Influence of Exogenous Melatonin on the Physiological Traits of Camellia hainanica Seedlings Under Polyethylene Glycol-Induced Drought Stress
Abstract
1. Introduction
2. Results
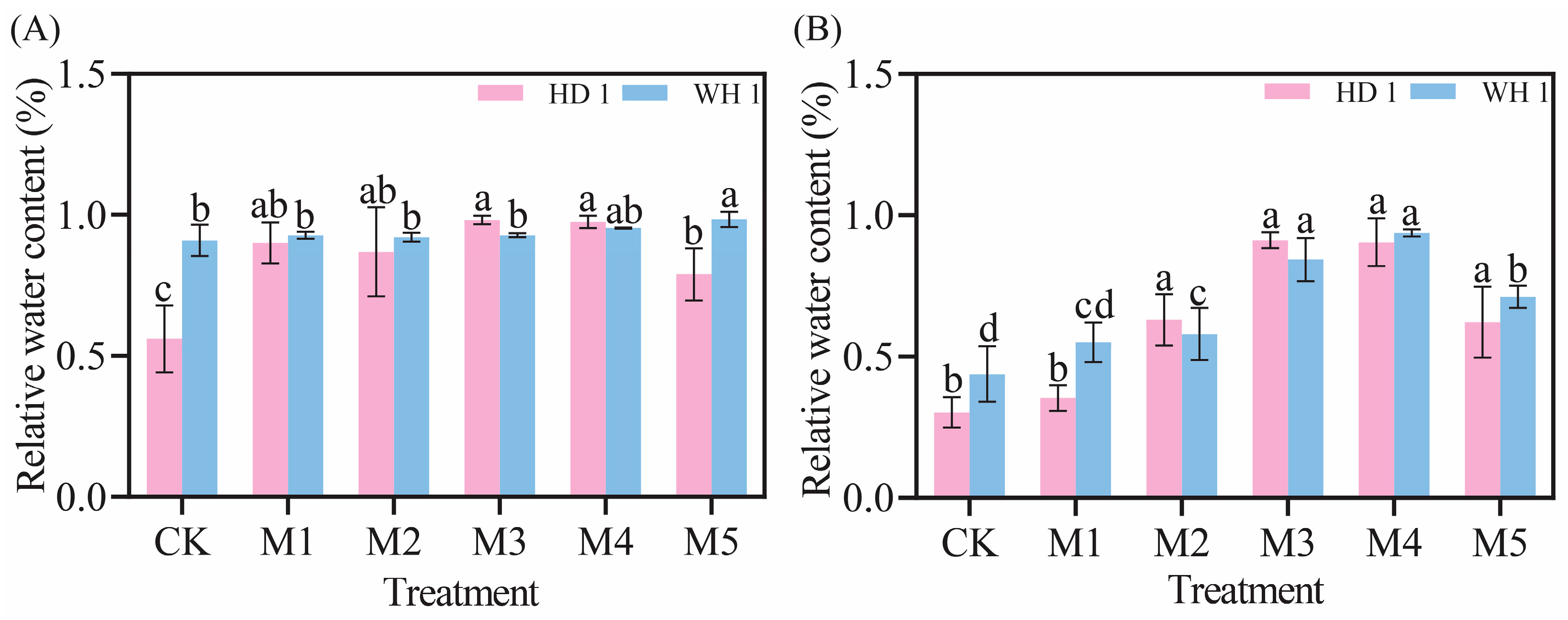
2.1. Effects of Exogenous MT on Growth and Leaf Water Content
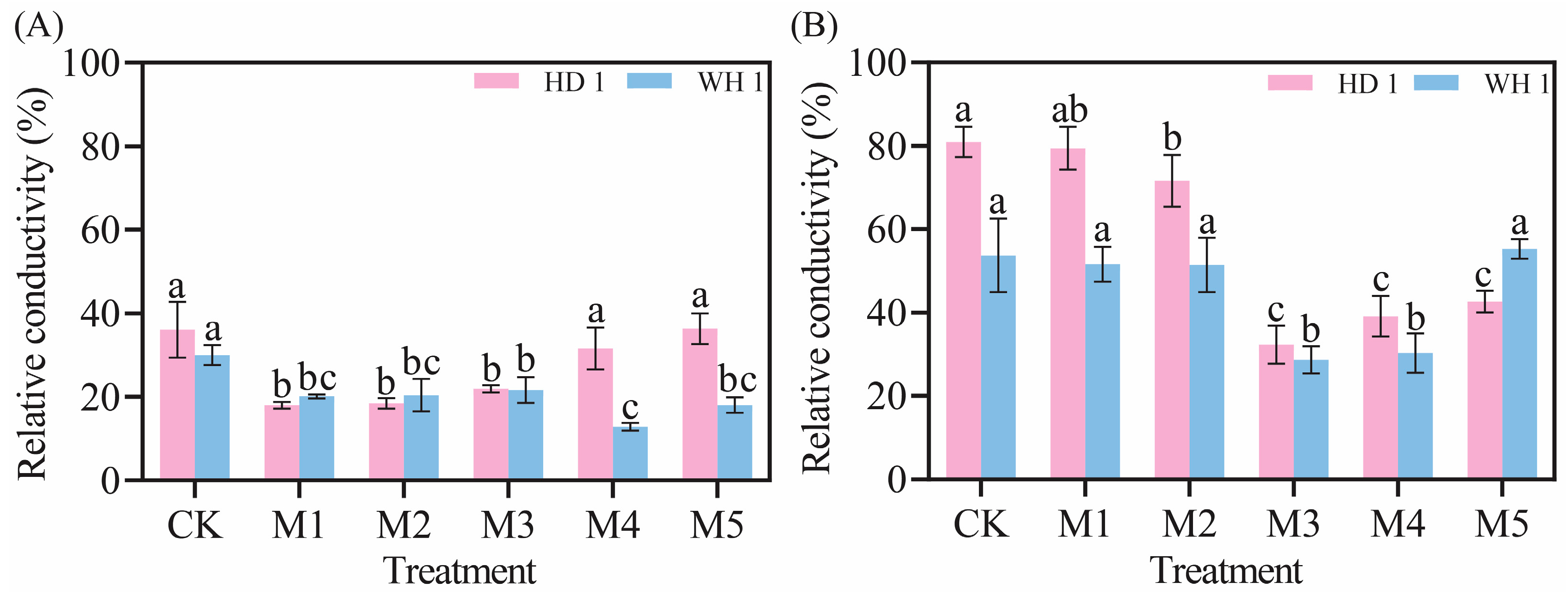
2.2. Effects of Exogenous MT on Leaf Electrical Conductivity
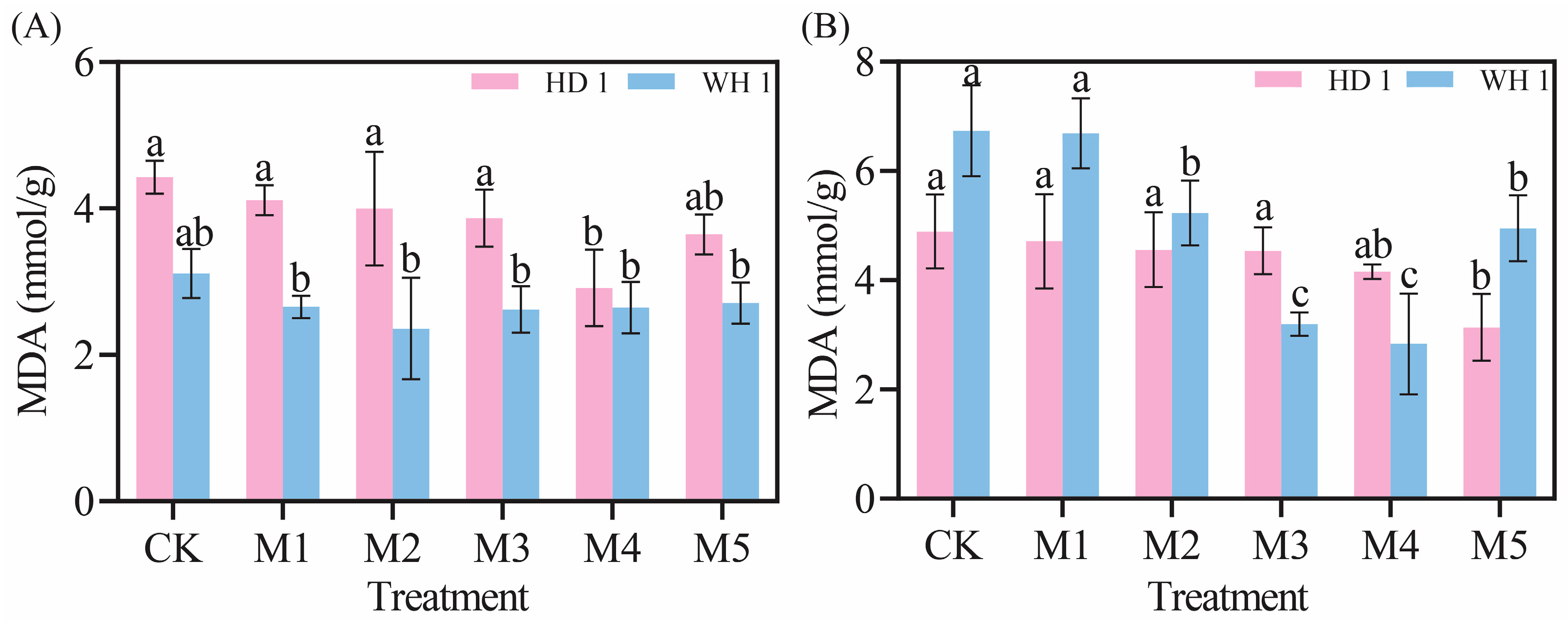
2.3. Effects of Exogenous MT on MDA Levels
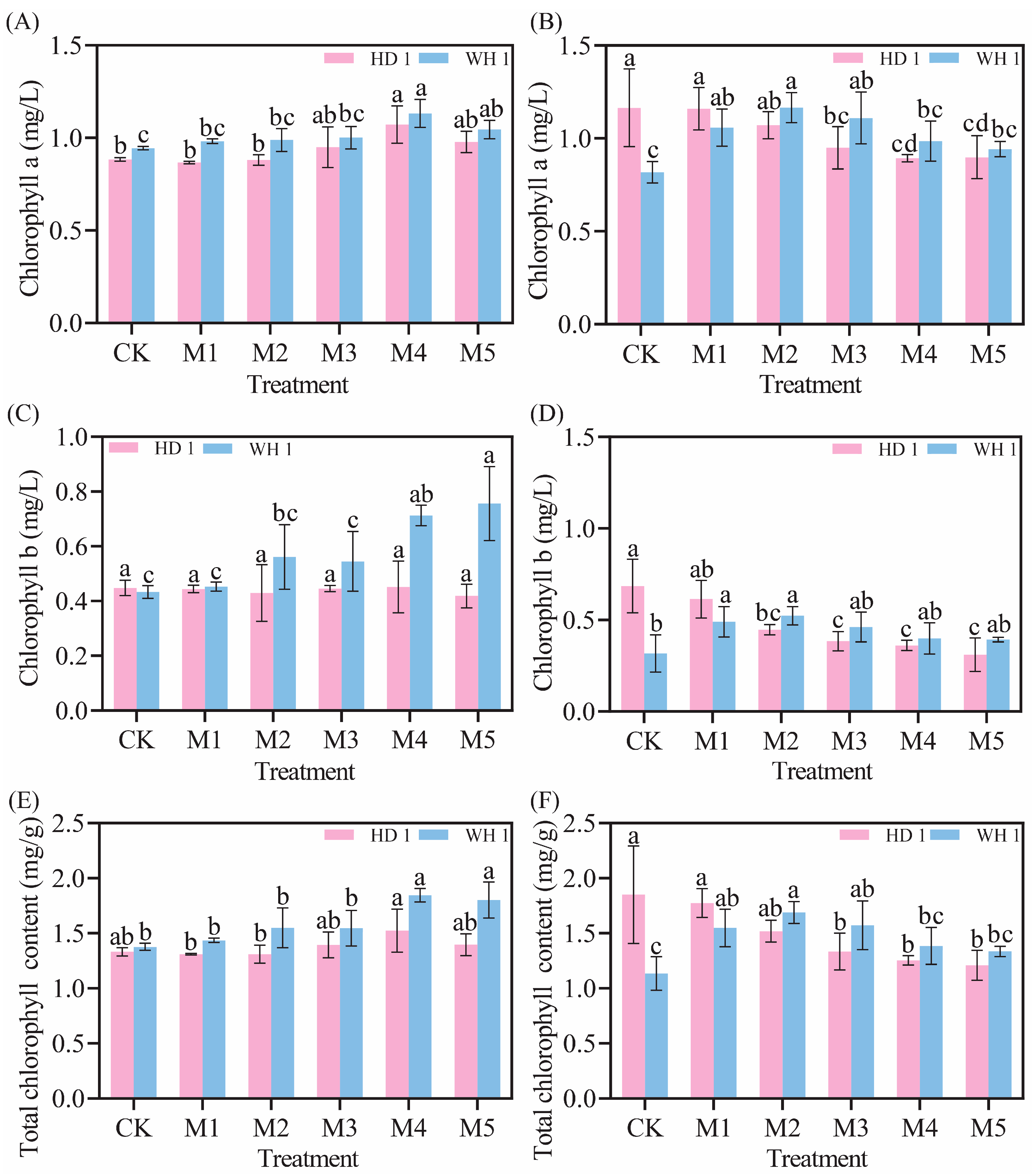
2.4. Effects of Exogenous MT on Leaf Chlorophyll Contents
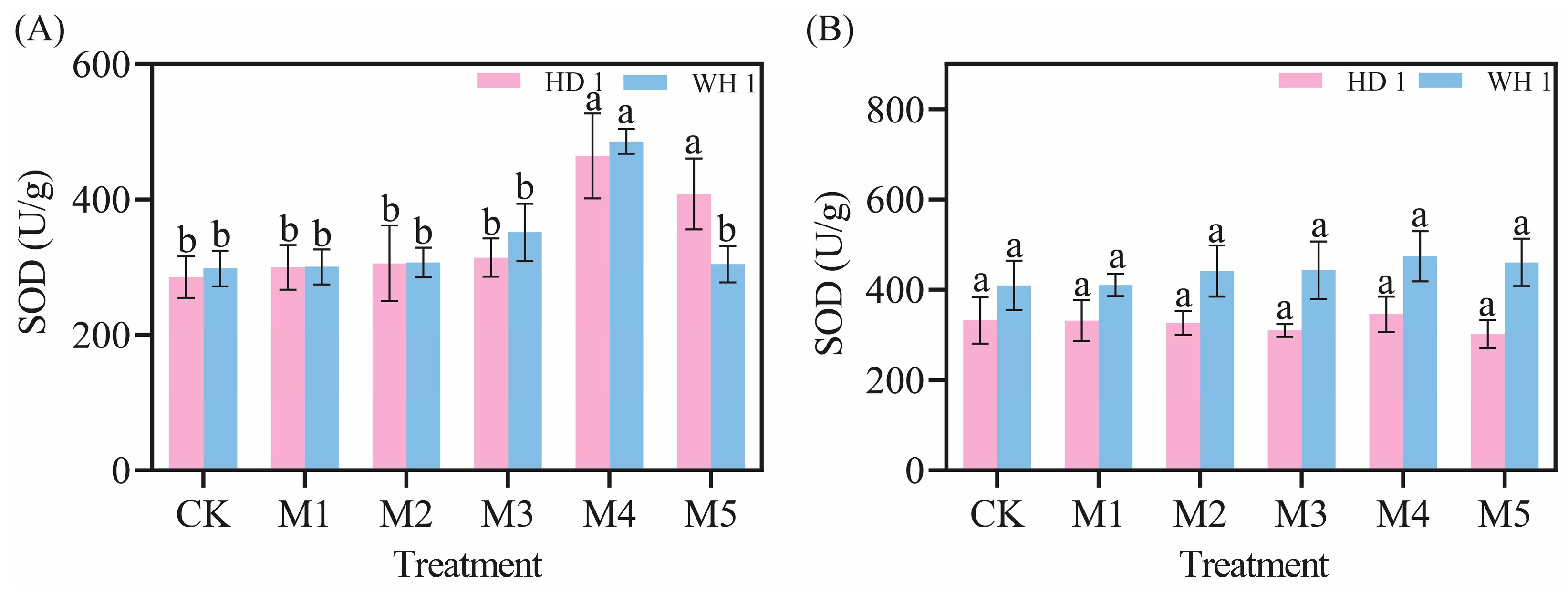
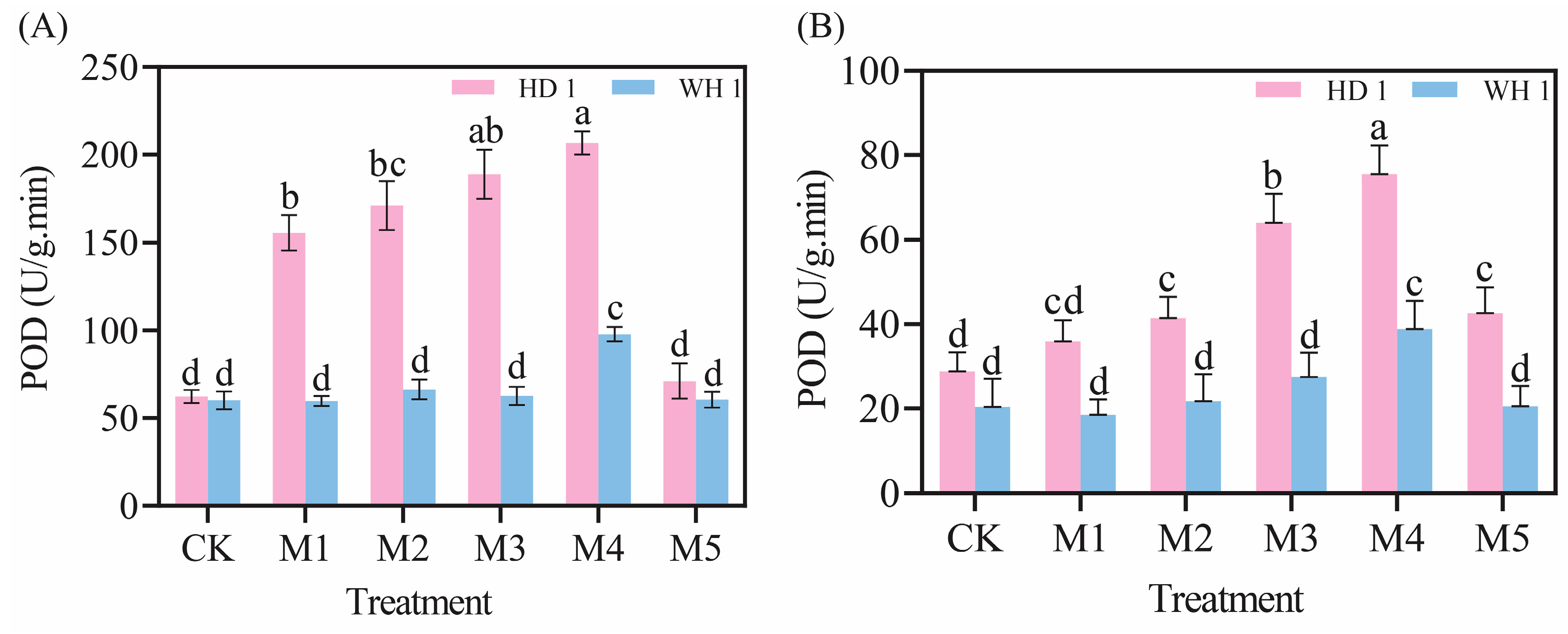
2.5. Effects of Exogenous MT on Antioxidant Enzymes
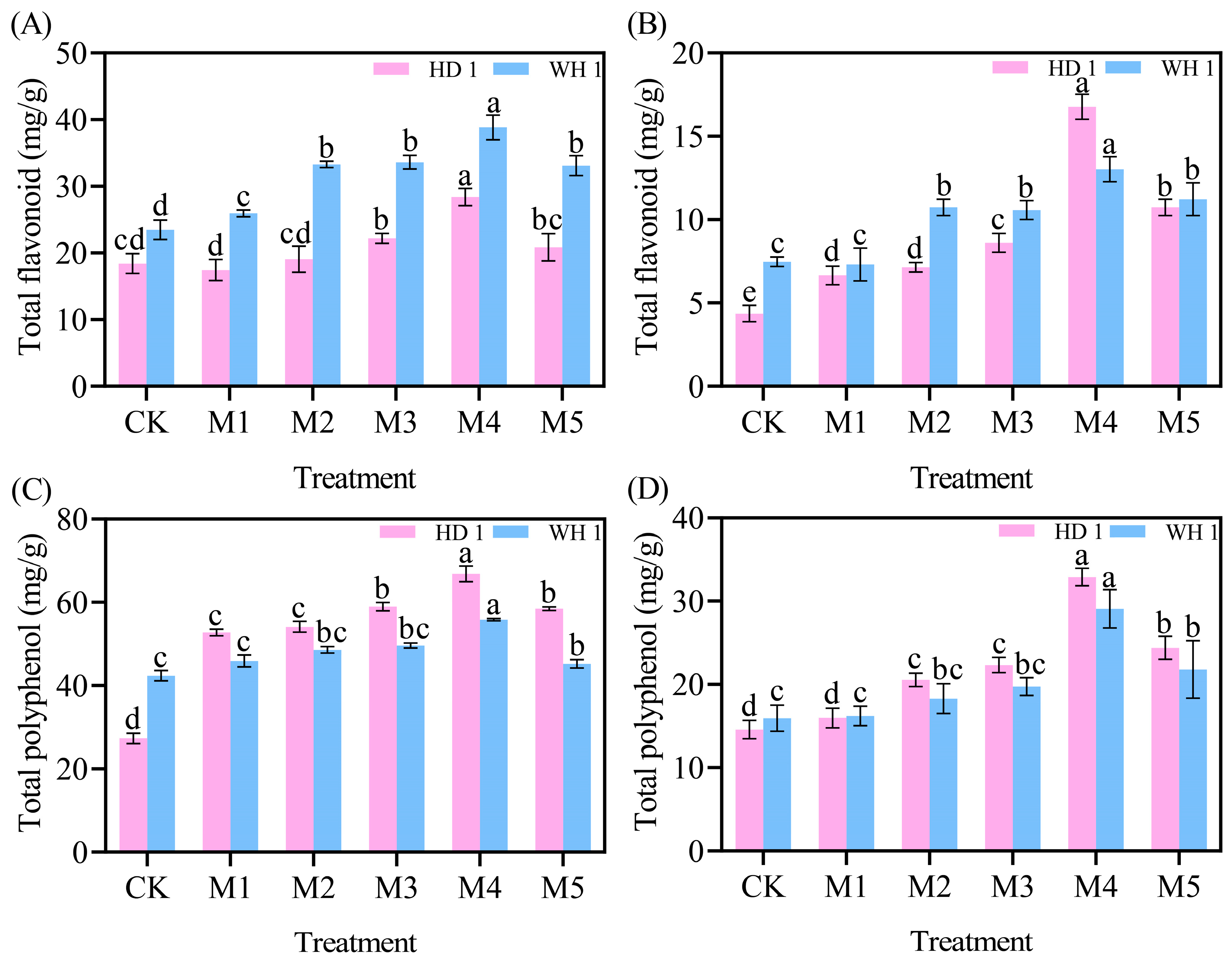
2.6. Effects of Exogenous MT on Secondary Metabolites
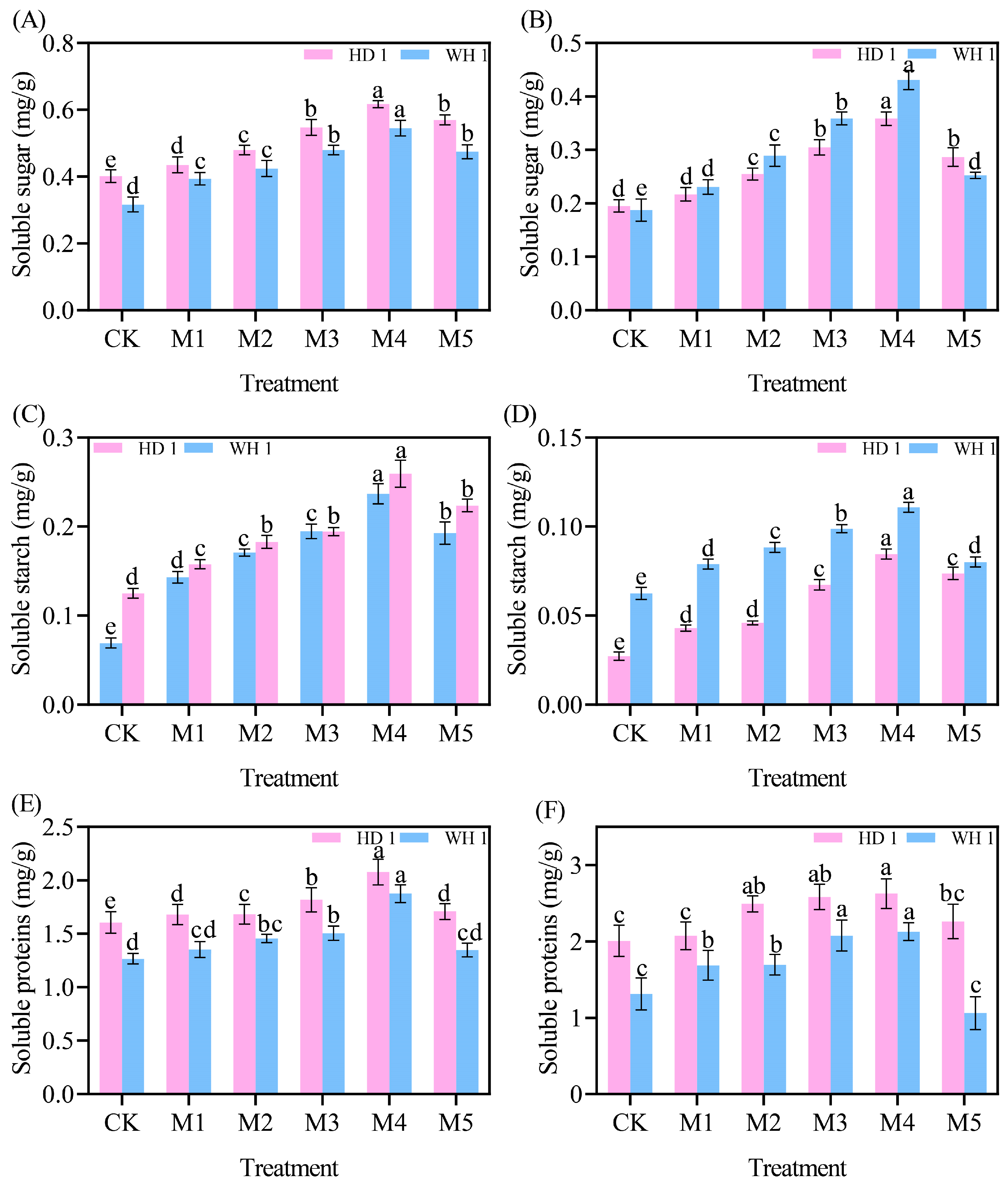
2.7. Effects of Exogenous MT on Carbohydrates
3. Discussion
3.1. Effects of MT on the Phenotype of C. hainanica Under Drought Stress
3.2. Effects of MT on Physiological Indices of C. hainanica Leaves
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Experimental Design and Treatment Management
4.3. Physiological Indexes Measurement
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Oguz, M.C.; Aycan, M.; Oguz, E.; Poyraz, I.; Yildiz, M. Drought Stress Tolerance in Plants: Interplay of Molecular, Biochemical and Physiological Responses in Important Development Stages. Physiologia 2022, 2, 180–197. [Google Scholar] [CrossRef]
- Fang, Y.; Xiong, L. General mechanisms of drought response and their application in drought resistance improvement in plants. Cell. Mol. Life Sci. 2015, 72, 673–689. [Google Scholar] [CrossRef]
- Li, Y.; Tan, B.; Wang, D.; Mu, Y.; Li, G.; Zhang, Z.; Pan, Y.; Zhu, L. Proteomic Analysis Revealed Different Molecular Mechanisms of Response to PEG Stress in Drought-Sensitive and Drought-Resistant Sorghums. Int. J. Mol. Sci. 2022, 23, 13297. [Google Scholar] [CrossRef]
- Zhao, Q.; Xiong, H.; Yu, H.; Wang, C.; Zhang, S.; Hao, J.; Wang, J.; Zhang, H.; Zhang, L. Function of MYB8 in larch under PEG simulated drought stress. Sci. Rep. 2024, 14, 11290. [Google Scholar] [CrossRef] [PubMed]
