Silicon Nano-Fertilizer-Enhanced Soybean Resilience and Yield Under Drought Stress
Abstract
1. Introduction
2. Results and Discussion
2.1. Impact of n-SiO2 on Silicon Accumulation and Soil Moisture Retention in Soybeans Under Drought Stress
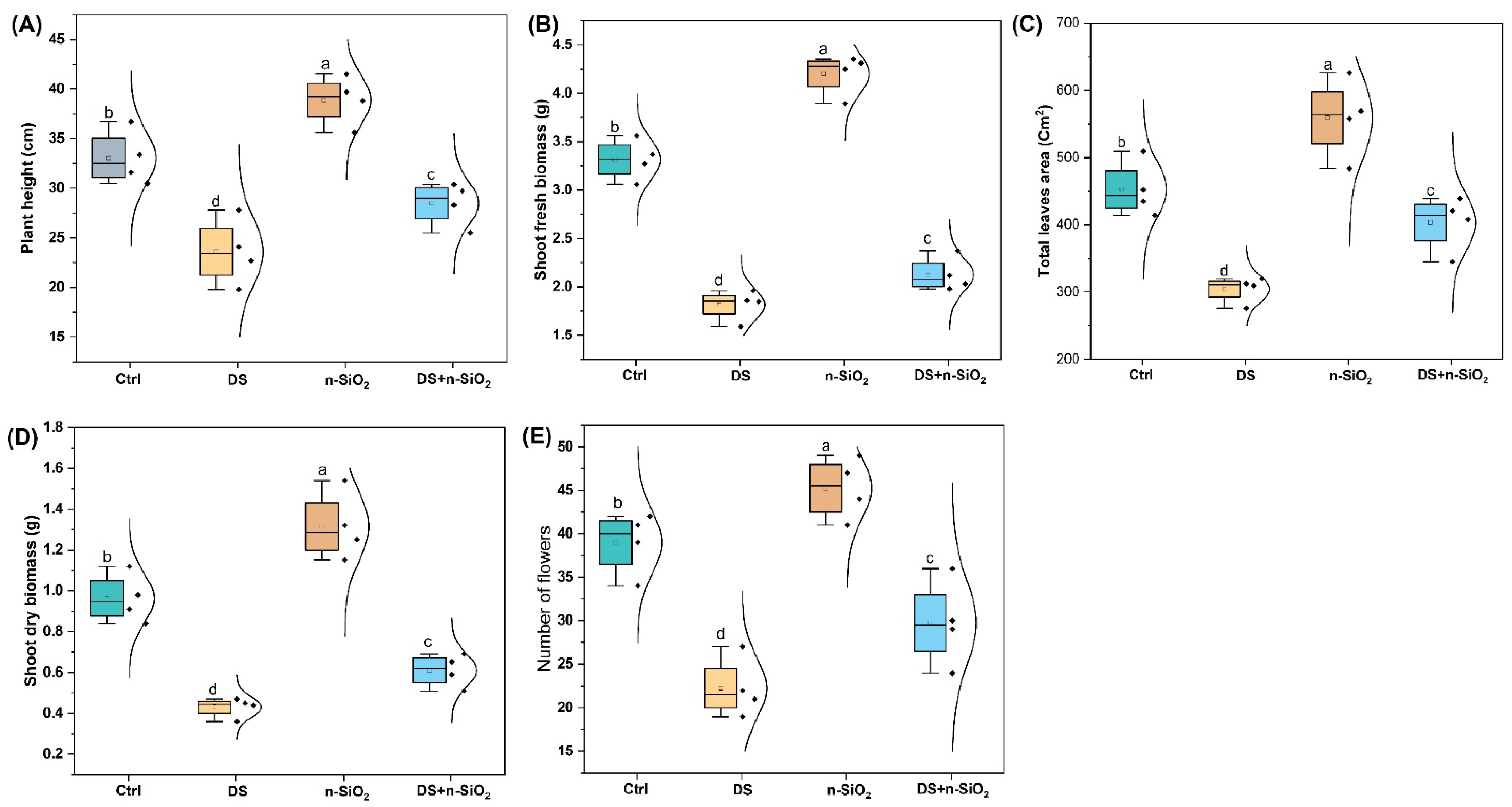
2.2. Low-Dose n-SiO2 Enhances Soybean Agronomic Traits Under Drought Stress
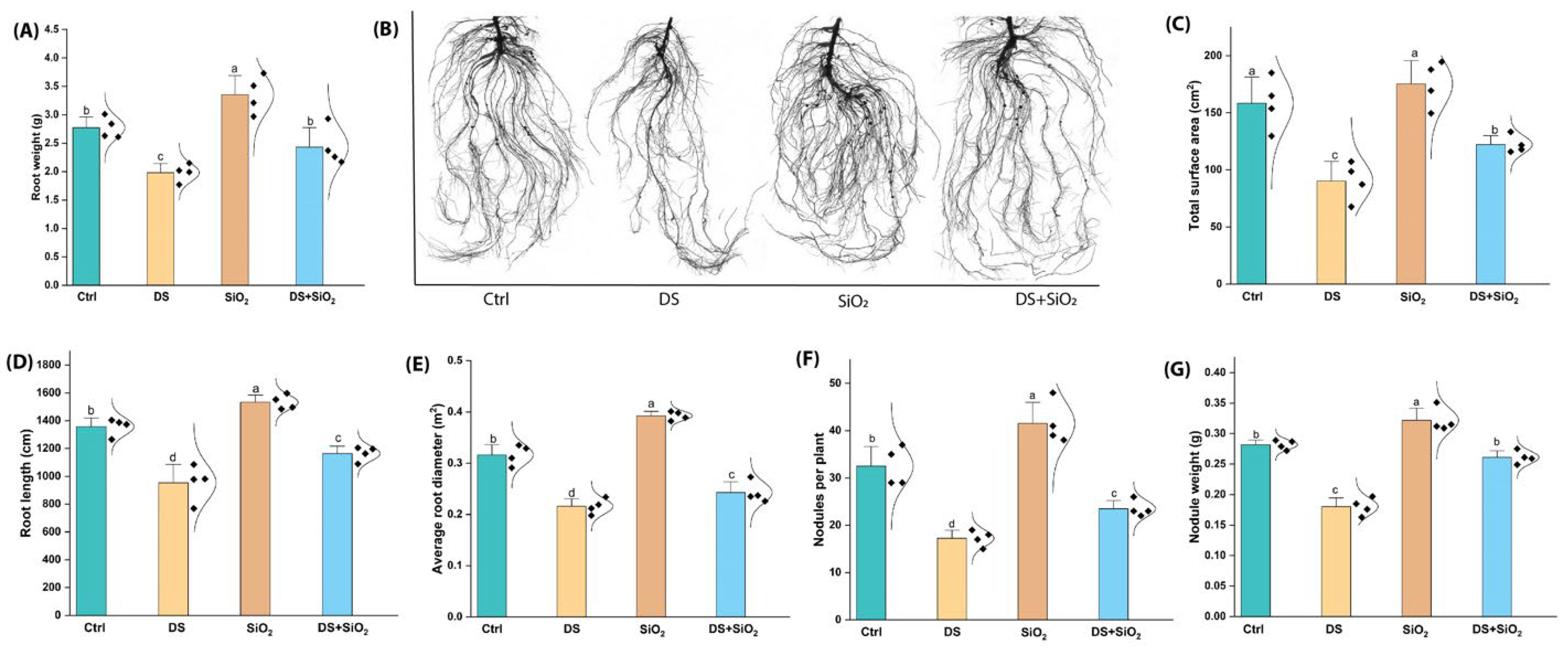
2.3. n-SiO2 Enhances Root and Nodule Physiological Health Under Drought Stress
2.4. Enhancement of Photosynthesis Activity in Soybean Under Drought Stress Induced by n-SiO2
2.5. Modulation of Enzyme Activity, Proline Content, and Na+/K+ Ratio in Soybean Under n-SiO2-Induced Drought Stress
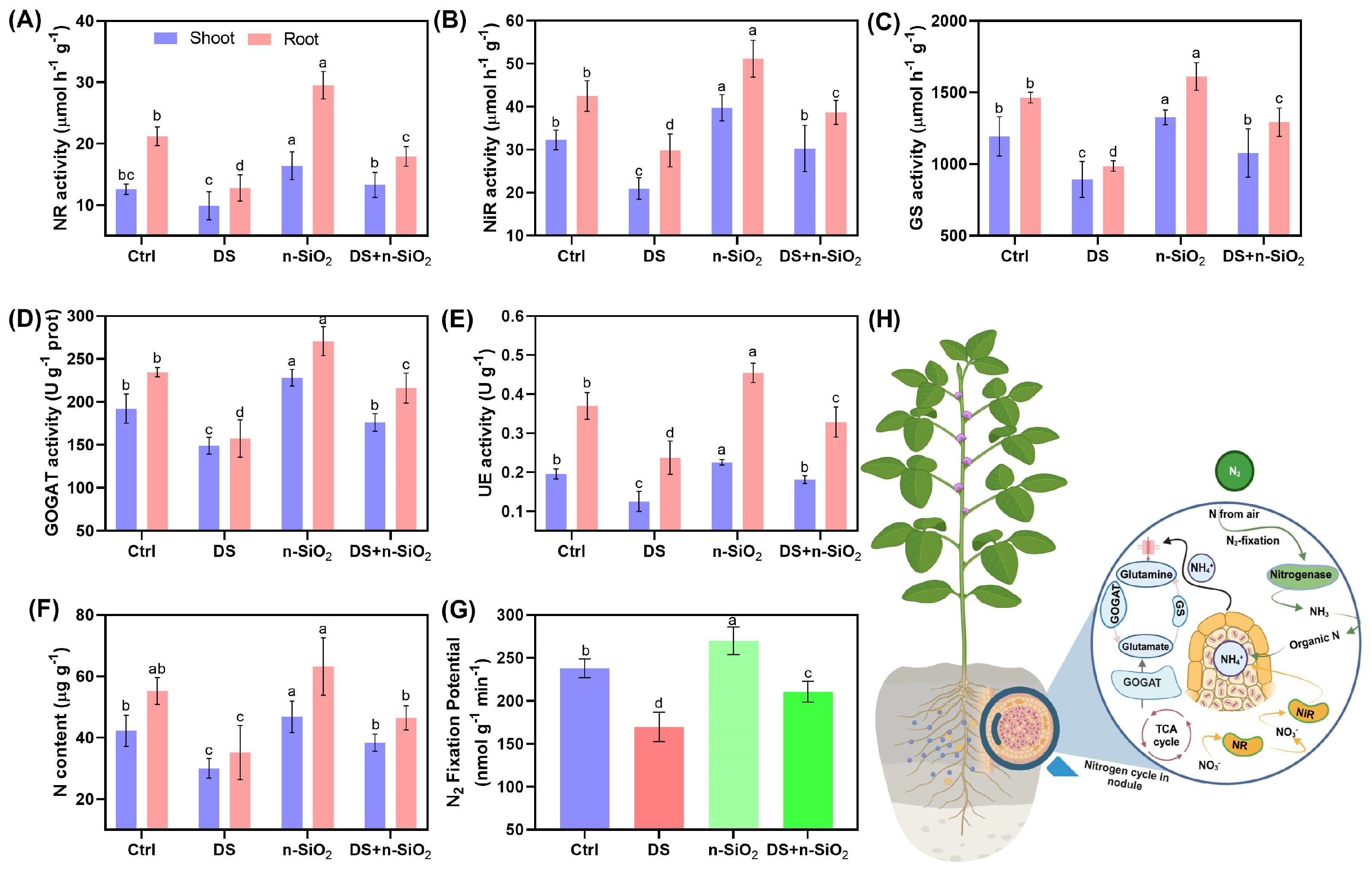
2.6. n-SiO2 Enhances Nitrogen Fixation and Assimilation in Soybean Under Drought Stress
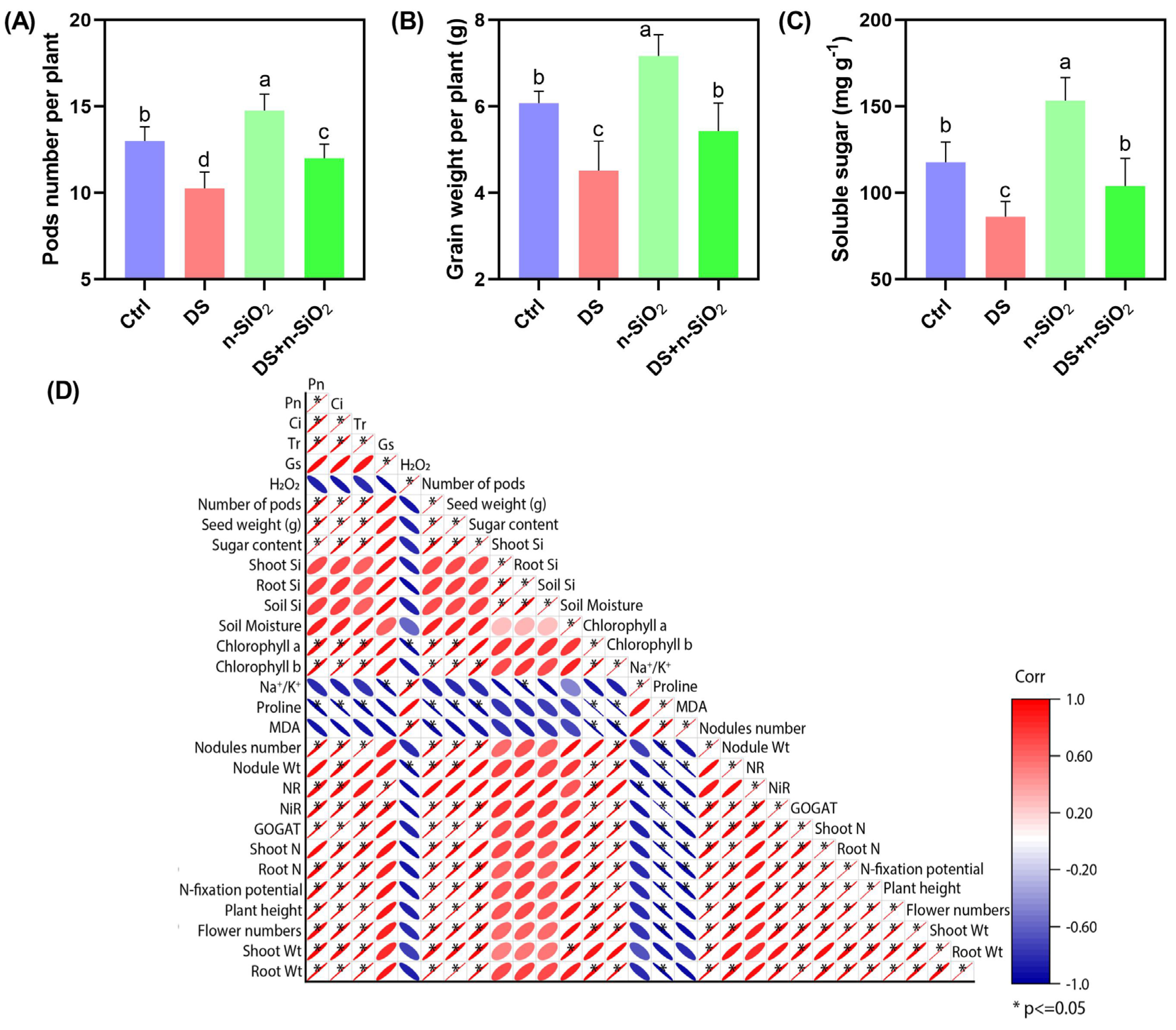
2.7. Nanoscale SiO2 Enhances Soybean Yield and Drought Resilience by Improving Physiological and Metabolic Functions
3. Materials and Methods
3.1. Characterization of Nanoscale Silicon Oxide
3.2. Experimental Design
3.3. Photosynthetic Activities and Pigments
3.4. Enzymatic Activities
3.5. Nitrogen Assimilation and Fixation Potential
3.6. Measurement of Si in Plant and Soil
3.7. Soluble Sugar Content Measurement in Seeds
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ahmadi, B.; Ahmadalipour, A.; Tootle, G.; Moradkhani, H. Remote Sensing of Water Use Efficiency and Terrestrial Drought Recovery across the Contiguous United States. Remote Sens. 2019, 11, 731. [Google Scholar] [CrossRef]
- Su, L.; Cao, Q.; Xiao, M.; Mocko, D.M.; Barlage, M.; Li, D.; Peters-Lidard, C.D.; Lettenmaier, D.P. Drought Variability over the Conterminous United States for the Past Century. J. Hydrometeorol. 2021, 22, 1153–1168. [Google Scholar] [CrossRef]
- Kim, W.; Iizumi, T.; Nishimori, M. Global Patterns of Crop Production Losses Associated with Droughts from 1983 to 2009. J. Appl. Meteorol. Climatol. 2019, 58, 1233–1244. [Google Scholar] [CrossRef]
- Rey, D.; Holman, I.P.; Knox, J.W. Developing drought resilience in irrigated agriculture in the face of increasing water scarcity. Reg. Environ. Change 2017, 17, 1527–1540. [Google Scholar] [CrossRef]
- Leng, G.; Hall, J. Crop yield sensitivity of global major agricultural countries to droughts and the projected changes in the future. Sci. Total Environ. 2019, 654, 811–821. [Google Scholar] [CrossRef]
- Sari, D.; Ferroudj, A.; Muthu, A.; Prokisch, J.; El-Ramady, H.; Elsakhawy, T.; Omara, A.E.-D.; Brevik, E.C. Nano-Enabled Agriculture Using Nano-Selenium for Crop Productivity: What Should be Addressed More? Environ. Biodivers. Soil Secur. 2023, 7, 85–99. [Google Scholar]
