Medicinal Potential of Broussonetia papyrifera: Chemical Composition and Biological Activity Analysis
Abstract
1. Introduction
2. Medicinal Active Ingredient
2.1. Polyphenolic Compounds
2.2. Terpenes and Alkaloids
2.3. Other Compounds
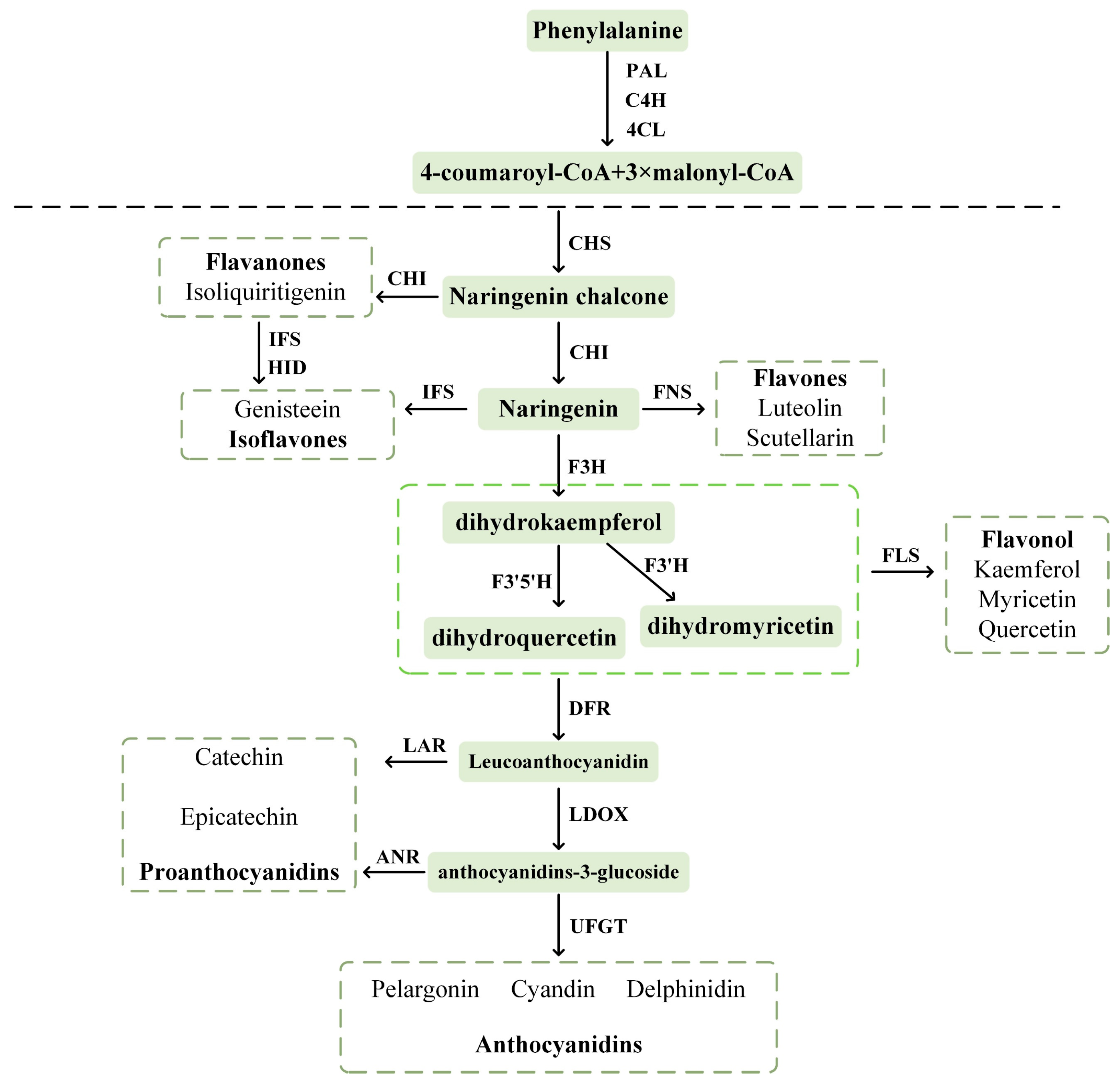
3. Metabolism of Flavonoids in B. papyrifera
3.1. Flavonoid Biosynthetic Metabolic Pathways and Related Enzyme-Encoding Genes
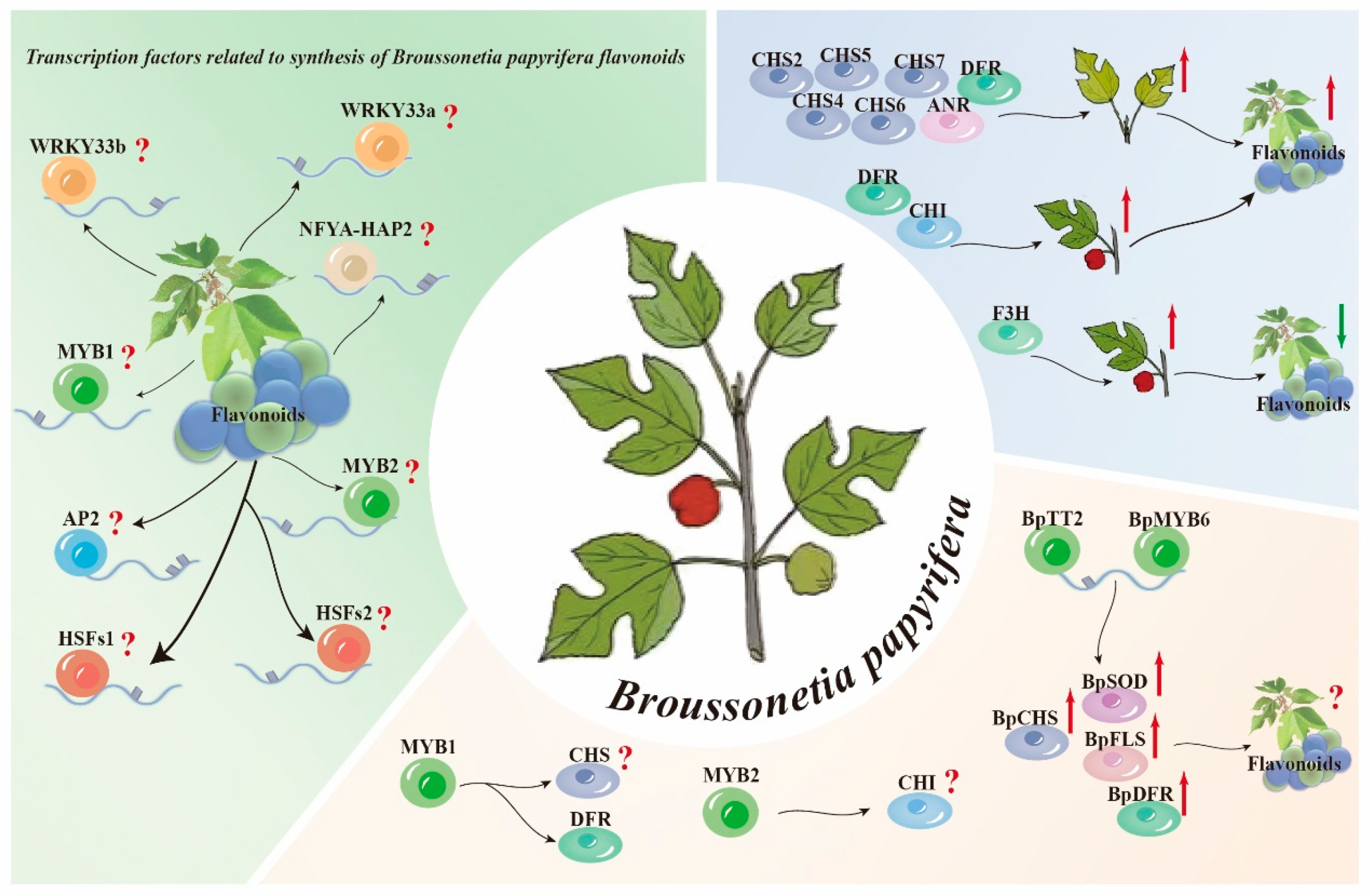
3.2. Transcription Factors Related to Flavonoid Biosynthesis in B. papyrifera
4. Flavonoid Extraction Method
| No. | Method | Extraction Conditions | Advantages and Disadvantages | Total Flavonoids | Reference |
|---|---|---|---|---|---|
| 1 | Ultrasonic-assisted ionic liquid extraction method. | Ionic liquid concentration 0.5 mol/L; ethanol concentration 60%; solid–liquid ratio 1:20; 60 °C; extraction 20 min. | Advantages: efficient extraction, short extraction time, environmentally friendly. Disadvantages: high cost, potential toxicity. | 0.4685 mg/g | [73] |
| 2 | Ultrasound-assisted method. | Sample powder 10 g; cold soak in 70% ethanol; ultrasonic for 20 min; power 300 W; recovery under reduced pressure; elution with 70% ethanol. | Advantages: short extraction time, little solvent, simple operation. Disadvantages: high equipment cost, solvent selection limit. | 2.18% | [74] |
| 3 | Ultrasound-assisted method. | Ethanol concentration 50%; material–liquid ratio 1:30; ultrasonic extraction at 50 °C for 40 min. | 35.36% | [74] | |
| 4 | Ultrasound-assisted method. | The ethanol concentration is 69%; the ultrasonic time is 37 min; the material-to-liquid ratio is 1:52. | 37.946 mg/g | [78] | |
| 5 | Alcohol extraction method. | Ethanol concentration 70%; material–liquid ratio 1:16; extraction temperature 75 °C; extraction time 117 min. | Advantages: low cost, wide scope of application, efficient extraction. Disadvantages: the extraction time is longer, solvent residue problem. | 23.93 mg/g | [75] |
| 6 | Alcohol extraction method. | Ethanol concentration 90%; solid–liquid ratio 1:35; extraction temperature 85 °C; extraction time 80 min. | 55.14 mg/g | [76] | |
| 7 | Microwave-assisted extraction method. | Ethanol concentration 55%; microwave time 15 min; power 450 W; material–liquid ratio 1:12. | Advantages: efficient and fast, extract efficiency, energy-saving, and environmental protection. Disadvantages: high equipment cost, potential component degradation. | 79.63 mg/g | [77] |
| 8 | Spectrophotometry. | Sample powder 2 g; petroleum ether 100 mL; degreasing with Soxhlet extractor for 6 h; Soxhlet extraction with 100 mL 60% methanol for 6 h; measurement at 505 nm. | Advantages: low cost, simple operation, high sensitivity. Disadvantages: poor selectivity, unable to provide structural information. | 6.05% | [79] |
| 9 | Spectrophotometry. | 5 g sample powder; extraction with 50 mL petroleum ether; 50 mL 95% ethanol; condensation reflux; measurement at 500 nm. | 3.13% | [79] | |
| 10 | Spectrophotometry. | Grind in 2 mL of concentrated HCl and ethanol; centrifuge at 12,000× g for 10 min; soak the supernatant in 80 °C water for 10 min; measure at 270, 300, and 330 nm. | [14] |
5. Pharmacological Effects
5.1. Antitumor
5.2. Antioxidants
5.3. Anti-Inflammatory Effects
5.4. Antibacterial and Antiviral Agents
5.5. Antidiabetic
5.6. Other Pharmacological Effects
6. Conclusions and Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Seelenfreund, D.; Clarke, A.; Oyanedel, N.; Piña, R.; Lobos, S.; Matisoo-Smith, E.; Seelenfreund, A. Paper Mulberry (Broussonetia Papyrifera) as a Commensal Model for Human Mobility in Oceania: Anthropological, Botanical and Genetic Considerations. N. Z. J. Bot. 2010, 48, 231–247. [Google Scholar] [CrossRef]
- Zhao, M.; Lv, D.; Hu, J.; He, Y.; Wang, Z.; Liu, X.; Ran, B.; Hu, J. Hybrid Broussonetia Papyrifera Fermented Feed Can Play a Role through Flavonoid Extracts to Increase Milk Production and Milk Fatty Acid Synthesis in Dairy Goats. Front. Vet. Sci. 2022, 9, 794443. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.S.; Qiu, Y.F.; Gao, L.; Qu, J.Q. Inhibitory effects of Broussonetia papyrifera alcohol extract and total flavonoids on isolated atria. J. Taishan Med. Coll. 1988, 2, 125–129. [Google Scholar]
- Shende, M.V.; Mahapatra, D.K.; Baravkar, A.A.; Nalawade, N.A. Looking Ahead to Reviewing Some Pharmacologically Active Phytoconstituents Present in Broussonetia Papyrifera (L.) Hert. Ex Vent. Int. J. Curr. Res. Rev. 2021, 13, 88–93. [Google Scholar] [CrossRef]
- Davinelli, S.; Ali, S.; Scapagnini, G.; Costagliola, C. Effects of Flavonoid Supplementation on Common Eye Disorders: A Systematic Review and Meta-Analysis of Clinical Trials. Front. Nutr. 2021, 8, 651441. [Google Scholar] [CrossRef]
- Ran, X.K.; Wang, X.T.; Liu, P.P.; Chi, Y.X.; Wang, B.J.; Dou, D.Q.; Kang, T.G.; Xiong, W. Cytotoxic Constituents from the Leaves of Broussonetia Papyrifera. Chin. J. Nat. Med. 2013, 11, 269–273. [Google Scholar] [CrossRef]
- Jin, J.H.; Lim, H.; Kwon, S.Y.; Son, K.H.; Kim, H.P. Anti-Inflammatory Activity of the Total Flavonoid Fraction from Broussonetia Papyrifera in Combination with Lonicera Japonica. Biomol. Ther. 2010, 18, 197–204. [Google Scholar] [CrossRef]
