zevallos, D.; Barreto, I.C.; Cabral De Holanda Cavalcanti, S.; Nunes, R.; Teodoro, A.V.; Xavier, H.S.; Barbosa Filho, J.M.; Guan, L.; et al. From Plant Scent Defense to Biopesticide Discovery: Evaluation of Toxicity and Acetylcholinesterase Docking Properties for Lamiaceae Monoterpenes. Crop Prot. 2023, 164, 106126. [Google Scholar] [CrossRef]
- Miranda, M.A.F.M.; Matos, A.P.; Volante, A.C.; Cunha, G.O.S.; Gualtieri, S.C.J. Insecticidal Activity from Leaves and Sesquiterpene Lactones of Tithonia diversifolia (Helms.) A. Gray (Asteraceae) on Spodoptera frugiperda (Lepidoptera: Noctuidae). S. Afr. J. Bot. 2022, 144, 377–379. [Google Scholar] [CrossRef]
- Pang, X.; Almaz, B.; Qi, X.-J.; Wang, Y.; Feng, Y.-X.; Geng, Z.-F.; Xi, C.; Du, S.-S. Bioactivity of Essential Oil from Atalantia buxifolia Leaves and Its Major Sesquiterpenes against Three Stored-Product Insects. J. Essent. Oil-Bear. Plants 2020, 23, 38–50. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Q.X.; Song, B. Pesticidal Activity and Mode of Action of Monoterpenes. J. Agric. Food Chem. 2022, 70, 4556–4571. [Google Scholar] [CrossRef]
- Rani, P.U. Plant Volatile Chemicals and Insect Responses. In Plant Biology and Biotechnology; Bahadur, B., Venkat Rajam, M., Sahijram, L., Krishnamurthy, K.V., Eds.; Springer: New Delhi, India, 2015; pp. 671–695. ISBN 978-81-322-2285-9. [Google Scholar]
- Pickett, J.A.; Aradottir, G.I.; Birkett, M.A.; Bruce, T.J.A.; Chamberlain, K.; Khan, Z.R.; Midega, C.A.O.; Smart, L.E.; Woodcock, C.M. Aspects of Insect Chemical Ecology, Exploitation of Reception and Detection as Tools for Deception of Pests and Beneficial Insects. Physiol. Entomol. 2012, 37, 2–9. [Google Scholar] [CrossRef]
- Bruce, T.J.A.; Pickett, J.A. Perception of Plant Volatile Blends by Herbivorous Insects—Finding the Right Mix. Phytochemistry 2011, 72, 1605–1611. [Google Scholar] [CrossRef] [PubMed]
- Casaña-Giner, V.; Gandía-Balaguer, A.; Hernández-Alamós, M.M.; Mengod-Puerta, C.; Garrido-Vivas, A.; Primo-Millo, J.; Primo-Yúfera, E. Attractiveness of 79 Compounds and Mixtures to Wild Ceratitis capitata (Diptera: Tephritidae) in Field Trials. J. Econ. Entomol. 2001, 94, 898–904. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Sánchez; Sanz-Berzosa; Casaña-Giner; Primo-Yúfera. Attractiveness for Ceratitis capitata (Wiedemann) (Dipt., Tephritidae) of Mango (Mangifera indica, Cv. Tommy Atkins) Airborne Terpenes. J. Appl. Entomol. 2001, 125, 189–192. [Google Scholar] [CrossRef]
- Shelly, T.E. Exposure to α-Copaene and α-Copaene-Containing Oils Enhances Mating Success of Male Mediterranean Fruit Flies (Diptera: Tephritidae). Ann. Entomol. Soc. Am. 2001, 94, 497–502. [Google Scholar] [CrossRef]
- Shelly, T.E.; Rendon, P.; Hernandez, E.; Salgado, S.; McInnis, D.; Villalobos, E.; Liedo, P. Effects of Diet, Ginger Root Oil, and Elevation on the Mating Competitiveness of Male Mediterranean Fruit Flies (Diptera: Tephritidae) from a Mass-Reared, Genetic Sexing Strain in Guatemala. J. Econ. Entomol. 2003, 96, 1132–1141. [Google Scholar] [CrossRef]