- Singh, M.; Goswami, S.P.; Ranjitha, G.; Sachan, P.; Sahu, D.K.; Beese, S.; Pandey, S.K. Nanotech for Fertilizers and Nutrients-Improving Nutrient use Efficiency with Nano-Enabled Fertilizers. J. Exp. Agric. Int. 2024, 46, 220–247. [Google Scholar] [CrossRef]
- Yan, G.; Huang, Q.; Zhao, S.; Xu, Y.; He, Y.; Nikolic, M.; Nikolic, N.; Liang, Y.; Zhu, Z. Silicon nanoparticles in sustainable agriculture: Synthesis, absorption, and plant stress alleviation. Front. Plant Sci. 2024, 15, 1393458. [Google Scholar] [CrossRef]
- Xu, X.; Guo, Y.; Hao, Y.; Cai, Z.; Cao, Y.; Fang, W.; Zhao, B.; Haynes, C.L.; White, J.C.; Ma, C. Nano-silicon fertiliser increases the yield and quality of cherry radish. Mod. Agric. 2023, 1, 152–165. [Google Scholar] [CrossRef]
- Khan, M.; Siddiqui, Z.A.; Parveen, A.; Khan, A.A.; Moon, I.S.; Alam, M. Elucidating the role of silicon dioxide and titanium dioxide nanoparticles in mitigating the disease of the eggplant caused by Phomopsis vexans, Ralstonia solanacearum, and root-knot nematode Meloidogyne incognita. Nanotechnol. Rev. 2022, 11, 1606–1619. [Google Scholar] [CrossRef]
- Rai-Kalal, P.; Tomar, R.S.; Jajoo, A. Seed nanopriming by silicon oxide improves drought stress alleviation potential in wheat plants. Funct. Plant Biol. 2021, 48, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Etesami, H.; Noori, F.; Jeong, B.R. Chapter 25—Directions for future research to use silicon and silicon nanoparticles to increase crops tolerance to stresses and improve their quality. In Silicon and Nano-Silicon in Environmental Stress Management and Crop Quality Improvement; Etesami, H., Al Saeedi, A.H., El-Ramady, H., Fujita, M., Pessarakli, M., Anwar Hossain, M., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 349–367. [Google Scholar]
- AlSaeedi, A.H. Enhancement of soil water characteristics curve (SWCC) and water use efficiency of cucumber (Cucumis sativus L.) in sandy soils by using silica nanoparticles. J. King Saud. Univ.—Sci. 2022, 34, 101926. [Google Scholar] [CrossRef]
- Ghorbanpour, M.; Mohammadi, H.; Kariman, K. Nanosilicon-based recovery of barley (Hordeum vulgare) plants subjected to drought stress. Environ. Sci. Nano 2020, 7, 443–461. [Google Scholar] [CrossRef]
- Foyer, C.H.; Lam, H.-M.; Nguyen, H.T.; Siddique, K.H.M.; Varshney, R.K.; Colmer, T.D.; Cowling, W.; Bramley, H.; Mori, T.A.; Hodgson, J.M.; et al. Neglecting legumes has compromised human health and sustainable food production. Nat. Plants 2016, 2, 16112. [Google Scholar] [CrossRef]
