Predicting Potential Suitable Habitats of Three Rare Wild Magnoliaceae Species (Michelia crassipes, Lirianthe coco, Manglietia insignis) Under Current and Future Climatic Scenarios Based on the Maxent Model
Abstract
1. Introduction
2. Result and Analysis
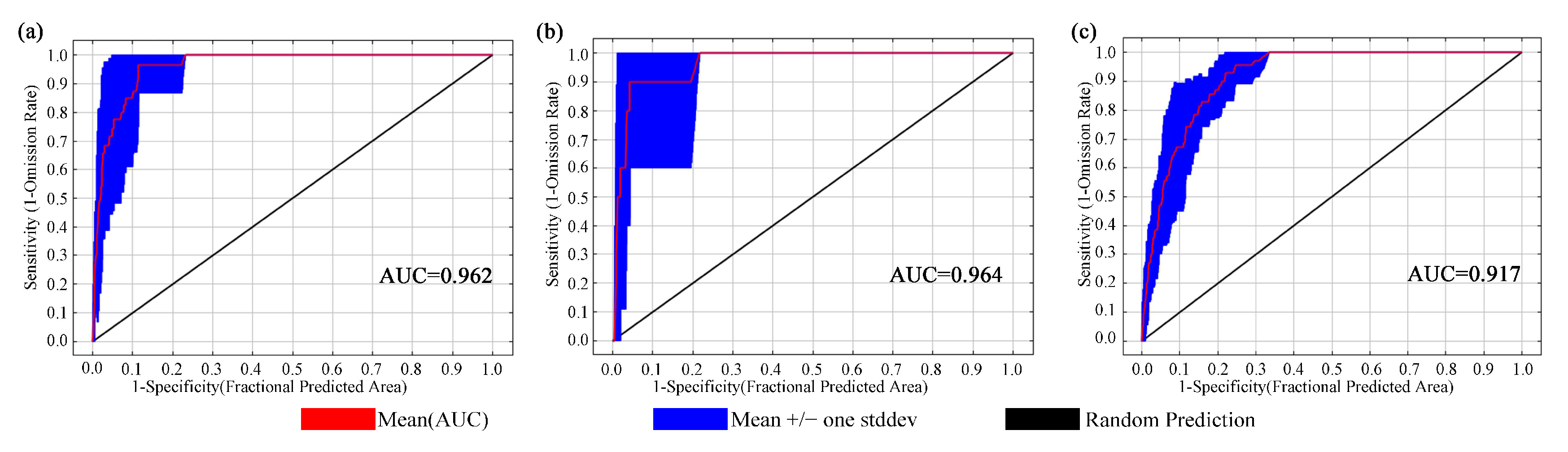
2.1. Model Accuracy Evaluation and Contribution of Environmental Variables
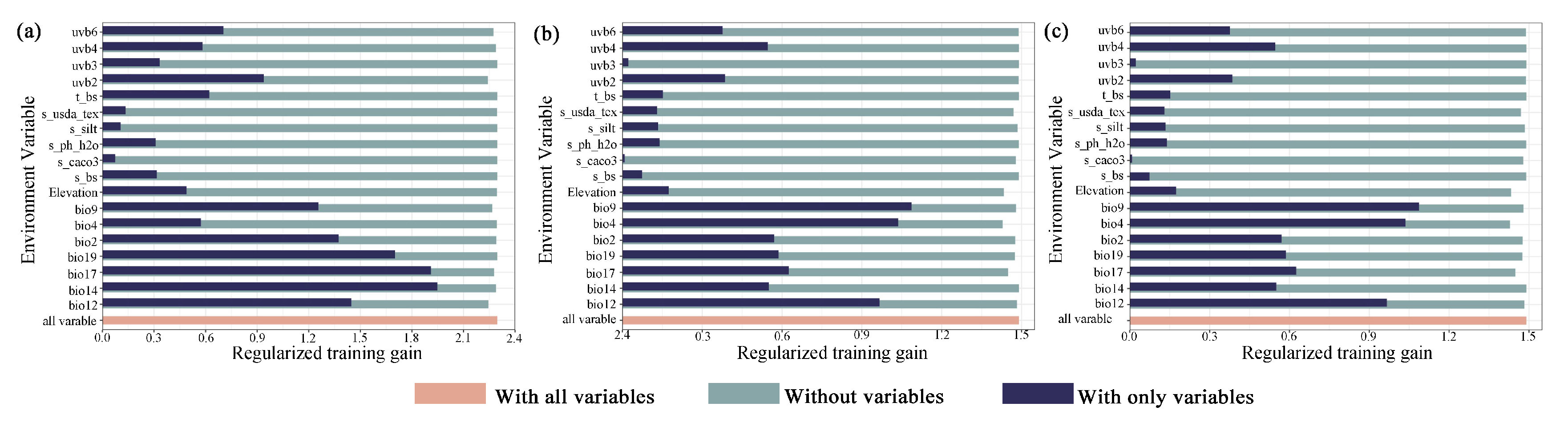
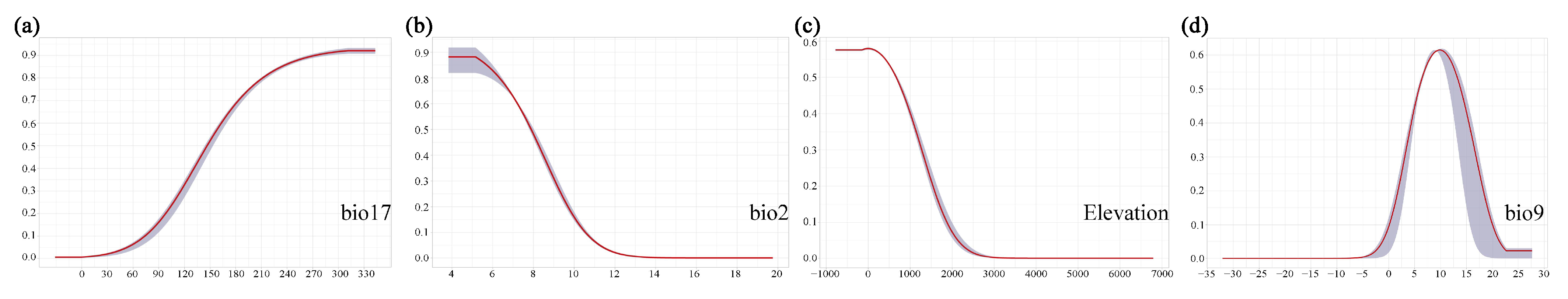
2.1.1. The Dominant Environmental Variables for the Distribution of M. crassipes
2.1.2. The Dominant Environmental Variables for the Distribution of L. coco
2.1.3. The Dominant Environmental Variables for the Distribution of M. insignis
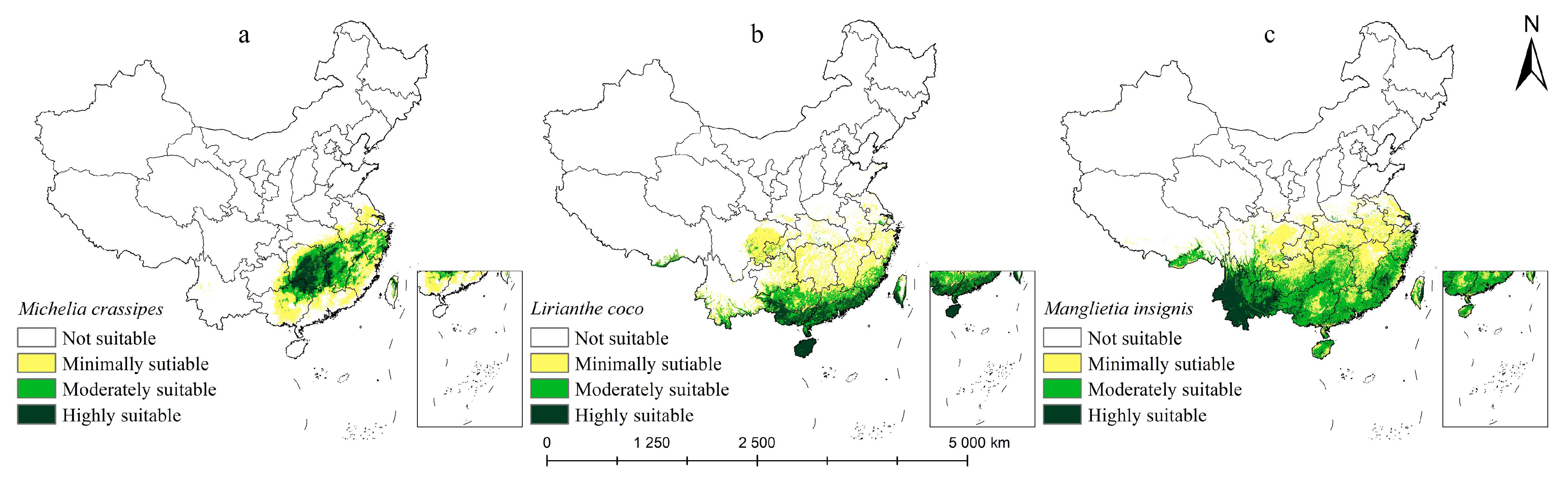
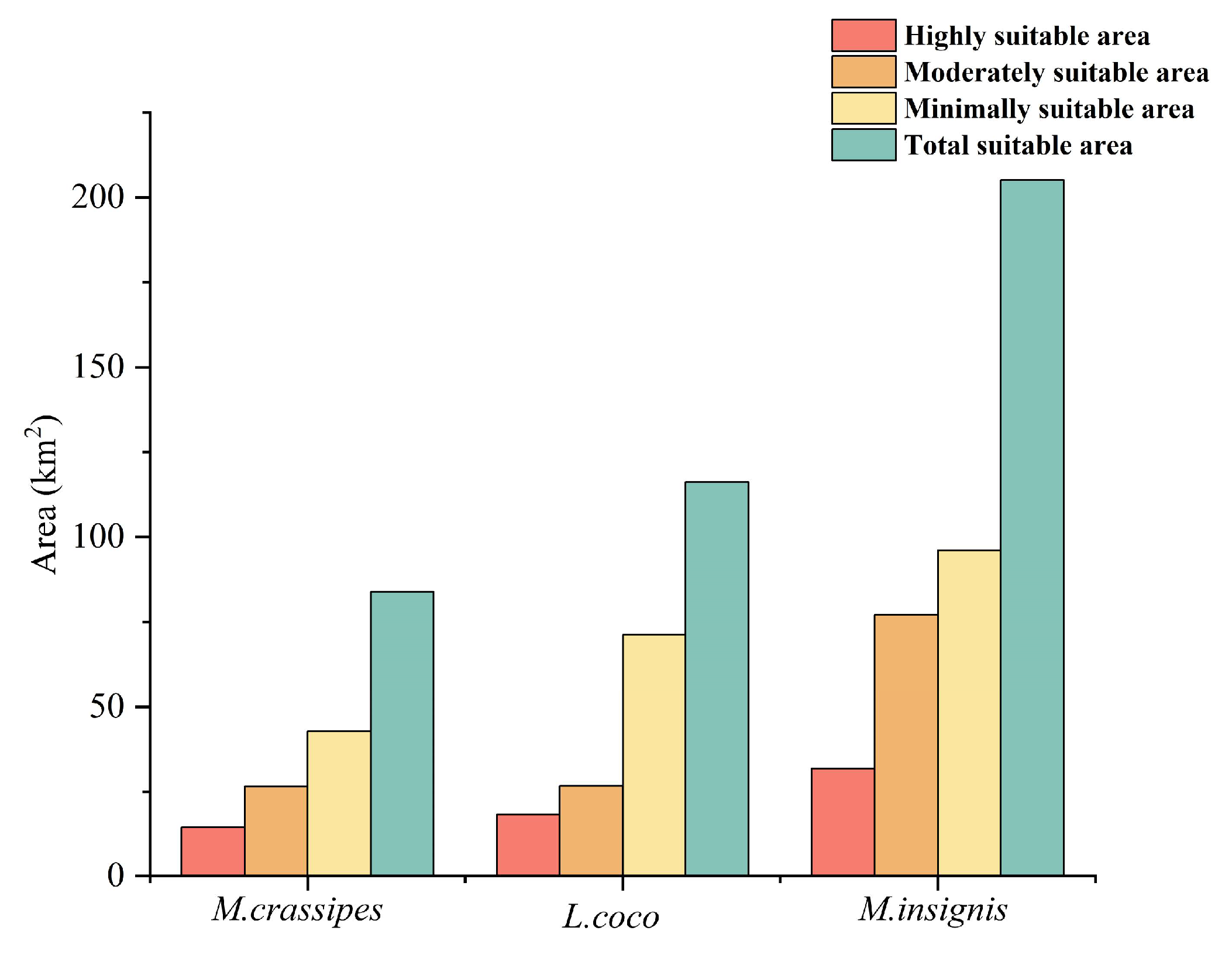
2.2. Potential Distribution Under Current Climate Conditions
2.3. Comparison of the Geographical Distribution and Ecological Niche
| Niche (Above the Diagonal)\Range Overlap (Below the Diagonal) | M. crassipes | L. coco | M. insignis | B2 |
|---|---|---|---|---|
| M. crassipes | 1 | 0.520 | 0.524 | 0.862 |
| L. coco | 0.059 | 1 | 0.639 | 0.879 |
| M. insignis | 0.522 | 0.780 | 1 | 0.913 |
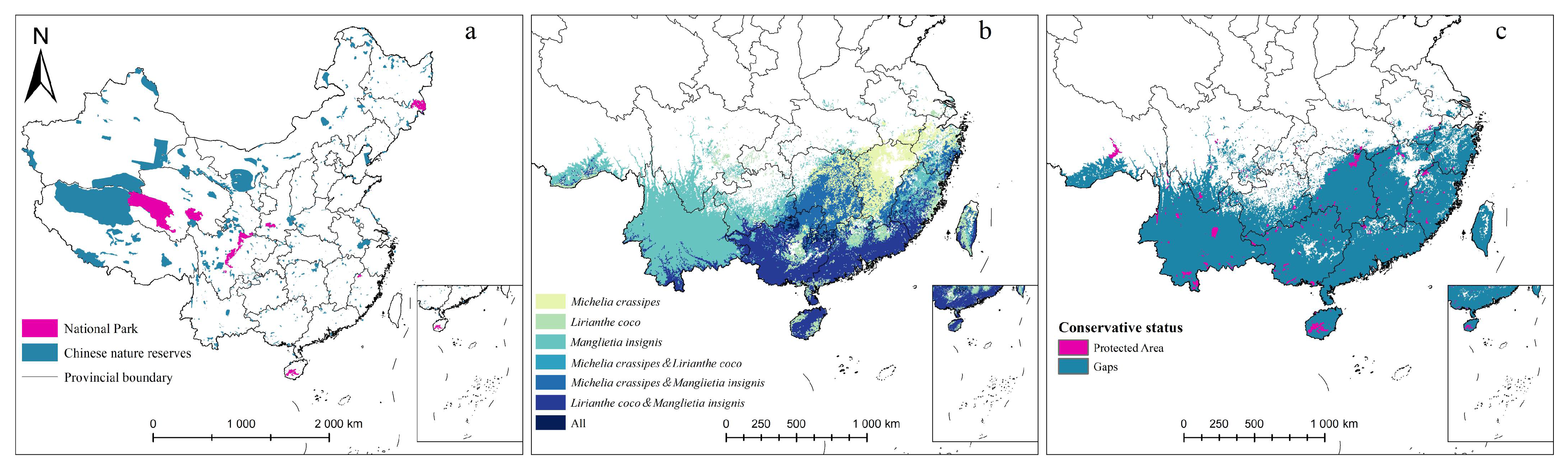
2.4. Conservation Gaps
2.5. Potential Distribution of Three Magnoliaceae Under Future Climate Conditions
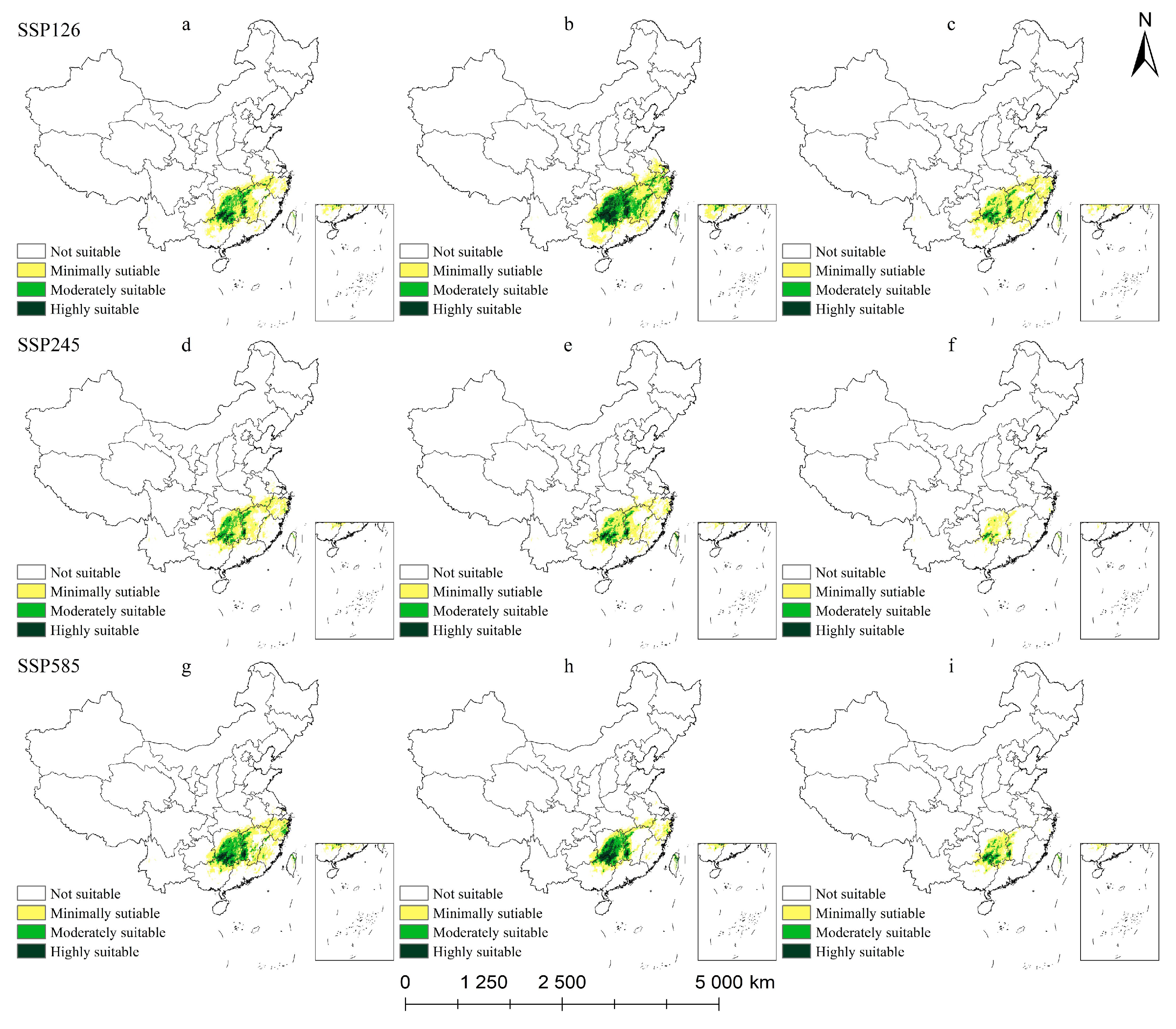
2.5.1. Potential Habitat for M. crassipes Under Climate Change Scenarios
2.5.2. Potential Habitat for L. coco Under Climate Change Scenarios
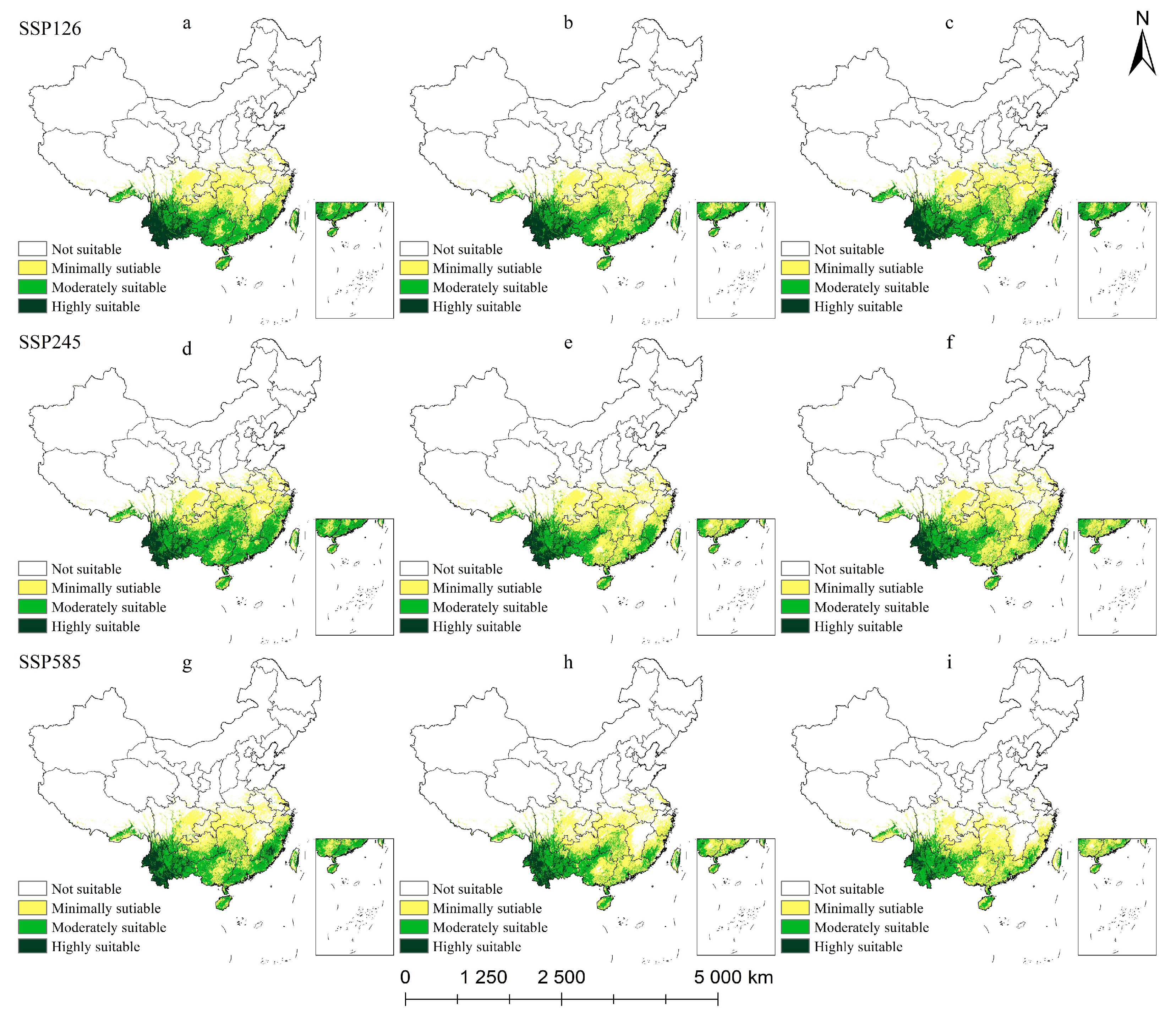
2.5.3. Potential Habitat for M. insignis Under Climate Change Scenarios
2.6. Centroid Shifts in Direction and Distance of Different Species
3. Discussion
3.1. Model Accuracy Analysis
3.2. The Predominant Environmental Variables Influencing Different Magnoliaceae Species
3.3. Suitable Habitat and Its Dynamics Change
3.4. Endangered Status and Conservation Recommendations
4. Materials and Methods
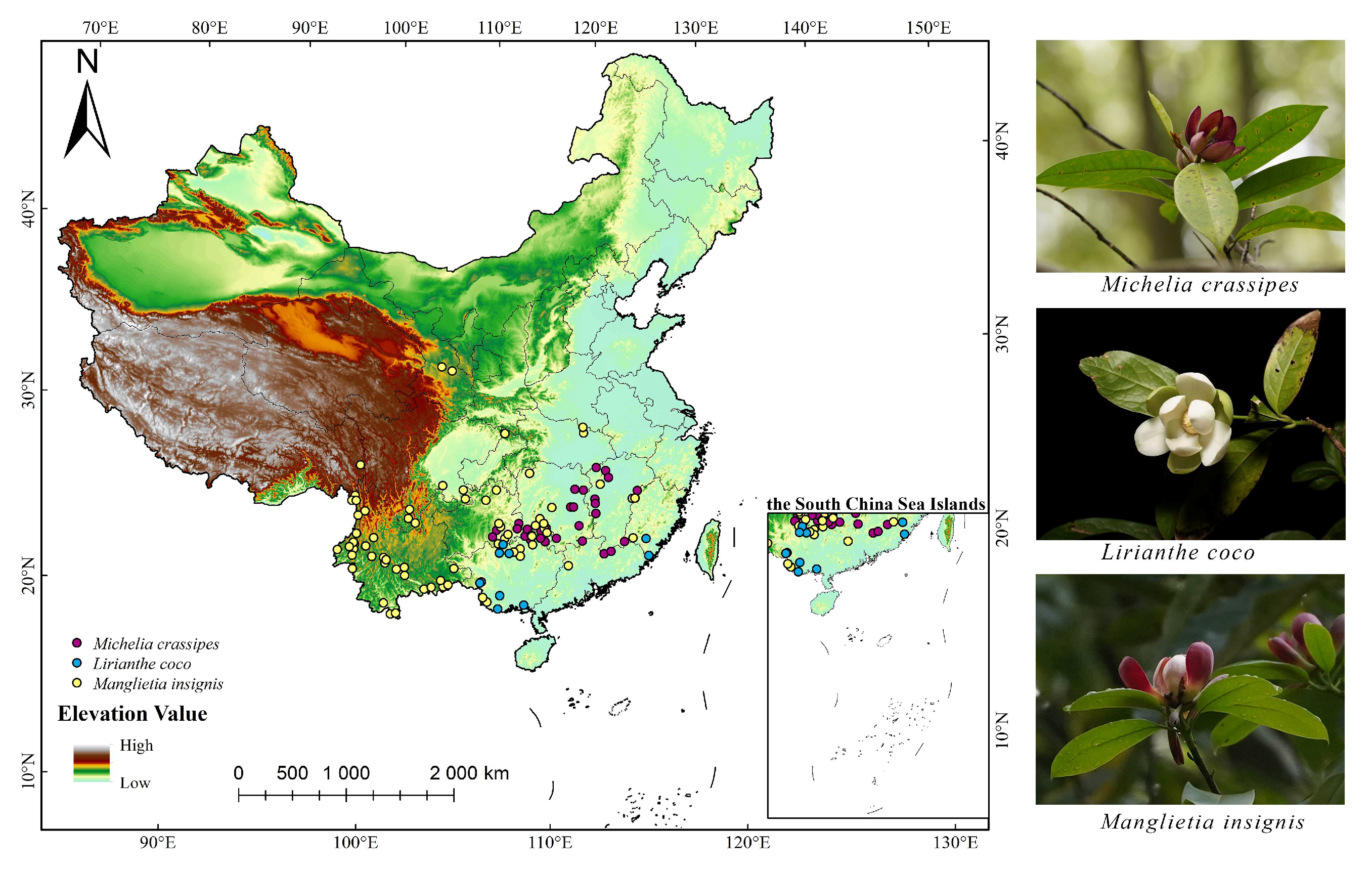
4.1. Species Data Sources
4.2. Environmental Variables and Processing
4.3. MaxEnt Model Construction and Threshold Selection
4.4. Measuring the Range Overlap, Ecological Niche Breadth and Overlap
4.5. Conservation Gap Analysis
4.6. Centroid Change Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, W.B.; Svenning, J.C.; Chen, G.K.; Zhang, M.G.; Huang, J.H.; Chen, B.; Ordonez, A.; Ma, K.P. Human activities have opposing effects on distributions of narrow-ranged and widespread plant species in China. Proc. Natl. Acad. Sci. USA 2019, 116, 26674–26681. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.B.; Bai, Y.; Alatalo, J.M.; Huang, Z.D.; Yang, F.; Pu, X.Y.; Wang, R.B.; Yang, W.; Guo, X.Y. Spatio-temporal variation in potential habitats for rare and endangered plants and habitat conservation based on the maximum entropy model. Sci. Total Environ. 2021, 784, 147080. [Google Scholar] [CrossRef]
