Prediction of Potential Distribution and Response of Changium smyrnioides to Climate Change Based on Optimized MaxEnt Model
Abstract
:1. Introduction
2. Results
2.1. Optimized Model and Performance Evaluation
2.2. Analysis of Key Environmental Variables and Response Curve
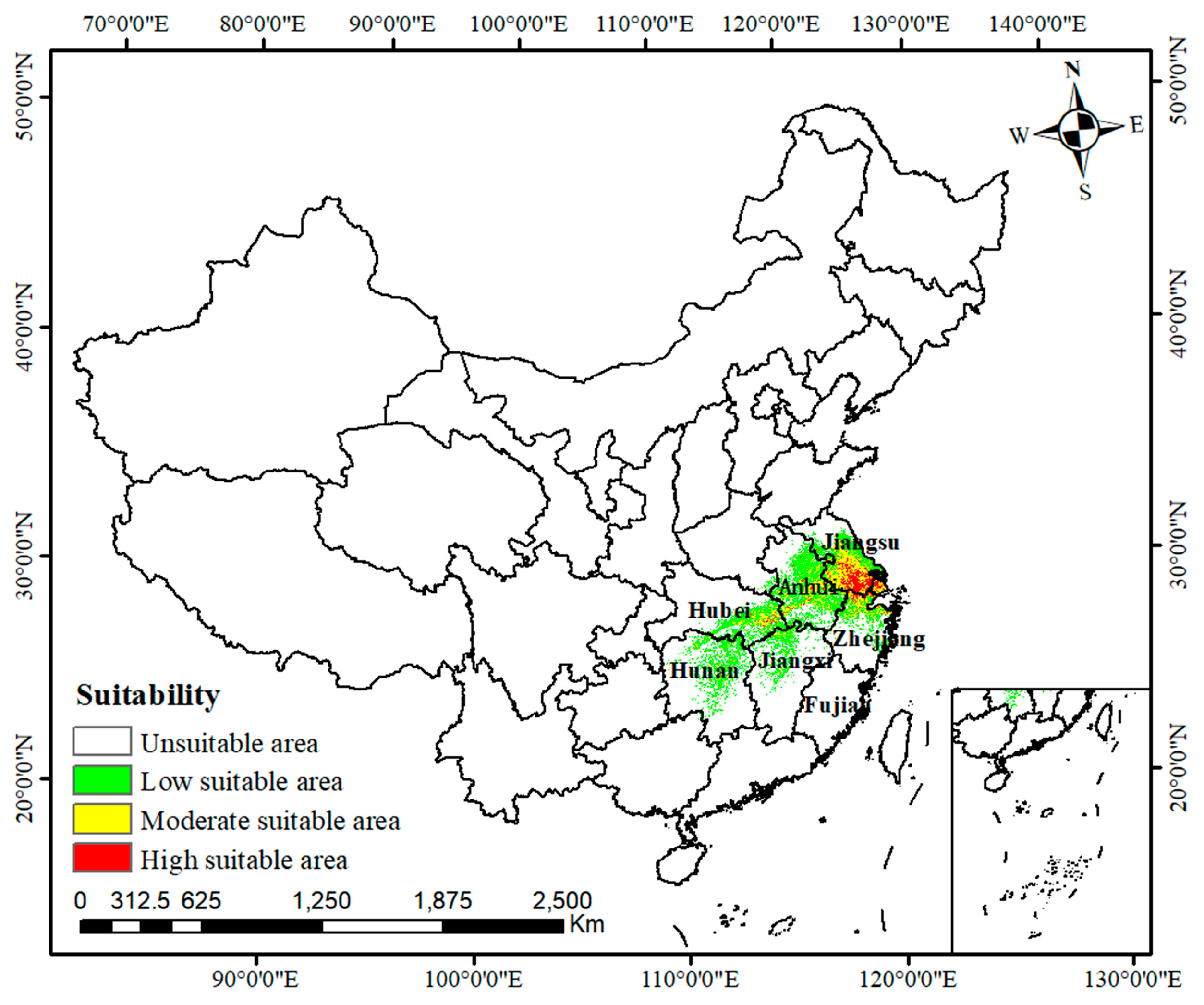
2.3. Modern Suitable Habitats for C. smyrnioides
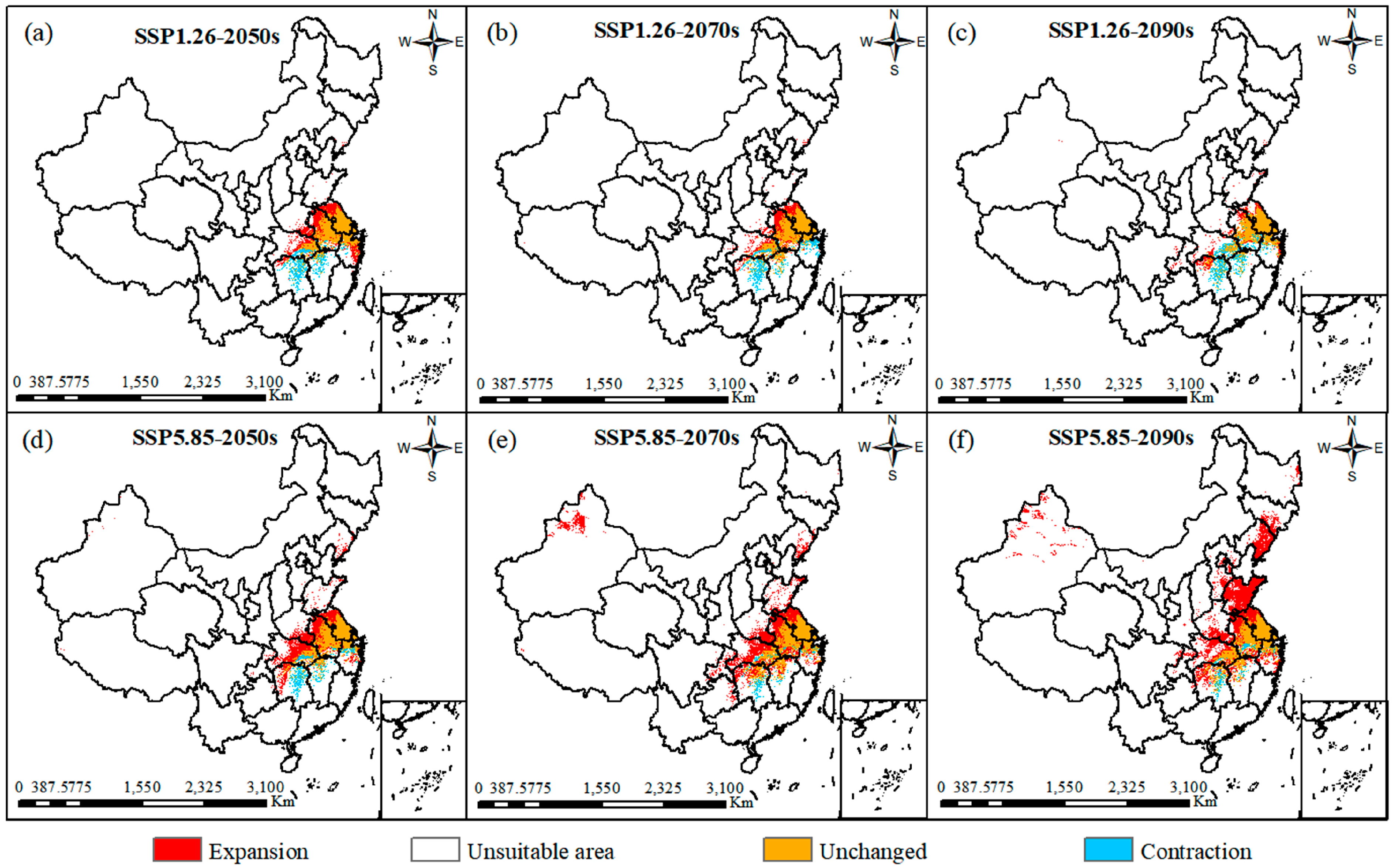
2.4. Prediction of Suitable Distribution in Future
2.5. Analysis of Dynamic Changes and Spatial Patterns
3. Discussion
3.1. Performance of Optimized MaxEnt Model
3.2. Analysis of Key Environmental Variables in Geographic Distribution of C. smyrnioides
3.3. Potential Distribution of C. smyrnioides Under Climate Changes
3.4. Conservation Strategies
- (1)
- Establishment of targeted small-scale protected areas: these areas should focus on in situ conservation, as they provide suitable living conditions for C. smyrnioides, even under future climate change scenarios.
- (2)
- Artificial cultivation in newly suitable areas: with the projected geographic expansion of suitable habitats under certain climate scenarios, provinces such as Liaoning and Shandong should be considered for the establishment of artificial cultivation areas to ensure species persistence.
- (3)
- Germplasm conservation for contracted areas: in regions like Jiangxi and Hunan, where suitable habitats are expected to contract, the establishment of germplasm banks is essential. These banks can support genetic research into the species’ adaptive mechanisms in response to climate change and promote cultivation strategies.
- (4)
- Strengthening legal frameworks and public awareness: comprehensive biodiversity protection laws and effective public outreach campaigns are critical for safeguarding C. smyrnioides and promoting sustainable conservation practices.
