Modeling Study on Optimizing Water and Nitrogen Management for Barley in Marginal Soils
Abstract
1. Introduction
2. Results and Discussion
2.1. Field Observations
2.2. Model Calibration and Validation
Soil Water Content
2.3. Leaf Area Index and Barley Development Stages
2.4. Partitioning of Total Above-Ground Biomass, Leaf, Stem, and Grain Yield
2.5. Estimated Water Stress
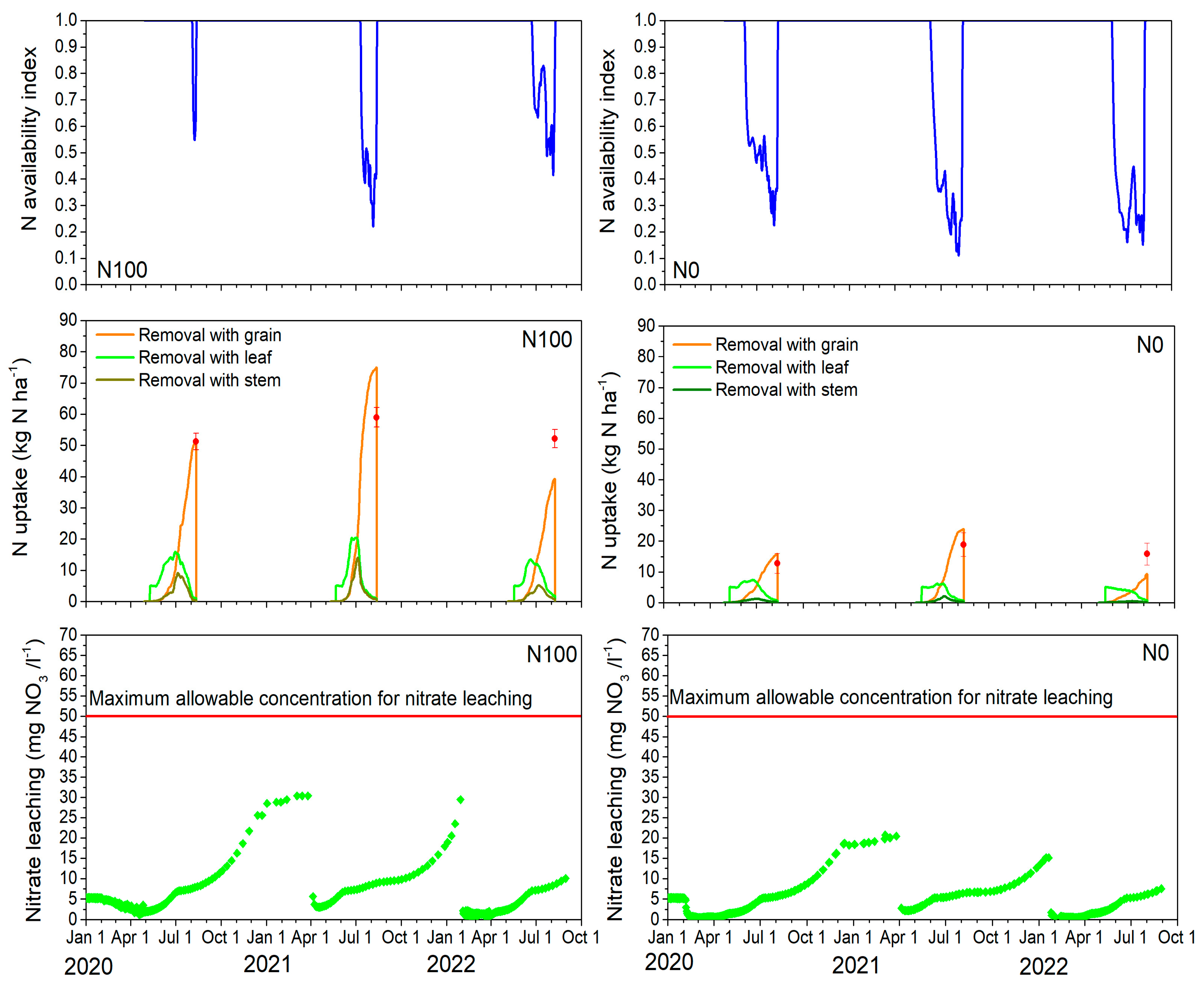
2.6. Estimated N Stress, Uptake, and Nitrate Leaching
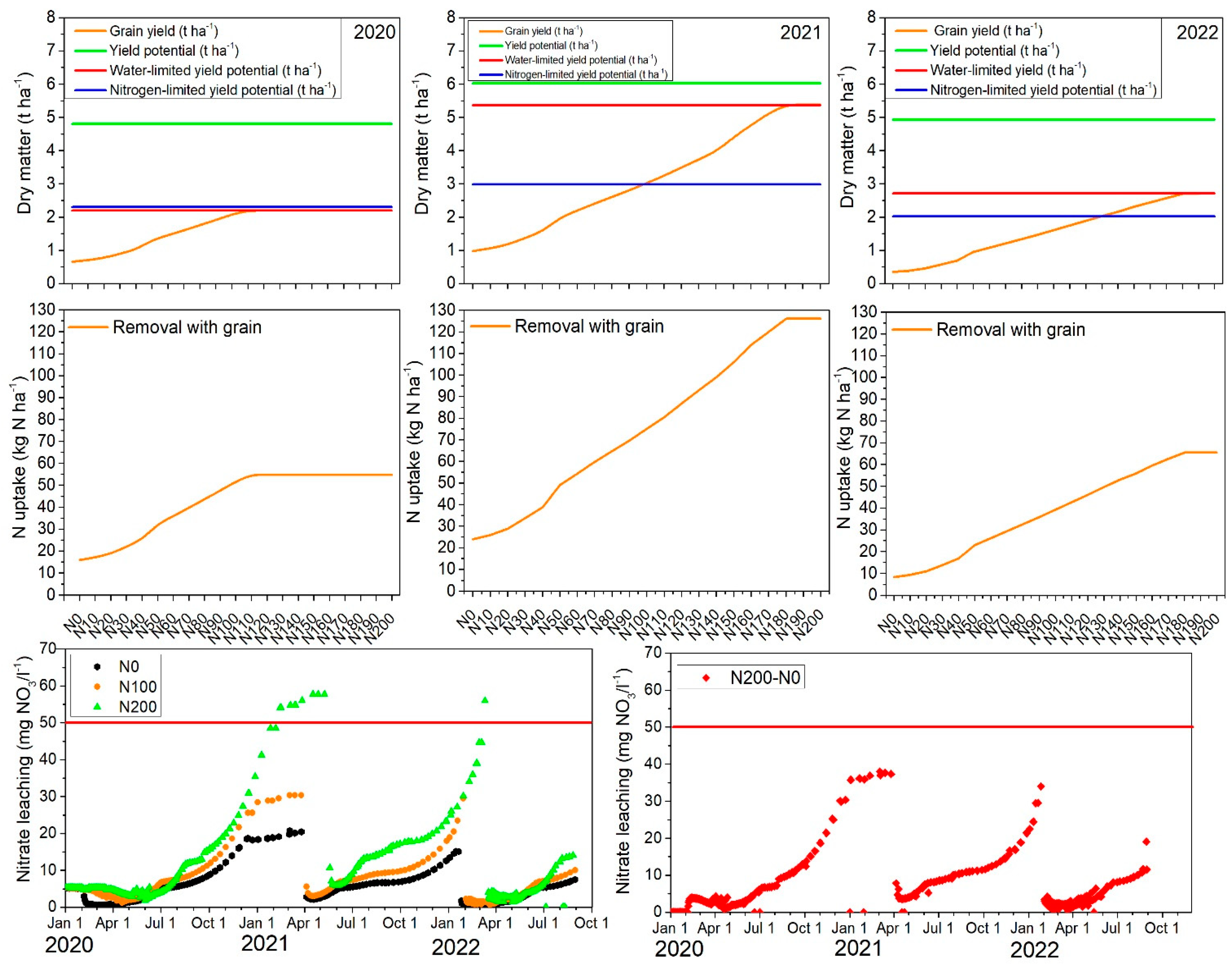
2.7. Model Application to Assess the Environmental Impact and Effect of Increasing Fertilizer on Barley Yields
2.7.1. Yield Potential, Water-Limited Yield, Nitrogen-Limited Yield, N Uptake, and N Leaching
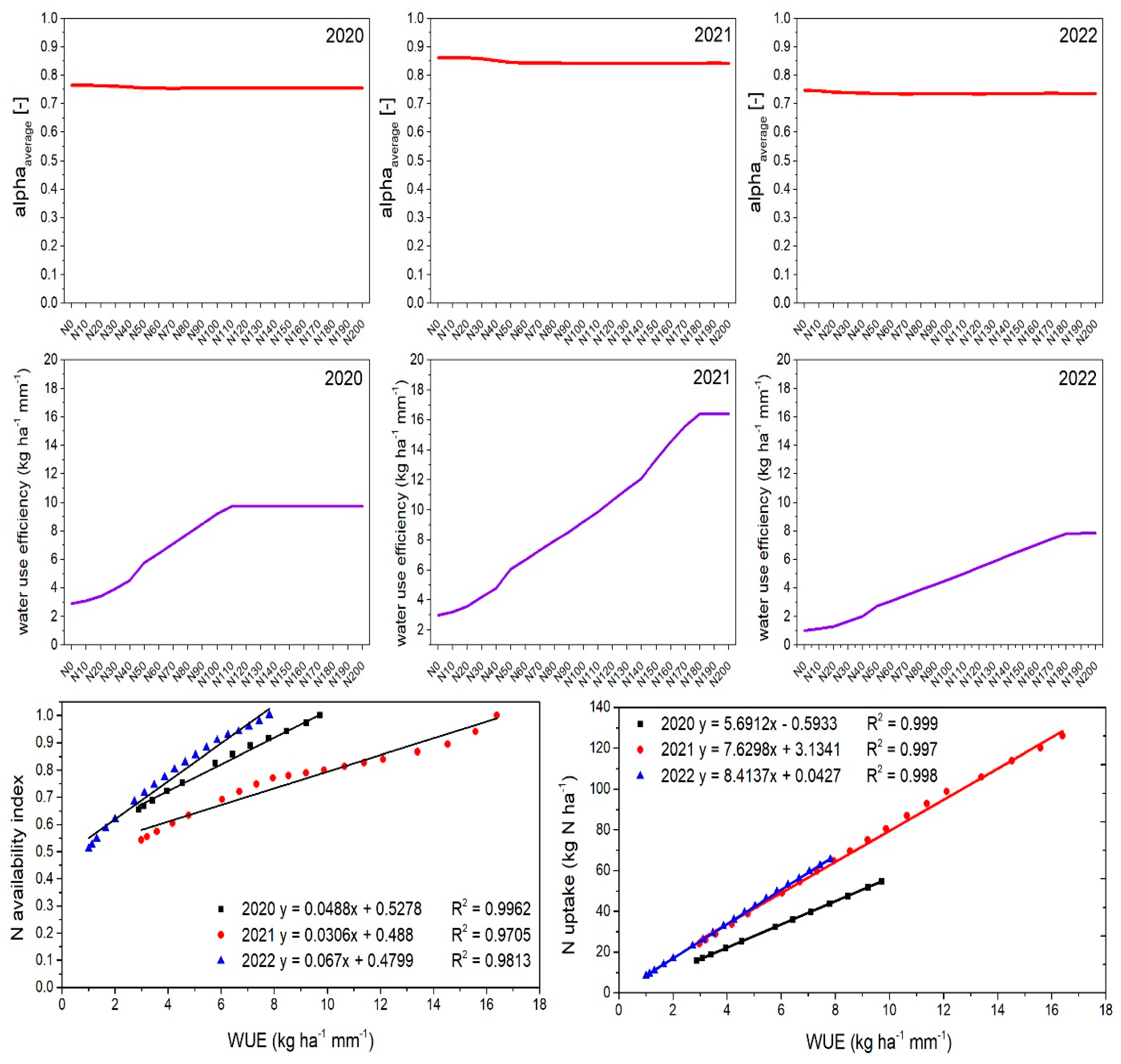
2.7.2. Estimated Nitrogen Stress and Nitrogen Use Efficiencies
2.7.3. Estimated Water Stress and Water Use Efficiency
3. Materials and Methods
3.1. Site Description
3.2. Experimental Design
3.3. Plant Measurements
3.4. Soil Measurements
3.4.1. Soil Chemical Properties
3.4.2. Soil Hydraulic Properties
3.4.3. Soil Water Content
3.5. AgroC Model Setup and Calibration
3.6. Climate Data
3.7. AgroC Model Application
3.7.1. Prediction of Barley Yield Potential and Yield Gap
3.7.2. Evaluation of N Management Practice Scenarios
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
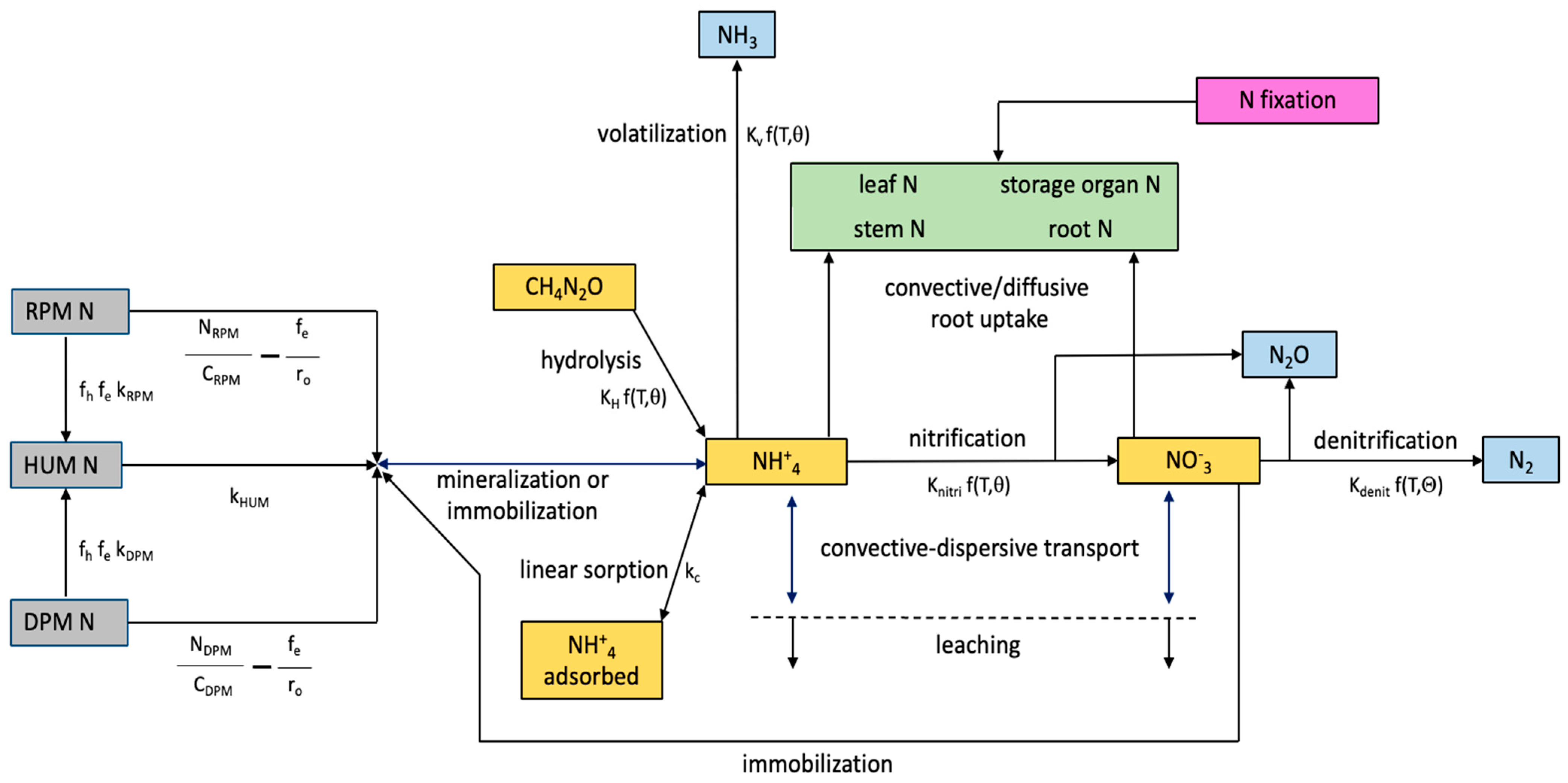
Appendix A.1. Mineralization of Soil Organic Nitrogen
Appendix A.2. Nitrification, Ammonia Volatilization and Urea Hydrolysis
Appendix A.3. Denitrification
Appendix A.4. Root Uptake of Nitrogen
Appendix A.5. N Stress
Appendix A.6. Water Uptake Stress
Appendix A.7. Effect of Stress on Growth