- Santachiara, G.; Borrás, L.; Salvagiotti, F.; Gerde, J.A.; Rotundo, J.L. Relative importance of biological nitrogen fixation and mineral uptake in high yielding soybean cultivars. Plant Soil 2017, 418, 191–203. [Google Scholar] [CrossRef]
- Direkvandy, S.; Eisvand, H.R.; Azizi, K.; Akbarpour, O.; Smith, D.L. Mitigation impact of SiO2 nanoparticles and mycorrhiza on wheat growth and yield under late-season drought stress. In Cereal Research Communications; Springer: Berlin/Heidelberg, Germany, 2024. [Google Scholar] [CrossRef]
- Sharf-Eldin, A.A.; Alwutayd, K.M.; El-Yazied, A.A.; El-Beltagi, H.S.; Alharbi, B.M.; Eisa, M.A.M.; Alqurashi, M.; Sharaf, M.; Al-Harbi, N.A.; Al-Qahtani, S.M.; et al. Response of Maize Seedlings to Silicon Dioxide Nanoparticles (SiO2NPs) under Drought Stress. Plants 2023, 12, 2592. [Google Scholar] [CrossRef]
- Oliveira, T.C.; Cabral, J.S.R.; Santana, L.R.; Tavares, G.G.; Santos, L.D.S.; Paim, T.P.; Müller, C.; Silva, F.G.; Costa, A.C.; Souchie, E.L.; et al. The arbuscular mycorrhizal fungus Rhizophagus clarus improves physiological tolerance to drought stress in soybean plants. Sci. Rep. 2022, 12, 9044. [Google Scholar] [CrossRef]
- Rahman, M.M.; Mostofa, M.G.; Das, A.K.; Anik, T.R.; Keya, S.S.; Ahsan, S.M.; Khan, M.A.R.; Ahmed, M.; Rahman, M.A.; Hossain, M.M.; et al. Ethanol Positively Modulates Photosynthetic Traits, Antioxidant Defense and Osmoprotectant Levels to Enhance Drought Acclimatization in Soybean. Antioxidants 2022, 11, 516. [Google Scholar] [CrossRef]
- Cao, X.; Wu, L.; Wu, M.; Zhu, C.; Jin, Q.; Zhang, J. Abscisic acid mediated proline biosynthesis and antioxidant ability in roots of two different rice genotypes under hypoxic stress. BMC Plant Biol. 2020, 20, 198. [Google Scholar] [CrossRef]
- Song, S.; Li, X.; Wang, X.; Zhou, Q.; Li, Y.; Wang, X.; Dong, S. Effects of Drought Stress on Key Enzymes of Carbon Metabolism, Photosynthetic Characteristics and Agronomic Traits of Soybean at the Flowering Stage under Different Soil Substrates. Phyton-Int. J. Exp. Bot. 2022, 91, 2475–2490. [Google Scholar] [CrossRef]
- Bhardwaj, S.; Sharma, D.; Singh, S.; Ramamurthy, P.C.; Verma, T.; Pujari, M.; Singh, J.; Kapoor, D.; Prasad, R. Physiological and molecular insights into the role of silicon in improving plant performance under abiotic stresses. Plant Soil 2023, 486, 25–43. [Google Scholar] [CrossRef]
