Genetic Variation of Growth Traits and Seed Production in a Patagonian Native Pasture in Semiarid Rangelands Under Different Environmental Settings
Abstract
1. Introduction
2. Results
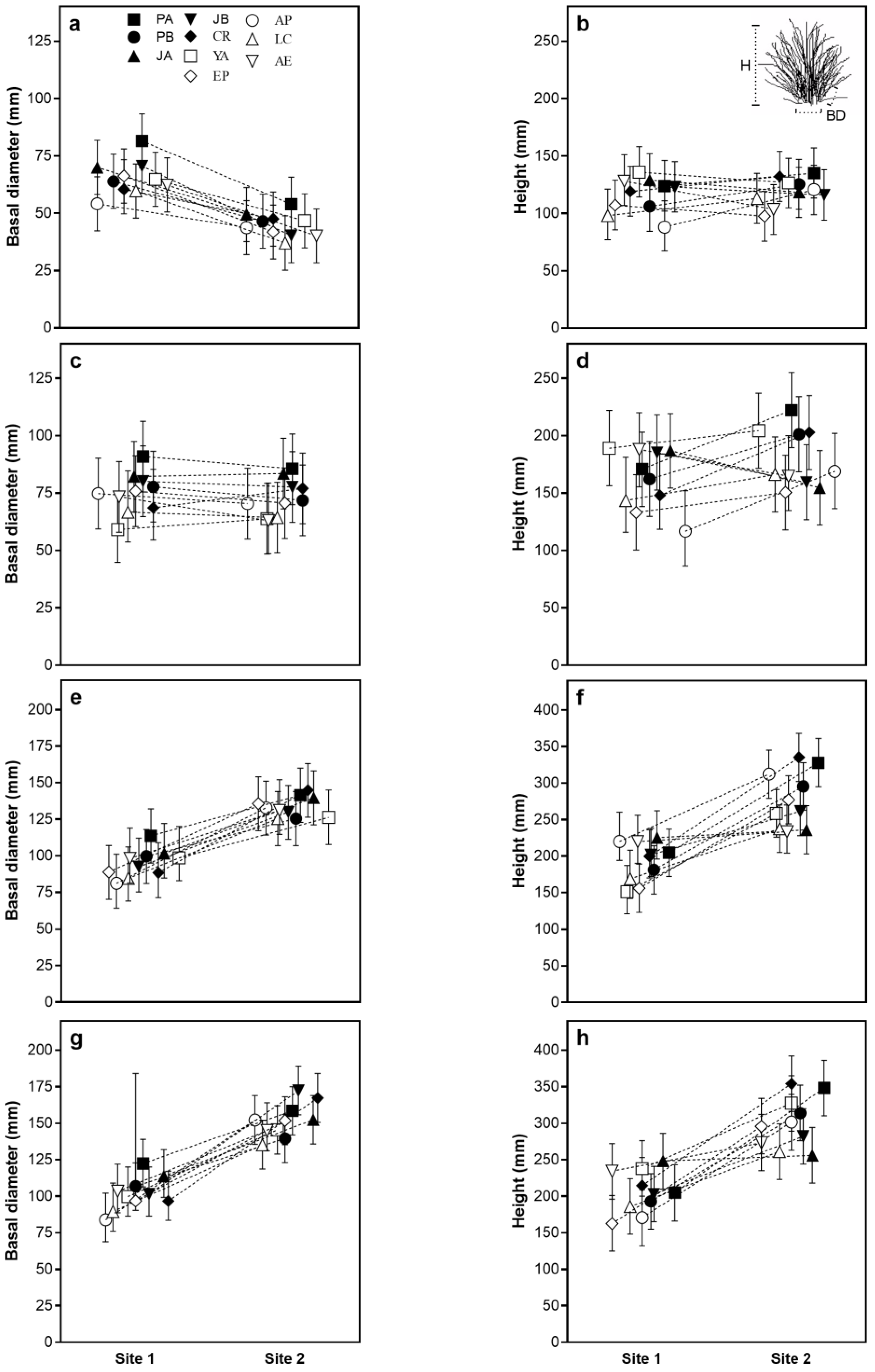
2.1. Growth Traits
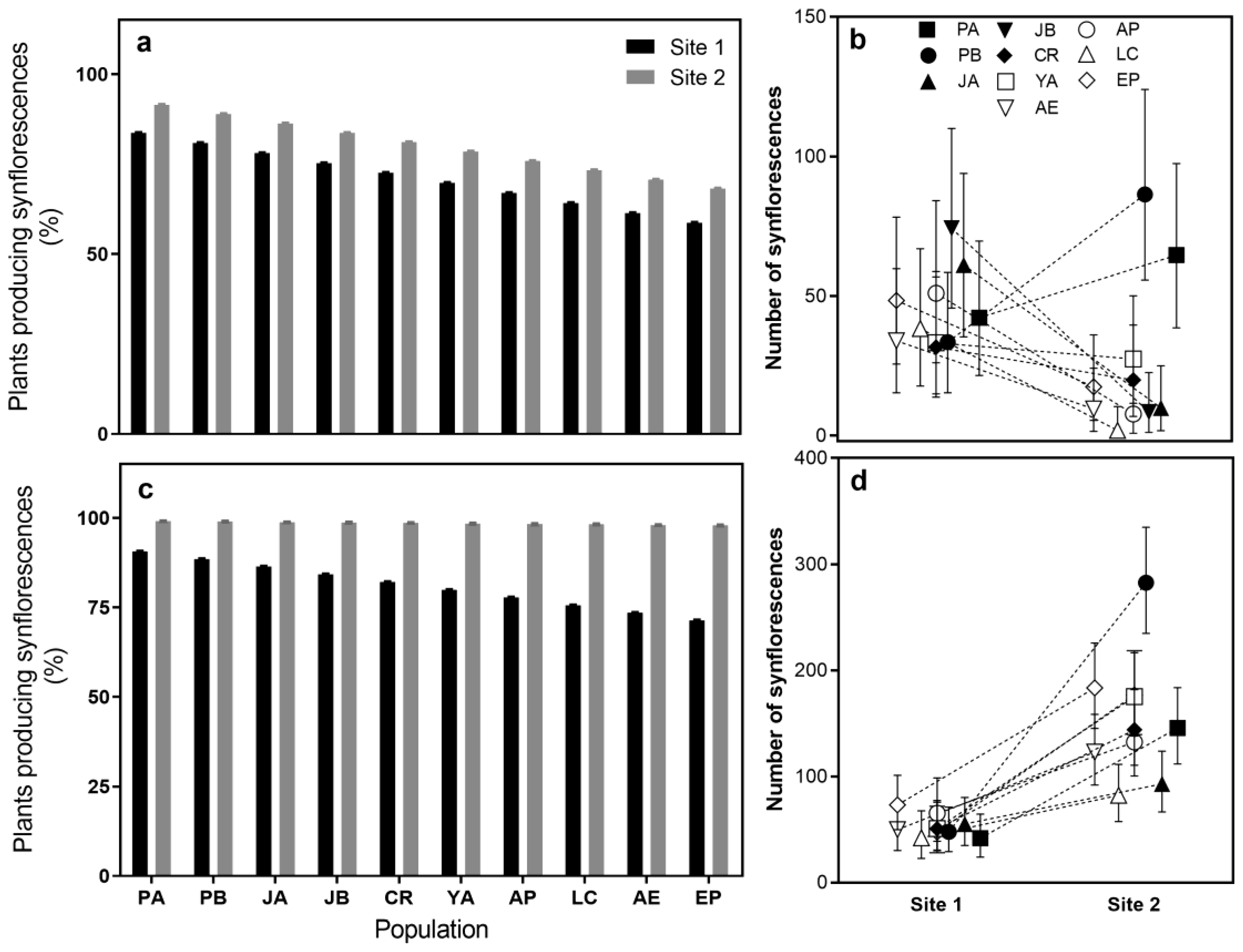
2.2. Reproductive Traits
3. Discussion
Implications for the Selection of Breeding Materials
4. Materials and Methods
4.1. Plant Material
4.2. Experimental Design
4.3. Growth and Reproductive Periods
4.4. Growth Traits Measurements
4.5. Reproductive Traits Measurements
4.6. Data Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Villagra, E.S.; Pelliza, A.; Willems, P.; Siffredi, G.; Bonvissuto, G. What do domestic livestock eat in northern Patagonian rangelands? Anim. Prod. Sci. 2013, 53, 360–367. [Google Scholar] [CrossRef]
- Barros, V.; Camilloni, I. La Argentina y el Cambio Climático. De la Física a la Política; Editorial Eudeba: Buenos Aires, Argentina, 2016. [Google Scholar]
- Morgan, J.W.; Salmon, K.L. Dominant C3 tussock grasses are resilient to the re-introduction of fire in long-unburned temperate grasslands. Appl. Veg. Sci. 2020, 23, 149–158. [Google Scholar] [CrossRef]
- Castillo, D.A.; Gaitán, J.J.; Villagra, E.S. Direct and indirect effects of climate and vegetation on sheep production across Patagonian rangelands (Argentina). Ecol. Indic. 2021, 124, 107417. [Google Scholar] [CrossRef]
- Carrera, A.L.; Bertiller, M.B. Relationships among plant litter, fine roots, and soil organic C and N across an aridity gradient in northern Patagonia, Argentina. Ecoscience 2010, 17, 276–286. [Google Scholar] [CrossRef]
- Derner, J.D.; Briske, D.D.; Polley, H.W. Tiller organization within the tussock grass Schizachyrium scoparium: A field assessment of competition–cooperation tradeoffs. Botany 2012, 90, 669–677. [Google Scholar] [CrossRef]
- Campanella, M.V.; Bisigato, A.J.; Rostagno, C.M. Plant production along a grazing gradient in a semiarid Patagonian rangeland, Argentina. Plant Ecol. 2016, 217, 1553–1562. [Google Scholar] [CrossRef]
- Gonzalez, S.L.; Ghermandi, L. Overgrazing causes a reduction in the vegetation cover and seed bank of Patagonian grasslands. Plant Soil 2021, 464, 75–87. [Google Scholar] [CrossRef]
- Reisch, C.; Schmid, C. Species and genetic diversity are not congruent in fragmented dry grasslands. Ecol. Evol. 2019, 9, 664–671. [Google Scholar] [CrossRef]