- Chen, P.; Shen, Y.; Shi, H.; Ma, X.; Lin, B.; Xiao, T.; Wu, F.; Zhu, J.; Li, Z.; Xiao, J.; et al. Gastroprotective Effects of Kangfuxin-against Ethanol-Induced Gastric Ulcer via Attenuating Oxidative Stress and ER Stress in Mice. Chem.-Biol. Interact. 2016, 260, 75–83. [Google Scholar] [CrossRef]
- Wang, Q.; Wei, Y.; Wang, Y.; Yu, Z.; Qin, H.; Zhao, L.; Cheng, J.; Shen, B.; Jin, M.; Feng, H. Total Flavonoids of Broussonetia Papyrifera Alleviate Non-Alcohol Fatty Liver Disease via Regulating Nrf2/AMPK/mTOR Signaling Pathways. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2024, 1869, 159497. [Google Scholar] [CrossRef]
- Lee, J.M.; Choi, S.S.; Park, M.H.; Jang, H.; Lee, Y.H.; Khim, K.W.; Oh, S.R.; Park, J.; Ryu, H.W.; Choi, J.H. Broussonetia Papyrifera Root Bark Extract Exhibits Anti-Inflammatory Effects on Adipose Tissue and Improves Insulin Sensitivity Potentially via AMPK Activation. Nutrients 2020, 12, 773. [Google Scholar] [CrossRef]
- Nguyen, L.T.H. Biological Activities of Paper Mulberry (Broussonetia Papyrifera): More than a Skin-Lightening Agent. Cosmetics 2022, 9, 112. [Google Scholar] [CrossRef]
- He, Q.; Zhou, W.; Chen, X.; Zhang, Q. Chemical and Bacterial Composition of Broussonetia Papyrifera Leaves Ensiled at Two Ensiling Densities with or without Lactobacillus Plantarum. J. Clean. Prod. 2021, 329, 129792. [Google Scholar] [CrossRef]
- Zheng, Z.P.; Cheng, K.W.; Chao, J.; Wu, J.; Wang, M. Tyrosinase Inhibitors from Paper Mulberry (Broussonetia Papyrifera). Food Chem. 2008, 106, 529–535. [Google Scholar] [CrossRef]
- Guo, P.; Huang, Z.; Li, X.; Zhao, W.; Wang, Y. Transcriptome Sequencing of Broussonetia Papyrifera Leaves Reveals Key Genes Involved in Flavonoids Biosynthesis. Plants 2023, 12, 563. [Google Scholar] [CrossRef]
- Jiao, P.; Chaoyang, L.; Wenhan, Z.; Jingyi, D.; Yunlin, Z.; Zhenggang, X. Integrative Metabolome and Transcriptome Analysis of Flavonoid Biosynthesis Genes in Broussonetia Papyrifera Leaves from the Perspective of Sex Differentiation. Front. Plant Sci. 2022, 13, 900030. [Google Scholar] [CrossRef]
- Geng, C.A.; Yan, M.H.; Zhang, X.M.; Chen, J.J. Anti-Oral Microbial Flavanes from Broussonetia Papyrifera Under the Guidance of Bioassay. Nat. Prod. Bioprospect. 2019, 9, 139–144. [Google Scholar] [CrossRef]
- Lee, Y.; Kwon, J.; Jeong, J.H.; Ryu, J.H.; Kim, K.I. Kazinol C from Broussonetia Kazinoki Stimulates Autophagy via Endoplasmic Reticulum Stress-Mediated Signaling. Anim. Cells Syst. 2022, 26, 28–36. [Google Scholar] [CrossRef]
- Ngoc, K.V.; Thi, T.L.; Woo, M.H.; Min, B.S. Anti-Inflammatory and Cytotoxic Activities of Phenolic Compounds from Broussonetia Kazinoki. NPS 2021, 27, 176–182. [Google Scholar] [CrossRef]
- Ghosh, R.; Chakraborty, A.; Biswas, A.; Chowdhuri, S. Identification of Polyphenols from Broussonetia Papyrifera as SARS CoV-2 Main Protease Inhibitors Using in Silico Docking and Molecular Dynamics Simulation Approaches. J. Biomol. Struct. Dyn. 2021, 39, 6747–6760. [Google Scholar] [CrossRef]
- Zeng, P.; Guo, Z.; Xiao, X.; Zhou, H.; Gu, J.; Liao, B. Tolerance Capacities of Broussonetia Papyrifera to Heavy Metal(Loid)s and Its Phytoremediation Potential of the Contaminated Soil. Int. J. Phytorem. 2022, 24, 580–589. [Google Scholar] [CrossRef]
- Haldar, A.G.M.; Dadure, K.M. Broussonetia Papyrifera: Reviewing Its Pharmacotherapeutic Potentials. Int. J. Med. Pharm. Sci. 2021, 11, 1–4. [Google Scholar] [CrossRef]
- Zhou, X.J.; Mei, R.Q.; Zhang, L.; Lu, Q.; Zhao, J.; Adebayo, A.H.; Cheng, Y.X. Antioxidant Phenolics from Broussonetia Papyrifera Fruits: Original Article. J. Asian Nat. Prod. Res. 2010, 12, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.W.; Park, M.H.; Kwon, O.K.; Kim, D.Y.; Hwang, J.Y.; Jo, Y.H.; Ahn, K.S.; Hwang, B.Y.; Oh, S.R. Anti-Inflammatory Flavonoids from Root Bark of Broussonetia Papyrifera in LPS-Stimulated RAW264.7 Cells. Bioorg. Chem. 2019, 92, 103233. [Google Scholar] [CrossRef]
- Zhuang, C.; Zhang, W.; Sheng, C.; Zhang, W.; Xing, C.; Miao, Z. Chalcone: A Privileged Structure in Medicinal Chemistry. Chem. Rev. 2017, 117, 7762–7810. [Google Scholar] [CrossRef] [PubMed]
- Baek, Y.S.; Ryu, Y.B.; Curtis-Long, M.J.; Ha, T.J.; Rengasamy, R.; Yang, M.S.; Park, K.H. Tyrosinase Inhibitory Effects of 1,3-Diphenylpropanes from Broussonetia Kazinoki. Bioorg. Med. Chem. 2009, 17, 35–41. [Google Scholar] [CrossRef]
- Lee, D.Y.; Lee, H.J.; Ryu, J.H. Prenylated Polyphenols from Broussonetia Kazinoki as Inhibitors of Nitric Oxide Production. Molecules 2018, 23, 639. [Google Scholar] [CrossRef]
- Li, F.; Wen, T.; Liu, J. Progress in Chemical Constituents and Bioactivities of Broussonetia Papyrifera (L.) Vent. Med. Res. 2019, 3, 190005. [Google Scholar] [CrossRef]
- Qureshi, H.; Anwar, T.; Kan, S.; Fatimah, H.; Waseem, M. Phytochemical Constituents of Broussonetia Papyrifera (L.) L’He’r. Ex Vent: An Overview. J. Indian Chem Soc 2020, 97, 55–65. [Google Scholar]
- Mei, R.Q.; Wang, Y.H.; Du, G.H.; Liu, G.M.; Zhang, L.; Cheng, Y.X. Antioxidant Lignans from the Fruits of Broussonetia Papyrifera. J. Nat. Prod. 2009, 72, 621–625. [Google Scholar] [CrossRef]