- Gamal, E.; Khdery, G.; Morsy, A.; Ali, M.; Hashim, A.; Saleh, H. GIS based modelling to aid conservation of two endangered plant species (Ebenus armitagei and Periploca angustifolia) at Wadi Al-Afreet, Egypt. Remote Sens. Appl. Soc. Environ. 2020, 19, 100336. [Google Scholar] [CrossRef]
- Yi, Y.J.; Zhou, Y.; Cai, Y.P.; Yang, W.; Li, Z.W.; Zhao, X. The influence of climate change on an endangered riparian plant species: The root of riparian Homonoia. Ecol. Indic. 2018, 92, 40–50. [Google Scholar] [CrossRef]
- Ouyang, X.; Lin, H.; Bai, S.; Chen, J.; Chen, A. Simulation the potential distribution of Dendrolimus houi and its hosts, Pinus yunnanensis and Cryptomeria fortunei, under climate change in China. Front. Plant Sci. 2022, 13, 1054710. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.P.; Lin, X.; Zhang, Y.; Gao, J.J.; Guo, L.; Li, J.S. Impacts of climate change on ecosystem in Priority Areas of Biodiversity Conservation in China. Chin. Sci. Bull. 2014, 59, 4668–4680. [Google Scholar] [CrossRef]
- Xie, C.P.; Li, M.; Chen, L.; Jim, C.Y. Climate-driven changes to the spatial–temporal pattern of endangered tree Toona ciliata Roem. in China. Theor. Appl. Climatol. 2024, 155, 2071–2085. [Google Scholar] [CrossRef]
- Walther, G.R.; Post, E.; Convey, P.; Menzel, A.; Parmesan, C.; Beebee, T.J.; Fromentin, J.M.; Hoegh-Guldberg, O.; Bairlein, F. Ecological responses to recent climate change. Nature 2002, 416, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Hansen, G.; Cramer, W. Global distribution of observed climate change impacts. Nat. Clim. Change 2015, 5, 182–185. [Google Scholar] [CrossRef]
- Tian, P.P.; Liu, Y.F.; Ou, J. Meta-analysis of the impact of future climate change on the area of woody plant habitats in China. Front. Plant Sci. 2023, 14, 1139739. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.H.; Tang, Y.T.; Fu, J.; Chi, X.L.; Du, W.H.; Dimitrov, D.; Liu, J.Q.; Xi, Z.X.; Wu, J.Y.; Xu, X.T. Diversity patterns and conservation gaps of Magnoliaceae species in China. Sci. Total Environ. 2022, 813, 152665. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.T.; Jiang, X.; Chen, H.; Jiang, P.; Liu, M.; Huang, Y. Predicting the Potential Distribution of the Endangered Plant Magnolia wilsonii Using MaxEnt Under Climate Change in China. Pol. J. Environ. Stud. 2022, 31, 4435–4445. [Google Scholar] [CrossRef]
- Xu, Y.; Zang, R. Conservation of rare and endangered plant species in China. iScience 2023, 26, 106008. [Google Scholar] [CrossRef]
- Jin, S.H.; Chi, Y.; Li, X.Q.; Shu, P.Z.; Zhu, M.X.; Yuan, Z.; Liu, Y.; Chen, W.J.; Han, Y.N. Predicting the Response of Three Common Subtropical Tree species in China to Climate Change with Maxent Modeling. Front. For. Glob. Change 2023, 6, 1299120. [Google Scholar] [CrossRef]