- Kölliker, R.; Stadelmann, F.J.; Reidy, B.; Nösberger, J. Fertilization and defoliation frequency affect genetic diversity of Festuca pratensis Huds. in permanent grasslands. Mol. Ecol. 1998, 7, 1557–1567. [Google Scholar] [CrossRef]
- Rouet, S.; Barillot, R.; Leclercq, D.; Bernicot, M.H.; Combes, D.; Escobar-Gutiérrez, A.; Durand, J.L. Interactions between environment and genetic diversity in perennial grass phenology: A review of processes at plant scale and modeling. Front. Plant Sci. 2021, 12, 672156. [Google Scholar] [CrossRef] [PubMed]
- Defossé, G.E.; Bertiller, M.B.; Ares, J.O. Above-ground phytomass dynamics in a grassland steppe of Patagonia, Argentina. Rangel. Ecol. Manag. J. Range Manag. Arch. 1990, 43, 157–160. [Google Scholar] [CrossRef]
- Ghermandi, L.; Gonzalez, S. Diversity and functional groups dynamics affected by drought and fire in Patagonian grasslands. Ecoscience 2009, 16, 408–417. [Google Scholar] [CrossRef]
- Oliva, G.; Collantes, M.; Humano, G. Reproductive effort and seed establishment in grazed tussock grass populations of Patagonia. Rangel. Ecol. Manag. 2013, 66, 164–173. [Google Scholar] [CrossRef]
- Mederos-Ramírez, A.; Ortiz-Pérez, R. Análisis de la interacción genotipo ambiente en el cultivo de la soya (Glycine max (L) Merrill). Cultiv. Trop. 2021, 42, e10. [Google Scholar]
- Ott, J.P.; Hartnett, D.C. Bud production and dynamics of flowering and vegetative tillers in Andropogon gerardii (Poaceae): The role of developmental constraints. Am. J. Bot. 2011, 98, 1293–1298. [Google Scholar] [CrossRef] [PubMed]
- Nelson, C.J.; Moore, K.J. Grass Morphology. Forages Sci. Grassl. Agric. 2020, 2, 23–49. [Google Scholar]
- Brummer, E.C.; Barber, W.T.; Collier, S.M.; Cox, T.S.; Johnson, R.; Murray, S.C.; Thro, A.M. Plant breeding for harmony between agriculture and the environment. Front. Ecol. Environ. 2011, 9, 561–568. [Google Scholar] [CrossRef]
- Karunarathne, P.; Feduzka, C.; Diego, H. Ecological setup, ploidy diversity, and reproductive biology of Paspalum modestum, a promising wetland forage grass from South America. Genet. Mol. Biol. 2020, 43, e20190101. [Google Scholar] [CrossRef]
- Marchelli, P.; López, A.S.; Azpilicueta, M.M.; Nagahama, N.; López, D.R.; Caballe, G.; Siffredi, G.L. Pastizales patagónicos: Avances en la domesticación del coirón blanco con fines productivos y de restauración ecosistémica. In Revista de Información sobre Investigación y Desarrollo Agrobioindustrial; Instituto Nacional de Tecnología Agropecuaria: Hurlingham, Argentina, 2021; Volume 1, pp. 55–62. [Google Scholar]