- Peršić, V.; Ament, A.; Antunović Dunić, J.; Drezner, G.; Cesar, V. PEG-induced physiological drought for screening winter wheat genotypes sensitivity—Integrated biochemical and chlorophyll a fluorescence analysis. Front. Plant Sci. 2022, 13, 987702. [Google Scholar] [CrossRef] [PubMed]
- Noman, M.; Jameel, A.; Qiang, W.-D.; Ahmad, N.; Liu, W.-C.; Wang, F.-W.; Li, H.-Y. Overexpression of GmCAMTA12 Enhanced Drought Tolerance in Arabidopsis and Soybean. Int. J. Mol. Sci. 2019, 20, 4849. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, S.; Kamruzzaman, M.; Bhattacharyya, S.; Alharbi, K.; Abd El Moneim, D.; Mostofa, M.G. Acetic acid positively modulates proline metabolism for mitigating PEG-mediated drought stress in Maize and Arabidopsis. Front. Plant Sci. 2023, 14, 1167238. [Google Scholar] [CrossRef]
- Lotfi, N.; Soleimani, A.; Çakmakçı, R.; Vahdati, K.; Mohammadi, P. Characterization of plant growth-promoting rhizobacteria (PGPR) in Persian walnut associated with drought stress tolerance. Sci. Rep. 2022, 12, 12725. [Google Scholar] [CrossRef]
- Huang, L.; Li, M.; Zhou, K.; Sun, T.; Hu, L.; Li, C.; Ma, F. Uptake and metabolism of ammonium and nitrate in response to drought stress in Malus prunifolia. Plant Physiol. Biochem. 2018, 127, 185–193. [Google Scholar] [CrossRef]
- Amnan, M.A.M.; Aizat, W.M.; Khaidizar, F.D.; Tan, B.C. Drought Stress Induces Morpho-Physiological and Proteome Changes of Pandanus amaryllifolius. Plants 2022, 11, 221. [Google Scholar] [CrossRef]
- Khodadadi, F.; Ahmadi, F.S.; Talebi, M.; Moshtaghi, N.; Matkowski, A.; Szumny, A.; Rahimmalek, M. Essential oil composition, physiological and morphological variation in Salvia abrotanoides and S. yangii under drought stress and chitosan treatments. Ind. Crops Prod. 2022, 187, 115429. [Google Scholar] [CrossRef]
- Song, W.; Song, R.; Zhao, Y.; Zhao, Y. Research on the characteristics of drought stress state based on plant stem water content. Sustain. Energy Technol. Assess. 2023, 56, 103080. [Google Scholar] [CrossRef]