- Kouloussis, N.A.; Gerofotis, C.D.; Ioannou, C.S.; Iliadis, I.V.; Papadopoulos, N.T.; Koveos, D.S. Towards Improving Sterile Insect Technique: Exposure to Orange Oil Compounds Increases Sexual Signalling and Longevity in Ceratitis capitata Males of the Vienna 8 GSS. PLoS ONE 2017, 12, e0188092. [Google Scholar] [CrossRef] [PubMed]
- Shelly, T.E.; Cowan, A.N.; Edu, J.; Pahio, E. Mating Success of Male Mediterranean Fruit Flies Following Exposure to Two Sources of α-Copaene, Manuka Oil and Mango. Fla. Entomol. 2008, 91, 9–15. [Google Scholar] [CrossRef]
- Papadopoulos, N.T.; Katsoyannos, B.I.; Kouloussis, N.A.; Hendrichs, J. Effect of Orange Peel Substances on Mating Competitiveness of Male Ceratitis capitata. Entomol. Exp. Appl. 2001, 99, 253–261. [Google Scholar] [CrossRef]
- Morelli, R.; Paranhos, B.J.; Coelho, A.M.; Castro, R.; Garziera, L.; Lopes, F.; Bento, J.M.S. Exposure of Sterile Mediterranean Fruit Fly (Diptera: Tephritidae) Males to Ginger Root Oil Reduces Female Remating. J. Appl. Entomol. 2013, 137, 75–82. [Google Scholar] [CrossRef]
- Socolsky, C.; Fascio, M.L.; D’Accorso, N.B.; Salvatore, A.; Willink, E.; Asakawa, Y.; Bardon, A. Effects of P-Vinylphenyl Glycosides and Other Related Compounds on the Oviposition Behavior of Ceratitis capitata. J. Chem. Ecol. 2008, 34, 539–548. [Google Scholar] [CrossRef]
- Ioannou, C.S.; Papadopoulos, N.T.; Kouloussis, N.A.; Tananaki, C.I.; Katsoyannos, B.I. Essential Oils of Citrus Fruit Stimulate Oviposition in the Mediterranean Fruit Fly Ceratitis capitata (Diptera: Tephritidae). Physiol. Entomol. 2012, 37, 330–339. [Google Scholar] [CrossRef]
- Hieu, T.T.; Choi, W.S.; Kim, S.-I.; Wang, M.; Ahn, Y.-J. Enhanced Repellency of Binary Mixtures of Calophyllum inophyllum Nut Oil Fatty Acids or Their Esters and Three Terpenoids to Stomoxys calcitrans. Pest Manag. Sci. 2015, 71, 1213–1218. [Google Scholar] [CrossRef] [PubMed]
- Bleeker, P.M.; Diergaarde, P.J.; Ament, K.; Guerra, J.; Weidner, M.; Schütz, S.; De Both, M.T.J.; Haring, M.A.; Schuurink, R.C. The Role of Specific Tomato Volatiles in Tomato-Whitefly Interaction. Plant Physiol. 2009, 151, 925–935. [Google Scholar] [CrossRef]
- Suárez, L.; Molina, A.; Murúa, F.; Acosta, J.C.; Moyano, B.; Escobar, J. Evaluación de colores para la oviposición de Ceratitis capitata (Diptera, Tephritidae) en Argentina. Rev. Per. Biol. 2007, 14, 291–293. [Google Scholar] [CrossRef][Green Version]
- Siskos, E.P.; Konstantopoulou, M.A.; Mazomenos, B.E. Insecticidal Activity of Citrus aurantium Peel Extract against Bactrocera oleae and Ceratitis capitata Adults (Diptera: Tephritidae). J. Appl. Entomol. 2009, 133, 108–116. [Google Scholar] [CrossRef]