- Stockwell, D. The GARP modelling system: Problems and solutions to automated spatial prediction. Int. J. Geogr. Inf. Sci. 1999, 13, 143–158. [Google Scholar] [CrossRef]
- Vincenzi, S.; Zucchetta, M.; Franzoi, P.; Pellizzato, M.; Pranovi, F.; De Leo, G.A.; Torricelli, P. Application of a Random Forest algorithm to predict spatial distribution of the potential yield of Ruditapes philippinarum in the Venice lagoon, Italy. Ecol. Model. 2011, 222, 1471–1478. [Google Scholar] [CrossRef]
- Engler, R.; Guisan, A. MigClim: Predicting plant distribution and dispersal in a changing climate. Divers. Distrib. 2009, 15, 590–601. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Lin, S.L.; Yao, D.D.; Jiang, H.X.; Qin, J.; Feng, Z.Y. Predicting current and future potential distributions of the greater bandicoot rat (Bandicota indica) under climate change conditions. Pest Manag. Sci. 2024, 80, 734–743. [Google Scholar] [CrossRef]
- Yang, W.J.; Sun, S.X.; Wang, N.X.; Fan, P.X.; You, C.; Wang, R.Q.; Zheng, P.M.; Wang, H. Dynamics of the distribution of invasive alien plants (Asteraceae) in China under climate change. Sci. Total Environ. 2023, 903, 166260. [Google Scholar] [CrossRef]
- Tang, X.G.; Yuan, Y.D.; Li, X.M.; Zhang, J.C. Maximum entropy modeling to predict the impact of climate change on pine wilt disease in China. Front. Plant Sci. 2021, 12, 652500. [Google Scholar] [CrossRef] [PubMed]
- Takhtadzhian, A.L. Diversity and Classification of Flowering Plants; Columbia University Press: New York, NY, USA, 1997. [Google Scholar]
- Liu, Y.H.; Xia, N.H.; Yang, H.Q. The origin, evolution and phytogeography of Magnoliaceae. J. Trop. Subtrop. Bot. 1995, 3, 1–12. [Google Scholar]
- Liu, Y.H. A Preliminary Study on the Taxonomy of the Family Magnoliaceae. J. Syst. Evol. 1984, 22, 89–109. [Google Scholar]
- Rivers, M.; Beech, E.; Murphy, L.; Oldfield, S. The Red List of Magnoliaceae-Revised and Extended; Botanic Gardens Conservation International: London, UK, 2016. [Google Scholar]
- Xu, F.; Rudall, P.J. Comparative floral anatomy and ontogeny in Magnoliaceae. Plant Syst. Evol. 2006, 258, 1–15. [Google Scholar] [CrossRef]
- Xu, F.X.; Kirchoff, B.K. Pollen morphology and ultrastructure of selected species of Magnoliaceae. Rev. Palaeobot. Palynol. 2008, 150, 140–153. [Google Scholar] [CrossRef][Green Version]
- Yang, X.; Yang, Z.; Li, H. Genetic diversity, population genetic structure and protection strategies for Houpoëa officinalis (Magnoliaceae), an endangered Chinese medical plant. J. Plant Biol. 2018, 61, 159–168. [Google Scholar] [CrossRef]
- Shi, X.D.; Yin, Q.; Sang, Z.Y.; Zhu, Z.L.; Jia, Z.K.; Ma, L.Y. Prediction of potentially suitable areas for the introduction of Magnolia wufengensis under climate change. Ecol. Indic. 2021, 127, 107762. [Google Scholar] [CrossRef]