- Moore, V.M.; Schlautman, B.; Fei, S.Z.; Roberts, L.M.; Wolfe, M.; Ryan, M.R.; Lorenz, A.J. Plant breeding for intercropping in temperate field crop systems: A review. Front. Plant Sci. 2022, 13, 843065. [Google Scholar] [CrossRef]
- Villellas, J.; Ehrlén, J.; Crone, E.; Csergő, A.; Garcia, M.; Laine, A.L.; Buckley, Y. Genetic Differentiation Can be Predicted from Observational Data for Reproductive but Not Vegetative Traits in a Widespread Short-Lived Plant. Authorea Preprints. 2021. Available online: https://d197for5662m48.cloudfront.net/documents/publicationstatus/55491/preprint_pdf/9a7fa980b6128254db29dd8fb8b05b01.pdf (accessed on 9 September 2021).
- Gaitán, J.J.; Bran, D.E.; Oliva, G. Patagonian Desert. In Encyclopedia of the World’s Biomes; Elsevier: Amsterdam, The Netherlands, 2020; pp. 163–180. [Google Scholar]
- Oliva, G.; Gaitan, J.; Ferrante, D. Humans cause deserts: Evidence of irreversible changes in Argentinian Patagonia rangelands. In The End of Desertification? Disputing Environmental Change in the Drylands; Springer: Berlin/Heidelberg, Germany, 2016; pp. 363–386. [Google Scholar]
- Oliva, G.; Paredes, P.; Ferrante, D.; Cepeda, C.; Rabinovich, J. Remotely sensed primary productivity shows that domestic and native herbivores combined are overgrazing Patagonia. J. Appl. Ecol. 2019, 56, 1575–1584. [Google Scholar] [CrossRef]
- Bertiller, M.B.; Irisarri, M.P.; Ares, J.O. Phenology of Festuca pallescens in relation to topography in north-western Patagonia. J. Veg. Sci. 1990, 1, 579–584. [Google Scholar] [CrossRef]
- Bertiller, M.B. Seasonal variation in the seed bank of a Patagonian grassland in relation to grazing and topography. J. Veg. Sci. 1992, 3, 47–54. [Google Scholar] [CrossRef]
- León, R.J.; Bran, D.; Collantes, M.; Paruelo, J.M.; Soriano, A. Grandes unidades de vegetación de la Patagonia extra andina. Ecol. Austral 1998, 8, 125–144. [Google Scholar]
- Franzese, J.; Ghermandi, L. Effect of fire on recruitment of two dominant perennial grasses with different palatability from semi-arid grasslands of NW Patagonia (Argentina). Plant Ecol. 2012, 213, 471–481. [Google Scholar] [CrossRef]
- Franzese, J.; Ghermandi, L. Early competition between the exotic herb Rumex acetosella and two native tussock grasses with different palatability and water stress tolerance. J. Arid Environ. 2014, 106, 58–62. [Google Scholar] [CrossRef]