4. Materials and Methods
4.1. Occurrence Data Collection
4.2. Environmental Variables
4.3. Establishment of MaxEnt Model and Performance Evaluation
4.4. Classification of Suitable Habitats and Statistics
4.5. Analysis of Distribution Change
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Editorial Committee of the Flora of China. Flora of China; Science Press: Beijing, China, 1979; Volume 55, p. 122. (In Chinese) [Google Scholar]
- Institute of Botany, Chinese Academy of Sciences (Ed.) Flora of Higher Plants of China; Science Press: Beijing, China, 1972; Volume 2. (In Chinese) [Google Scholar]
- Qiu, Y.X. Conservation biology study of the endangered plant Changium smyrnioides, endemic to China. Doctoral Dissertation, Zhejiang University, Hangzhou, China, 2003. [Google Scholar]
- Chen, Y.H.; Lu, Y.W.; Yin, X.J. Predicting habitat suitability due to climate change of southwest coniferous forest tree species. J. Nanjing Univ. 2019, 43, 1–10. [Google Scholar]
- Urban, M.C. Accelerating extinction risk from climate change. Science 2015, 348, 571–573. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.D.; Yin, Q.; Sang, Z.Y.; Zhu, Z.L.; Jia, Z.K.; Ma, L.Y. Prediction of potentially suitable areas for the introduction of Magnolia wufengensis under climate change. Ecol. Indic. 2021, 127, 107762. [Google Scholar] [CrossRef]
- Shukla, P.R.; Skeg, J.; Buendia, E.C.; Masson-Delmotte, V.; Pörtner, H.-O.; Roberts, D.C.; Zhai, P.; Slade, R.; Connors, S.; van Diemen, R.; et al. Climate Change and Land: An IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems; IPCC: Geneva, Switzerland, 2019. [Google Scholar]
- Trenberth, K.E. Climate change caused by human activities is happening and it already has major consequences. J. Energy Nat. Resour. Law 2018, 36, 463–481. [Google Scholar] [CrossRef]
- Jump, A.S.; Peñuelas, J. Running to stand still: Adaptation and the response of plants to rapid climate change. Ecol. Lett. 2005, 8, 1010–1020. [Google Scholar] [CrossRef]
- Pio, D.V.; Engler, R.; Linder, H.P.; Monadjem, A.; Cotterill, P.D.F.; Taylor, J.P.; Schoeman, M.C.; Price, B.W.; Villet, M.H.; Eick, G.; et al. Climate change effects on animal and plant phylogenetic diversity in southern Africa. Glob. Chang. Biol. 2014, 20, 1538–1549. [Google Scholar] [CrossRef]
- Dieleman, C.M.; Branfireun, B.A.; McLaughlin, J.W.; Lindo, Z. Climate change drives a shift in peatland ecosystem plant community: Implications for ecosystem function and stability. Glob. Chang. Biol. 2015, 21, 388–395. [Google Scholar] [CrossRef]
- Zhao, G.H.; Cui, X.y.; Sun, J.J.; Li, T.T.; Wang, Q.; Ye, X.Z.; Fan, B.G. Analysis of the distribution pattern of Chinese Ziziphus jujuba under climate change based on optimized biomod2 and MaxEnt models. Ecol. Indic. 2021, 132, 108256. [Google Scholar] [CrossRef]
- Kong, F.; Tang, L.; He, H.; Yang, F.X.; Tao, J.; Wang, W.C. Assessing the impact of climate change on the distribution of Osmanthus fragrans using Maxent. Environ. Sci. Pollut. Res. 2021, 28, 34655–34663. [Google Scholar] [CrossRef]