- Zhou, T.; Huang, X.J.; Zhang, S.Z.; Wang, Y.; Wang, Y.J.; Liu, W.Z.; Wang, Y.L.; Zou, J.B.; Li, Z.H. Population demographic history of a rare and endangered tree Magnolia sprengeri Pamp. in east Asia revealed by molecular data and ecological niche analysis. Forests 2021, 12, 931. [Google Scholar] [CrossRef]
- Li, J.J.; Fan, G.; He, Y. Predicting the current and future distribution of three Coptis herbs in China under climate change conditions, using the MaxEnt model and chemical analysis. Sci. Total Environ. 2020, 698, 134141. [Google Scholar] [CrossRef]
- Liu, S.J.; Chen, T.T.; Ye, D.; Chen, Q.T.; Ni, J.; Rao, M.D. Prediction of distributional patterns of four major Camellia oilseed species in China under climate and land use changes. Ecol. Indic. 2023, 155, 110996. [Google Scholar] [CrossRef]
- Zhuang, H.F.; Zhang, Y.B.; Wang, W.; Ren, Y.H.; Liu, F.Z.; Du, J.H.; Zhou, Y. Optimized hot spot analysis for probability of species distribution under different spatial scales based on MaxEnt model: Manglietia insignis case. Biodivers. Sci. 2018, 26, 931. [Google Scholar] [CrossRef]
- Huang, L.; Li, S.; Huang, W.; Jin, J.; Oskolski, A.A. Late Pleistocene glacial expansion of a low-latitude species Magnolia insignis: Megafossil evidence and species distribution modeling. Ecol. Indic. 2024, 158, 111519. [Google Scholar] [CrossRef]
- Zhai, X.Y.; Shen, Y.F.; Zhu, S.H.; Tu, T.H.; Zhang, C.G.; Li, H.G. Potential Impacts of Climate Change in Future on the Geographical Distributions of Relic Liriodendron chinense. J. Trop. Subtrop. Bot. 2021, 29, 151–161. [Google Scholar]
- Yu, D.P.; Wen, X.Y.; Li, C.H.; Xiong, T.Y.; Peng, Q.X.; Li, X.J.; Liu, H.; Ren, H. Integrated conservation for Parakmeria omeiensis (Magnoliaceae), a Critically Endangered plant species endemic to south-west China. Oryx 2020, 54, 460–465. [Google Scholar] [CrossRef]
- Shen, Y.F.; Tu, Z.H.; Zhang, Y.L.; Zhong, W.P.; Xia, H.; Hao, Z.Y.; Zhang, C.G.; Li, H.G. Predicting the impact of climate change on the distribution of two relict Liriodendron species by coupling the MaxEnt model and actual physiological indicators in relation to stress tolerance. J. Environ. Manag. 2022, 322, 116024. [Google Scholar] [CrossRef]
- Liu, H.M.; Gao, J.X.; Song, C.Y.; Yu, S.X. Conservation status and human disturbance of the habitats of Michelia crassipes Law in China. China Environ. Sci. 2019, 39, 3976–3981. [Google Scholar]
- Song, C.Y.; Liu, H.M. Habitat differentiation and conservation gap of Magnolia biondii, M. denudata, and M. sprengeri in China. PeerJ 2019, 6, e6126. [Google Scholar] [CrossRef] [PubMed]
- Huan, Z.Q.; Geng, X.M.; Xu, X.R.; Liu, W.; Zhu, Z.L.; Tang, M. Potential geographical distribution of Michelia martini under different climate change scenarios based on MaxEnt model. J. Ecol. Rural. Environ. 2023, 39, 1277–1287. [Google Scholar]