- Ahmed, U.; Rao, M.J.; Qi, C.; Xie, Q.; Noushahi, H.A.; Yaseen, M.; Shi, X.; Zheng, B. Expression Profiling of Flavonoid Biosynthesis Genes and Secondary Metabolites Accumulation in Populus under Drought Stress. Molecules 2021, 26, 5546. [Google Scholar] [CrossRef]
- Ru, C.; Hu, X.; Chen, D.; Wang, W.; Zhen, J. Photosynthetic, antioxidant activities, and osmoregulatory responses in winter wheat differ during the stress and recovery periods under heat, drought, and combined stress. Plant Sci. 2022, 327, 111557. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, S.A.; Alharby, H.F.; Bamagoos, A.A.; Zaki, S.-n.S.; Abu El-Hassan, A.M.A.; Desoky, E.-S.M.; Mohamed, I.A.A.; Rady, M.M. Rebalancing Nutrients, Reinforcing Antioxidant and Osmoregulatory Capacity, and Improving Yield Quality in Drought-Stressed Phaseolus vulgaris by Foliar Application of a Bee-Honey Solution. Plants 2023, 12, 63. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Yan, W.; Xie, S.; Yu, J.; Yao, G.; Xia, P.; Wu, Y.; Yang, H. Physiological and transcriptional analysis reveals the response mechanism of camellia vietnamensis huang to drought stress. Int. J. Mol. Sci. 2022, 23, 11801. [Google Scholar] [CrossRef]
- Back, K. Melatonin metabolism, signaling and possible roles in plants. Plant J. 2021, 105, 376–391. [Google Scholar] [CrossRef]
- Liu, G.-Q.; Hu, Q.; Zhang, X.; Jiang, J.; Zhang, Y.; Zhang, Z. Melatonin biosynthesis and signal transduction in plants in response to environmental conditions. J. Exp. Bot. 2022, 73, 5818–5827. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Xie, Y.; Zhang, Z.; Chen, L. Melatonin: A Multifunctional Factor in Plants. Int. J. Mol. Sci. 2018, 19, 1528. [Google Scholar] [CrossRef] [PubMed]
- Debnath, B.; Islam, W.; Li, M.; Sun, Y.; Lu, X.; Mitra, S.; Hussain, M.; Liu, S.; Qiu, D. Melatonin Mediates Enhancement of Stress Tolerance in Plants. Int. J. Mol. Sci. 2019, 20, 1040. [Google Scholar] [CrossRef]
- Tiwari, R.K.; Lal, M.K.; Kumar, R.; Chourasia, K.N.; Naga, K.C.; Kumar, D.; Das, S.; Zinta, G. Mechanistic insights on melatonin mediated drought stress mitigation in plants. Physiol. Plant. 2020, 172, 1212–1226. [Google Scholar] [CrossRef] [PubMed]