References
- IPCC. Climate Change 2014: Impacts, Adaptation, and Vulnerability; Intergovernmental Panel on Climate Change, IPPC Secretariat: Geneva, Switzerland, 2014. [Google Scholar]
- United Nations Department of Economic and Social Affairs. World Population Prospects 2024: Summary of Results. Available online: https://desapublications.un.org/publications/world-population-prospects-2024-summary-results (accessed on 10 September 2024).
- Schröder, P.; Mench, M.; Povilaitis, V.; Rineau, F.; Rutkowska, B.; Schloter, M.; Szulc, W.; Žydelis, R.; Loit, E. Relaunch cropping on marginal soils by incorporating amendments and beneficial trace elements in an interdisciplinary approach. Sci. Total Environ. 2022, 803, 149844. [Google Scholar] [CrossRef] [PubMed]
- Appiah, M.; Abdulai, I.; Schulman, A.; Moshelion, M.; Dewi, E.S.; Daszkowska-Golec, A.; Bracho-Mujica, G.; Rötter, R.P. Drought response of water-conserving and non-conserving spring barley cultivars. Front. Plant Sci. 2023, 14, 1247853. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Martre, P.; Zhao, Z.; Ewert, F.; Maiorano, A.; Rötter, R.P.; Kimball, B.A.; Ottman, M.J.; Wall, G.W.; White, J.W.; et al. The uncertainty of crop yield projections is reduced by improved temperature response functions. Nat. Plants 2017, 3, 17102. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Prentice, I.C.; Keenan, T.F.; Davis, T.W.; Wright, I.J.; Cornwell, W.K.; Evans, B.J.; Peng, C. Towards a universal model for carbon dioxide uptake by plants. Nat. Plants 2017, 3, 734–741. [Google Scholar] [CrossRef] [PubMed]