- Navarro-Cerrillo, R.M.; Rodriguez-Vallejo, C.; Silveiro, E.; Hortal, A.; Palacios-Rodríguez, G.; Duque-Lazo, J.; Camarero, J.J. Cumulative Drought Stress Leads to a Loss of Growth Resilience and Explains Higher Mortality in Planted than in Naturally Regenerated Pinus pinaster Stands. Forests 2018, 9, 358. [Google Scholar] [CrossRef]
- Elsalahy, H.H.; Bellingrath-Kimura, S.D.; Roß, C.-L.; Kautz, T.; Döring, T.F. Crop resilience to drought with and without response diversity. Front. Plant Sci. 2020, 11, 721. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhou, X.; Qu, Z.; Yan, C.; Ma, C.; Liu, J.; Dong, S. Regulation of soybean drought response by mepiquat chloride pretreatment. Front. Plant Sci. 2023, 14, 1149114. [Google Scholar] [CrossRef]
- Wang, X.; Wu, Z.; Zhou, Q.; Wang, X.; Song, S.; Dong, S. Physiological Response of Soybean Plants to Water Deficit. Front. Plant Sci. 2021, 12, 809692. [Google Scholar] [CrossRef]
- Rahman, M.; Mostofa, M.G.; Keya, S.S.; Rahman, A.; Das, A.K.; Islam, R.; Abdelrahman, M.; Bhuiyan, S.U.; Naznin, T.; Ansary, M.U.; et al. Acetic acid improves drought acclimation in soybean: An integrative response of photosynthesis, osmoregulation, mineral uptake and antioxidant defense. Physiol. Plant. 2021, 172, 334–350. [Google Scholar] [CrossRef]
- Ahmed, T.; Guo, J.; Noman, M.; Lv, L.; Manzoor, N.; Qi, X.; Li, B. Metagenomic and biochemical analyses reveal the potential of silicon to alleviate arsenic toxicity in rice (Oryza sativa L.). Environ. Pollut. 2024, 345, 123537. [Google Scholar] [CrossRef]
- Naidu, S.; Pandey, J.; Mishra, L.C.; Chakraborty, A.; Roy, A.; Singh, I.K.; Singh, A. Silicon nanoparticles: Synthesis, uptake and their role in mitigation of biotic stress. Ecotoxicol. Environ. Saf. 2023, 255, 114783. [Google Scholar] [CrossRef]
- Hussain, S.; Mumtaz, M.; Manzoor, S.; Shuxian, L.; Ahmed, I.; Skalicky, M.; Brestic, M.; Rastogi, A.; Ulhassan, Z.; Shafiq, I.; et al. Foliar application of silicon improves growth of soybean by enhancing carbon metabolism under shading conditions. Plant Physiol. Biochem. 2021, 159, 43–52. [Google Scholar] [CrossRef]
- Sigit Tri Pamungkas, S.; Suwarto; Suprayogi; Farid, N. Drought Stress: Responses and Mechanism in Plants. Rev. Agric. Sci. 2022, 10, 168–185. [Google Scholar] [CrossRef]
- Wang, M.; Wang, R.; Mur, L.A.J.; Ruan, J.; Shen, Q.; Guo, S. Functions of silicon in plant drought stress responses. Hortic. Res. 2021, 8, 254. [Google Scholar] [CrossRef] [PubMed]
- Valkov, V.T.; Sol, S.; Rogato, A.; Chiurazzi, M. The functional characterization of LjNRT2.4 indicates a novel, positive role of nitrate for an efficient nodule N2-fixation activity. New Phytol. 2020, 228, 682–696. [Google Scholar] [CrossRef] [PubMed]