- Yang, C.; Li, F.; Du, B.; Chen, B.; Wang, F.; Wang, M. Isolation and Characterization of New Phenolic Compounds with Estrogen Biosynthesis-Inhibiting and Antioxidation Activities from Broussonetia Papyrifera Leaves. PLoS ONE 2014, 9, e94198. [Google Scholar] [CrossRef]
- Malaník, M.; Treml, J.; Leláková, V.; Nykodýmová, D.; Oravec, M.; Marek, J.; Šmejkal, K. Anti-Inflammatory and Antioxidant Properties of Chemical Constituents of Broussonetia Papyrifera. Bioorg. Chem. 2020, 104, 104298. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Chen, X.; Li, Y.; Guo, S.; Wang, Z.; Yu, X. Advances in Pharmacological Activities of Terpenoids. Nat. Prod. Commun. 2020, 15, 1934578X2090355. [Google Scholar] [CrossRef]
- Tholl, D. Biosynthesis and Biological Functions of Terpenoids in Plants. In Biotechnology of Isoprenoids; Schrader, J., Bohlmann, J., Eds.; Advances in Biochemical Engineering/Biotechnology; Springer International Publishing: Cham, Switzerland, 2015; Volume 148, pp. 63–106. ISBN 978-3-319-20106-1. [Google Scholar]
- Bergman, M.E.; Davis, B.; Phillips, M.A. Medically useful plant terpenoids: Biosynthesis, occurrence, and mechanism of action. Molecules 2019, 24, 3961. [Google Scholar] [CrossRef]
- Page, J.E.; Hause, G.; Raschke, M.; Gao, W.; Schmidt, J.; Zenk, M.H.; Kutchan, T.M. Functional Analysis of the Final Steps of the 1-Deoxy-D-Xylulose 5-Phosphate (DXP) Pathway to Isoprenoids in Plants Using Virus-Induced Gene Silencing. Plant Physiol. 2004, 134, 1401–1413. [Google Scholar] [CrossRef][Green Version]
- Zhong, H.T.; Li, F.; Chen, B.; Wang, M.K. Euphane Triterpenes from the Bark of Broussonetia Papyrifera. Helv. Chim. Acta 2011, 94, 2061–2065. [Google Scholar] [CrossRef]
- Ko, H.H.; Chang, W.L.; Lu, T.M. Antityrosinase and Antioxidant Effects of Ent -Kaurane Diterpenes from Leaves of Broussonetia Papyrifera. J. Nat. Prod. 2008, 71, 1930–1933. [Google Scholar] [CrossRef]
- Song, F.; Bor-Jinn, S. Chung Phenolic Constituents of Formosan Broussonetia Papyrifera. Phytochemistry 1994, 37, 851–853. [Google Scholar] [CrossRef]
- Wang, Y.; Hou, H.; Ren, Q.; Hu, H.; Yang, T.; Li, X. Natural Drug Sources for Respiratory Diseases from Fritillaria: Chemical and Biological Analyses. Chin. Med. 2021, 16, 40. [Google Scholar] [CrossRef]
- Wen, J.; Xiang, Q.; Guo, J.; Zhang, J.; Yang, N.; Huang, Y.; Chen, Y.; Hu, T.; Rao, C. Pharmacological Activities of Zanthoxylum L. Plants and Its Exploitation and Utilization. Heliyon 2024, 10, e33207. [Google Scholar] [CrossRef]
- Duan, H.; Erbu, A.; Dongzhi, Z.; Xie, H.; Ye, B.; He, J. Alkaloids from Dendrobium and Their Biosynthetic Pathway, Biological Activity and Total Synthesis. Phytomedicine 2022, 102, 154132. [Google Scholar] [CrossRef]
- Wang, L.; Chen, S.; Gao, X.; Liang, X.; Lv, W.; Zhang, D.; Jin, X. Recent Progress in Chemistry and Bioactivity of Monoterpenoid Indole Alkaloids from the Genus Gelsemium: A Comprehensive Review. J. Enzym. Inhib. Med. Chem. 2023, 38, 2155639. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A. Broussonetia Papyrifera Linn: Deep Insights into Its Dominant Pharmacological Perspectives. World J. Pharm. Res. 2021, 10, 577–590. [Google Scholar]
- Barreca, D.; Bellocco, E.; Leuzzi, U.; Gattuso, G. First Evidence of C- and O-Glycosyl Flavone in Blood Orange (Citrus Sinensis (L.) Osbeck) Juice and Their Influence on Antioxidant Properties. Food Chem. 2014, 149, 244–252. [Google Scholar] [CrossRef]
- Peng, M.; Shahzad, R.; Gul, A.; Subthain, H.; Shen, S.; Lei, L.; Zheng, Z.; Zhou, J.; Lu, D.; Wang, S.; et al. Differentially Evolved Glucosyltransferases Determine Natural Variation of Rice Flavone Accumulation and UV-Tolerance. Nat. Commun. 2017, 8, 1975. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.M.; Šamec, D.; Tomczyk, M.; Milella, L.; Russo, D.; Habtemariam, S.; Suntar, I.; Rastrelli, L.; Daglia, M.; Xiao, J.; et al. Flavonoid Biosynthetic Pathways in Plants: Versatile Targets for Metabolic Engineering. Biotechnol. Adv. 2020, 38, 107316. [Google Scholar] [CrossRef]
- Wen, W.; Alseekh, S.; Fernie, A.R. Conservation and Diversification of Flavonoid Metabolism in the Plant Kingdom. Curr. Opin. Plant Biol. 2020, 55, 100–108. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef]
- Gharibi, S.; Sayed Tabatabaei, B.E.; Saeidi, G.; Talebi, M.; Matkowski, A. The Effect of Drought Stress on Polyphenolic Compounds and Expression of Flavonoid Biosynthesis Related Genes in Achillea Pachycephala Rech.f. Phytochemistry 2019, 162, 90–98. [Google Scholar] [CrossRef]
- Jaakola, L.; Hohtola, A. Effect of Latitude on Flavonoid Biosynthesis in Plants. Plant Cell Environ. 2010, 33, 1239–1247. [Google Scholar] [CrossRef]
- Abusaliya, A.; Ha, S.E.; Bhosale, P.B.; Kim, H.H.; Park, M.Y.; Vetrivel, P.; Kim, G.S. Glycosidic Flavonoids and Their Potential Applications in Cancer Research: A Review. Mol. Cell. Toxicol. 2022, 18, 9–16. [Google Scholar] [CrossRef]
- Peng, X.; Liu, H.; Chen, P.; Tang, F.; Hu, Y.; Wang, F.; Pi, Z.; Zhao, M.; Chen, N.; Chen, H.; et al. A Chromosome-Scale Genome Assembly of Paper Mulberry (Broussonetia Papyrifera) Provides New Insights into Its Forage and Papermaking Usage. Mol. Plant 2019, 12, 661–677. [Google Scholar] [CrossRef] [PubMed]