- Tan, C.; Ferguson, D.K.; Yang, Y. Distribution and Conservation of Ephedra rhytidosperma. Ecol. Evol. 2025, 15, e70762. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Z.Z.; Meng, S.; Jiang, Q.; Hu, G.M.; Zhang, L.; Yao, X.H. Potential distribution under climate change and ecological niche differences between Actinidia chinensis complex. Sci. Hortic. 2024, 337, 113533. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species distribution models: Ecological explanation and prediction across space and time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Yu, H.; Fang, G.J.; Rose, K.A.; Tang, Y.L.; Song, X.F. Examining epibenthic assemblages associated with artificial reefs using a species archetype approach. Mar. Coast. Fish. 2022, 14, e10206. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, W.-J.; Zhu, Y.-Z.; Cao, Y.-T.; Yang, X.-M.; Geng, Y.; Zhang, F.-J.; Sun, R.-Q.; Jia, R.-W.; Yam, C.-L.; et al. Impacts of Climate Changes on Geographic Distribution of Primula filchnerae, an Endangered Herb in China. Plants 2023, 12, 3561. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Dudík, M.; Schapire, R.E.; Blair, M.E. Opening the black box: An open-source release of Maxent. Ecography 2017, 40, 887–893. [Google Scholar] [CrossRef]
- Graham, C.H.; Elith, J.; Hijmans, R.J.; Guisan, A.; Peterson, A.T.; Loiselle, B.A. The influence of spatial errors in species occurrence data used in distribution models. J. Appl. Ecol. 2008, 45, 239–247. [Google Scholar] [CrossRef]
- Merow, C.; Smith, M.J.; Silander, J.A., Jr. A practical guide to MaxEnt for modeling species’ distributions: What it does, and why inputs and settings matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Kass, J.M.; Muscarella, R.; Galante, P.J.; Bohl, C.L.; Pinilla-Buitrago, G.E.; Boria, R.A.; Soley-Guardia, M.; Anderson, R.P. ENMeval 2.0: Redesigned for customizable and reproducible modeling of species’ niches and distributions. Methods Ecol. Evol. 2021, 12, 1602–1608. [Google Scholar] [CrossRef]
- Cobos, M.E.; Peterson, A.T.; Barve, N.; Olvera, L.O. kuenm: An R package for detailed development of ecological niche models using Maxent. PeerJ 2019, 7, e6281. [Google Scholar] [CrossRef]
- Chen, N.N.; Yuan, L.; Wu, H.X.; Xue, J.Z. Climate change’s influence on Chelydra serpentina’s global and Chinese distribution and invasion: A MaxEnt model-based prediction. Glob. Ecol. Conserv. 2024, 54, e03137. [Google Scholar] [CrossRef]
- Yan, J.; Han, J.Z.; Chen, C.; Sun, Y.J.; Xiang, Y.N.; Xu, X.Y.; Liu, J.; Li, H.R.; Wang, Q.; Qi, Z.C.; et al. Identification and invasive potential of Opuntia humifusa complex in China: Insights from morphology, plastid genomics, and niche modeling. Glob. Ecol. Conserv. 2024, 56, e03344. [Google Scholar] [CrossRef]
- Zhang, Q.J.; Wu, X.Y.; Wang, X.; Yang, A.S.; Zhang, X.Y.; Zhao, W.M.; Li, J.F.; Li, Z.H. Evolutionary history and population dynamics of a rare and endangered medicinal plant Bergenia scopulosa (Saxifragaceae): Evidences from chloroplast genomes and ecological niche analysis. Glob. Ecol. Conserv. 2024, 54, e03097. [Google Scholar] [CrossRef]
- Meng, W.; Chen, J.W.; Li, X. Chemical Constituents of Supercritical Carbon Dioxide Extraction from the Root Bark of Changium smyrnioides Wolff. Nat. Prod. Res. 2012, 24, 764. [Google Scholar]
- Zhang, Y.; Chen, J.W.; Li, X.; Gu, Y.Y. Accumulation and Distribution of Furanocoumarins in Rare Medicinal Plants Changium smyrnioides Wolff. Nat. Prod. Res. 2012, 24, 510. [Google Scholar]
- Tao, X.Y.; Gui, X.Q.; Fu, C.X. Analysis of genetic differentiation and phylogenetic relationship between Changium smyrnioides and Chuanminshen violaceum using molecular markers and ITS sequences. J. Zhejiang Univ.-Agric. Life Sci. 2008, 34, 473–481. [Google Scholar]
