Seasonal Variation in Nutritional and Chemical Profiles of Wild Opuntia ficus-indica Fruits
Abstract
1. Introduction
2. Methodology
2.1. Sample Gathering and Processing
2.2. Moisture, Ash, and Macronutrients
2.3. Extraction of the Lipidic Fraction
2.4. Tocopherol Determination
2.5. Fatty Acid Profile
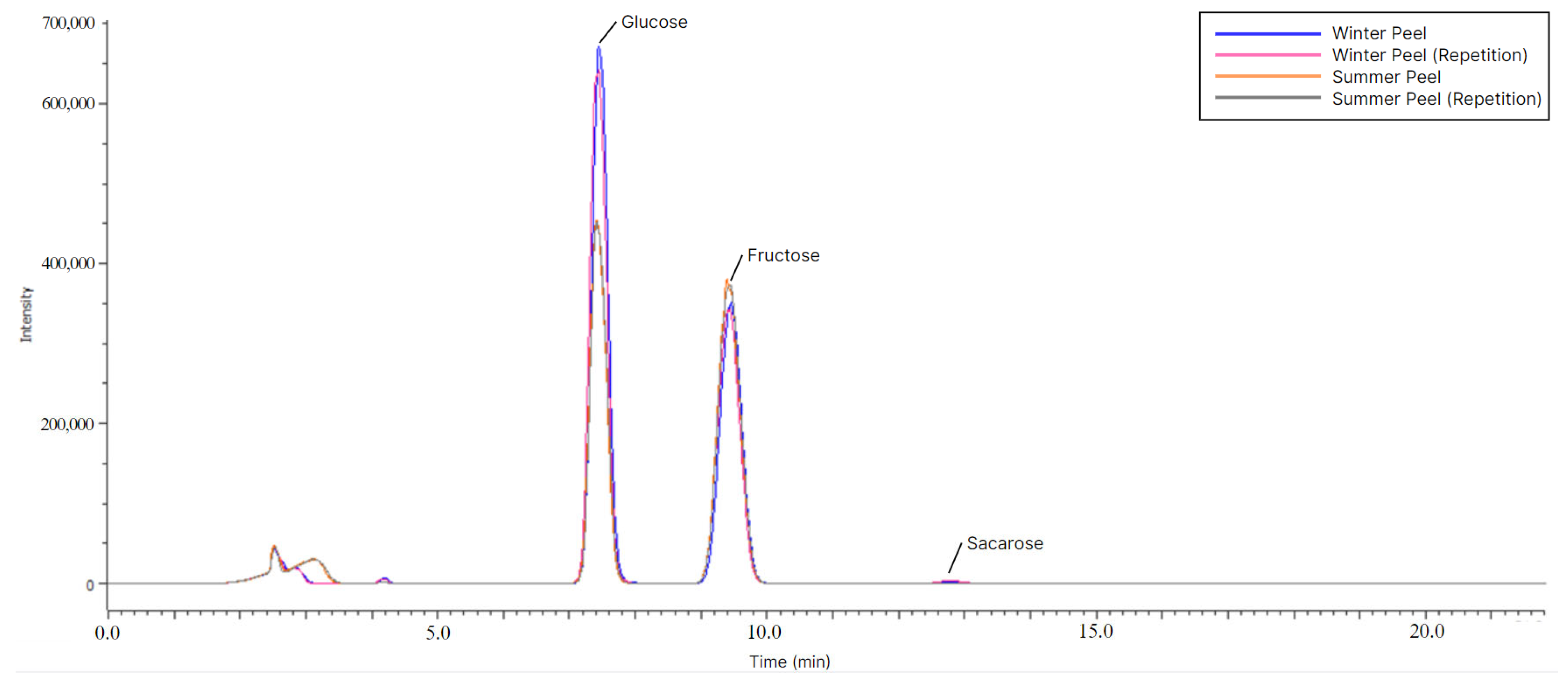
2.6. Free Sugar Determination
2.7. Determination of Total Phenol Content, Flavonoid Content, and In Vitro Antioxidant Activities
2.8. Statistical Analysis
3. Results and Discussion
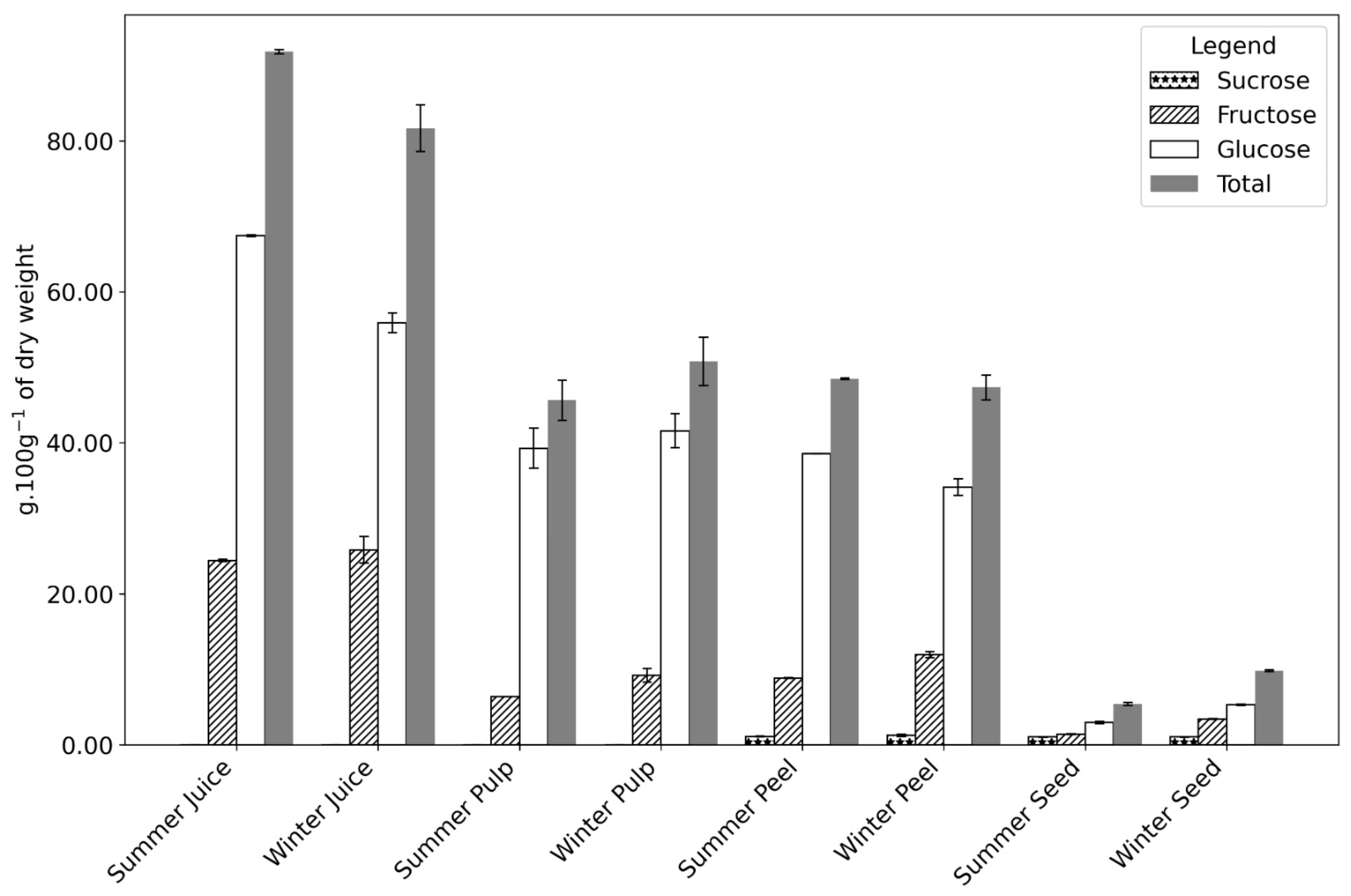
3.1. Macronutrients and Free Sugars
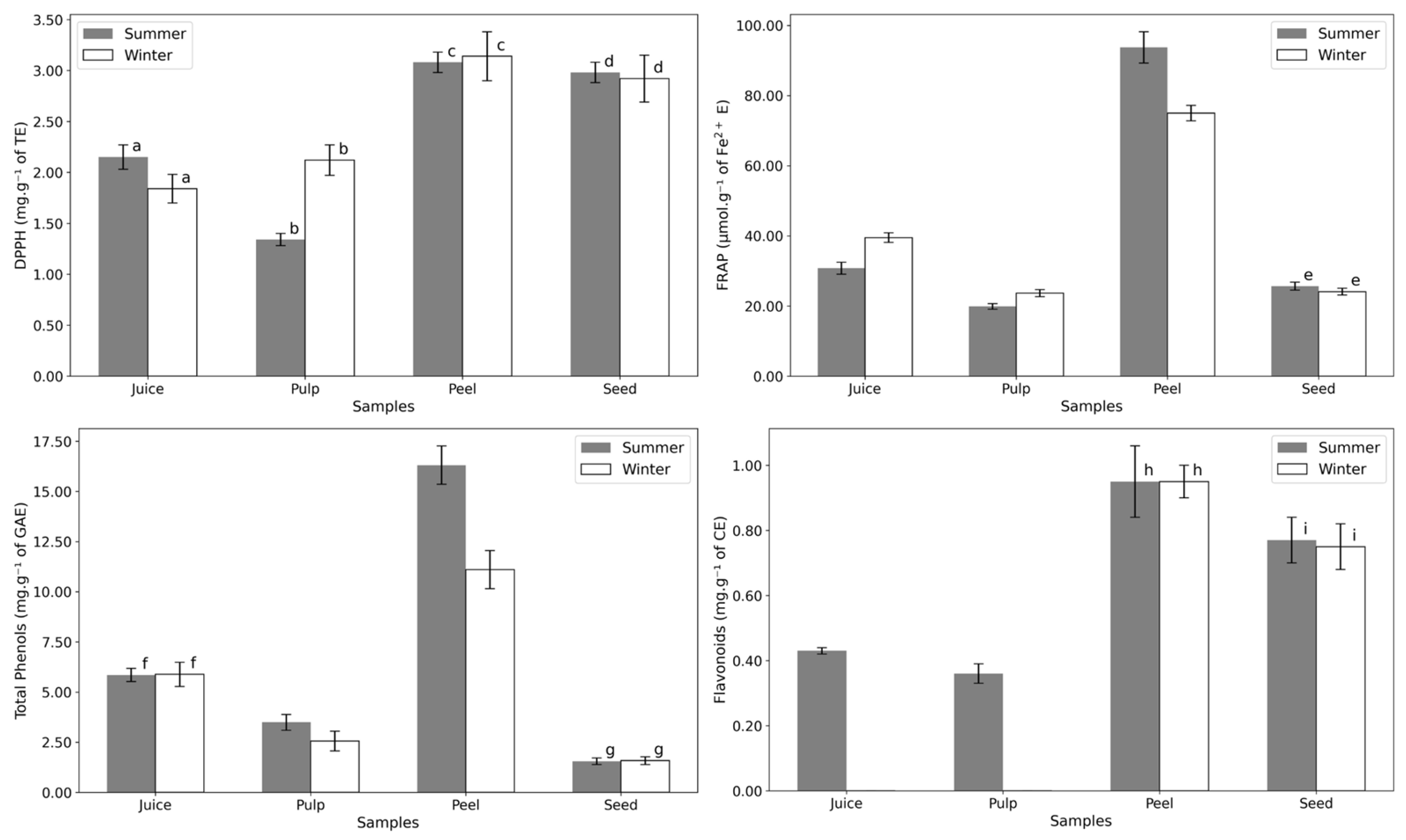
3.2. Antioxidants
3.3. Vitamin E
3.4. Fatty Acids
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Samples | Fructose | Glucose | Sucrose | Total | |
|---|---|---|---|---|---|
| Juice | Summer | 24.40 ± 0.15 a | 67.43 ± 0.13 | n.d. | 91.83 ± 0.28 |
| Winter | 25.82 ± 1.76 a | 55.85 ± 1.31 | n.d. | 81.67 ± 3.07 | |
| Pulp | Summer | 6.38 ± 0.01 | 39.27 ± 2.65 | n.d. | 45.65 ± 2.66 |
| Winter | 9.21 ± 0.91 | 41.57 ± 2.27 | n.d. | 50.78 ± 3.18 | |
| Peel | Summer | 8.86 ± 0.05 | 38.51 ± 0.02 | 1.11 ± 0.04 | 48.48 ± 0.11 |
| Winter | 11.95 ± 0.39 | 34.10 ± 1.09 | 1.27 ± 0.15 | 47.32 ± 1.63 | |
| Seed | Summer | 1.43 ± 0.06 | 2.96 ± 0.15 | 1.05 ± 0.02 b | 5.44 ± 0.21 |
| Winter | 3.45 ± 0.05 | 5.32 ± 0.07 | 1.05 ± 0.02 b | 9.82 ± 0.14 | |
| DPPH | FRAP | Total Phenols | Flavonoids | ||
|---|---|---|---|---|---|