- Velasco, V.; Siffredi, G.L. Guía para el reconocimiento de especies de los pastizales de sierras y mesetas occidentales de Patagonia. 2009. Ley Ovina UEP Río Negro; Proyecto Manejo Sustentable de Ecosistemas Áridos y Semiáridos para el control de la Desertificación en la Patagonia (GEF Patagonia); Ediciones INTA e INTA EEA Bariloche–Proyecto Regional “Gestión de sistemas productivos sustentables en el área de secano de Río Negro y Neuquén”. pp. 18–19. Available online: https://www.academia.edu/40090760/Instituto_Nacional_de_Tecnolog%C3%ADa_Agropecuaria_Ediciones_Gu%C3%ADa_para_el_reconocimiento_de_especies_de_los_pastizales_de_sierras_y_mesetas_occidentales_de_Patagonia_Ley_Ovina. (accessed on 20 July 2021).
- López, A.S.; Marchelli, P.; Batlla, D.; López, D.R.; Arana, M.V. Seed responses to temperature indicate different germination strategies among Festuca pallescens populations from semi-arid environments in North Patagonia. Agric. For. Meteorol. 2019, 272, 81–90. [Google Scholar] [CrossRef]
- López, A.S.; López, D.R.; Caballé, G.; Siffredi, G.L.; Marchelli, P. Local adaptation along a sharp rainfall gradient occurs in a native Patagonian grass, Festuca pallescens, regardless of extensive gene flow. Environ. Exp. Bot. 2020, 171, 103933. [Google Scholar] [CrossRef]
- López, A.S.; López, D.R.; Arana, M.V.; Batlla, D.; Marchelli, P. Germination response to water availability in populations of Festuca pallescens along a Patagonian rainfall gradient based on hydrotime model parameters. Sci. Rep. 2021, 11, 10653. [Google Scholar] [CrossRef] [PubMed]
- López, A.S.; López, D.R.; Caballe, G.; Edwards, P.; Marchelli, P. Do populations of Festuca pallescens from a rainfall gradient differ in the expression of morpho-physiological traits under drought stress? Environ. Exp. Bot. 2023, 210, 105335. [Google Scholar] [CrossRef]
- Quiroga, R.E.; Fernández, R.J.; Golluscio, R.A.; Blanco, L.J. Differential water-use strategies and drought resistance in Trichloris crinita plants from contrasting aridity origins. Plant Ecol. 2013, 214, 1027–1035. [Google Scholar] [CrossRef]
- Kozub, P.C.; Cavagnaro, J.B.; Cavagnaro, P.F. Exploiting genetic and physiological variation of the native forage grass Trichloris crinita for revegetation in arid and semi-arid regions: An integrative review. Grass Forage Sci. 2018, 73, 257–271. [Google Scholar] [CrossRef]