- Zhao, R.F.; Wang, S.J.; Chen, S.Y. Predicting the potential habitat suitability of saussurea species in China under future climate scenarios using the optimized maximum entropy (maxent) model. J. Clean. Prod. 2024, 474, 143552. [Google Scholar] [CrossRef]
- Frenne, D.P.; Lenoir, J.; Luoto, M.; Scheffers, B.R.; Zellweger, F.; Aalto, J.; Ashcroft, M.B.; Christiansen, D.M.; Decocq, G.; De Pauw, K.; et al. Forest microclimates and climate change: Importance, drivers and future research agenda. Glob. Chang. Biol. 2021, 27, 2279–2297. [Google Scholar] [CrossRef]
- Ma, L.; Pan, J.H. Spatial identification and priority conservation areas determination of wilderness in China. J. Clean. Prod. 2024, 451, 142069. [Google Scholar] [CrossRef]
- Hu, W.J.; Wang, Y.Y.; Zhang, D.; Yu, W.W.; Chen, G.C.; Xie, T.; Liu, Z.H.; Ma, Z.Y.; Du, J.G.; Chao, B.X.; et al. Mapping the potential of mangrove forest restoration based on species distribution models: A case study in China. Sci. Total Environ. 2020, 748, 142321. [Google Scholar] [CrossRef]
- Yu, Y.; Li, Z.Q. Predicting the Potential Distribution of Cheirotonus jansoni (Coleoptera: Scarabaeidae) Under Climate Change. Insects 2024, 15, 1012. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Z.; Feng, J.N.; Hong, Y.; Du, H.R.; Zhang, M.H.; Zhang, W.Q. The Future Migration Direction of Deer and Japanese Yew Is Consistent Under Climate Change. Forests 2024, 15, 1983. [Google Scholar] [CrossRef]
- Zhang, X.F.; Nizamani, M.M.; Jiang, C.; Fang, F.Z.; Zhao, K.K. Potential planting regions of Pterocarpus santalinus (Fabaceae) under current and future climate in China based on MaxEnt modeling. Ecol. Evol. 2024, 14, e11409. [Google Scholar] [CrossRef]
- Zhao, Y.; Deng, X.W.; Xiang, W.H.; Chen, L.; Ouyang, S. Predicting potential suitable habitats of Chinese fir under current and future climatic scenarios based on Maxent model. Ecol. Inform. 2021, 64, 101393. [Google Scholar] [CrossRef]
- Wang, Y.J.; Xie, L.y.; Zhou, X.Y.; Chen, R.F.; Zhao, G.H.; Zhang, F.G. Prediction of the potentially suitable areas of Leonurus japonicus in China based on future climate change using the optimized MaxEnt model. Ecol. Evol. 2023, 13, e10597. [Google Scholar] [CrossRef]
- Zheng, Y.X.; Yuan, C.; Matsushita, N.; Lian, C.L.; Geng, Q.F. Analysis of the distribution pattern of the ectomycorrhizal fungus Cenococcum geophilum under climate change using the optimized MaxEnt model. Ecol. Evol. 2023, 13, e10565. [Google Scholar] [CrossRef]
- Fang, H.Q.; Jiang, Z.X.; Chen, S.M.; Yu, X.; Song, J.; Yang, Q.S. Predicting the distribution of potentially suitable habitat in China for Cirsium japonicum Fisch. ex DC. under future climate scenarios based on the R-optimized MaxEnt model. Ecol. Evol. 2024, 14, e11653. [Google Scholar] [CrossRef]
- Yang, J.T.; Huang, Y.; Jiang, X.; Chen, H.; Mei, L.; Wang, R.L. Potential geographical distribution of the edangred plant Isoetes under human activities using MaxEnt and GARP. Glob. Ecol. Conserv. 2022, 38, e02186. [Google Scholar] [CrossRef]
- Zhang, F.G.; Liang, F.R.; Wu, K.F.; Xie, L.Y.; Zhao, G.H.; Wang, Y.J. The potential habitat of Angelica dahurica in China under climate change scenario predicted by Maxent model. Front. Plant Sci. 2024, 15, 1388099. [Google Scholar] [CrossRef]
- Wang, C.L.; Guo, Q.S.; Zhu, Z.B.; Cheng, B.X. Physiological characteristics, dry matter, and active component accumulation patterns of Changium smyrnioides in response to a light intensity gradient. Pharm. Biol. 2017, 55, 581–589. [Google Scholar] [CrossRef]