- González Lorca, J.; Rivera Hutinel, A.; Moncada, X.; Lobos, S.; Seelenfreund, D.; Seelenfreund, A. Ancient and Modern Introduction of Broussonetia Papyrifera ([L.] Vent.; Moraceae) into the Pacific: Genetic, Geographical and Historical Evidence. N. Z. J. Botan. 2015, 53, 75–89. [Google Scholar] [CrossRef]
- Winkel Shirley, B. Flavonoid Biosynthesis. A Colorful Model for Genetics, Biochemistry, Cell Biology, and Biotechnology. Plant Physiol. 2001, 126, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Austin, M.B.; Noel, J.P. The Chalcone Synthase Superfamily of Type III Polyketide Synthases. Nat. Prod. Rep. 2003, 20, 79–110. [Google Scholar] [CrossRef]
- Karak, P. Biological Activities of Flavonoids: An Overview. Int. J. Pharm. Sci. Res. 2019, 10, 1567–1574. [Google Scholar] [CrossRef]
- Kreuzaler, F.; Ragg, H.; Heller, W.; Tesch, R.; Witt, I.; Hammer, D.; Hahlbrock, K. Flavanone Synthase from Petroselinum Hortense: Molecular Weight, Subunit Composition, Size of Messenger RNA, and Absence of Pantetheinyl Residue. Eur. J. Biochem. 1979, 99, 89–96. [Google Scholar] [CrossRef]
- Aharoni, A.; De Vos, C.H.R.; Wein, M.; Sun, Z.; Greco, R.; Kroon, A.; Mol, J.N.M.; O’Connell, A.P. The Strawberry FaMYB1 Transcription Factor Suppresses Anthocyanin and Flavonol Accumulation in Transgenic Tobacco. Plant J. 2001, 28, 319–332. [Google Scholar] [CrossRef]
- Moriguchi, T.; Kita, M.; Tomono, Y.; Endo-Inagaki, T.; Omura, M. Gene Expression in Flavonoid Biosynthesis: Correlation with Flavonoid Accumulation in Developing Citrus Fruit. Physiol. Plant. 2001, 111, 66–74. [Google Scholar] [CrossRef]
- Fischer, T.C.; Gosch, C.; Pfeiffer, J.; Halbwirth, H.; Halle, C.; Stich, K.; Forkmann, G. Flavonoid Genes of Pear (Pyrus Communis). Trees 2007, 21, 521–529. [Google Scholar] [CrossRef]
- Xu, Z.; Dong, M.; Peng, X.; Ku, W.; Zhao, Y.; Yang, G. New Insight into the Molecular Basis of Cadmium Stress Responses of Wild Paper Mulberry Plant by Transcriptome Analysis. Ecotoxicol. Environ. Saf. 2019, 171, 301–312. [Google Scholar] [CrossRef]
- Riaz, M.; Kamran, M.; Fang, Y.; Wang, Q.; Cao, H.; Yang, G.; Deng, L.; Wang, Y.; Zhou, Y.; Anastopoulos, I.; et al. Arbuscular Mycorrhizal Fungi-Induced Mitigation of Heavy Metal Phytotoxicity in Metal Contaminated Soils: A Critical Review. J. Hazard. Mater. 2021, 402, 123919. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Pan, L.; Ke, T.; Liang, J.; Zhang, R.; Chen, H.; Tang, M.; Hu, W. Rhizophagus Irregularis Regulates Flavonoids Metabolism in Paper Mulberry Roots under Cadmium Stress. Mycorrhiza 2024, 34, 317–339. [Google Scholar] [CrossRef] [PubMed]
- Terrier, N.; Torregrosa, L.; Ageorges, A.; Vialet, S.; Verriès, C.; Cheynier, V.; Romieu, C. Ectopic Expression of VvMybPA2 Promotes Proanthocyanidin Biosynthesis in Grapevine and Suggests Additional Targets in the Pathway. Plant Physiol. 2009, 149, 1028–1041. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zhao, X.; Li, Y.; Wang, Y.; Li, J.; Meng, J. Cloning and Expression Analysis of MaMYB308 Gene in Mulberry. Mol. Plant Breed. 2023, 14, 1–10. [Google Scholar] [CrossRef]
- Vu, N.K.; Ha, M.T.; Kim, C.S.; Gal, M.; Kim, J.A.; Woo, M.H.; Lee, J.H.; Min, B.S. Structural Characterization of Prenylated Compounds from Broussonetia Kazinoki and Their Antiosteoclastogenic Activity. Phytochemistry 2021, 188, 112791. [Google Scholar] [CrossRef]
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef]
- Rahman, N.R.A.; Yunus, N.A.; Mustaffa, A.A. Selection of Optimum Ionic Liquid Solvents for Flavonoid and Phenolic Acids Extraction. IOP Conf. Ser. Mater. Sci. Eng. 2017, 206, 012061. [Google Scholar] [CrossRef]
- Rodríguez De Luna, S.L.; Ramírez-Garza, R.E.; Serna Saldívar, S.O. Environmentally Friendly Methods for Flavonoid Extraction from Plant Material: Impact of Their Operating Conditions on Yield and Antioxidant Properties. Sci. World J. 2020, 6792069. [Google Scholar] [CrossRef]
- Yusoff, I.M.; Mat Taher, Z.; Rahmat, Z.; Chua, L.S. A Review of Ultrasound-Assisted Extraction for Plant Bioactive Compounds: Phenolics, Flavonoids, Thymols, Saponins and Proteins. Food Res. Int. 2022, 157, 111268. [Google Scholar] [CrossRef]
- Chen, R.; Zhou, X.; Deng, Q.; Yang, M.; Li, S.; Zhang, Q.; Sun, Y.; Chen, H. Extraction, Structural Characterization and Biological Activities of Polysaccharides from Mulberry Leaves: A Review. Int. J. Biol. Macromol. 2024, 257, 128669. [Google Scholar] [CrossRef]
- Xiao, J.; Chen, G.; Li, N. Ionic Liquid Solutions as a Green Tool for the Extraction and Isolation of Natural Products. Molecules 2018, 23, 1765. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jin, G.M.; Qian, L.S.; Guo, B.; Zhou, Y.Y. Study on the ionic liquids-based extraction technology of total flavonoids from Broussonetia papyrifera leaves. Chem. Ind. Eng. Prog. 2016, 35, 328–331. [Google Scholar] [CrossRef]
- Chu, F.B.; Wu, X.Y.; Ding, L.X. Process optimization of extracting and separating flavonoids from Broussonetia papyrifera leaves. J. Anhui Agric. Sci. 2015, 88, 84–86. [Google Scholar] [CrossRef]