- Vogel, E.; Donat, M.G.; Alexander, L.V.; Meinshausen, M.; Ray, D.K.; Karoly, D.; Meinshausen, N.; Frieler, K. The effects of climate extremes on global agricultural yields. Environ. Res. Lett. 2019, 14, 054010. [Google Scholar] [CrossRef]
- Žydelis, R.; Dechmi, F.; Isla, R.; Weihermüller, L.; Lazauskas, S. CERES-Maize model performance under mineral and organic fertilization in nemoral climate conditions. Agron. J. 2021, 113, 2474–2490. [Google Scholar] [CrossRef]
- Malik, W.; Isla, R.; Dechmi, F. DSSAT-CERES-maize modelling to improve irrigation and nitrogen management practices under Mediterranean conditions. Agric. Water Manag. 2019, 213, 298–308. [Google Scholar] [CrossRef]
- Povilaitis, A.; Šileika, A.; Deelstra, J.; Gaigalis, K.; Baigys, G. Nitrogen losses from small agricultural catchments in Lithuania. Agric. Ecosyst. Environ. 2014, 195, 54–64. [Google Scholar] [CrossRef]
- Bijay-Singh; Craswell, E. Fertilizers and nitrate pollution of surface and ground water: An increasingly pervasive global problem. SN Appl. Sci. 2021, 3, 518. [Google Scholar] [CrossRef]
- Yawson, D.O.; Adu, M.O.; Armah, F.A. Impacts of climate change and mitigation policies on malt barley supplies and associated virtual water flows in the UK. Sci. Rep. 2020, 10, 376. [Google Scholar] [CrossRef] [PubMed]
- Dawson, I.K.; Russell, J.; Powell, W.; Steffenson, B.; Thomas, W.T.B.; Waugh, R. Barley: A translational model for adaptation to climate change. New Phytol. 2015, 206, 913–931. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT. Statictical Database; Food and Agriculture Organization of the United Nations: Rome, Italy, 2023; Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 12 February 2025).