- Bao, Z.Z.; Zhu, Z.Y.; Gai, Y.A. The complete chloroplast genome of Changium smyrnioides Wolff. Mitochondrial DNA Part B 2019, 4, 4081–4082. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.L.; Tian, F.P.; Ren, G.H.; Liu, Z.H. Seed mass increase along altitude within four Saussurea (Asteraceae) species in Tibetan Plateau. Pol. J. Ecol. 2011, 59, 617–622. [Google Scholar]
- He, Z.H.; Webster, S.; He, S.Y. Growth–defense trade-offs in plants. Curr. Biol. 2022, 32, R634–R639. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.X.; Fu, C.X. Studies on the endangerment mechanism of and conservation strategies for Changium smyrnioides. Sheng Wu Duo Yang Xing 2001, 9, 151. [Google Scholar]
- O’Donnell, M.S.; Ignizio, D.A. Bioclimatic Predictors for Supporting Ecological Applications in the Conterminous United States; U.S. Geological Survey Data Series 691; U.S. Geological Survey: Reston, VA, USA, 2012; 10p. [Google Scholar]
- Nwoko, O.E.; Manyangadze, T.; Chimbari, M.J. Predicted changes in habitat suitability for human schistosomiasis intermediate host snails for modelled future climatic conditions in KwaZulu-Natal, South Africa. Front. Environ. Sci. 2023, 11, 1243777. [Google Scholar] [CrossRef]
- Arnold, P.A.; Wang, S.; Catling, A.A.; Kruuk, L.E.B.; Nicotra, A.B. Patterns of phenotypic plasticity along a thermal gradient differ by trait type in an alpine plant. Funct. Ecol. 2022, 36, 2412–2428. [Google Scholar] [CrossRef]
- Sheldon, K.S.; Leaché, A.D.; Cruz, F.B. The influence of temperature seasonality on elevational range size across latitude: A test using Liolaemus lizards. Glob. Ecol. Biogeogr. 2015, 24, 632–641. [Google Scholar] [CrossRef]
- Devireddy, A.R.; Tschaplinski, T.J.; Tuskan, G.A.; Muchero, W.; Chen, J.G. Role of reactive oxygen species and hormones in plant responses to temperature changes. Int. J. Mol. Sci. 2021, 22, 8843. [Google Scholar] [CrossRef]
- Sharafati, A.; Pezeshki, E. A strategy to assess the uncertainty of a climate change impact on extreme hydrological events in the semi-arid Dehbar catchment in Iran. Theor. Appl. Climatol. 2020, 139, 389–402. [Google Scholar] [CrossRef]
- Huntley, B.; Collingham, Y.C.; Green, R.E.; Hilton, G.M.; Rahbek, C.; Willis, S.G. Potential impacts of climatic change upon geographical distributions of birds. Ibis 2006, 148, 8–28. [Google Scholar] [CrossRef]
- Trew, B.T.; Maclean, I.M.D. Vulnerability of global biodiversity hotspots to climate change. Glob. Ecol. Biogeogr. 2021, 30, 768–783. [Google Scholar] [CrossRef]
- Chen, I.C.; Hill, J.K.; Ohlemüller, R.; Roy, D.B.; Thomas, C.D. Rapid range shifts of species associated with high levels of climate warming. Science 2011, 333, 1024–1026. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tang, J.S.; Ren, G.; Zhao, K.x.; Wang, X.F. Global potential distribution prediction of Xanthium italicum based on Maxent model. Sci. Rep. 2021, 11, 16545. [Google Scholar] [CrossRef]
- Khalaf, S.M.H.; Alqahtani, M.S.M.; Ali, M.R.M.; Abdelalim, I.T.T.; Hodhod, M.S. Using maxent modeling to analyze climate change impacts on Pseudomonas syringae van Hall, 1904 distribution on the global scale. Heliyon 2024, 10, e41017. [Google Scholar] [CrossRef]
- Gao, X.Q.; Lin, F.R.; Li, M.; Mei, Y.J.; Li, Y.X.; Bai, Y.L.; He, X.L.; Zheng, Y.Q. Prediction of the potential distribution of a raspberry (Rubus idaeus) in China based on MaxEnt model. Sci. Rep. 2024, 14, 24438. [Google Scholar] [CrossRef]