- Zhang, M.J.; Duan, X.W.; Wang, Y.; Yang, H.W.; Liu, B.; Xiang, W.J.; You, T.H. Optimization of ethanol extraction process for active components from Broussonetia papyrifera root bark and its antioxidant activity. Sci. Technol. Food Ind. 2023, 44, 196–203. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Hu, P.H.; Huang, Z.; Cheng, R.B.; Zhong, X.M. Extraction process optimization and anti-oxidant, whitening activities of total flavonoids from Broussonetia kazinoki. Chin. Tradit. Pat. Med. 2020, 42, 842–848. [Google Scholar]
- Zhu, K.M.; Gu, S.J.; Yao, L.X.; Yao, L.X.; Liu, J.N.; Liu, J.N.; Ou, Y.; Ou, Y.; Tang, S.D.; Tang, S.D. Research on the extraction technology of total flavonoids from Broussonetia papyrifera by microwave-assisted method. Med. Plant 2012, 3, 26–29. [Google Scholar]
- Tao, Y.Z. Isolation and Identification of Flavonoid from Broussonetia Papyrifera and Synthesis of Luteolin Selenium Complex; Guangxi University: Guangxi, China, 2022. [Google Scholar]
- Chi, Y.X. Determination of total flavonoids content in the leaves of Pueraria vinifera and Structure leaves. Mod. Chin. Med. 2018, 10, 16–17. [Google Scholar] [CrossRef]
- Wang, L. Anti-Inflammatory and Anticancer Properties of Dichloromethane and Butanol Fractions from the Stem Bark of Broussonetia Papyrifera. J. Korean Soc. Appl. Bi. 2010, 53, 297–303. [Google Scholar] [CrossRef]
- Li, Y.; Li, H.; Zhang, Y.; Li, L.; Qin, C. In Vitro Antioxidant and Anticancer Activities of the Extract from Paper Mulberry (Broussonetia Papyrifera L.) Fruit. Asian J. Chem. 2013, 25, 5453. [Google Scholar] [CrossRef]
- Guo, M.; Wang, M.; Zhang, X.; Deng, H.; Wang, Z.-Y. Broussoflavonol B Restricts Growth of ER-Negative Breast Cancer Stem-like Cells. Anticancer Res. 2013, 33, 1873–1879. [Google Scholar]
- Dou, C.Z.; Liu, Y.F.; Zhang, L.L.; Chen, S.H.; Hu, C.Y.; Liu, Y.; Zhao, Y.T. Polyphenols from Broussonetia Papyrifera Induce Apoptosis of HepG2 Cells via Inactivation of ERK and AKT Signaling Pathways. Evid-Based. Compl. Alt. 2021, 2021, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.L.; Chen, Y.C.; Hsu, H.Y. Kazinol Q from Broussonetia Kazinoki Enhances Cell Death Induced by Cu(Ll) through Increased Reactive Oxygen Species. Molecules 2011, 16, 3212–3221. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Lee, J.; Shon, J.C.; Phuc, N.M.; Jee, J.G.; Liu, K.H. The Inhibitory Potential of Broussochalcone a for the Human Cytochrome P450 2J2 Isoform and Its Anti-Cancer Effects via FOXO3 Activation. Phytomedicine 2018, 42, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Son, Y.; Liu, K.H.; Kang, W.; Oh, S. Cytotoxic Activity of Broussochalcone a against Colon and Liver Cancer Cells by Promoting Destruction Complex-Independent β-Catenin Degradation. Food Chem. Toxicol. 2019, 131, 110550. [Google Scholar] [CrossRef]
- Meerson, A.; Khatib, S.; Mahajna, J. Natural Products Targeting Cancer Stem Cells for Augmenting Cancer Therapeutics. Int. J. Mol. Sci. 2021, 22, 13044. [Google Scholar] [CrossRef]
- Wang, Q.; Zhong, S.; Wu, H.; Wu, Q. In Vitro Anti-Cancer Effect of Marmesin by Suppression of PI3K/Akt Pathway in Esophagus Cancer Cells. Esophagus-Tokyo 2022, 19, 163–174. [Google Scholar] [CrossRef]
- Xiao-jing, Q.U.; Fang-fang, P.; Ying-fu, Y.I.N.; Ying-ying, T.a.N.; Chun-shan*, Q. Ultrasonic Extraction and Antioxidant Activity Investigation of Broussonetia Papyrifer Seed Oil. Nat. Prod. Res. Dev. 2014, 26, 1685. [Google Scholar]
- Gulcin, İ. Antioxidants and Antioxidant Methods: An Updated Overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef]
- Husain, S.R.; Ahmad, M.S.; Ahmad, F.; Ahmad, M.; Osman, S.M. Hibiscus Mutabilis Seed Oil–Characterization of HBr-reactive Acids. Lipid Fett. 1989, 91, 167–168. [Google Scholar] [CrossRef]
- Shah, P.; Modi, H.A. Comparative Study of DPPH, ABTS and FRAP Assays for Determination of Antioxidant Activity. Int. J. Res. Appl. Sci. Eng. Technol. 2015, 3, 636–641. [Google Scholar]
- Flieger, J.; Flieger, W.; Baj, J.; Maciejewski, R. Antioxidants: Classification, Natural Sources, Activity/Capacity Measurements, and Usefulness for the Synthesis of Nanoparticles. Materials 2021, 14, 4135. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Liu, S.; Zhang, C.; Yu, L.; Bi, J.; Zhu, F.; Yang, Q. Chemical Composition and Antioxidant Activities of Broussonetia Papyrifera Fruits. PLoS ONE 2012, 7, e32021. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, C.S.; Yu, L.N.; Bi, J.; Liu, S.F.; Zhu, F.; Yang, Q.L. Antioxidant Activity and Total Phenolics of Broussonetia Papyrifera Flower Extracts. Appl. Mech. Mater. 2011, 140, 263–267. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, L.; Liu, X.; Wang, F.; An, Y.; Zhao, W.; Tian, J.; Kong, D.; Zhang, W.; Xu, Y.; et al. The Genus Broussonetia: An Updated Review of Phytochemistry, Pharmacology and Applications. Molecules 2022, 27, 5344. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.J.; Lin, C.N.; Hwang, T.L.; Teng, C.M. Broussochalcone a, a Potent Antioxidant and Effective Suppressor of Inducible Nitric Oxide Synthase in Lipopolysaccharide-Activated Macrophages. Biochemical Pharmacology. 2000, 61, 939–946. [Google Scholar] [CrossRef]
- Kwak, W.J.; Moon, T.C.; Lin, C.X.; Rhyn, H.G.; Jung, H.; Lee, E.; Kwon, D.Y.; Son, K.H.; Kim, H.P.; Kang, S.S.; et al. Papyriflavonol a from Broussonetia Papyrifera Inhibits the Passive Cutaneous Anaphylaxis Reaction and Has a Secretory Phospholipase A2-Inhibitory Activity. Biol. Pharm. Bull. 2003, 26, 299–302. [Google Scholar] [CrossRef]