- Olesen, J.E.; Trnka, M.; Kersebaum, K.C.; Skjelvåg, A.O.; Seguin, B.; Peltonen-Sainio, P.; Rossi, F.; Kozyra, J.; Micale, F. Impacts and adaptation of European crop production systems to climate change. Eur. J. Agron. 2011, 34, 96–112. [Google Scholar] [CrossRef]
- Khanna, M.; Chen, L.; Basso, B.; Cai, X.; Field, J.L.; Guan, K.; Jiang, C.; Lark, T.L.; Richard, T.L.; Spawn-Lee, S.A.; et al. Redefining marginal land for bioenergy crop production. GCB Bioenergy 2021, 13, 1590–1609. [Google Scholar] [CrossRef]
- Gerwin, W.; Repmann, F.; Galatsidas, S.; Vlachaki, D.; Gounaris, N.; Baumgarten, W.; Volkmann, C.; Keramitzis, D.; Kiourtsis, F.; Freese, D. Assessment and quantification of marginal lands for biomass production in Europe using soil-quality indicators. Soil 2018, 4, 267–290. [Google Scholar] [CrossRef]
- Persson, T.; Höglind, M.; Wallsten, J.; Nadeau, E.; Huang, X.; Rustas, B. Adapting the grassland model BASGRA to simulate yield and nutritive value of whole-crop barley. Eur. J. Agron. 2024, 154, 127075. [Google Scholar] [CrossRef]
- Kersebaum, K.C.; Boote, K.J.; Jorgenson, J.S.; Nendel, C.; Bindi, M.; Frühauf, C.; Gaiser, T.; Hoogenboom, G.; Kollas, C.; Olesen, J.E.; et al. Analysis and classification of data sets for calibration and validation of agro-ecosystem models. Environ. Model. Softw. 2015, 72, 402–417. [Google Scholar] [CrossRef]
- Žydelis, R.; Herbst, M.; Weihermüller, L.; Ruzgas, R.; Volungevičius, J.; Barčauskaitė, K.; Tilvikienė, V. Yield potential and factor influencing yield gap in industrial hemp cultivation under nemoral climate conditions. Eur. J. Agron. 2022, 139, 126576. [Google Scholar] [CrossRef]
- Herbst, M.; Hellebrand, H.J.; Bauer, J.; Huisman, J.A.; Šimůnek, J.; Weihermüller, L.; Graf, A.; Vanderborght, J.; Vereecken, H. Multiyear heterotrophic soil respiration: Evaluation of a coupled CO2 transport and carbon turnover model. Ecol. Model. 2008, 214, 271–281. [Google Scholar] [CrossRef]
- Klosterhalfen, A.; Herbst, M.; Weihermüller, L.; Graf, A.; Schmidt, M.; Stadler, A.; Schneider, K.; Subke, J.A.; Huisman, J.A.; Vereecken, H. Multi-site calibration and validation of a net ecosystem carbon exchange model for croplands. Ecol. Model. 2017, 363, 137–156. [Google Scholar] [CrossRef]
- Official Statistics Portal. Agricultural Production Methods and Irrigation. 2024. Available online: https://osp.stat.gov.lt/zus2020-rezultatai/zemes-ukio-gamybos-metodai-ir-drekinimas (accessed on 12 February 2025).
- Wang, N.; Wang, J.; Wang, E.; Yu, Q.; Shi, Y.; He, D. Increased uncertainty in simulated maize phenology with more frequent supra-optimal temperature under climate warming. Eur. J. Agron. 2015, 71, 19–33. [Google Scholar] [CrossRef]
- Usowicz, B.; Lipiec, J. Spatial variability of soil properties and cereal yield in a cultivated field on sandy soil. Soil Tillage Res. 2017, 174, 241–250. [Google Scholar] [CrossRef]
- Castaldi, F.; Koparan, M.H.; Wetterlind, J.; Žydelis, R.; Vinci, I.; Savas, A.O.; Kivrak, C.; Tuncay, T.; Volungevičius, J.; Obber, S.; et al. Assessing the capability of Sentinel-2 time-series to estimate soil organic carbon and clay content at local scale in croplands. ISPRS J. Photogramm. Remote Sens. 2023, 199, 40–60. [Google Scholar] [CrossRef]
- Wiegmann, M.; Maurer, A.; Pham, A.; March, J.T.; Al-Abdallat, A.; Thomas, W.T.B.; Bull, H.J.; Shahid, M.; Eglinton, J.; Baum, M.; et al. Barley yield formation under abiotic stress depends on the interplay between flowering time genes and environmental cues. Sci. Rep. 2019, 9, 6397. [Google Scholar] [CrossRef] [PubMed]
- Baik, B.K.; Newman, C.W.; Newman, R.K. Barley: Production, Improvement, and Uses; Ullrich, S.E., Ed.; Wiley-Blackwell: Oxford, UK, 2011. [Google Scholar] [CrossRef]
- Schils, R.; Olesen, J.E.; Kersebaum, K.C.; Rijk, B.; Oberforster, M.; Kalyada, V.; Khitrykau, M.; Gobin, A.; Kirchev, H.; Manolova, V.; et al. Cereal yield gaps across Europe. Eur. J. Agron. 2018, 101, 109–120. [Google Scholar] [CrossRef]
- Hoffman, M.P.; Haakana, M.; Asseng, S.; Höhn, J.G.; Palosuo, T.; Ruiz-Ramos, M.; Fronzek, S.; Ewert, F.; Gaiser, T.; Kassie, B.T.; et al. How does inter-annual variability of attainable yield affect the magnitude of yield gaps for wheat and maize? An analysis at ten sites. Agric. Syst. 2018, 159, 199–208. [Google Scholar] [CrossRef]
- Žydelis, R.; Weihermüller, L.; Herbst, M.; Klosterhalfen, A.; Lazauskas, S. A model study on the effect of water and cold stress on maize development under nemoral climate. Agric. For. Meteorol. 2018, 263, 169–179. [Google Scholar] [CrossRef]
- Van Grinsvel, H.J.M.; Ebanyat, P.; Glendining, M.; Gu, B.; Hijbeek, R.; Lam, S.K.; Lassaletta, L.; Mueller, N.D.; Pacheco, F.S.; Quemada, M.; et al. Establishing long-term nitrogen response of global cereals to assess sustainable fertilizer rates. Nat. Food 2022, 3, 122–132. [Google Scholar] [CrossRef] [PubMed]
- EC. Council Directive 91/676/EEC of 12 December 1991 Concerning the Protection of Waters Against Pollution Caused by Nitrates from Agricultural Sources; European Commission (EC): Brussels, Belgium, 1991. [Google Scholar]
- Valkama, E.; Salo, T.; Esala, M.; Turtola, E. Nitrogen balances and yields of spring cereals as affected bu nitrogen fertilization in northern conditions: A meta-analysis. Agric. Ecosyst. Environ. 2013, 164, 1–13. [Google Scholar] [CrossRef]
- Tręšimo Plano Sudarymas; Lietuvos Žemės Ūkio Konsultavimo Tarnyba: Kėdainių, Lithuania, 2002; ISBN 9986-540-83-6.