- Shan, Y.X.; Lu, Z.R.; Yan, L.M.; Rosseleena, E.R.; Du, F.P.; Fan, Z.W.; Han, R.C.; Tong, X.H. Prediction of Platycodon grandiflorus distribution in China using MaxEnt model concerning current and future climate change. Nord. J. Bot. 2024, 2024, e04502. [Google Scholar] [CrossRef]
- Ye, X.Z.; Zhao, G.H.; Zhang, M.Z.; Cui, X.Y.; Fan, H.H.; Liu, B. Distribution pattern of endangered plant Semiliquidambar cathayensis (Hamamelidaceae) in response to climate change after the last interglacial period. Forests 2020, 11, 434. [Google Scholar] [CrossRef]
- Chi, Y.; Wang, G.G.; Zhu, M.X.; Jin, P.; Hu, Y.; Shu, P.Z.; Wang, Z.X.; Fan, A.F.; Qian, P.H.; Han, Y.; et al. Potentially suitable habitat prediction of Pinus massoniana Lamb. in China under climate change using Maxent model. Front. For. Glob. Chang. 2023, 6, 1144401. [Google Scholar] [CrossRef]
- Wang, X.Y.; Li, Z.S.; Zhang, L.J.; Wang, Y.L.; Liu, Y.; Ma, Y.S. The optimized Maxent model reveals the pattern of distribution and changes in the suitable cultivation areas for Reaumuria songarica being driven by climate change. Ecol. Evol. 2024, 14, e70015. [Google Scholar] [CrossRef]
- Wu, Y.M.; Shen, X.L.; Tong, L.; Lei, L.T.; Mu, X.Y.; Zhang, Z.X. Impact of past and future climate change on the potential distribution of an endangered montane shrub Lonicera oblata and its conservation implications. Forests 2021, 12, 125. [Google Scholar] [CrossRef]
- Dong, Z.Y.; Ma, L.; Gao, S.H.; Han, P.; Zhang, Q.; Bao, Y.F. Potential habitat prediction of Tetraena mongolica and its GAP analysis with nature reserves. Arid. Land Geogr. 2023, 46, 595–603. [Google Scholar]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Fonseca, G.A.B.D.; Ken, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Song, C.F.; Liu, Y.L.; Chu, X.F.; Liu, Q.X. Investigation on the Population Distribution of Changium smyrnioides and Chuanminshen violaceum. Chin. Wild Plant Resour. 2012, 31, 58–61. (In Chinese) [Google Scholar]
- Warren, D.L.; Matzke, N.J.; Cardillo, M.; Baumgarner, J.B.; Beaumont, L.J.; Turelli, M.; Glor, R.E.; Huron, N.A.; Simões, M.; Iglesias, T.L.; et al. ENMTools 1.0: An R package for comparative ecological biogeography. Ecography 2021, 44, 504–511. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2.1: New 1-Km Spatial Resolution Climate Surfaces for Global Land Areas. Available online: https://worldclim.org/data/index.html (accessed on 5 October 2024).
- Wu, T.W.; Lu, Y.X.; Fang, Y.J.; Xin, X.G.; Li, L.; Li, W.P.; Jie, W.H.; Zhang, J.; Liu, Y.M.; Zhang, L.; et al. The Beijing climate center climate system model (BCC-CSM): The main progress from CMIP5 to CMIP6. Geosci. Model Dev. 2019, 12, 1573–1600. [Google Scholar] [CrossRef]
- GSCloud. Available online: https://www.gscloud.cn/#page1/2 (accessed on 5 September 2024).
- Food and Agriculture Organization of the United Nations. Available online: http://www.fao.org/faostat/en/#data (accessed on 6 September 2024).
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Shi, X.D.; Wang, J.W.; Zhang, L.; Chen, S.X.; Zhao, A.L.; Ning, X.D.; Fan, G.R.; Wu, N.S.; Zhang, L.; Wang, Z.D. Prediction of the potentially suitable areas of Litsea cubeba in China based on future climate change using the optimized MaxEnt model. Ecol. Indic. 2023, 148, 110093. [Google Scholar] [CrossRef]