| Samples | mg·g−1 of TE | µmol·g−1 of Fe2+ E | mg·g−1 of GAE | mg·g−1 of CE | |
| Juice | Summer | 2.15 ± 0.12 a | 30.77 ± 1.68 | 5.85 ± 0.33 f | 0.43 ± 0.01 |
| Winter | 1.84 ± 0.14 a | 39.49 ± 1.36 | 5.88 ± 0.60 f | n.d. | |
| Pulp | Summer | 1.34 ± 0.06 b | 19.88 ± 0.81 | 3.49 ± 0.39 | 0.36 ± 0.03 |
| Winter | 2.12 ± 0.15 b | 23.66 ± 0.99 | 2.56 ± 0.49 | n.d. | |
| Peel | Summer | 3.08 ± 0.10 c | 93.72 ± 4.47 | 16.31 ± 0.96 | 0.95 ± 0.11 h |
| Winter | 3.14 ± 0.24 c | 74.96 ± 2.20 | 11.10 ± 0.95 | 0.95 ± 0.05 h | |
| Seed | Summer | 2.98 ± 0.10 d | 25.64 ± 1.13 e | 1.55 ± 0.16 g | 0.77 ± 0.07 i |
| Winter | 2.92 ± 0.23 d | 24.10 ± 0.99 e | 1.58 ± 0.19 g | 0.75 ± 0.07 i | |
References
- Jorge, A.O.S.; Costa, A.S.G.; Oliveira, M.B.P.P. Adapting to Climate Change with Opuntia. Plants 2023, 12, 2907. [Google Scholar] [CrossRef] [PubMed]
- Sipango, N.; Ravhuhali, K.E.; Sebola, N.A.; Hawu, O.; Mabelebele, M.; Mokoboki, H.K.; Moyo, B. Prickly Pear (Opuntia spp.) as an Invasive Species and a Potential Fodder Resource for Ruminant Animals. Sustainability 2022, 14, 3719. [Google Scholar] [CrossRef]
- Nobel, P.S. Environmental Biology of Agaves and Cacti. Choice Rev. Online 1989, 26, 2710. [Google Scholar] [CrossRef]
- Nobel, P.S.; De la Barrera, E. Tolerances and Acclimation to Low and High Temperatures for Cladodes, Fruits and Roots of a Widely Cultivated Cactus, Opuntia ficus-indica. New Phytol. 2003, 157, 271–279. [Google Scholar] [CrossRef]