- Hurtt, G.C.; Chini, L.; Sahajpal, R.; Frolking, S.; Bodirsky, B.L.; Calvin, K.; Doelman, J.C.; Fisk, J.; Fujimori, S.; Goldewijk, K.K.; et al. Harmonization of global land use change and management for the period 850–2100 (LUH2) for CMIP6. Geosci. Model Dev. 2020, 13, 5425–5464. [Google Scholar] [CrossRef]
- Zhang, K.L.; Yao, L.J.; Meng, J.S.; Tao, J. Maxent modeling for predicting the potential geographical distribution of two peony species under climate change. Sci. Total Environ. 2018, 634, 1326–1334. [Google Scholar] [CrossRef] [PubMed]
- Rahbek, C.; Borregaard, M.K.; Colwell, R.K.; Dalsgaard, B.O.; Holt, B.G.; Morueta-Holme, N.; Nogues-Bravo, D.; Whittaker, R.J.; Fjeldså, J. Humboldt’s enigma: What causes global patterns of mountain biodiversity? Science 2019, 365, 1108–1113. [Google Scholar] [CrossRef]
- Wu, H.Y.; Liu, Y.H.; He, Q.X.; Ye, J.W.; Tian, B. Differential distribution shifts in two subregions of East Asian subtropical evergreen broadleaved forests—A case of Magnoliaceae. Front. Plant Sci. 2024, 14, 1326207. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Zhang, Z.Y.; Chen, L.D.; Song, P.Y.; Wang, X. Changes in potential geographical distribution of Tsoongiodendron odorum since the Last Glacial Maximum. Chin. J. Plant Ecol. 2020, 44, 44–55. [Google Scholar] [CrossRef]
- Hernández, M.; Palmarola, A.; Veltjen, E.; Asselman, P.; Testé, E.; Larridon, I.; Samain, M.; González-Torres, L.R. Population structure and genetic diversity of Magnolia cubensis subsp. acunae (Magnoliaceae): Effects of habitat fragmentation and implications for conservation. Oryx 2020, 54, 451–459. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Da Fonseca, G.A.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Ye, P.C.; Zhang, G.F.; Wu, J.Y. Hotspots and conservation gaps: A case study of key higher plant species from Northwest Yunnan, China. Glob. Ecol. Conserv. 2020, 23, e01005. [Google Scholar] [CrossRef]
- Liu, D.T.; Yang, J.B.; Chen, S.Y.; Sun, W.B. Potential distribution of threatened maples in China under climate change: Implications for conservation. Glob. Ecol. Conserv. 2022, 40, e02337. [Google Scholar] [CrossRef]
- Yang, F.M.; Cai, L.; Dao, Z.L.; Sun, W.B. Genomic data reveals population genetic and demographic history of Magnolia fistulosa (Magnoliaceae), a plant species with extremely small populations in Yunnan Province, China. Front. Plant Sci. 2022, 13, 811312. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Wang, R.L.; Hou, K.; Wang, X.Y.; Wu, W. Predicting the current and future cultivation regions of Carthamus tinctorius L. using MaxEnt model under climate change in China. Glob. Ecol. Conserv. 2018, 16, e00477. [Google Scholar] [CrossRef]