- López, S.; Lima, B.; Aragón, L.; Espinar, L.A.; Tapia, A.; Zacchino, S.; Zygadlo, J.; Feresin, G.E.; López, M.L. Essential Oil of Azorella cryptantha Collected in Two Different Locations from San Juan Province, Argentina: Chemical Variability and Anti-Insect and Antimicrobial Activities. Chem. Biodivers. 2012, 9, 1452–1464. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, C.; Qin, Y.; Yin, X.; Dong, X.; Desneux, N.; Zhou, H. Monitoring the Methyl Eugenol Response and Non-Responsiveness Mechanisms in Oriental Fruit Fly Bactrocera Dorsalis in China. Insects 2022, 13, 1004. [Google Scholar] [CrossRef] [PubMed]
- López, M.D.; Pascual-Villalobos, M.J. Mode of Inhibition of Acetylcholinesterase by Monoterpenoids and Implications for Pest Control. Ind. Crops Prod. 2010, 31, 284–288. [Google Scholar] [CrossRef]
- Miyazawa, M.; Watanabe, H.; Kameoka, H. Inhibition of Acetylcholinesterase Activity by Monoterpenoids with a p-Menthane Skeleton. J. Agric. Food Chem. 1997, 45, 677–679. [Google Scholar] [CrossRef]
- Cortez-Vega, A.; Jofré-Barud, F.; Andino, N.; Gómez, M.P.; López, M.L. Toxicological Interactions between Spinosad and Essential Oils in the Mediterranean Fruit Fly, Ceratitis capitata. J. Appl. Entomol. 2023, 147, 834–842. [Google Scholar] [CrossRef]
- Martínez, L.C.; Plata-Rueda, A.; Colares, H.C.; Campos, J.M.; Dos Santos, M.H.; Fernandes, F.L.; Serrão, J.E.; Zanuncio, J.C. Toxic Effects of Two Essential Oils and Their Constituents on the Mealworm Beetle, Tenebrio molitor. Bull. Entomol. Res. 2018, 108, 716–725. [Google Scholar] [CrossRef]
- Jung, W.-C.; Jang, Y.-S.; Hieu, T.T.; Lee, C.-K.; Ahn, Y.-J. Toxicity of Myristica fragrans Seed Compounds Against Blattella germanica (Dictyoptera: Blattellidae). J. Med. Entomol. 2007, 44, 524–529. [Google Scholar] [CrossRef]
- Ali, A.; Tabanca, N.; Ozek, G.; Ozek, T.; Aytac, Z.; Bernier, U.R.; Agramonte, N.M.; Baser, K.H.C.; Khan, I.A. Essential Oils of Echinophora lamondiana (Apiales: Umbelliferae): A Relationship Between Chemical Profile and Biting Deterrence and Larvicidal Activity Against Mosquitoes (Diptera: Culicidae). J. Med. Entomol. 2015, 52, 93–100. [Google Scholar] [CrossRef]
- Aboalhaija, A.; Amro, R.; Abaza, I.; Khalil, E.; Al-Aboudi, A.; Abu-Zarga, M.; Afifi, F.U. Schinus molle L. Collected from Jordan and Turkey: Essential Oil Composition and Anticholinesterase Activity. J. Essent. Oil-Bear. Plants 2019, 22, 704–716. [Google Scholar] [CrossRef]
| Chemical Group | Area (%) |
|---|---|
| Aliphatic hydrocarbons | 0.2 |
| Monoterpene hydrocarbons | 65.4 |
| Oxygenated monoterpenes | 2.2 |
| Sesquiterpene hydrocarbons | 18.8 |
| Oxygenated sesquiterpenes | 7.3 |
| Not identified | 3.6 |
| Total | 97.5 |
| Sex | Variable | Y-Tube Arm | Estimator a,b | p c | |
|---|---|---|---|---|---|
| EO | Control | ||||
| Females | Initial Choice * | 58 | 38 | 4.17 | 0.040 * |
| Final Choice ≠ | 55 | 38 | 3.11 | 0.080 | |
| Time Spent (s) | 68.8 | 43 | 4.31 | 0.040 * | |
| Males | Initial Choice | 60 | 44 | 2.46 | 0.12 |
| Final Choice | 62 | 40 | 4.75 | 0.03 * | |