- Snyman, H.A.; Fouche, H.; Avenant, P.; Ratsele, C. Frost Sensitivity of Opuntia ficus-indica and O. robusta in a Semiarid Climate of South Africa. J. Prof. Assoc. Cactus Dev. 2007, 9, 1–21. [Google Scholar]
- Bensadón, S.; Hervert-Hernández, D.; Sáyago-Ayerdi, S.G.; Goñi, I. By-Products of Opuntia ficus-indica as a Source of Antioxidant Dietary Fiber. Plant Foods Hum. Nutr. 2010, 65, 210–216. [Google Scholar] [CrossRef]
- Zhong, X.K.; Jin, X.; Lai, F.Y.; Lin, Q.S.; Jiang, J.G.C.A.; Activities, A. In Vitro of Polysaccharide Extracted from Opuntia ficus indica Mill. Cultiv. China 2010, 82, 722. [Google Scholar]
- Chiteva, R.; Wairagu, N. Chemical and Nutritional Content of Opuntia-ficus (L.). Afr. J. Biotechnol. 2013, 12, 3309–3312. [Google Scholar]
- Galati, E.M.; Mondello, M.R.; Giuffrida, D.; Dugo, G.; Miceli, N.; Pergolizzi, S.; Taviano, M.F. Chemical Characterization and Biological Effects of Sicilian Opuntia ficus indica (L.) Mill. Fruit Juice: Antioxidant and Antiulcerogenic Activity. J. Agric. Food Chem. 2003, 51, 4903–4908. [Google Scholar] [CrossRef]
- Fernandez, M.L.; Lin, E.C.K.; Trejo, A.; McNamara, D.J. Prickly Pear (Opuntia sp.) Pectin Reverses Low Density Lipoprotein Receptor Suppression Induced by a Hypercholesterolemic Diet in Guinea Pigs. J. Nutr. 1992, 122, 2330–2340. [Google Scholar] [CrossRef]
- Shedbalkar, U.U.; Adki, V.S.; Jadhav, J.P.; Bapat, V.A. Opuntia and Other Cacti: Applications and Biotechnological Insights. Trop. Plant Biol. 2010, 3, 136–150. [Google Scholar] [CrossRef]