- Xing, X.; Du, H.; Yang, Z.; Li, X.; Kong, Y.; Li, W.; Zhang, C. GmSPX8, a nodule-localized regulator confers nodule development and nitrogen fixation under phosphorus starvation in soybean. BMC Plant Biol. 2022, 22, 161. [Google Scholar] [CrossRef] [PubMed]
- Dhami, N.; Prasad, B.N. Increase in root nodulation and crop yield of soybean by native Bradyrhizobium japonicum strains. Bot. Orient. J. Plant Sci. 2010, 6, 1–3. [Google Scholar] [CrossRef][Green Version]
- Elhady, A.; Hallmann, J.; Heuer, H. Symbiosis of soybean with nitrogen fixing bacteria affected by root lesion nematodes in a density-dependent manner. Sci. Rep. 2020, 10, 1619. [Google Scholar] [CrossRef]
- Chen, D.; Wang, S.; Yin, L.; Deng, X. How Does Silicon Mediate Plant Water Uptake and Loss Under Water Deficiency? Front. Plant Sci. 2018, 9, 281. [Google Scholar] [CrossRef]
- Raza, M.A.S.; Zulfiqar, B.; Iqbal, R.; Muzamil, M.N.; Aslam, M.U.; Muhammad, F.; Amin, J.; Aslam, H.M.U.; Ibrahim, M.A.; Uzair, M.; et al. Morpho-physiological and biochemical response of wheat to various treatments of silicon nano-particles under drought stress conditions. Sci. Rep. 2023, 13, 2700. [Google Scholar] [CrossRef]
- Rezayian, M.; Ebrahimzadeh, H.; Niknam, V. Nitric Oxide Stimulates Antioxidant System and Osmotic Adjustment in Soybean Under Drought Stress. J. Soil Sci. Plant Nutr. 2020, 20, 1122–1132. [Google Scholar] [CrossRef]
- Yan, Q.; Cui, X.; Lin, S.; Gan, S.; Xing, H.; Dou, D. GmCYP82A3, a Soybean Cytochrome P450 Family Gene Involved in the Jasmonic Acid and Ethylene Signaling Pathway, Enhances Plant Resistance to Biotic and Abiotic Stresses. PLoS ONE 2016, 11, e0162253. [Google Scholar] [CrossRef]
- Ahmad, P.; Abdel Latef, A.A.; Hashem, A.; Abd Allah, E.F.; Gucel, S.; Tran, L.S. Nitric Oxide Mitigates Salt Stress by Regulating Levels of Osmolytes and Antioxidant Enzymes in Chickpea. Front. Plant Sci. 2016, 7, 347. [Google Scholar] [CrossRef]
- Adie, M.M.; Krisnawati, A. Agronomic characters of drought-tolerant soybeans at the reproductive stage. Ber. Biol. 2019, 18, 339–349. [Google Scholar] [CrossRef]
- Kuczyński, J.; Gracz-Bernaciak, J.; Twardowski, T.; Karłowski, W.M.; Tyczewska, A. Cold stress-induced miRNA and degradome changes in four soybean varieties differing in chilling resistance. J. Agron. Crop Sci. 2022, 208, 777–794. [Google Scholar] [CrossRef]