- Toledo, S.; Peri, P.L.; Fontenla, S.B. Environmental conditions and grazing exerted effects on arbuscular mycorrhizal in plants at southern Patagonia rangelands. Rangel. Ecol. Manag. 2022, 81, 44–54. [Google Scholar] [CrossRef]
- Chaudhry, S.; Sidhu GP, S. Climate change regulated abiotic stress mechanisms in plants: A comprehensive review. Plant Cell Rep. 2022, 41, 1–31. [Google Scholar] [CrossRef]
- Oñatibia, G.R.; Aguiar, M.R. Grasses and grazers in arid rangelands: Impact of sheep management on forage and non-forage grass populations. J. Environ. Manag. 2019, 235, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Gil-Báez, C.G.; Agüero, R.O.; Ernst, R.D.; Ruiz, M.A. Caracterización morfológica, biomasa aérea y calidad en distintas poblaciones de Trichloris crinita. Arch. De Zootec. 2015, 64, 49–56. [Google Scholar] [CrossRef][Green Version]
- Godagnone, R.; Bran, D.E. Inventario Integrado de los Recursos Naturales de la Provincia de Río Negro: Geología, Hidrogeología, Geomorfología, Suelos, Clima, Vegetación Y Fauna; INTA: Buenos Aires, Argentina, 2009. [Google Scholar]
- Dahlgren, R.A.; Saigusa, M.; Ugolini, F.C. The nature, properties and management of volcanic soils. Adv. Agron. 2004, 82, 113–182. [Google Scholar]
- Münzbergová, Z.; Hadincová, V. Transgenerational plasticity as an important mechanism affecting response of clonal species to changing climate. Ecol. Evol. 2017, 7, 5236–5247. [Google Scholar] [CrossRef]
- Reyes, M.F.; Aguiar, M.R. Mind the gap among patches in arid plant communities: Rapid root proliferation in response to N addition. J. Plant Ecol. 2019, 12, 89–95. [Google Scholar] [CrossRef]
- Bertiller, M.B.; Elissalde, N.O.; Rostagno, C.M.; Defossé, G.E. Environmental patterns and plant distribution along aprecipitation gradient in western Patagonia. J. Arid Environ. 1995, 29, 85–97. [Google Scholar] [CrossRef]
- Fernández, M.E.; Gyenge, J.E.; Schlichter, T.M. Growth of Festuca pallescens in silvopastoral systems in Patagonia, Part 1: Positive balance between competition and facilitation. Agrofor. Syst. 2006, 66, 259–269. [Google Scholar] [CrossRef]
- Moreno, L.; Bertiller, M.B. Phenotypic plasticity of morpho-chemical traits of perennial grasses from contrasting environments of arid Patagonia. J. Arid Environ. 2015, 116, 96–102. [Google Scholar] [CrossRef]
- Vilela, A.E.; González-Paleo, L. Changes in resource-use strategy and phenotypic plasticity associated with selection for yield in wild species native to arid environments. J. Arid Environ. 2015, 113, 51–58. [Google Scholar] [CrossRef]