- Avila-Nava, A.; Calderón-Oliver, M.; Medina-Campos, O.N.; Zou, T.; Gu, L.; Torres, N.; Tovar, A.R.; Pedraza-Chaverri, J. Extract of Cactus (Opuntia ficus indica) Cladodes Scavenges Reactive Oxygen Species in Vitro and Enhances Plasma Antioxidant Capacity in Humans. J. Funct. Foods 2014, 10, 13–24. [Google Scholar] [CrossRef]
- Osuna-Martínez, L.; Reyes Esparza, J.; Rodríguez-Fragoso, L. Cactus (Opuntia ficus-indica): A Review on Its Antioxidants Properties and Potential Pharmacological Use in Chronic Diseases. Nat. Prod. Chem. Res. 2014, 2, 153–160. [Google Scholar] [CrossRef]
- El-Mostafa, K.; El Kharrassi, Y.; Badreddine, A.; Andreoletti, P.; Vamecq, J.; El Kebbaj, M.S.; Latruffe, N.; Lizard, G.; Nasser, B.; Cherkaoui-Malki, M. Nopal Cactus (Opuntia ficus-indica) as a Source of Bioactive Compounds for Nutrition, Health and Disease. Molecules 2014, 19, 14879–14901. [Google Scholar] [CrossRef]
- Coria Cayupán, Y.S.; Ochoa, M.J.; Nazareno, M.A. Health-Promoting Substances and Antioxidant Properties of Opuntia sp. Fruits. Changes in Bioactive-Compound Contents during Ripening Process. Food Chem. 2011, 126, 514–519. [Google Scholar] [CrossRef]
- Akkol, E.K.; Ilhan, M.; Karpuz, B.; Genç, Y.; Sobarzo-Sánchez, E. Sedative and Anxiolytic Activities of Opuntia ficus indica (L.) Mill.: An Experimental Assessment in Mice. Molecules 2020, 25, 1844. [Google Scholar] [CrossRef]