- Mohammadi, P.P.; Moieni, A.; Hiraga, S.; Komatsu, S. Organ-specific proteomic analysis of drought-stressed soybean seedlings. J. Proteom. 2012, 75, 1906–1923. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, D.; Bertin, D.; Sinclair, T.R.; Nogueira, M.A.; Livingston, D.; Carter, T. Microsphere stem blockage as a screen for nitrogen-fixation drought tolerance in soybean. Physiol. Plant. 2021, 172, 1376–1381. [Google Scholar] [CrossRef]
- Shakoor, N.; Hussain, M.; Adeel, M.; Azeem, I.; Ahmad, M.A.; Zain, M.; Zhang, P.; Li, Y.; Quanlong, W.; Horton, R.; et al. Lithium-induced alterations in soybean nodulation and nitrogen fixation through multifunctional mechanisms. Sci. Total Environ. 2023, 904, 166438. [Google Scholar] [CrossRef]
- Azeem, I.; Wang, Q.; Adeel, M.; Shakoor, N.; Zain, M.; khan, A.A.; Li, Y.; Azeem, K.; Nadeem, M.; Zhu, G.; et al. Assessing the combined impacts of microplastics and nickel oxide nanomaterials on soybean growth and nitrogen fixation potential. J. Hazard. Mater. 2024, 480, 136062. [Google Scholar] [CrossRef]
- Shakoor, N.; Adeel, M.; Ahmad, M.A.; Zain, M.; Waheed, U.; Javaid, R.A.; Haider, F.U.; Azeem, I.; Zhou, P.; Li, Y.; et al. Reimagining safe lithium applications in the living environment and its impacts on human, animal, and plant system. Environ. Sci. Ecotechnol. 2023, 15, 100252. [Google Scholar] [CrossRef]
- Du, Y.; Zhao, Q.; Chen, L.; Yao, X.; Zhang, H.; Wu, J.; Xie, F. Effect of Drought Stress during Soybean R2–R6 Growth Stages on Sucrose Metabolism in Leaf and Seed. Int. J. Mol. Sci. 2020, 21, 618. [Google Scholar] [CrossRef]
- Chen, Z.; Fang, X.; Yuan, X.; Zhang, Y.; Li, H.; Zhou, Y.; Cui, X. Overexpression of Transcription Factor GmTGA15 Enhances Drought Tolerance in Transgenic Soybean Hairy Roots and Arabidopsis Plants. Agronomy 2021, 11, 170. [Google Scholar] [CrossRef]
- Dong, S.; Zhou, X.; Qu, Z.; Wang, X. Effects of drought stress at different stages on soluble sugar content of soybeans. Plant Soil Environ. 2023, 69, 500–511. [Google Scholar] [CrossRef]
- Sintaha, M.; Man, C.-K.; Yung, W.-S.; Duan, S.; Li, M.-W.; Lam, H.-M. Drought Stress Priming Improved the Drought Tolerance of Soybean. Plants 2022, 11, 2954. [Google Scholar] [CrossRef] [PubMed]
- Guzzo, M.C.; Costamagna, C.; Salloum, M.S.; Rotundo, J.L.; Monteoliva, M.I.; Luna, C.V. Morpho-physiological Traits Associated With Drought Responses in Soybean. Crop Sci. 2020, 61, 672–688. [Google Scholar] [CrossRef]
- Yu, Q.; Sun, G.-Z.; Liu, Y.; Zhou, Y.; Chen, M.; Ma, Y.-Z.; Xu, Z.S.; Lan, J. Genome-Wide Analysis of the Soybean Calmodulin-Binding Protein 60 Family and Identification of GmCBP60A-1 Responses to Drought and Salt Stresses. Int. J. Mol. Sci. 2021, 22, 13501. [Google Scholar] [CrossRef]