- Huang, S.P.; Guan, X.; Kai, G.Y.; Xu, Y.Z.; Xu, Y.; Wang, H.J.; Pang, T.; Zhang, L.Y.; Liu, Y. Broussonin E Suppresses LPS-Induced Inflammatory Response in Macrophages via Inhibiting MAPK Pathway and Enhancing JAK2-STAT3 Pathway. Chin. J. Nat. Med. 2019, 17, 372–380. [Google Scholar] [CrossRef]
- Lin, L.W.; Chen, H.Y.; Wu, C.R.; Liao, P.M.; Lin, Y.T.; Hsieh, M.T.; Ching, H. Comparison with Various Parts of Broussonetia Papyrifera as to the Antinociceptive and Anti-Inflammatory Activities in Rodents. Biosci. Biotechnol. Biochem. 2008, 72, 2377–2384. [Google Scholar] [CrossRef]
- Tian, J.L.; Liu, T.L.; Xue, J.J.; Hong, W.; Zhang, Y.; Zhang, D.X.; Cui, C.C.; Liu, M.C.; Niu, S.L. Flavanoids Derivatives from the Root Bark of Broussonetia Papyrifera as a Tyrosinase Inhibitor. Ind. Crops Prod. 2019, 138, 111445. [Google Scholar] [CrossRef]
- Sohn, H.Y.; Son, K.H.; Kwon, C.S.; Kwon, G.S.; Kang, S.S. Antimicrobial and Cytotoxic Activity of 18 Prenylated Flavonoids Isolated from Medicinal Plants: Morus Alba L., Morus Mongolica Schneider, Broussnetia Papyrifera (L.) Vent, Sophora Flavescens Ait and Echinosophora Koreensis Nakai. Phytomedicine 2004, 11, 666–672. [Google Scholar] [CrossRef]
- Sohn, H.Y. Fungicidal Effect of Prenylated Flavonol, Papyriflavonol a, Isolated from Broussonetia Papyrifera (L.) Vent. Against Candida Albicans. J. Microbiol. Biotechnol. 2010, 20, 1397–1402. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.N.; Ramakrishnaiah, H.; Krishna, V.; Deepalakshmi, A.P. Gc-Ms Analysis and Antimicrobial Activity of Seed Oil of Broussonetia Papyrifera (L.) Vent. Int. J. Pharm. Sci. Res. 2015, 6, 3954. [Google Scholar]
- Han, Q.; Wu, Z.; Huang, B.; Sun, L.; Ding, C.; Yuan, S.; Zhang, Z.; Chen, Y.; Hu, C.; Zhou, L.; et al. Extraction, Antioxidant and Antibacterial Activities of Broussonetia Papyrifera Fruits Polysaccharides. Int. J. Biol. Macromol. 2016, 92, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Yuk, H.J.; Ryu, H.W.; Lim, S.H.; Kim, K.S.; Park, K.H.; Ryu, Y.B.; Lee, W.S. Evaluation of Polyphenols from Broussonetia Papyrifera as Coronavirus Protease Inhibitors. J. Enzym. Inhib. Med. Chem. 2017, 32, 504–512. [Google Scholar] [CrossRef]
- Ryu, H.W.; Lee, J.H.; Kang, J.E.; Jin, Y.M.; Park, K.H. Inhibition of Xanthine Oxidase by Phenolic Phytochemicals from Broussonetia Papyrifera. J. Korean Soc. Appl. Biol. Chem. 2012, 55, 587–594. [Google Scholar] [CrossRef]
- Bae, U.J.; Lee, D.Y.; Song, M.Y.; Lee, S.M.; Park, J.W.; Ryu, J.H.; Park, B.H. A Prenylated Flavan from Broussonetia Kazinoki Prevents Cytokine- Induced b-Cell Death through Suppression of Nuclear Factor-kB Activity. Biol. Pharm. Bull. 2011, 34, 1026–1031. [Google Scholar] [CrossRef][Green Version]
- Eleftheriou, P.; Geronikaki, A.; Petrou, A. PTP1b Inhibition, a Promising Approach for the Treatment of Diabetes Type II. Curr. Top. Med. Chem. 2019, 19, 246–263. [Google Scholar] [CrossRef]
- Xu, B.; Hao, K.; Chen, X.; Wu, E.; Nie, D.; Zhang, G.; Si, H. Broussonetia Papyrifera Polysaccharide Alleviated Acetaminophen-Induced Liver Injury by Regulating the Intestinal Flora. Nutrients 2022, 14, 2636. [Google Scholar] [CrossRef]
- Kim, J. The Potential Efficacy of Broussonetia Kazinoki Stem Extract to Show Antioxidant Property or Suppress Collagenase Activity. Biomed. J. Sci. Tech. Res. 2019, 17, 12802–12804. [Google Scholar] [CrossRef]
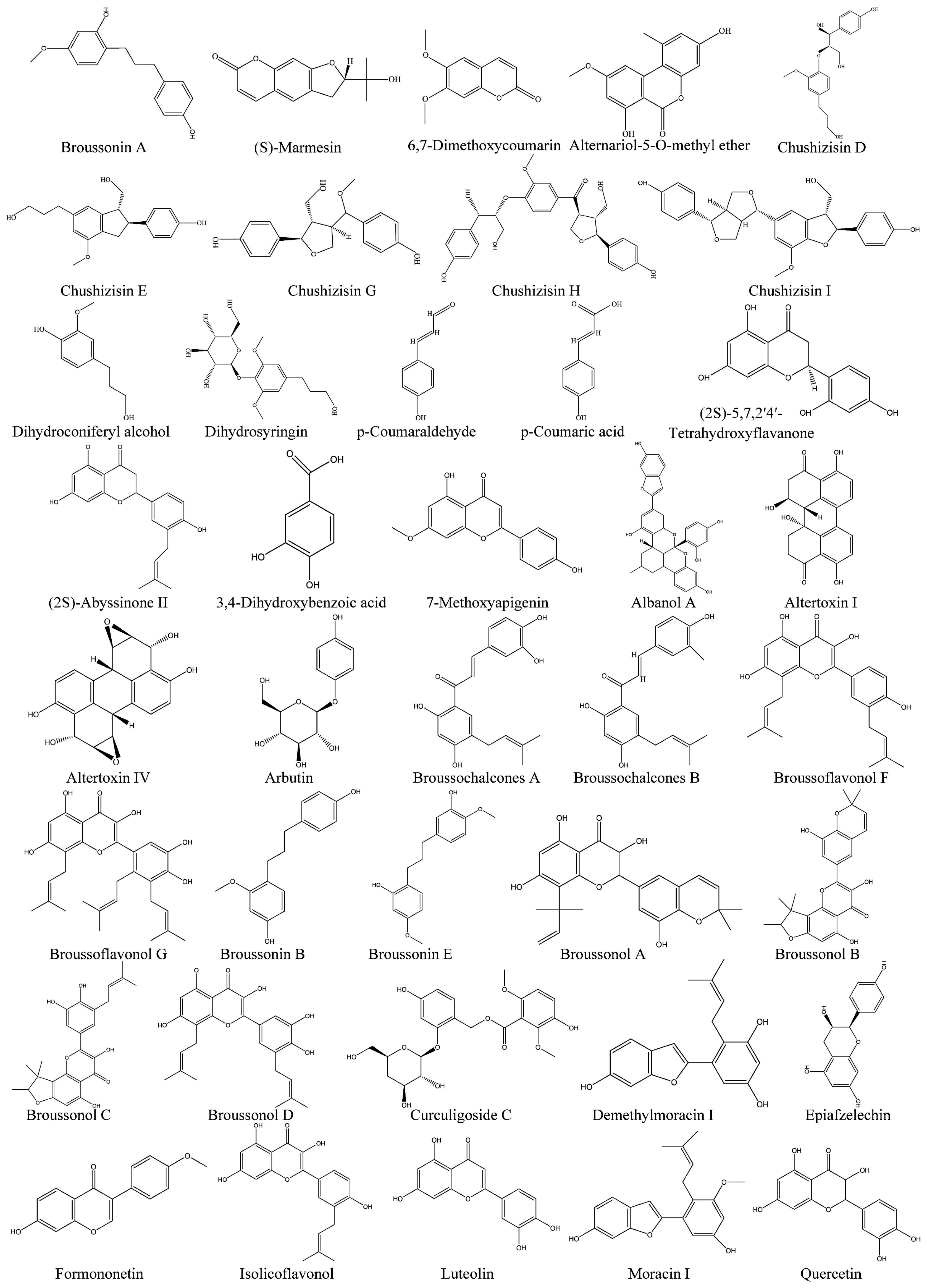
| No. | Compound Name | Source | Classification | Reference |
|---|---|---|---|---|
| 1 | Broussonin A | Leaves | Chalcones | [24] |
| 2 | Moracin N | Whole plants | Isoflavonoids | [24] |
| 3 | Demethylmoracin I | Whole plants | Isoflavonoids | [24] |
| 4 | 1-(2,4-Dihydroxyphenyl)-3-(4-hydroxyphenyl)-propane | Whole plants | Diphenylpropane polyphenol | [24] |
| 5 | Albanol A | Whole plants | Chalcone- Isoflavonoids | [24] |
| 6 | Broussonin B | Roots | Chalcones | [25] |
| 7 | Kazinol F | Whole plants | Lignans | [25] |
| 8 | Kazinol C | Roots\Twigs | Lignans | [25] |
| 9 | Kazinol D | Roots\Twigs | Lignans | [25] |
| 10 | Curculigoside C | Fruits | Phenylethanoid Glycosides | [22] |
| 11 | 3,4-Dihydroxybenzoic acid | Fruits | Phenolic Acids | [22] |
| 12 | (7R,8S) -3-Methoxy-4,9,9-trihydroxy-4,7-epoxy-5,8-neolignan | Whole plants | Lignans | [26] |
| 13 | (7R,8S,8R)-7,8-Threo-3-methoxy-7-oxo-4,4,7,9,9-pentahydroxy-4,8:7,9-bis-epoxy-8,8-sesquineolignan | Fruits | Lignans | [22] |
| 14 | Kazinol W | Roots | Lignans | [26] |
| 15 | Kazinol J | Roots\Leaves | Lignans | [26] |
| 16 | Kazinol V | Roots | Lignans | [26] |
| 17 | Broussonin E | Roots | Lignans | [26] |
| 18 | Broussoside D | Leaves | Lignans | [26] |
| 19 | 3,5,4-Trihydroxy-bibenzyl-3-O-β-D-glucoside | Leaves | Lignans | [26] |
| 20 | Broussofluorenone A | Roots | Benzopyrone polyphenols | [26] |
| 21 | Moracin I | Whole plants | Lignans | [26] |
| 22 | Moracin D | Whole plants | Lignans | [26] |
| 23 | Moracin M | Whole plants | Lignans | [26] |
| 24 | Altertoxin I | Whole plants | Furanone polyphenols | [24] |
| 25 | Altertoxin IV | Whole plants | Furanone polyphenols | [24] |