- Kaur, M. Pharmacological Actions of Opuntia Ficus Indica: A Review. J. Appl. Pharm. Sci. 2012, 2, 15–18. [Google Scholar] [CrossRef]
- Butera, D.; Tesoriere, L.; Di Gaudio, F.; Bongiorno, A.; Allegra, M.; Pintaudi, A.M.; Kohen, R.; Livrea, M.A. Antioxidant Activities of Sicilian Prickly Pear (Opuntia ficus indica) Fruit Extracts and Reducing Properties of Its Betalains: Betanin and Indicaxanthin. J. Agric. Food Chem. 2002, 50, 6895–6901. [Google Scholar] [CrossRef]
- Albano, C.; Negro, C.; Tommasi, N.; Gerardi, C.; Mita, G.; Miceli, A.; De Bellis, L.; Blando, F. Betalains, Phenols and Antioxidant Capacity in Cactus Pear [Opuntia ficus-indica (L.) Mill.] Fruits from Apulia (South Italy) Genotypes. Antioxidants 2015, 4, 269–280. [Google Scholar] [CrossRef]
- Instituto Nacional de Investigação Agrária E Veterinária. Base de Dados INFOSOLO. Available online: https://portalgeo.iniav.pt/portal/apps/webappviewer/index.html?id=17574ca60800415dace9a6369ac53208 (accessed on 10 January 2025).
- Liguori, G.; Di Miceli, C.; Gugliuzza, G.; Inglese, P. Physiological and Technical Aspects of Cactus Pear [Opuntia ficus-indica (L.) Mill.] Double Rellowering and Out-of-Season Winter Fruit Cropping. Int. J. Fruit Sci. 2007, 6, 23–34. [Google Scholar] [CrossRef]
- Centro de Informação Geoespacial do Exército. Carta Militar Itinerária de Portugal Continental. Available online: https://www.igeoe.pt/index.php?id=86 (accessed on 20 November 2024).