- Allen, J.L.; Lendemer, J.C. Climate change impacts on endemic, high-elevation lichens in a biodiversity hotspot. Biodivers. Conserv. 2016, 25, 555–568. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Su, B.D.; Huang, J.L.; Mondal, S.K.; Zhai, J.Q.; Wang, Y.J.; Wen, S.S.; Gao, M.N.; Lv, Y.R.; Jiang, S.; Jiang, T.; et al. Insight from CMIP6 SSP-RCP scenarios for future drought characteristics in China. Atmos. Res. 2021, 250, 105375. [Google Scholar] [CrossRef]
- Wu, T.W.; Lu, Y.X.; Fang, Y.J.; Xin, X.G.; Li, L.; Li, W.P.; Jie, W.H.; Zhang, J.; Liu, Y.M.; Zhang, L.; et al. The Beijing climate center climate system model (BCC-CSM): The main progress from CMIP5 to CMIP6. Geosci. Model Dev. 2019, 12, 1573–1600. [Google Scholar] [CrossRef]
- Beckmann, M.; Václavík, T.; Manceur, A.M.; Šprtová, L.; von Wehrden, H.; Welk, E.; Cord, A.F. gl UV: A global UV-B radiation data set for macroecological studies. Methods Ecol. Evol. 2014, 5, 372–383. [Google Scholar] [CrossRef]
- Chi, Y.; Wang, G.G.; Zhu, M.X.; Jin, P.; Hu, Y.; Shu, P.Z.; Wang, Z.X.; Fan, A.F.; Qian, P.H.; Han, Y.N.; et al. Potentially suitable habitat prediction of Pinus massoniana Lamb. in China under climate change using Maxent model. Front. For. Glob. Change 2023, 6, 1144401. [Google Scholar] [CrossRef]
- Muscarella, R.; Galante, P.J.; Soley-Guardia, M.; Boria, R.A.; Kass, J.M.; Uriarte, M.; Anderson, R.P. ENM eval: An R package for conducting spatially independent evaluations and estimating optimal model complexity for Maxent ecological niche models. Methods Ecol. Evol. 2014, 5, 1198–1205. [Google Scholar] [CrossRef]
- Zhao, X.F.; Lei, M.; Wei, C.H.; Guo, X.X. Assessing the suitable regions and the key factors for three Cd-accumulating plants (Sedum alfredii, Phytolacca americana, and Hylotelephium spectabile) in China using MaxEnt model. Sci. Total Environ. 2022, 852, 158202. [Google Scholar] [CrossRef]
- Warren, D.L.; Glor, R.E.; Turelli, M. ENMTools: A toolbox for comparative studies of environmental niche models. Ecography 2010, 33, 607–611. [Google Scholar] [CrossRef]
- Schoener, T.W. The Anolis lizards of Bimini: Resource partitioning in a complex fauna. Ecology 1968, 49, 704–726. [Google Scholar] [CrossRef]
- Levins, R. Evolution in Changing Environments: Some Theoretical Explorations; Princeton University Press: Princeton, NJ, USA, 1968. [Google Scholar]
- Yang, B.; Qin, S.Y.; Xu, W.S.; Busch, J.; Yang, X.Y.; Gu, X.D.; Yang, Z.S.; Wang, B.; Dai, Q.; Xu, Y. Gap analysis of giant panda conservation as an example for planning China’s national park system. Curr. Biol. 2020, 30, 1287–1291. [Google Scholar] [CrossRef] [PubMed]




| Period | SSP126 | SSP245 | SSP585 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 2050 | 2070 | 2090 | 2050 | 2070 | 2090 | 2050 | 2070 | 2090 | |
| Minimally suitable area/km2 | 32.85 | 42.45 | 41.6 | 32.21 | 29.78 | 11.72 | 33.27 | 20.55 | 15.76 |
| Moderately suitable area/km2 | 10.6 | 20.54 | 8.94 | 9.00 | 6.63 | 1.38 | 13.87 | 10.06 | 4.72 |