- World Health Organization. Guidelines for Drinking-Water Quality; WHO: Geneva, Switzerland, 2012. [Google Scholar]
- Abascal, E.; Gómez-Coma, L.; Ortiz, I.; Ortiz, A. Global diagnosis of nitrate pollution in groundwater and review of removal technologies. Sci. Total Environ. 2022, 810, 152233. [Google Scholar] [CrossRef]
- Goyal, M.R.; Harmsen, E.W. Evapotranspiration; Apple Academic Press: New York, NY, USA, 2013. [Google Scholar] [CrossRef]
- Metzger, M.J.; Shkaruba, A.D.; Jongman, R.H.G.; Bunce, R.G.H. Description of the European Environmental Zones and Strata; Alterra Report 2281; Alterra: Wageningen, The Nederland, 2012. [Google Scholar]
- Kavaliauskas, A.; Žydelis, R.; Castaldi, F.; Auškalnienė, O.; Povilaitis, V. Predicting Maize Theoretical Methane Yield in Combination with Ground and UAV Remote Data Using Machine Learning. Plants 2023, 12, 1823. [Google Scholar] [CrossRef]
- Bukantis, A. Agroclimatic Zoning. In Lithuanian National Atlas; National Land Service under the Ministry of Agriculture: Vilniaus, Lithuania, 2009. [Google Scholar]
- WRB. World Reference Base for Soil Resources 2014. International soil classification system for naming soils and creating legends for soil maps; World Soil Resources Reports No. 106; FAO: Rome, Italy, 2014. [Google Scholar]
- Meier, U. Growth Stages of Mono- and Dicotyledonous Plants. BBCH Monograph. Julius Kühn-Institut (JKI) Quedlinburg. 2018. Available online: https://www.julius-kuehn.de/media/Veroeffentlichungen/bbch%20epaper%20en/page.pdf (accessed on 10 January 2023).
- Mažeika, R.; Arbačiauskas, J.; Masevičienė, A.; Narutytė, I.; Šumskis, D.; Žičkienė, L.; Rainys, K.; Drapanauskaitė, D.; Staugaitis, G.; Baltrusaitis, J. Nutrient Dynamics and Plant Response in Soil to Organic Chicken Manure-Based Fertilizers. Waste Biomass Valorization 2020, 12, 371–382. [Google Scholar] [CrossRef]
- Dobermann, A.R. Nitrogen use efficiency-state of the art. In Proceedings of the IFA International Workshop on Enhanced-Efficiency Fertilizers, Frankfurt, Germany, 28–30 June 2005. [Google Scholar]
- Schindler, U.; Durner, W.; von Unold, G.; Mueller, L. Evaporation method for measuring unsaturated hydraulic properties of soils: Extending the measurement range. Soil Sci. Soc. Am. J. 2010, 74, 1071–1083. [Google Scholar] [CrossRef]
- van Genuchten, M.T. A close-form equation for predicting the hydraulic conductivity of unsaturated soils. Soil Sci. Soc. Am. J. 1980, 44, 892–898. [Google Scholar] [CrossRef]
- Müller, H.W.; Dohrmann, R.; Klosa, D.; Rehder, S.; Eckelmann, W. Comparison of two procedures for particle-size analysis: Köhn pipette and X-ray granulometry. J. Plant Nutr. Soil Sci. 2009, 172, 172–179. [Google Scholar] [CrossRef]
- Bogena, H.R.; Huisman, J.A.; Schilling, B.; Weuthen, A.; Vereecken, H. Effective Calibration of Low-Cost Soil Water Content Sensors. Sensors 2017, 17, 208. [Google Scholar] [CrossRef]
- Howell, T.A. Enhancing Water Use Efficiency in Irrigated Agriculture. Agron. J. 2001, 93, 281–289. [Google Scholar] [CrossRef]
- Žydelis, R.; Weihermüller, L.; Herbst, M. Future climate change will accelerate maize phenological development and increase yield in the Nemoral climate. Sci. Total Environ. 2021, 784, 147175. [Google Scholar] [CrossRef] [PubMed]
- Herbst, M.; Pohlig, P.; Graf, A.; Weihermüller, L.; Schmidt, M.; Vanderborght, J.; Vereecken, H. Quantification of water stress induced within-field variability of carbon dioxide fluxes in a sugar beet stand. Agric. For. Meteorol. 2021, 297, 108242. [Google Scholar] [CrossRef]
- Groh, J.; Diamantopoulos, E.; Duan, X.; Ewert, F.; Heinlein, F.; Herbst, M.; Holbak, M.; Kamali, B.; Kersebaum, K.C.; Kuhnert, M.; et al. Same soil, different climate: Crop model intercomparison on translocated lysimeters. Vadose Zone J. 2022, 21, e20202. [Google Scholar] [CrossRef]
- Wallach, D.; Palosuo, T.; Thorburn, P.; Mielenz, H.; Buis, S.; Hochman, Z.; Gourdain, E.; Andrianasolo, F.; Dumont, B.; Ferrise, R.; et al. Proposal and extensive test of a calibration protocol for crop phenology models. Agron. Sustain. Dev. 2023, 43, 46. [Google Scholar] [CrossRef]
- Brogi, C.; Huisman, J.A.; Weihermüller, L.; Klosterhalfen, A.; Montzka, C.; Reichenau, T.G.; Verrecken, H. Simulation of spatial variability in crop leaf area index and yield using agroecosystem modeling and geophysics-based quantitative soil information. Vadose Zone J. 2020, 19, e20009. [Google Scholar] [CrossRef]
- Duan, Q.; Sorooshian, S.; Gupta, V. Effective and efficient global optimization for conceptual rainfall-runoff models. Water Resour. Res. 1992, 28, 1015–1031. [Google Scholar] [CrossRef]
- Duan, Q.Y.; Gupta, V.K.; Sorooshian, S. Shuffled complex evolution approach for effective and efficient global minimization. J. Optim. Theory Appl. 1993, 76, 501–502. [Google Scholar] [CrossRef]
- Wallach, D.; Palosuo, T.; Thorburn, P.; Hochman, Z.; Andrianasolo, F.; Asseng, S.; Basso, B.; Buis, S.; Crout, N.; Dumont, B.; et al. Multi-model evaluation of phenology prediction for wheat in Australia. Agric. For. Meteorol. 2021, 298−299, 108289. [Google Scholar] [CrossRef]
- Wallach, D. Evaluating Crop Models. In Working with Dynamic Crop Models; Wallach, D., Makowski, D., Jones, J.W., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; pp. 11–55. [Google Scholar]
- Willmott, C.J. On the Validation of Models. Phys. Geogr. 1981, 2, 184–194. [Google Scholar] [CrossRef]
- Coleman, K.; Prout, J.M.; Milne, A.E. RothC—A Model for the Turnover of Carbon in Soil. Model Description (February 2024). Rothamsted Research, Harpenden, 2024, p. 24. Available online: https://www.rothamsted.ac.uk/sites/default/files/Documents/RothC_description.pdf (accessed on 12 February 2025).