- Instituto Português do Mar e da Atmosfera. Índice PDSI—Palmer Drought Severity Index. Available online: http://www.ipma.pt/pt/oclima/observatorio.secas/pdsi/monitorizacao/servico.situacaoatual/ (accessed on 20 November 2024).
- AOAC International. Official Method 920.15. In Official Methods of Analysis of AOAC International, 19th ed.; AOAC International: Gaithersburg, MD, USA, 2012. [Google Scholar]
- AOAC International. Official Method 982.08. In Official Methods of Analysis of AOAC International, 19th ed.; AOAC International: Gaithersburg, MD, USA, 2012. [Google Scholar]
- AOAC International. Official Method 991.36. In Official Methods of Analysis of AOAC International, 19th ed.; AOAC International: Gaithersburg, MD, USA, 2012. [Google Scholar]
- AOAC International. Official Method 985.29. In Official Methods of Analysis of AOAC International, 19th ed.; AOAC International: Gaithersburg, MD, USA, 2012. [Google Scholar]
- ISO 12966-2:2011; Animal and Vegetable Fats and Oils—Gas Chromatography of Fatty Acid Methyl Esters—Part 2: Preparation of Methyl Esters of Fatty Acids. International Organization for Standardization: Geneva, Switzerland, 2011.
- Montesano, D.; Cossignani, L.; Giua, L.; Urbani, E.; Simonetti, M.S.; Blasi, F. A Simple HPLC-ELSD Method for Sugar Analysis in Goji Berry. J. Chem. 2016, 2016, 6271808. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Costa, A.S.G.; Alves, R.C.; Vinha, A.F.; Barreira, S.V.P.; Nunes, M.A.; Cunha, L.M.; Oliveira, M.B.P.P. Optimization of Antioxidants Extraction from Coffee Silverskin, a Roasting by-Product, Having in View a Sustainable Process. Ind. Crops Prod. 2014, 53, 350–357. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Urbiola, M.I.; Contreras-Padilla, M.; Pérez-Torrero, E.; Hernández-Quevedo, G.; Rojas-Molina, J.I.; Cortes, M.E.; Rodríguez-García, M.E. Study of Nutritional Composition of Nopal (Opuntia ficus indica cv. Redonda) at Different Maturity Stages. Open Nutr. J. 2010, 4, 11–16. [Google Scholar] [CrossRef]
- El Mannoubi, I.; Barrek, S.; Skanji, T.; Casabianca, H.; Zarrouk, H. Characterization of Opuntia ficus indica Seed Oil from Tunisia. Chem. Nat. Compd. 2009, 45, 616–620. [Google Scholar] [CrossRef]
- Ennouri, M.; Evelyne, B.; Laurence, M.; Hamadi, A. Fatty Acid Composition and Rheological Behaviour of Prickly Pear Seed Oils. Food Chem. 2005, 93, 431–437. [Google Scholar] [CrossRef]
- Salim, N. Chemical Composition of Opuntia ficus-indica (L.) Fruit. Afr. J. Biotechnol. 2010, 8, 1630–1636. [Google Scholar]
- Özcan, M.M.; Al Juhaimi, F.Y. Nutritive Value and Chemical Composition of Prickly Pear Seeds (Opuntia ficus indica L.) Growing in Turkey. Int. J. Food Sci. Nutr. 2011, 62, 533–536. [Google Scholar] [CrossRef]
- Coşkuner, Y.n.; Tekin, A. Monitoring of Seed Composition of Prickly Pear (Opuntia ficus-indica L.) Fruits during Maturation Period. J. Sci. Food Agric. 2003, 83, 846–849. [Google Scholar] [CrossRef]