- Leva, P.E.; Aguiar, M.R.; Premoli, A.C. Latitudinal variation of genecological traits in native grasses of Patagonian rangelands. Aust. J. Bot. 2013, 61, 475–485. [Google Scholar] [CrossRef]
- Oñatibia, G.R.; Aguiar, M.R. Continuous moderate grazing management promotes biomass production in Patagonian arid rangelands. J. Arid Environ. 2016, 125, 73–79. [Google Scholar] [CrossRef]
- Pittaro, M.G.; Duchini, P.G.; Guzatti, G.C.; Sbrissia, A.F. Unraveling the forage productivity puzzle: Comparing fast and slow-growing grasses. PLoS ONE 2024, 19, e0306692. [Google Scholar] [CrossRef]
- López, A.S.; Azpilicueta, M.M.; López, D.R.; Siffredi, G.L.; Marchelli, P. Phylogenetic relationships and intraspecific diversity of a North Patagonian Fescue: Evidence of differentiation and interspecific introgression at peripheral populations. Folia Geobot. 2018, 53, 115–131. [Google Scholar] [CrossRef]
- Guidalevich, V.; Nagahama, N.; López, A.S.; Angeli, J.P.; Marchelli, P.; Azpilicueta, M.M. Intraspecific phylogeny of a Patagonian fescue: Differentiation at molecular markers and morphological traits suggests hybridization at peripheral populations. Ann. Bot. 2023, 131, 1011–1023. [Google Scholar] [CrossRef]
- Bran, D.; Ayesa, J.; López, C. Regiones Ecológicas de Río Negro. Comunicación Técnica No 59 EEA INTA San Carlos de Bariloche–Río Negro; INTA: Bariloche, Argentina, 2000. [Google Scholar]
- Walter, A.; Studer, B.; Kölliker, R. Advanced phenotyping offers opportunities for improved breeding of forage and turf species. Ann. Bot. 2012, 110, 1271–1279. [Google Scholar] [CrossRef]
- DeLacy, I.H.; Basford, K.E.; Cooper, M.; Bull, J.K.; McLaren, C.G. Analysis of multi-environment trials–an historical perspective. Plant Adapt. Crop Improv. 1996, 39124, 39–124. [Google Scholar]
- La Manna, L.; Carabelli, F.; Gómez, M.; Matteucci, S.D. Disposición espacial de parches de Austrocedrus chilensis con síntomas de defoliación y mortalidad en el Valle 16 de Octubre (Chubut, Argentina). Bosque 2008, 29, 23–32. [Google Scholar] [CrossRef]
- Fariña, C.M. Pastoreo intensivo en distintas estaciones del año: Efectos a escala de planta y de comunidad en una estepa de Patagonia Norte. Master’s Thesis, Universidad de Buenos Aires, Facultad de Agronomía, Escuela para Graduados, Buenos Aires, Argentina, 2018. Available online: http://ri.agro.uba.ar/greenstone3/library/collection/tesis/document/2019farinaclaramaria (accessed on 15 June 2021).