- Altaf, M.A.; Shahid, R.; Ren, M.-X.; Naz, S.; Altaf, M.M.; Khan, L.U.; Tiwari, R.K.; Lal, M.K.; Shahid, M.A.; Kumar, R.; et al. Melatonin Improves Drought Stress Tolerance of Tomato by Modulating Plant Growth, Root Architecture, Photosynthesis, and Antioxidant Defense System. Antioxidants 2022, 11, 309. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Zheng, B. Melatonin Mediated Regulation of Drought Stress: Physiological and Molecular Aspects. Plants 2019, 8, 190. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.; Zhao, X.; Liu, S.; Sun, F.; Zhang, C.; Xi, Y. Beneficial effects of melatonin in overcoming drought stress in wheat seedlings. Plant Physiol. Biochem. 2017, 118, 138–149. [Google Scholar] [CrossRef]
- Su, X.; Fan, X.; Shao, R.; Guo, J.; Wang, Y.; Yang, J.; Yang, Q.; Guo, L. Physiological and iTRAQ-based proteomic analyses reveal that melatonin alleviates oxidative damage in maize leaves exposed to drought stress. Plant Physiol. Biochem. 2019, 142, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, I.; Ahmad, S.; Shen, W. Melatonin-mediated molecular responses in plants: Enhancing stress tolerance and mitigating environmental challenges in cereal crop production. Int. J. Mol. Sci. 2024, 25, 4551. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Ali, S.; Yan, K.; Zhao, W.; Wang, S.; Zhou, Z.; Hu, W. Melatonin alleviates drought-induced cotton pollen abortion by improving carbohydrate and energy metabolism in anthers. Ind. Crops Prod. 2025, 224, 120387. [Google Scholar] [CrossRef]
- Chen, J.; Guo, Y.; Hu, X.; Zhou, K. Comparison of the Chloroplast Genome Sequences of 13 Oil-Tea Camellia Samples and Identification of an Undetermined Oil-Tea Camellia Species From Hainan Province. Front. Plant Sci. 2022, 12, 798581. [Google Scholar] [CrossRef]
- Wu, K.; Liu, Y.; Xu, Y.; Yu, Z.; Cao, Q.; Gong, H.; Yang, Y.; Ye, J.; Jia, X. Unveiling the Molecular Mechanisms of Browning in Camellia hainanica Callus through Transcriptomic and Metabolomic Analysis. Int. J. Mol. Sci. 2024, 25, 11021. [Google Scholar] [CrossRef]
- Carson, C.F.; Hammer, K.A.; Riley, T.V. Melaleuca alternifolia (Tea Tree) Oil: A Review of Antimicrobial and Other Medicinal Properties. Clin. Microbiol. Rev. 2006, 19, 50–62. [Google Scholar] [CrossRef]