| 26 | Erythro-1-(4-hydroxyphenyl)-2-{4-[(E)-3-hydroxy-1-propenyl]-2-methoxyphenoxy}-1,3-propanediol | Whole plants | Polyphenolic | [27] |
| 27 | erythro-1-(4-hydroxyphenyl)-2-[4-(3-hydroxy-1-propyl)-2-methoxyphenoxy]-1,3-propanediol | Whole plants | Polyphenolic | [27] |
| 28 | Threo-1-(4-hydroxyphenyl)-2-{4-[(E)-3-hydroxy1-propenyl]-2-methoxyphenoxy}-1,3-propanediol | Whole plants | Polyphenolic | [27] |
| 29 | threo-1-(4-hydroxyphenyl)-2-[4-(3-hydroxy-1-propyl)-2-methoxyphenoxy]-1,3-propanediol | Whole plants | Polyphenolic | [27] |
| No. | Compound Name | Source | Classification | Reference |
|---|---|---|---|---|
| 1 | Chushizisin A | Fruits | Flavonols | [29] |
| 2 | Chushizisin B | Fruits | Flavonols | [29] |
| 3 | Chushizisin C | Fruits | Flavonols | [29] |
| 4 | Chushizisin D | Fruits | Flavonols | [29] |
| 5 | Chushizisin E | Fruits | Flavonols | [29] |
| 6 | Chushizisin F | Fruits | Flavonols | [29] |
| 7 | Chushizisin G | Fruits | Flavonols | [29] |
| 8 | Chushizisin H | Fruits | Flavonols | [29] |
| 9 | Chushizisin I | Fruits | Flavonols | [29] |
| 10 | Threo-1-(4-hydroxy-3-methoxyphenyl)-2-{4-(E)-3-hydroxy-1-propenyl-2-methoxyphenoxy}-1,3-propanediol | Fruits | Phenolics | [29] |
| 11 | Erythro-1-(4-hydroxy-3-methoxyphenyl)-2-{4-(E)-3-hydroxy-1-propenyl-2-methoxy-phenoxy}-1,3-propanediol | Fruits | Phenolics | [29] |
| 12 | 3-2-(4-Hydroxyphenyl)-3-hydroxymethyl-2,3-dihydro-1-benzofuran-5-ylpropan-1-ol | Fruits | Phenolics | [29] |
| 13 | p-Coumaraldehyde | Fruits | Phenolics | [22] |
| 14 | Cissyringin | Fruits | Phenolics | [22] |
| 15 | Cisconiferin | Fruits | Phenolics | [22] |
| 16 | Erythro-1-(4-hydroxyphenyl) glycerol | Fruits | Phenolics | [22] |
| 17 | Threo-1-(4-hydroxyphenyl) glycerol | Fruits | Phenolics | [22] |
| 18 | Dihydroconiferyl alcohol | Fruits | Phenylpropanoids | [22] |
| 19 | Dihydrosyringin | Leaves | Phenylpropanoids | [30] |
| 20 | Syringaresinol-4-O-β-D-glucoside | Leaves | Lignans | [30] |
| 21 | Pinoresinol-4-O-β-D-glucopyranoside | Leaves | Lignans | [30] |
| 22 | p-Coumaric acid | Leaves | Phenylpropanoids | [30] |
| 23 | 6,7-Dimethoxycoumarin | Whole plants | Coumarins | [27] |
| 24 | Iariciresinol-9-O-β-D-glucopyranoside | Leaves | Lignans | [27] |
| 25 | 3,4,5-Trihy- droxy-5-methoxy-6H-benzo [c] chromen-6-one | Whole plants | Phenylpropanoids | [27] |
| 26 | Alternariol-4-O-methyl ether | Whole plants | Phenylpropanoids | [27] |
| 27 | Alternariol-5-O-methyl ether | Whole plants | Phenylpropanoids | [27] |
| 28 | (S)-Marmesin | Twigs | Phenylpropanoids | [31] |
| 29 | (S)-8-Methoxymarmesin | Twigs | Phenylpropanoids | [31] |
| 30 | 7,8-Dihydroxy-6-(3-methylbut-2-en-1yl)-2H-chromen-2-one | Roots | Phenylpropanoids | [28] |
| 31 | (+)-Pinoresinol-4-O-β-D-glucopyranosyl-4-O-β-D-apiofuranoside | Leaves | Phenylpropanoids | [28] |
| No. | Compound Name | Source | Reference |
|---|---|---|---|
| 1 | Broussonetone A | Leaves | [37] |
| 2 | Broussonetone B | Leaves | [37] |
| 3 | Broussonetone C | Leaves | [37] |
| 4 | Taraxerol acetate | Leaves | [37] |
| 5 | Squalene | Roots/Fruits | [38] |
| 6 | Butyrospermol acetate | Whole plants | [38] |
| 7 | Augustic acid | Whole plants | [27] |
| 8 | Oleanolic acid | Roots | [27] |
| 9 | Lupeol acetate | Whole plants | [27] |
| 10 | 3β-Acetoxy-tirucalla-7-en-24S,25-diol | Roots | [27] |
| 11 | (3β)-3-(Acetyloxy)-eupha-7,25-dien-24-one | Roots | [27] |
| 12 | (3β,24R)-3-(Acetyloxy)-eupha-7,25-dien-24-ol | Roots | [27] |
| 13 | (3β,24S)-Eupha-7,25-diene-3,24-diol | Roots | [27] |
| 14 | (3β,24R)-Eupha-7,25-diene3,24-diol | Roots | [36] |
| 15 | α-Amyrin acetate | Roots | [36] |
| 16 | β-Amyrin | Roots | [36] |
| 17 | Lupeol | Roots | [36] |
| No. | Compound Name | Source | Reference |
|---|---|---|---|
| 1 | Broussonpapyrine | Fruits | [43] |
| 2 | Liriodenine | Fruits | [43] |
| 3 | Oxyavicine | Fruits | [43] |
| 4 | Nitidine | Fruits | [43] |
| 5 | Dihydrosanguinarine | Fruits | [43] |
| 6 | N-Norchelerythrine | Fruits | [43] |
| 7 | 2-Deoxyuridine | Whole plants | [28] |
| 8 | 2-Deoxyadenosine | Whole plants | [28] |
| 9 | Thymidine | Whole plants | [28] |
| 10 | Isoterihanine | Whole plants | [43] |
| 11 | Chelerythrine | Whole plants | [43] |
| 12 | Erythrinasinate | Roots | [43] |
| No. | Compound Name | Source | Classification | Reference |
|---|---|---|---|---|
| 1 | Fucosterol | Whole plants | Sterol | [43] |
| 2 | Ergosterol peroxide | Whole plants | Steroid peroxide | [43] |
| 3 | β-Sitosterol | Whole plants | Phytosterol | [30] |
| 4 | β-Daucosterol | Whole plants | Sterol | [30] |
| 5 | Ergosta-4,6,8,22-tetraen-3-one | Whole plants | Steroid | [30] |
| 6 | D-Galacitol | Whole plants | Sugar alcohol | [30] |
| 7 | Daucosterol palmitate | Whole plants | Sterol ester | [27] |
| 8 | Palmitic acid | Whole plants | Saturated fatty acid | [27] |
| 9 | Phytol | Whole plants | Carotenoid derivative | [27] |
| 10 | Physcion | Whole plants | Anthraquinone | [27] |
| 11 | Palmitic acid ethyl ester | Whole plants | Fatty acid ester | [27] |
| 12 | Linoleic acid | Whole plants | Polyunsaturated fatty acid | [27] |
| 13 | 8,11-Octadecadienoic acid | Whole plants | Polyunsaturated fatty acid | [27] |