| Highly suitable area/km2 | 2.58 | 9.15 | 1.3 | 0.85 | 1.57 | 0.02 | 3.87 | 5.46 | 0.86 |
| Total suitable area/km2 | 46.03 | 72.14 | 51.84 | 42.05 | 37.98 | 13.12 | 51.01 | 36.07 | 21.34 |
| Suitable area changes/km2 | −37.93 | 26.11 | −20.31 | −41.91 | −4.07 | −24.86 | −32.95 | −14.94 | −14.73 |
| Rate of change (%) | −45.18 | 56.72 | −28.15 | −49.92 | −9.68 | −65.46 | −39.24 | −29.29 | −40.84 |
| Period | SSP126 | SSP245 | SSP585 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 2050 | 2070 | 2090 | 2050 | 2070 | 2090 | 2050 | 2070 | 2090 | |
| Minimally suitable area/km2 | 60.61 | 56.35 | 71.23 | 76.73 | 65.87 | 68.5 | 65.9 | 56.56 | 49.64 |
| Moderately suitable area/km2 | 25.01 | 24.39 | 27.71 | 28.89 | 24.54 | 25.74 | 25.43 | 23.78 | 22.43 |
| Highly suitable area/km2 | 17.88 | 17.93 | 20.92 | 19.91 | 16.52 | 17.64 | 18.05 | 16.14 | 14.24 |
| Total suitable area/km2 | 103.49 | 98.67 | 119.86 | 125.54 | 106.93 | 111.87 | 109.39 | 96.48 | 86.31 |
| Suitable area changes/km2 | −12.73 | −4.82 | 21.19 | 9.32 | −18.61 | 4.94 | −6.83 | −12.91 | −10.17 |
| Rate of change (%) | −10.96 | −4.66 | 21.48 | 7.77 | −14.82 | 4.62 | −6.11 | −11.80 | −10.54 |
| Period | SSP126 | SSP245 | SSP585 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 2050 | 2070 | 2090 | 2050 | 2070 | 2090 | 2050 | 2070 | 2090 | |
| Minimally suitable area/km2 | 96.33 | 104.64 | 104.67 | 102.98 | 116.68 | 115.03 | 99.41 | 101.71 | 94.15 |
| Moderately suitable area/km2 | 62.38 | 62.95 | 71.79 | 80.90 | 52.92 | 55.81 | 66.61 | 51.45 | 45.92 |
| Highly suitable area/km2 | 27.21 | 23.89 | 26.13 | 23.43 | 18.62 | 19.01 | 20.94 | 16.58 | 10.85 |
| Total suitable area/km2 | 185.92 | 191.48 | 202.58 | 207.31 | 188.85 | 189.85 | 186.95 | 169.74 | 150.92 |
| Suitable area changes/km2 | −19.11 | 5.56 | 11.10 | 2.28 | −18.46 | 1.00 | −18.08 | −17.21 | −18.82 |
| Rate of change (%) | −9.32 | 2.99 | 5.80 | 1.11 | −8.90 | 0.53 | −8.82 | −9.21 | −11.09 |
| Period | M. crassipes | L. coco | M. insignis | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Lon (E) | Lat (N) | Dist (km) | Lon (E) | Lat (N) | Dist (km) | Lon (E) | Lat (N) | Dist (km) | |
| Current | 115.26 | 27.46 | - | 110.52 | 26.61 | - | 109.10 | 27.28 | - |
| SSP126-2050 | 114.23 | 27.40 | 103.66 | 110.63 | 26.45 | 21.00 | 108.59 | 27.35 | 51.85 |
| SSP126-2070 | 115.08 | 27.53 | 86.66 | 110.46 | 26.39 | 17.92 | 108.87 | 27.31 | 28.65 |
| SSP126-2090 | 114.99 | 26.96 | 64.70 | 110.74 | 26.66 | 41.30 | 108.91 | 27.38 | 9.09 |
| SSP245-2050 | 114.62 | 27.76 | 72.55 | 110.82 | 26.84 | 40.58 | 108.81 | 27.47 | 35.69 |
| SSP245-2070 | 115.03 | 27.13 | 81.73 | 110.50 | 26.66 | 38.86 | 108.30 | 27.28 | 55.57 |
| SSP245-2090 | 113.63 | 26.47 | 159.39 | 110.79 | 26.76 | 31.53 | 108.68 | 27.56 | 49.53 |
| SSP585-2050 | 114.62 | 27.13 | 73.79 | 110.69 | 26.52 | 19.57 | 108.95 | 27.24 | 15.90 |