- Hasan, M.M.; Ali, M.A.; Soliman, M.H.; Alqarawi, A.A.; Fang, X.W. Insights Into 28-Homobrassinolide (HBR)-mediated Redox Homeostasis, AsA–GSH Cycle, and Methylglyoxal Detoxification in Soybean Under Drought-Induced Oxidative Stress. J. Plant Interact. 2020, 15, 371–385. [Google Scholar] [CrossRef]
- Xuan, H.; Huang, Y.; Zhou, L.; Deng, S.; Wang, C.; Xu, J.; Wang, R.; Zhao, J.; Guo, N.; Han, X. Key Soybean Seedlings Drought-Responsive Genes and Pathways Revealed by Comparative Transcriptome Analyses of Two Cultivars. Int. J. Mol. Sci. 2022, 23, 2893. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Dong, S. Screening and Identification of Drought Tolerance of Spring Soybean at Seedling Stage Under Climate Change. Front. Sustain. Food Syst. 2022, 6, 988319. [Google Scholar] [CrossRef]
- Dong, S.; Jiang, Y.; Dong, Y.; Wang, L.; Wang, W.; Ma, Z.; Yan, C.; Ma, C.; Liu, L. A Study on Soybean Responses to Drought Stress and Rehydration. Saudi J. Biol. Sci. 2019, 26, 2006–2017. [Google Scholar] [CrossRef]
- Qu, Z.; Tian, Y.; Zhou, X.; Li, X.; Zhou, Q.; Wang, X.; Dong, S. Effects of Exogenous Sodium Nitroprusside Spraying on Physiological Characteristics of Soybean Leaves at the Flowering Stage Under Drought Stress. Plants 2023, 12, 1598. [Google Scholar] [CrossRef]
- Zain, M.; Ma, H.; Nuruzzaman, M.; Chaudhary, S.; Nadeem, M.; Shakoor, N.; Azeem, I.; Duan, A.; Sun, C.; Ahamad, T. Nanotechnology based precision agriculture for alleviating biotic and abiotic stress in plants. Plant Stress 2023, 10, 100239. [Google Scholar] [CrossRef]
- Gai, Z.; Zhang, M.; Zhang, P.; Zhang, J.; Liu, J.; Cai, L.; Xu, Y.; Zhang, N.; Yan, Z.; Liu, L.; et al. 2-Oxoglutarate Contributes to the Effect of Foliar Nitrogen on Enhancing Drought Tolerance During Flowering and Grain Yield of Soybean. Sci. Rep. 2023, 13, 7274. [Google Scholar] [CrossRef]
- Nader, A.A. Drought-Tolerant Bacteria and Arbuscular Mycorrhizal Fungi Mitigate the Detrimental Effects of Drought Stress Induced by Withholding Irrigation at Critical Growth Stages of Soybean (Glycine max L.). Microorganisms 2024, 12, 1123. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Zhao, Y.; Zhao, Y.; Chen, F.; Zhang, Y.; Wang, F.; Li, X.; Gao, H.; Liu, W.; Jin, Y.; et al. Soybean F-Box-Like Protein GmFBL144 Interacts With Small Heat Shock Protein and Negatively Regulates Plant Drought Stress Tolerance. Front. Plant Sci. 2022, 13, 823529. [Google Scholar] [CrossRef] [PubMed]