- Curasi, S.R.; Fetcher, N.; Wright, K.S.; Weldon, D.P.; Rocha, A.V. Insights into the tussock growth form with model–data fusion. New Phytol. 2023, 239, 562–575. [Google Scholar] [CrossRef]
- Anandhi, A. Growing degree days–Ecosystem indicator for changing diurnal temperatures and their impact on corn growth stages in Kansas. Ecol. Indic. 2016, 61, 149–158. [Google Scholar] [CrossRef]
- Wilhelm, W.W.; McMaster, G.S. Importance of the phyllochron in studying development and growth in grasses. Crop Sci. 1995, 35, 1–3. [Google Scholar] [CrossRef]
- Ordoñez, I.; Oyaneder, P.; Radic, S.; Valenzuela, J.; Ivelic, J.; Yagello, J. Respuesta Fisiológica de Festuca Gracillima a la Altura de Defoliación y Estrés Hídrico Severo; Informativo INIA Kampenaike: Punta Arenas, Chile, 2022. [Google Scholar]
- Posit team. RStudio: Integrated Development Environment for R. Posit Software; PBC: Boston, MA, USA, 2024. Available online: http://www.posit.co/ (accessed on 1 June 2021).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Navure Team. Navure (2.7.1): A Data-Science-Statistic Oriented Application for Making Evidence-Based Decisions. 2023. Available online: http://www.navure.com (accessed on 1 June 2021).


| Year | 2018 | 2019 | 2018–2019 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Growth Period | Spring | Early Summer | Spring | Early Summer | Growth Rate | ||||||||||
| Main Effects | Main Effects | Main Effects | Main Effects | Main Effects | |||||||||||
| Growth traits | S | P | S × P | S | P | S × P | S | P | S × P | S | P | S × P | S | P | S × P |
| df = 1 | df = 9 | df = 18 | df = 1 | df = 9 | df = 18 | df = 1 | df = 9 | df = 18 | df = 1 | df = 9 | df = 18 | df = 1 | df = 9 | df = 18) | |
| Basal diameter | 56.37 * | 1.85 * | 0.54 | 0.29 | 2.27 * | 0.24 | 53.72 * | 0.99 | 0.67 | 125.94 * | 2.30 * | 1.58 | 50.43 * | 0.71 | 1.98 |
| Height | 0.24 | 1.87 | 1.35 | 2.03 | 3.45 * | 2.54 * | 126.7 * | 4.48 * | 3.88 * | 72.96 * | 2.92 * | 3.30 * | 24.67 * | 2.81 * | 4.9 * |
| Harvest 2018–2019 | Harvest 2019–2020 | ||||||||||||||
| Main effects | Main effects | ||||||||||||||
| Reproductive traits | S | P | S × P | S | P | S × P | |||||||||
| df = 1 | df = 9 | df = 18 | df = 1 | df = 9 | df = 18 | ||||||||||
| Synflorescence production | 15.34 * | 3.4 * | 5.86 * | 131.14 * | 5.23 * | 4.69 * | |||||||||
| Plants producing synflorescences | 4.75 | 6.54 * | 0.83 | 0.98 | 4.71 * | 3.69 | |||||||||
| ID | Population | Lat | Long | Alt | Pp | FPT |
|---|---|---|---|---|---|---|
| PA | Experimental Field Pilcaniyeu, RN | −41.06 | −70.52 | 1260 | 264 | Shrub-grass steppe |
| PB | Experimental Field Pilcaniyeu RN | −41.07 | −70.58 | 970 | 264 | Meadow |