- Cai, J.; Liu, S.; Li, Y.; Liu, Q.; Xu, M.; Mo, C.; Mai, T.; Xu, X.; Tang, X.; Chen, Q. Effects of oil tea on obesity and dyslipidemia: A cross-sectional study in China. Diabetes Metab. Syndr. Obes. 2021, 14, 3173–3185. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Xiong, Z.; Sun, X.-q.; Chen, J.; Wang, C.; Chen, Y.; Zheng, D. Metabolomic profiles and health-promoting functions of Camellia drupifera mature-seeds were revealed relate to their geographical origins using comparative metabolomic analysis and network pharmacology approach. Food Chem. 2023, 426, 136619. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gu, Y.; Lin, W.; Zhang, Q. Rapid quantitative authentication and analysis of camellia oil adulterated with edible oils by electronic nose and FTIR spectroscopy. Curr. Res. Food Sci. 2024, 8, 100732. [Google Scholar] [CrossRef]
- Ye, C.; He, Z.; Peng, J.; Wang, R.; Wang, X.; Fu, M.; Zhang, Y.; Wang, A.; Liu, Z.; Jia, G.; et al. Genomic and genetic advances of oiltea-camellia (Camellia oleifera). Front. Plant Sci. 2023, 14, 1101766. [Google Scholar] [CrossRef]
- Pham, Y.H.; Reardon-Smith, K.; Deo, R.C. Evaluating management strategies for sustainable crop production under changing climate conditions: A system dynamics approach. J. Environ. Manag. 2021, 292, 112790. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Li, A.; Sun, H.; Li, P.; Liu, X.; Guo, C.; Zhang, Y.; Zhang, K.; Bai, Z.; Dong, H. The effect of exogenous melatonin on root growth and lifespan and seed cotton yield under drought stress. Ind. Crops Prod. 2023, 204, 117344. [Google Scholar] [CrossRef]
- Marino, G.; Haworth, M.; Scartazza, A.; Tognetti, R.; Centritto, M. A Comparison of the Variable J and Carbon-Isotopic Composition of Sugars Methods to Assess Mesophyll Conductance from the Leaf to the Canopy Scale in Drought-Stressed Cherry. Int. J. Mol. Sci. 2020, 21, 1222. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.-R.; Wu, L.-L.; Wang, Y.; Liu, D.; Li, J.-A. Effects of Drought Stress on the Morphological Structure and Flower Organ Physiological Characteristics of Camellia oleifera Flower Buds. Plants 2023, 12, 2585. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, M.; Wani, S.P. Rhizobacterial-plant interactions: Strategies ensuring plant growth promotion under drought and salinity stress. Agric. Ecosyst. Environ. 2016, 231, 68–78. [Google Scholar] [CrossRef]