- Purwantoro, P.; Suhartina, S.; Nugrahaeni, N.; Sulistyo, A. Response of Soybean Genotypes Introduced From South Korea to Drought Stress During Reproductive Stage. Biodiversitas J. Biol. Divers. 2017, 18, 15–19. [Google Scholar] [CrossRef]
- Sun, M.; Li, Y.; Zheng, J.; Wu, D.; Li, C.; Li, Z.; Zang, Z.; Zhang, Y.; Fang, Q.; Li, W.; et al. A Nuclear Factor Y-B Transcription Factor, GmNFYB17, Regulates Resistance to Drought Stress in Soybean. Int. J. Mol. Sci. 2022, 23, 7242. [Google Scholar] [CrossRef]
- Jabborova, D.; Annapurna, K.; Davranov, K.; Narimanov, A.A.; Enakiev, Y.I.; Syed, A.; Elgorban, A.M.; Bahkali, A.H.; Wirth, S.; Sayyed, R.Z.; et al. Co-Inoculation of Rhizobacteria Promotes Growth, Yield, and Nutrient Contents in Soybean and Improves Soil Enzymes and Nutrients Under Drought Conditions. Sci. Rep. 2021, 11, 22081. [Google Scholar] [CrossRef]
- Shakoor, N.; Adeel, M.; Ahmad, M.A.; Hussain, M.; Azeem, I.; Zain, M.; Zhou, P.; Li, Y.; Xu, M.; Rui, Y. Environment relevant concentrations of lithium influence soybean development via metabolic reprogramming. J. Hazard. Mater. 2023, 441, 129898. [Google Scholar] [CrossRef]
- Cao, X.; Yue, L.; Wang, C.; Luo, X.; Zhang, C.; Zhao, X.; Wu, F.; White, J.C.; Wang, Z.; Xing, B. Foliar Application with Iron Oxide Nanomaterials Stimulate Nitrogen Fixation, Yield, and Nutritional Quality of Soybean. ACS Nano 2022, 16, 1170–1181. [Google Scholar] [CrossRef]
- Zhou, P.; Jiang, Y.; Adeel, M.; Shakoor, N.; Zhao, W.; Liu, Y.; Li, Y.; Li, M.; Azeem, I.; Rui, Y.; et al. Nickel Oxide Nanoparticles Improve Soybean Yield and Enhance Nitrogen Assimilation. Environ. Sci. Technol. 2023, 57, 7547–7558. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, J.; Liu, L.; Wei, Z.; Qin, Q.; Bai, Q.; Zhao, C.; Zhang, S.; Wang, H. Silicon Nano-Fertilizer-Enhanced Soybean Resilience and Yield Under Drought Stress. Plants 2025, 14, 751. https://doi.org/10.3390/plants14050751
Wei J, Liu L, Wei Z, Qin Q, Bai Q, Zhao C, Zhang S, Wang H. Silicon Nano-Fertilizer-Enhanced Soybean Resilience and Yield Under Drought Stress. Plants. 2025; 14(5):751. https://doi.org/10.3390/plants14050751
Chicago/Turabian StyleWei, Jian, Lu Liu, Zihan Wei, Qiushi Qin, Qianyue Bai, Chungang Zhao, Shuheng Zhang, and Hongtao Wang. 2025. "Silicon Nano-Fertilizer-Enhanced Soybean Resilience and Yield Under Drought Stress" Plants 14, no. 5: 751. https://doi.org/10.3390/plants14050751
APA StyleWei, J., Liu, L., Wei, Z., Qin, Q., Bai, Q., Zhao, C., Zhang, S., & Wang, H. (2025). Silicon Nano-Fertilizer-Enhanced Soybean Resilience and Yield Under Drought Stress. Plants, 14(5), 751. https://doi.org/10.3390/plants14050751