- Giraldo, D.C.; Kucuker, D.M. Ecological niche modeling of Lactarius deliciosus using kuenm R package: Insights into habitat preferences. Fungal Biol. 2024, 128, 2022–2031. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Modell. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Yan, H.Y.; Feng, L.; Zhao, Y.F.; Feng, L.; Wu, D.; Zhu, C.P. Prediction of the spatial distribution of Alternanthera philoxeroides in China based on ArcGIS and MaxEnt. Glob. Ecol. Conserv. 2020, 21, e00856. [Google Scholar] [CrossRef]
- Wang, L.R.; Ma, L.Y.; Wang, X.Q.; He, S.C.; Liu, X. Seedling techniques of Magnolia wufengensis and M. wufengensis var. multitepala. J. Zhejiang Agric. For. Univ. 2007, 24, 242–246. [Google Scholar]
- Li, N.Q.; Xu, G.Y. Grid analysis of land use based on natural breaks (jenks) classification. Bull. Surv. Mapp. 2020, 106–110, 156. [Google Scholar] [CrossRef]
- Brown, J.L. SDM toolbox: A python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. Methods Ecol. Evol. 2014, 5, 694–700. [Google Scholar] [CrossRef]
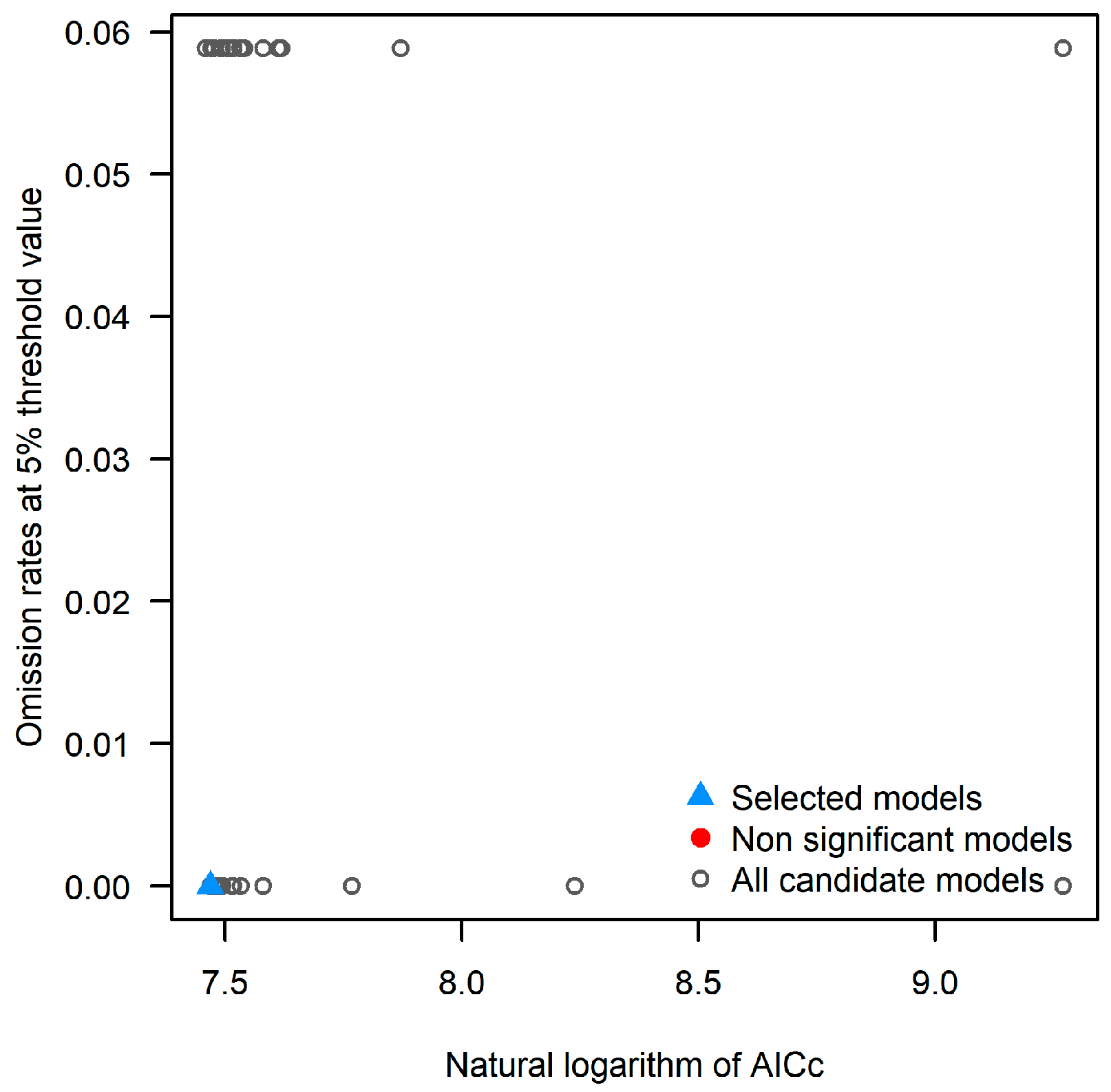
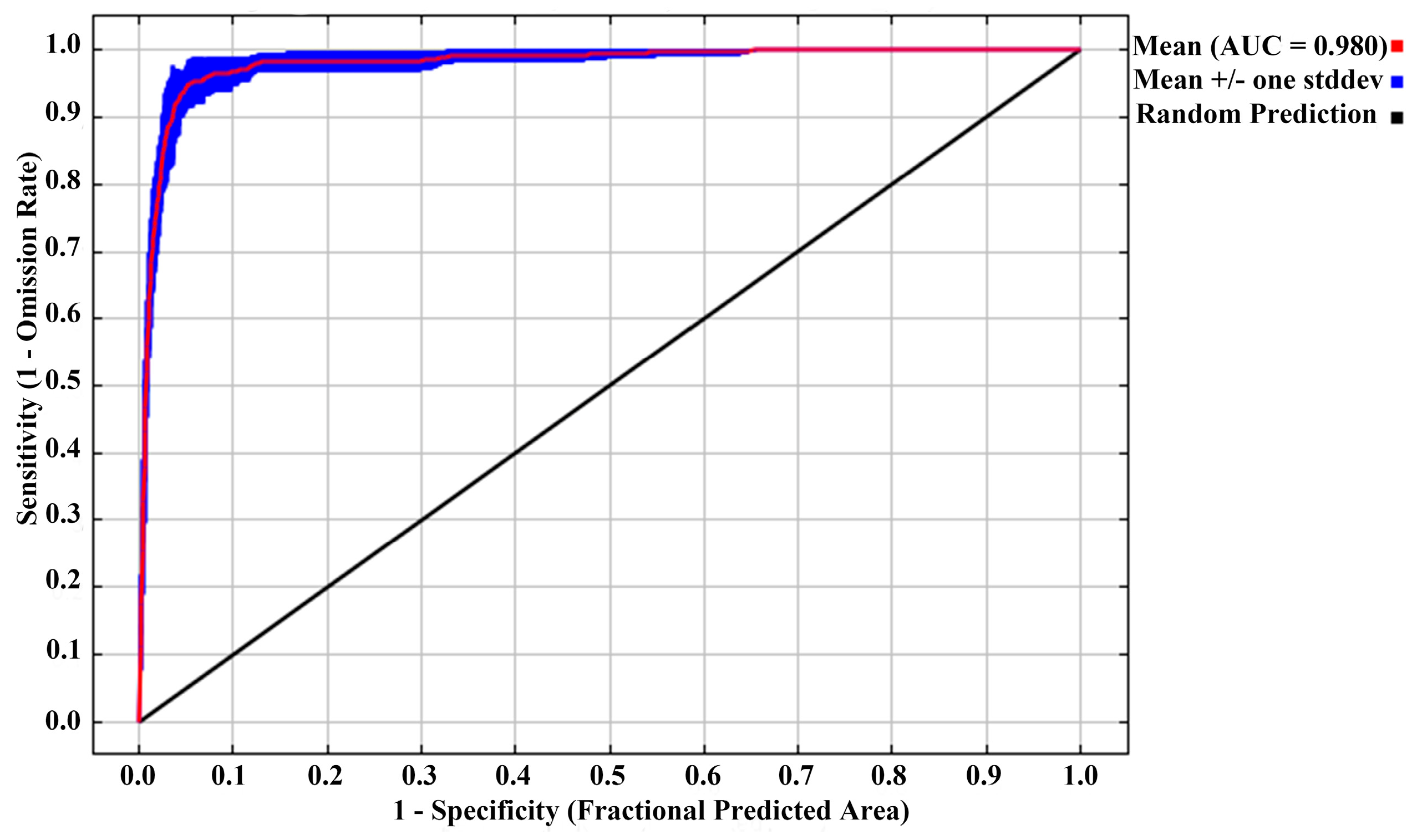





| Type | RM | FC | Mean AUC Ratio | Omission Rate at 5% | Delta AICc |
|---|---|---|---|---|---|
| Default | 1 | lqph | 1.961 | 0 | 2048.245 |
| Optimization | 0.5 | lq | 1.973 | 0.059 | 0 |
| Climate Scenarios | Unsuitable Area (106 Km) | Low-Suitability Area (104 Km) | Moderately Suitable Area (104 Km) | Highly Suitable Area (104 Km) | Total Suitable Area (104 Km) |
|---|---|---|---|---|---|
| Current | 930.43 | 20.11 | 7.35 | 2.11 | 29.57 |
| SSP1.26-2050s | 922.23 | 12.88 | 7.87 | 17.03 | 37.78 |
| SSP1.26-2070s | 925.91 | 14.23 | 7.71 | 12.14 | 34.08 |
| SSP1.26-2090s | 929.68 | 16.28 | 8.25 | 5.79 | 30.32 |
| SSP5.85-2050s | 912.33 | 20.02 | 12.32 | 15.34 | 47.68 |
| SSP5.85-2070s | 893.09 | 38.17 | 11.09 | 17.66 | 66.92 |
| SSP5.85-2090s | 872.53 | 55.27 | 22.02 | 10.18 | 87.47 |
| Climate Scenarios | Expansion (105 Km) | Stable (105 Km) | Contraction (104 Km) |
|---|---|---|---|
| Current_SSP1.26-2050s | 1.539 | 2.19 | 7.32 |
| Current_SSP1.26-2070s | 1.16 | 2.20 | 7.19 |
| Current_SSP1.26-2090s | 0.89 | 2.10 | 8.21 |
| Current_SSP5.85-2050s | 2.38 | 2.33 | 5.94 |
| Current_SSP5.85-2070s | 3.99 | 2.61 | 3.11 |
| Current_SSP5.85-2090s | 6.04 | 2.60 | 3.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, X.; Jiang, X.; Chen, Y.; Li, C.; Ding, S.; Zhang, X.; Luo, L.; Jia, Y.; Zhao, G. Prediction of Potential Distribution and Response of Changium smyrnioides to Climate Change Based on Optimized MaxEnt Model. Plants 2025, 14, 743. https://doi.org/10.3390/plants14050743
Zhu X, Jiang X, Chen Y, Li C, Ding S, Zhang X, Luo L, Jia Y, Zhao G. Prediction of Potential Distribution and Response of Changium smyrnioides to Climate Change Based on Optimized MaxEnt Model. Plants. 2025; 14(5):743. https://doi.org/10.3390/plants14050743
Chicago/Turabian StyleZhu, Xingyu, Xin Jiang, Ying Chen, Congcong Li, Shi Ding, Xuejiao Zhang, Lulu Luo, Yun Jia, and Gang Zhao. 2025. "Prediction of Potential Distribution and Response of Changium smyrnioides to Climate Change Based on Optimized MaxEnt Model" Plants 14, no. 5: 743. https://doi.org/10.3390/plants14050743
APA StyleZhu, X., Jiang, X., Chen, Y., Li, C., Ding, S., Zhang, X., Luo, L., Jia, Y., & Zhao, G. (2025). Prediction of Potential Distribution and Response of Changium smyrnioides to Climate Change Based on Optimized MaxEnt Model. Plants, 14(5), 743. https://doi.org/10.3390/plants14050743