- Yang, Y.; Nan, R.; Mi, T.; Song, Y.; Shi, F.; Liu, X.; Wang, Y.; Sun, F.; Xi, Y.; Zhang, C. Rapid and Nondestructive Evaluation of Wheat Chlorophyll under Drought Stress Using Hyperspectral Imaging. Int. J. Mol. Sci. 2023, 24, 5825. [Google Scholar] [CrossRef] [PubMed]
- Anwar, T.; Shehzadi, A.; Qureshi, H.; Shah, M.N.; Danish, S.; Salmen, S.H.; Ansari, M.J. Alleviation of cadmium and drought stress in wheat by improving growth and chlorophyll contents amended with GA3 enriched deashed biochar. Sci. Rep. 2023, 13, 18503. [Google Scholar] [CrossRef] [PubMed]
- Sadak, M.S.; Bakry, B.A. Alleviation of drought stress by melatonin foliar treatment on two flax varieties under sandy soil. Physiol. Mol. Biol. Plants 2020, 26, 907–919. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ma, X.; Hu, Y.; Hu, Q.; Wen, J.; Chen, Y.; Qian, R.; Zheng, J. Effects of exogenous spraying of melatonin on the growth of Platycrater arguta under drought stress. Front. Plant Sci. 2025, 15, 1516302. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Wang, J.; Xu, D.; Tao, S.; Chong, S.; Yan, D.; Li, Z.; Yuan, H.; Zheng, B. Melatonin regulates the functional components of photosynthesis, antioxidant system, gene expression, and metabolic pathways to induce drought resistance in grafted Carya cathayensis plants. Sci. Total Environ. 2020, 713, 136675. [Google Scholar] [CrossRef] [PubMed]
- Sabaragamuwa, R.; Perera, C.O. Total Triterpenes, Polyphenols, Flavonoids, and Antioxidant Activity of Bioactive Phytochemicals of Centella asiatica by Different Extraction Techniques. Foods 2023, 12, 3972. [Google Scholar] [CrossRef]
- Lee, C.-D.; Cho, H.; Shim, J.; Tran, G.H.; Lee, H.-D.; Ahn, K.H.; Yoo, E.; Chung, M.J.; Lee, S. Characteristics of Phenolic Compounds in Peucedanum japonicum According to Various Stem and Seed Colors. Molecules 2023, 28, 6266. [Google Scholar] [CrossRef] [PubMed]