| JA | Ing. Jacobacci, RN | −41.92 | −69.22 | 1400 | 170 | Grass steppe |
| JB | Ing. Jacobacci, RN | −41.92 | −69.22 | 970 | 170 | Salty Meadow |
| CR | Cronómetro, CH | −41.23 | −71.07 | 875 | 500 | Shrub grass steppe |
| YA | Yagüe, CH | −42.95 | −71.20 | 748 | 450 | Shrub-grass steppe |
| AP | A. Pescado, CH | −43.03 | −70.97 | 679 | 350 | Shrub-grass steppe |
| LC | La Cancha, CH | −42.78 | −70.95 | 778 | 300 | Shrub-grass steppe |
| AE | Aeropuerto, CH | −42.90 | −71.13 | 800 | 500 | Shrub-grass steppe |
| EP | El Puesto, CH. | −45.32 | −70.22 | 493 | 150 | Meadow |
| Environmental Conditions | Site 1 | Site 2 |
|---|---|---|
| Mean annual temperature | 8.6 | 10 |
| Mean minimum temperature | 3.5 | 4.9 |
| Mean maximum temperature | 13.8 | 15.1 |
| Mean annual precipitation | 840 | 976 |
| Soil type | Mollisol | Andisol * |
| pH | 7.25 | 6.09 |
| E.C. | 0.04 | 0.105 |
| O.M. | 4.3 | 6.1 |
| N | 0.25 | 0.185 |
| P | 2.14 | 42 |
| K | 342 | 807 |
| During the trial | site 1 | site 2 |
| Mean annual temperature | 9.4 | 10.41 |
| Mean accumulated precipitation | 62.3 | 71 |
| Irrigation | 21.9 m3/ha three times a week, sprinkler | 10.9 m3/ha every day, drip |
| Irrigation period | 2017–2019 | 2018–2020 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López, A.S.; Nagahama, N.; Aparicio, A.; Azpilicueta, M.M.; Guidalevich, V.; Angeli, J.P.; Marchelli, P. Genetic Variation of Growth Traits and Seed Production in a Patagonian Native Pasture in Semiarid Rangelands Under Different Environmental Settings. Plants 2025, 14, 736. https://doi.org/10.3390/plants14050736
López AS, Nagahama N, Aparicio A, Azpilicueta MM, Guidalevich V, Angeli JP, Marchelli P. Genetic Variation of Growth Traits and Seed Production in a Patagonian Native Pasture in Semiarid Rangelands Under Different Environmental Settings. Plants. 2025; 14(5):736. https://doi.org/10.3390/plants14050736
Chicago/Turabian StyleLópez, Aldana Soledad, Nicolás Nagahama, Alejandro Aparicio, María Marta Azpilicueta, Verónica Guidalevich, Juan Pablo Angeli, and Paula Marchelli. 2025. "Genetic Variation of Growth Traits and Seed Production in a Patagonian Native Pasture in Semiarid Rangelands Under Different Environmental Settings" Plants 14, no. 5: 736. https://doi.org/10.3390/plants14050736
APA StyleLópez, A. S., Nagahama, N., Aparicio, A., Azpilicueta, M. M., Guidalevich, V., Angeli, J. P., & Marchelli, P. (2025). Genetic Variation of Growth Traits and Seed Production in a Patagonian Native Pasture in Semiarid Rangelands Under Different Environmental Settings. Plants, 14(5), 736. https://doi.org/10.3390/plants14050736