- Vereecken, H.; Vanclooster, M.; Swerts, M.; Diels, J. Simulating water and nitrogen behaviour in soil cropped with winter wheat. Fert. Res. 1991, 27, 233–243. [Google Scholar] [CrossRef]
- Aulakh, M.S.; Doran, J.W.; Mosier, A.R. Soil denitrification significance, measurements and effects of management. Adv. Soil Sci. 1992, 18, 2–42. [Google Scholar]
- McIsaac, D.M.; Watts, D. Users Guide to NITWAT—A Nitrogen and Water Management Model; Agricultural Engineering Deptartment, University of Nebraska: Lincoln, NE, USA, 1985. [Google Scholar]
- Huwe, B.; Van der Ploeg, R. Modelle zur Simulation des Stickstoffhaushaltes von Standorten mit Unterschiedlicher landwirtschaftlicher Nutzung; Eigenverlag des Instituts für Wasserbau der Universität Stuttgart: Stuugart, Germany, 1988; Volume 69, p. 213. [Google Scholar]
- Feddes, R.A.; Kowalik, P.J.; Zaradny, H. Simulation of Field Water Use and Crop Yield; Simulation Monographs: Wageningen, The Netherlands, 1978; p. 188. [Google Scholar]
- Vanclooster, M.; Viaene, P.; Diels, J.; Christiaens, K. WAVE: A Mathematical Model for Simulating Water and Agrochemicals in the Soil and Vadose Environment: Reference and User’s Manual (Release 2.0); Institute for Land and Water Management, Katholieke Universiteit: Leuven, Belgium, 1995; p. 154. [Google Scholar]
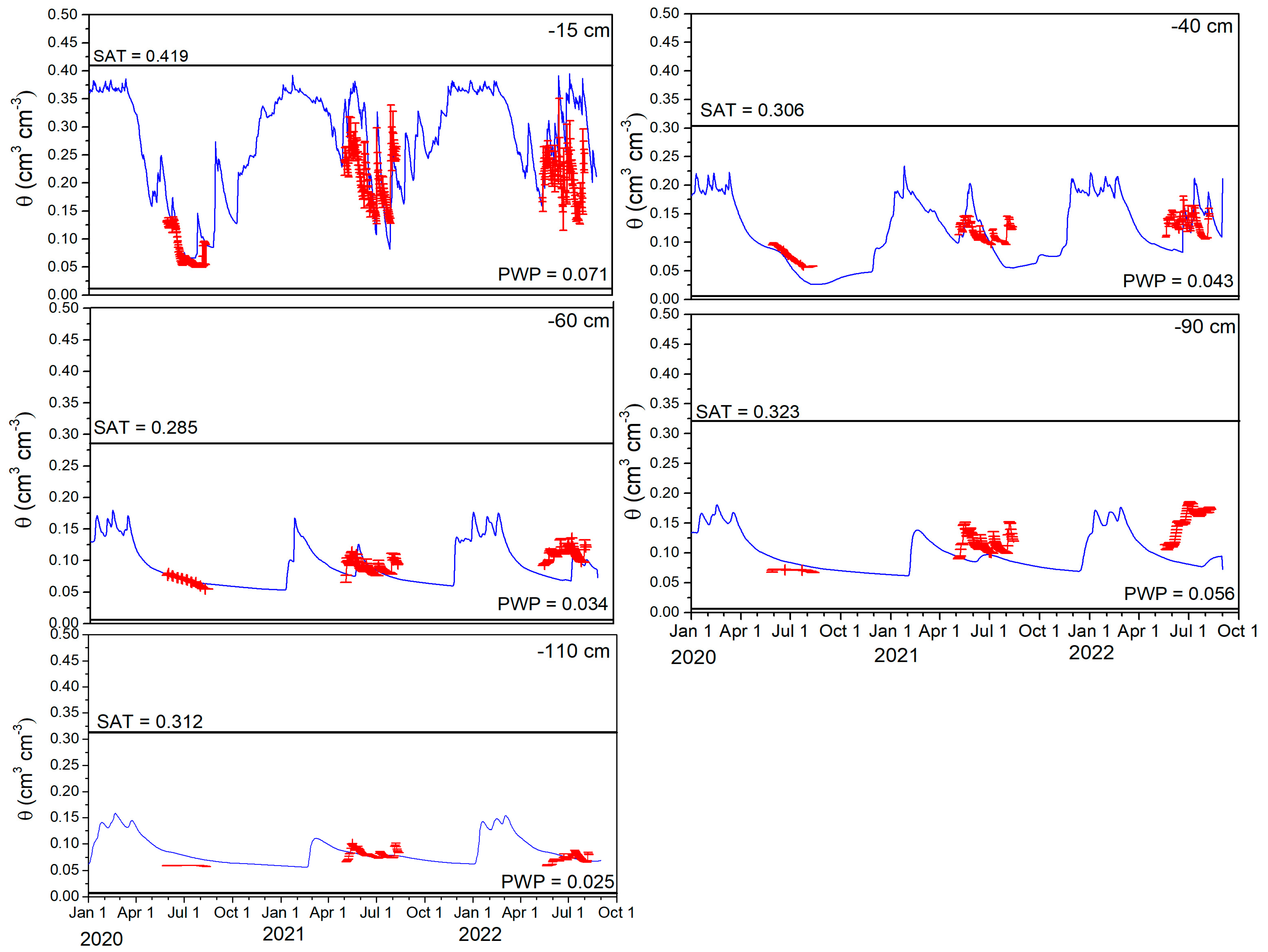
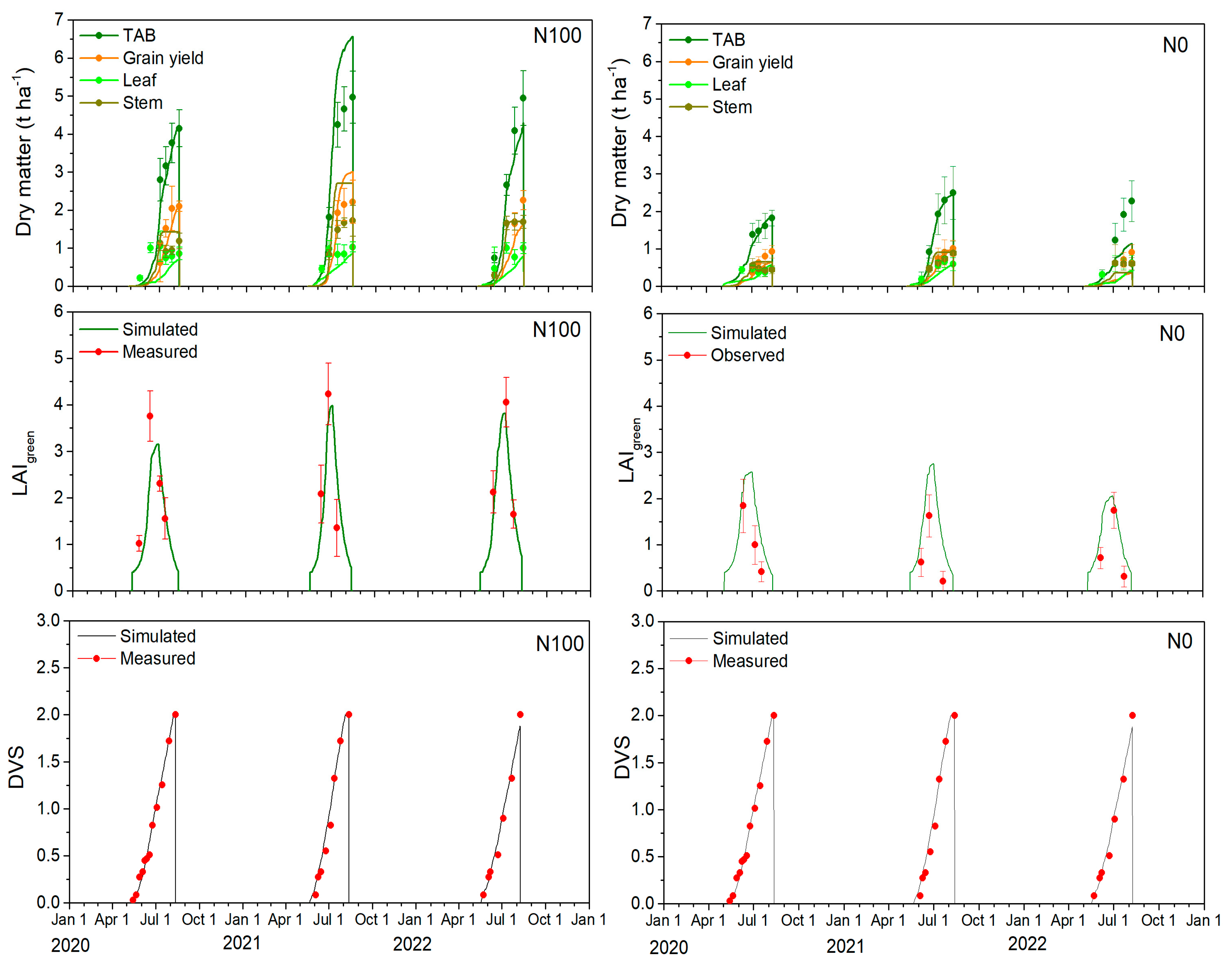
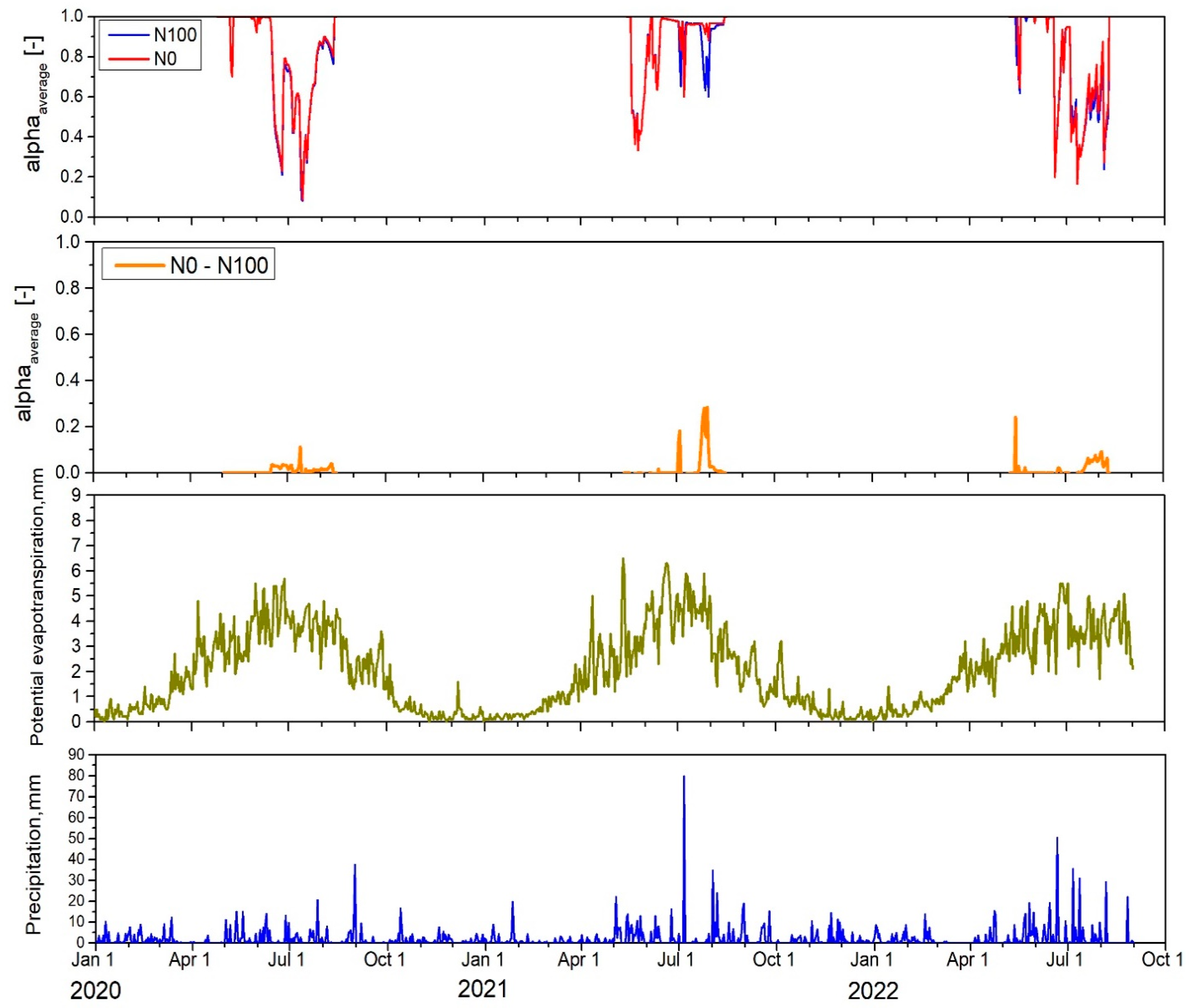

| Parameters | d | RMSE | BIAS | |||
|---|---|---|---|---|---|---|
| N0 | N100 | N0 | N100 | N0 | N100 | |
| 2020 | ||||||
| Leaf area index | 0.704 | 0.834 | 0.663 | 0.535 | −0.619 | 0.286 |
| TAB (t ha−1) | 0.982 | 0.980 | 0.128 | 0.422 | −0.029 | −0.321 |
| Leaf (t ha−1) | 0.702 | 0.513 | 0.193 | 0.444 | 0.074 | 0.347 |
| Stem (t ha−1) | 0.892 | 0.587 | 0.172 | 0.446 | −0.163 | −0.420 |
| Storage organs and GY (t ha−1) | 0.725 | 0.928 | 0.241 | 0.315 | 0.258 | 0.242 |
| SWC at 15 cm (cm3 cm−3) | − | 0.788 | − | 0.030 | − | 0.040 |
| SWC at 40 cm (cm3 cm−3) | − | 0.775 | − | 0.019 | − | −0.017 |
| SWC at 60 cm (cm3 cm−3) | − | 0.907 | − | 0.004 | − | 0.002 |
| SWC at 90 cm (cm3 cm−3) | − | 0.159 | − | 0.013 | − | 0.012 |
| SWC at 110 cm (cm3 cm−3) | − | 0.101 | − | 0.019 | − | 0.018 |
| 2021 | ||||||
| Leaf area index | 0.719 | 0.806 | 0.795 | 0.875 | −0.787 | 0.037 |
| TAB (t ha−1) | 0.998 | 0.925 | 0.051 | 1.197 | 0.034 | −0.973 |
| Leaf (t ha−1) | 0.728 | 0.755 | 0.196 | 0.365 | 0.169 | 0.323 |