- Orsavová, J.; Juríková, T.; Bednaříková, R.; Mlček, J. Total Phenolic and Total Flavonoid Content, Individual Phenolic Compounds and Antioxidant Activity in Sweet Rowanberry Cultivars. Antioxidants 2023, 12, 913. [Google Scholar] [CrossRef] [PubMed]
- Jafari, M.; Shahsavar, A. The Effect of Foliar Application of Melatonin on Changes in Secondary Metabolite Contents in Two Citrus Species Under Drought Stress Conditions. Front. Plant Sci. 2021, 12, 692735. [Google Scholar] [CrossRef] [PubMed]
- Lili, X.; Qianyu, Y.; Feng’e, B.; Heng, Z.; Yuxin, Y. Melatonin treatment enhances the polyphenol content and antioxidant capacity of red wine. Hortic. Plant J. 2018, 4, 144–150. [Google Scholar]
- Wang, N.; Luo, X.; Wang, Z.; Liu, J. Mitigating Effect of Exogenous Melatonin on Salt and Drought Stress in Cyperus esculentus L.during the Tillering Stage. Agronomy 2024, 14, 1009. [Google Scholar]
- Hu, W.; Cao, Y.; Loka, D.A.; Harris-Shultz, K.R.; Reiter, R.J.; Ali, S.; Liu, Y.; Zhou, Z. Exogenous melatonin improves cotton (Gossypium hirsutum L.) pollen fertility under drought by regulating carbohydrate metabolism in male tissues. Plant Physiol. Biochem. 2020, 151, 579–588. [Google Scholar]
- Xie, F.; Liu, Y.; Zhao, Q.; Liu, X.; Wang, C.; Wang, Q.; Wei, Q.; Zhao, X.; Jiang, J.; Liu, R.; et al. Exogenous Application of Melatonin and Strigolactone by Regulating Morphophysiological Responses and Gene Expression to Improve Drought Resistance in Fodder Soybean Seedlings. Agronomy 2024, 14, 1803. [Google Scholar] [CrossRef]
- Liu, L.; Cao, X.; Zhai, Z.; Ma, S.; Tian, Y.; Cheng, J. Direct evidence of drought stress memory in mulberry from a physiological perspective: Antioxidative, osmotic and phytohormonal regulations. Plant Physiol. Biochem. 2022, 186, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhou, X.; Zhou, J.; Shang, R.; Wang, Y.; Jing, P. Comparative Study on Several Determination Methods of Chlorophyll Content in Plants. IOP Conf. Ser. Mater. Sci. Eng. 2020, 730, 012066. [Google Scholar] [CrossRef]