| Time Spent (s) | 60.7 | 46.8 | 3.18 | 0.077 | |
| Tsl Males | Wild Males | W | p | |||
|---|---|---|---|---|---|---|
| Median | Q1–Q3 | Median | Q1–Q3 | |||
| S. areira | 126 | 17–155 | 28 | 13–95 | 695.5 | 0.0418 |
| Control | 75 | 12–105 | 35 | 11–98 | 1190 | 0.7163 |
| Tsl Males | Wild Males | W | p | |||
|---|---|---|---|---|---|---|
| Median | Q1–Q3 | Median | Q1–Q3 | |||
| S. areira | 122 | 99–161 | 189 | 156–210 | 191.50 | 0.0127 |
| Control | 128 | 104–143 | 190 | 174–216 | 179.50 | 0.0001 |
| Males | χ2 (d.f = 1) | p | |||
|---|---|---|---|---|---|
| Treatment | Location | Tsl | Wild | ||
| S. areira | Tree | 5 | 71 | 8.37 | 0.0038 |
| Cage | 9 | 25 | |||
| Control | Tree | 8 | 91 | 27.30 | <0.0001 |
| Cage | 11 | 9 | |||
| Males | χ2 (d.f. Height = 1S.areira; 2control; d.f. Leaf Side = 1) | p | ||||
|---|---|---|---|---|---|---|
| Tsl | Wild | |||||
| S. areira | Height | High | 15 | 95 | 0.63 | 0.4256 |
| Medium | 0 | 4 | ||||
| Low | - | - | ||||
| Leaf side | Inf | 4 | 69 | 1.64 | 0.1997 | |
| Sup | 1 | 4 | ||||
| Control | Height | High | 15 | 92 | 6.61 | 0.0366 |
| Medium | 3 | 8 | ||||
| Low | 1 | 0 | ||||
| Leaf side | Inf | 7 | 91 | 11.49 | 0.0007 | |
| Sup | 1 | 0 | ||||
| Sex | Time (h) | LD50 µg/fly | Slope ± SE | χ2 | df |
|---|---|---|---|---|---|
| Males ♂ | 24 | 30.65 (24.84–37.48) | 2.05 ± 0.18 | 23.89 | 14 |
| 48 | 27.34 (21.49–34.00) | 1.94 ± 0.18 | 25.81 | ||
| 72 | 23.84 (17.98–30.20) | 1.94 ± 0.18 | 28.30 | ||
| Females ♀ | 24 | 28.63 (23.00–35.09) | 2.41 ± 0.19 | 31.43 | 14 |
| 48 | 24.43 (19.78–29.44) | 2.27 ± 0.19 | 24.18 | ||
| 72 | 21.83 (18.73–25.03) | 2.19 ± 0.19 | 19.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jofré Barud, F.; Gomez, M.P.; Ruiz, M.J.; Bachmann, G.; Segura, D.F.; Vera, M.T.; López, M.L. Effect of Schinus areira L. Essential Oil on Attraction, Reproductive Behavior, and Survival of Ceratitis capitata Wiedemann. Plants 2025, 14, 794. https://doi.org/10.3390/plants14050794
Jofré Barud F, Gomez MP, Ruiz MJ, Bachmann G, Segura DF, Vera MT, López ML. Effect of Schinus areira L. Essential Oil on Attraction, Reproductive Behavior, and Survival of Ceratitis capitata Wiedemann. Plants. 2025; 14(5):794. https://doi.org/10.3390/plants14050794
Chicago/Turabian StyleJofré Barud, Flavia, María Pía Gomez, María Josefina Ruiz, Guillermo Bachmann, Diego Fernando Segura, María Teresa Vera, and María Liza López. 2025. "Effect of Schinus areira L. Essential Oil on Attraction, Reproductive Behavior, and Survival of Ceratitis capitata Wiedemann" Plants 14, no. 5: 794. https://doi.org/10.3390/plants14050794
APA StyleJofré Barud, F., Gomez, M. P., Ruiz, M. J., Bachmann, G., Segura, D. F., Vera, M. T., & López, M. L. (2025). Effect of Schinus areira L. Essential Oil on Attraction, Reproductive Behavior, and Survival of Ceratitis capitata Wiedemann. Plants, 14(5), 794. https://doi.org/10.3390/plants14050794