| Stem (t ha−1) | 0.802 | 0.570 | 0.159 | 0.938 | −0.100 | −0.786 |
| Storage organs and GY (t ha−1) | 0.975 | 0.643 | 0.166 | 0.624 | 0.027 | −0.430 |
| SWC at 15 cm (cm3 cm−3) | − | 0.788 | − | 0.067 | − | 0.040 |
| SWC at 40 cm (cm3 cm−3) | − | 0.391 | − | 0.039 | − | −0.006 |
| SWC at 60 cm (cm3 cm−3) | − | 0.468 | − | 0.019 | − | −0.005 |
| SWC at 90 cm (cm3 cm−3) | − | 0.339 | − | 0.031 | − | −0.025 |
| SWC at 110 cm (cm3 cm−3) | − | 0.455 | − | 0.008 | − | −0.003 |
| 2022 | ||||||
| Leaf area index | 0.882 | 0.949 | 0.384 | 0.429 | −0.349 | 0.295 |
| TAB (t ha−1) | 0.629 | 0.972 | 0.772 | 0.527 | 0.656 | 0.485 |
| Leaf (t ha−1) | 0.389 | 0.582 | 0.334 | 0.423 | 0.314 | 0.385 |
| Stem (t ha−1) | 0.438 | 0.992 | 0.248 | 0.113 | 0.248 | 0.069 |
| Storage organs and GY (t ha−1) | 0.408 | 0.914 | 0.411 | 0.470 | 0.266 | 0.057 |
| SWC at 15 cm (cm3 cm−3) | − | 0.452 | − | 0.071 | − | 0.093 |
| SWC at 40 cm (cm3 cm−3) | − | 0.308 | − | 0.043 | − | −0.006 |
| SWC at 60 cm (cm3 cm−3) | − | 0.293 | − | 0.035 | − | −0.027 |
| SWC at 90 cm (cm3 cm−3) | − | 0.108 | − | 0.072 | − | −0.065 |
| SWC at 110 cm (cm3 cm−3) | − | 0.751 | − | 0.011 | − | 0.004 |
| 2020 | 2021 | 2022 | |
|---|---|---|---|
| Soil (FAO classification) | Endocalcaric Eutric Brunic Arenosol (Geoabruptic, Aric) | ||
| Soil pH (1 N KCl extraction) | 5.5 | 6.0 | 6.3 |
| Soil P2O5 (mg kg−1) (Egner-Riehm-Domingo (A-L)) | 170 | 205 | 192 |
| Soil K2O (mg kg−1) (Egner-Riehm-Domingo (A-L)) | 324 | 174 | 180 |
| Soil organic carbon (%) | 1.34 | 1.16 | 1.21 |
| Soil N total (%) (Kjeldahl) | 0.103 | 0.101 | 0.103 |
| Previous crop | Buckwheat | Barley | Barley |
| Barley cultivar | KWS Fantex | KWS Fantex | KWS Fantex |
| Barley seeding dates | 27 April 2020 | 10 May 2021 | 04 May 2022 |
| Seeding density (seeds per m2) | 450 | 450 | 450 |
| Plot size | 3 m × 10 m = 30 m2 | 3 m × 10 m = 30 m2 | 3 m × 10 m = 30 m2 |
| Fertilization | 27 April 2020—N50P80K140; 8 June 2020—N50 | 7 May 2021—N50P80K140; 11 June 2021—N50 | 3 May 2022—N50P80K140; 6 June—N50 |
| Pesticides, fungicides, insecticides | – | – | – |
| Barley harvesting | 12 August 2020 | 13 August 2021 | 9 August 2022 |
| Horizon Description | SOC | pH | Ntotal | P205 | K20 | SMN |
|---|---|---|---|---|---|---|
| (NO3−-N + NH4+-N) | ||||||
| % | - | % | mg kg−1 | mg kg−1 | kg ha−1 | |
| Ap (0–30 cm) | 1.34 | 5.5 | 0.103 | 170 | 324 | 71.3 ± 11.5 |
| B1 (30–50 cm) | 0.46 | 5.8 | 0.013 | 120 | 153 | – |