- Wang, Z.-L.; Wu, D.; Hui, M.; Wang, Y.; Han, X.; Yao, F.; Cao, X.; Li, Y.-H.; Li, H.; Wang, H. Screening of cold hardiness-related indexes and establishment of a comprehensive evaluation method for grapevines (V. vinifera). Front. Plant Sci. 2022, 13, 1014330. [Google Scholar] [CrossRef] [PubMed]
- Zhenggang, X.; Li, F.; Mengxi, Z.; Yunlin, Z.; Huimin, H.; Guiyan, Y. Physiological dynamics as indicators of plant response to manganese binary effect. Front. Plant Sci. 2023, 14, 1145427. [Google Scholar] [CrossRef]
- Matić, P.; Sabljić, M.; Jakobek, L. Validation of Spectrophotometric Methods for the Determination of Total Polyphenol and Total Flavonoid Content. J. AOAC Int. 2017, 100, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Nayak, D.; Sahoo, S.; Barik, S.; Sanghamitra, P.; Sangeeta, S.; Pandit, E.; Reshmi Raj, K.; Basak, N.; Pradhan, S. Association mapping for protein, total soluble sugars, starch, amylose and chlorophyll content in rice. BMC Plant Biol. 2022, 22, 620. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, L.; Zeeshan Ul Haq, M.; Yao, Y.; Yang, D.; Liu, Y.; Yang, H.; Wu, Y. Influence of Exogenous Melatonin on the Physiological Traits of Camellia hainanica Seedlings Under Polyethylene Glycol-Induced Drought Stress. Plants 2025, 14, 676. https://doi.org/10.3390/plants14050676
Ge L, Zeeshan Ul Haq M, Yao Y, Yang D, Liu Y, Yang H, Wu Y. Influence of Exogenous Melatonin on the Physiological Traits of Camellia hainanica Seedlings Under Polyethylene Glycol-Induced Drought Stress. Plants. 2025; 14(5):676. https://doi.org/10.3390/plants14050676
Chicago/Turabian StyleGe, Liyan, Muhammad Zeeshan Ul Haq, Yanqiang Yao, Dongmei Yang, Ya Liu, Huageng Yang, and Yougen Wu. 2025. "Influence of Exogenous Melatonin on the Physiological Traits of Camellia hainanica Seedlings Under Polyethylene Glycol-Induced Drought Stress" Plants 14, no. 5: 676. https://doi.org/10.3390/plants14050676
APA StyleGe, L., Zeeshan Ul Haq, M., Yao, Y., Yang, D., Liu, Y., Yang, H., & Wu, Y. (2025). Influence of Exogenous Melatonin on the Physiological Traits of Camellia hainanica Seedlings Under Polyethylene Glycol-Induced Drought Stress. Plants, 14(5), 676. https://doi.org/10.3390/plants14050676