| 14 | 9-Octadecenoic acid | Whole plants | Monounsaturated fatty acid | [27] |
| 15 | α-Monopalmitin | Whole plants | Glyceride | [27] |
| 16 | Monoheptadecanoin | Whole plants | Glyceride | [27] |
| 17 | Heptadecanoic acid | Whole plants | Saturated fatty acid | [27] |
| 18 | Altersolanol A | Whole plants | Phenolic quinone | [27] |
| 19 | Altersolanol C | Whole plants | Phenolic quinone | [27] |
| 20 | δ-Tocopherol | Whole plants | Tocopherol | [27] |
| 21 | (4R,5S,10S)-8,9,10-Trihydroxy-4-[3-methoxy-4-hydroxyphenyl]-1,6-dioxaspiro [4,5]decan-2-one | Whole plants | heterocyclic compound | [27] |
| 22 | 4-Hydroxyacetophenone | Whole plants | Phenolic | [27] |
| 23 | (7R,8S)-3-Methoxy-7-oxo-4,9,9-trihydroxy-4,7-epoxy-5,8-neolignan | Whole plants | Neolignans | [27] |
| 24 | (7R,8S)-3-Methoxy-4,9,9-trihydroxy-4,7-epoxy-5,8-neolignan | Whole plants | Neolignans | [27] |
| 25 | Benzylbenzoate-2,6-di-O-β-D-glucopyranoside | Whole plants | Benzylbenzoate | [27] |
| 26 | Broussoside A | Leaves | Phenylethanoid | [30] |
| 27 | Broussoside C | Leaves | Phenylethanoid | [30] |
| 28 | Broussoside E | Leaves | Phenylethanoid | [30] |
| 29 | Poliothyrsoside | Leaves | Phenylethanoid | [30] |
| 30 | 4-Hydroxybenzaldehyde | Fruits | Aromatic aldehydes | [22] |
| 31 | Curculigoside I | Fruits | Phenylethanoid | [22] |
| 32 | 2-(4-Hydroxyphenyl) propane-1,3-diol-1-O-β-D-glucopyranoside | Fruits | Phenylpropanoid | [22] |
| 33 | (2R,3R,5R,6S,9R)-3-Hydroxy-5,6-epoxyb-ionol-2-O-β-D-glucopyranoside | Leaves | Carotenoid derivative | [22] |
| 34 | Lignoceric acid | Roots | Saturated fatty acid | [43] |
| 35 | Octacosan-1-ol | Roots | long-chain alcohol | [43] |
| 36 | 4-Hydroxycis-cinnamic acid octacosyl ester | Roots | Cinnamic acid ester | [43] |
| No. | Compound Name | Source | Classification | Reference |
|---|---|---|---|---|
| 1 | Papyriflavonol A | Roots\Twigs | Flavonoids | [28] |
| 2 | 5,7,3′,4′-Tetrahydroxy-6,5′-di-(γ,γ-dimethylallyl)-flavonol | Leaves | Flavonols | [28] |
| 3 | 8-(1,1-Dimethylallyl)-5′-(3 methylbut-2-enyl)-3′,4′,5,7-tetrahydroxyflavonol | Barks | Flavonols | [27] |
| 4 | 3′-(3-Methylbut-2-enyl)-3′,4′,7-trihydroxyflavane | Barks | Flavans | [27] |
| 5 | 5,7,3′,4′-Tetrahydroxy-3-methoxy-8-ger anylflavone | Barks | Flavonoids | [31] |
| 6 | 5,7,3′,4′-Tetrahydroxy-3-me thoxy-8,5′-diprenylflavone | Barks | Flavonoids | [31] |
| 7 | 3,5,7,4′-Tetrahydroxy- 3′-(2-hydroxy-3-methylbut-3-enyl | Barks | Flavonoids | [31] |
| 8 | Quercetin | Twigs | Flavonols | [43] |
| 9 | Dihydroquercetin | Whole plants | Flavonols | [43] |
| 10 | Isolicoflavonol | Whole plants | Flavonols | [43] |
| 11 | Broussoflavonol G | Whole plants | Flavonols | [43] |
| 12 | Broussoflavonol F | Roots\Twigs | Flavonols | [43] |
| 13 | Broussoflavonol E | Roots\Twigs | Flavonols | [43] |
| 14 | 4′7-Dihydroxyflavone | Leaves | Flavonoids | [28] |
| 15 | Broussonol A | Leaves | Flavonols | [43] |
| 16 | Broussonol B | Leaves | Flavonols | [43] |
| 17 | Broussonol C | Roots\Leaves | Flavonols | [43] |
| 18 | Broussonol D | Roots\Leaves | Flavonols | [43] |
| 19 | Broussonol E | Twigs | Flavonols | [43] |
| 20 | Apigenin | Leaves | Flavonols | [43] |
| 21 | Cosmosiin | Leaves | Flavonoids | [43] |
| 22 | Luteolin | Leaves\Twigs | Flavonoids | [43] |
| 23 | Luteolin-7-O-β-D-glucopyranoside | Leaves | Flavonoids | [43] |
| 24 | 7-Methoxyapigenin | Leaves | Flavonoids | [43] |
| 25 | 5,7,3′,4′-Tetrahydroxy-3-methoxy-6-geranylflavone | Barks | Flavonoids | [43] |
| 26 | 5,7,2′,4′-Tetrahydroxy-3-geranylflavone | Barks | Flavonoids | [43] |
| 27 | (2S)-Abyssinone II | Whole plants | Flavonoids | [43] |
| 28 | (2S)-5,7,2′4′-Tetrahydroxyflavanone | Barks | Flavans | [43] |
| 29 | Broussochalcones A | Whole plants | Chalcones | [43] |
| 30 | Broussochalcones B | Whole plants | Chalcones | [43] |
| 31 | 3′,4′,7-Trihydroxyfiavone | Barks | Flavonoids | [43] |
| 32 | Formononetin | Leaves\Twigs | Isoflavones | [43] |
| 33 | Epiafzelechin | Leaves\Twigs | Flavanols | [43] |
| 34 | Arbutin | Whole plants | Flavonoids | [43] |
| 35 | Broussoside B | Whole plants | Flavonoids | [43] |
| 36 | Flacourtin | Leaves | Flavonols | [30] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Huang, R.; Zhang, W.; Chen, Q.; Wang, Q.; Ye, J.; Xu, F. Medicinal Potential of Broussonetia papyrifera: Chemical Composition and Biological Activity Analysis. Plants 2025, 14, 523. https://doi.org/10.3390/plants14040523
Li Y, Huang R, Zhang W, Chen Q, Wang Q, Ye J, Xu F. Medicinal Potential of Broussonetia papyrifera: Chemical Composition and Biological Activity Analysis. Plants. 2025; 14(4):523. https://doi.org/10.3390/plants14040523
Chicago/Turabian StyleLi, Ying, Renhua Huang, Weiwei Zhang, Qiangwen Chen, Qijian Wang, Jiabao Ye, and Feng Xu. 2025. "Medicinal Potential of Broussonetia papyrifera: Chemical Composition and Biological Activity Analysis" Plants 14, no. 4: 523. https://doi.org/10.3390/plants14040523
APA StyleLi, Y., Huang, R., Zhang, W., Chen, Q., Wang, Q., Ye, J., & Xu, F. (2025). Medicinal Potential of Broussonetia papyrifera: Chemical Composition and Biological Activity Analysis. Plants, 14(4), 523. https://doi.org/10.3390/plants14040523