| B2 (50–78 cm) | 0.54 | 6.4 | 0.014 | 259 | 122 | – |
| 2C(k) (78–105 cm) | 0.37 | 7.6 | 0.003 | 195 | 43.5 | – |
| 2C (105–120 cm) | 0.29 | 7.6 | 0.003 | 208 | 38 | – |
| Horizon Description | PWP | θr | θs | α | n | Ks |
|---|---|---|---|---|---|---|
| (cm3 cm−3) | (cm−1) | (-) | (cm day−1) | |||
| Ap (0–30 cm) | 0.071 | 0.0145 | 0.419 | 0.011 | 1.395 | 58.87 |
| B1 (30–50 cm) | 0.043 | 0.0260 | 0.306 | 0.033 | 1.980 | 9.34 |
| B2 (50–78 cm) | 0.034 | 0.0205 | 0.285 | 0.040 | 3.647 | 5.88 |
| 2C(k) (78–105 cm) | 0.056 | 0.0240 | 0.323 | 0.088 | 2.821 | 5.05 |
| 2C (105–120 cm) | 0.026 | 0.0260 | 0.312 | 0.047 | 4.575 | 1.95 |
| Horizon Description | Particle Size % | Textural Class | Bulk Density | ||
|---|---|---|---|---|---|
| Sand | Silt | Clay | (g cm−3) | ||
| Ap (0–30 cm) | 45.2 | 44.3 | 10.5 | Sandy Silt Loam | 1.47 |
| B1 (30–50 cm) | 88.0 | 7.4 | 4.6 | Sand | 1.59 |
| B2 (50–78 cm) | 81.9 | 11.4 | 6.7 | Loamy Sand | 1.64 |
| 2C(k) (78–105 cm) | 90.9 | 5.9 | 3.2 | Sand | 1.76 |
| 2C (105–120 cm) | 95.4 | 2.9 | 1.7 | Sand | 1.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Žydelis, R.; Chiarella, R.; Weihermüller, L.; Herbst, M.; Loit-Harro, E.; Szulc, W.; Schröder, P.; Povilaitis, V.; Mench, M.; Rineau, F.; et al. Modeling Study on Optimizing Water and Nitrogen Management for Barley in Marginal Soils. Plants 2025, 14, 704. https://doi.org/10.3390/plants14050704
Žydelis R, Chiarella R, Weihermüller L, Herbst M, Loit-Harro E, Szulc W, Schröder P, Povilaitis V, Mench M, Rineau F, et al. Modeling Study on Optimizing Water and Nitrogen Management for Barley in Marginal Soils. Plants. 2025; 14(5):704. https://doi.org/10.3390/plants14050704
Chicago/Turabian StyleŽydelis, Renaldas, Rafaella Chiarella, Lutz Weihermüller, Michael Herbst, Evelin Loit-Harro, Wieslaw Szulc, Peter Schröder, Virmantas Povilaitis, Michel Mench, Francois Rineau, and et al. 2025. "Modeling Study on Optimizing Water and Nitrogen Management for Barley in Marginal Soils" Plants 14, no. 5: 704. https://doi.org/10.3390/plants14050704
APA StyleŽydelis, R., Chiarella, R., Weihermüller, L., Herbst, M., Loit-Harro, E., Szulc, W., Schröder, P., Povilaitis, V., Mench, M., Rineau, F., Bakšienė, E., Volungevičius, J., Rutkowska, B., & Povilaitis, A. (2025). Modeling Study on Optimizing Water and Nitrogen Management for Barley in Marginal Soils. Plants, 14(5), 704. https://doi.org/10.3390/plants14050704