| SSP585-2070 | 115.19 | 27.17 | 57.05 | 110.29 | 26.26 | 50.44 | 107.73 | 27.11 | 124.00 |
| SSP585-2090 | 113.69 | 26.47 | 170.51 | 110.39 | 26.14 | 17.33 | 107.34 | 26.82 | 50.82 |
| Variable | Description | % Contribution | ||
|---|---|---|---|---|
| M. crassipes | L. coco | M. insignis | ||
| Ele | Elevation | 3.8 | 14 | 6.1 |
| bio2 | Mean Diurnal Range (Mean of monthly (max temp–min temp)) | 5.5 | 23.4 | 1.1 |
| bio4 | Temperature Seasonality (standard deviation × 100) | 0.1 | 11.3 | 42.1 |
| bio9 | Mean Temperature in the Driest Quarter | 3.7 | 34.8 | 30.5 |
| bio12 | Annual Precipitation | 3.7 | 0.1 | 1.7 |
| bio14 | Precipitation in the Driest Month | 1.8 | 0.4 | 0.6 |
| bio17 | Precipitation in the Driest Quarter | 70.8 | 0 | 3.2 |
| bio19 | Precipitation in the Coldest Quarter | 0.7 | 1.2 | 0.7 |
| T_BS | Topsoil Base Saturation | 3.6 | 0 | 0 |
| S_BS | Subsoil Base Saturation | 0.7 | 0 | 0.2 |
| S_CACO3 | Subsoil Calcium Carbonate | 0 | 0 | 0.5 |
| S_PH_H2O | Subsoil pH (H2O) | 0 | 0 | 0.7 |
| S_SILT | Subsoil Silt Fraction | 0 | 0.7 | 2.5 |
| S_USDA_TEX | Subsoil USDA Texture Classification | 0.2 | 6.4 | 1.3 |
| UVB2 | UV-B Seasonality | 3.6 | 4.8 | 3.4 |
| UVB3 | Mean UV-B of the Highest Month | 0 | 0 | 0 |
| UVB4 | Mean UV-B of the Lowest Month | 0.2 | 0.7 | 4.6 |
| UVB6 | Sum of Monthly Mean UV-B during Lowest Quarter | 1.5 | 2.2 | 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Y.; Yao, W.; Wang, Z.; Fan, X.; Hu, S.; Wang, H.; Ou, J. Predicting Potential Suitable Habitats of Three Rare Wild Magnoliaceae Species (Michelia crassipes, Lirianthe coco, Manglietia insignis) Under Current and Future Climatic Scenarios Based on the Maxent Model. Plants 2025, 14, 506. https://doi.org/10.3390/plants14040506
Fan Y, Yao W, Wang Z, Fan X, Hu S, Wang H, Ou J. Predicting Potential Suitable Habitats of Three Rare Wild Magnoliaceae Species (Michelia crassipes, Lirianthe coco, Manglietia insignis) Under Current and Future Climatic Scenarios Based on the Maxent Model. Plants. 2025; 14(4):506. https://doi.org/10.3390/plants14040506
Chicago/Turabian StyleFan, Yu, Weihao Yao, Zenghui Wang, Xinyue Fan, Shuyue Hu, Hongfei Wang, and Jing Ou. 2025. "Predicting Potential Suitable Habitats of Three Rare Wild Magnoliaceae Species (Michelia crassipes, Lirianthe coco, Manglietia insignis) Under Current and Future Climatic Scenarios Based on the Maxent Model" Plants 14, no. 4: 506. https://doi.org/10.3390/plants14040506
APA StyleFan, Y., Yao, W., Wang, Z., Fan, X., Hu, S., Wang, H., & Ou, J. (2025). Predicting Potential Suitable Habitats of Three Rare Wild Magnoliaceae Species (Michelia crassipes, Lirianthe coco, Manglietia insignis) Under Current and Future Climatic Scenarios Based on the Maxent Model. Plants, 14(4), 506. https://doi.org/10.3390/plants14040506