- Andreu, L.; Nuncio-Jáuregui, N.; Carbonell-Barrachina, Á.A.; Legua, P.; Hernández, F. Antioxidant Properties and Chemical Characterization of Spanish Opuntia ficus-indica Mill. Cladodes and Fruits. J. Sci. Food Agric. 2018, 98, 1566–1573. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Beckles, D.M. Dynamic Changes in the Starch-Sugar Interconversion within Plant Source and Sink Tissues Promote a Better Abiotic Stress Response. J. Plant Physiol. 2019, 234, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Teper-Bamnolker, P.; Roitman, M.; Katar, O.; Peleg, N.; Aruchamy, K.; Suher, S.; Doron-Faigenboim, A.; Leibman, D.; Omid, A.; Belausov, E.; et al. An Alternative Pathway to Plant Cold Tolerance in the Absence of Vacuolar Invertase Activity. Plant J. 2023, 113, 327–341. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Zhang, F.; Song, S.; Yu, X.; Ren, Y.; Zhao, X.; Liu, H.; Liu, G.; Wang, Y.; He, H. CsSWEET2, a Hexose Transporter from Cucumber (Cucumis sativus L.), Affects Sugar Metabolism and Improves Cold Tolerance in Arabidopsis. Int. J. Mol. Sci. 2022, 23, 3886. [Google Scholar] [CrossRef] [PubMed]
- Pontis, H.G. Fructans and Cold Stress. J. Plant Physiol. 1989, 134, 148–150. [Google Scholar] [CrossRef]
- Valluru, R.; Van den Ende, W. Plant Fructans in Stress Environments: Emerging Concepts and Future Prospects. J. Exp. Bot. 2008, 59, 2905–2916. [Google Scholar] [CrossRef]
- Chiappero, J.; Cappellari, L.d.R.; Palermo, T.B.; Giordano, W.; Banchio, E. A Simple Method to Determine Antioxidant Status in Aromatic Plants Subjected to Drought Stress. Biochem. Mol. Biol. Educ. 2021, 49, 483–491. [Google Scholar] [CrossRef]
- Bouaouich, A.; Bouguerche, F.; Mahiaoui, H.; Peron, G.; Bendif, H. Phytochemical Elucidation and Antioxidant Activity of Seeds from Three Prickly Pear (Opuntia ficus-indica L.) Cultivars from Algeria. Appl. Sci. 2023, 13, 1444. [Google Scholar] [CrossRef]
- Gonzalez, F.P.H.; Saucedo, V.C.; Guerra, R.D.; Suarez, E.J.; Soto, H.R.M.; Lopez, J.A.; Garcia, C.E.; Hernández, R.G. Post-Harvest Quality and Quantification of Betalains, Phenolic Compounds and Antioxidant Activity in Fruits of Three Cultivars of Prickly Pear (Opuntia ficus-indica L. Mill). J. Hortic. Sci. 2021, 16, 91–102. [Google Scholar] [CrossRef]
- Al-Nablsi, S.; El-Keblawy, A.; Ali, M.A.; Mosa, K.A.; Hamoda, A.M.; Shanableh, A.; Almehdi, A.M.; Soliman, S.S.M. Phenolic Contents and Antioxidant Activity of Citrullus Colocynthis Fruits, Growing in the Hot Arid Desert of the UAE, Influenced by the Fruit Parts, Accessions, and Seasons of Fruit Collection. Antioxidants 2022, 11, 656. [Google Scholar] [CrossRef]
- Brigelius-Flohé, R.; Traber, M.G. Vitamin E: Function and Metabolism. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 1999, 13, 1145–1155. [Google Scholar]
- Michalak, M.; Pierzak, M.; Kręcisz, B.; Suliga, E. Bioactive Compounds for Skin Health: A Review. Nutrients 2021, 13, 203. [Google Scholar] [CrossRef] [PubMed]
- Munné-Bosch, S.; Puig, S.; Fenollosa, E.; Casadesús, A.; Fernández, E. Vitamin E Protects from Lipid Peroxidation during Winter Stress in the Seagrass Cymodocea Nodosa. Planta 2022, 255, 41. [Google Scholar] [CrossRef] [PubMed]
- Al-Naqeb, G.; Fiori, L.; Ciolli, M.; Aprea, E. Prickly Pear Seed Oil Extraction, Chemical Characterization and Potential Health Benefits. Molecules 2021, 26, 5018. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, M.; Akram, N.A.; Ashraf, M.; Al-Qurainy, F.; Ahmad, P. Alpha-Tocopherol-Induced Regulation of Growth and Metabolism in Plants Under Non-Stress and Stress Conditions. J. Plant Growth Regul. 2019, 38, 1325–1340. [Google Scholar] [CrossRef]
- Maeda, H.; DellaPenna, D. Tocopherol Functions in Photosynthetic Organisms. Curr. Opin. Plant Biol. 2007, 10, 260–265. [Google Scholar] [CrossRef]
- Khémiri, I.; Bitri, L. Effectiveness of Opuntia Ficus Indica L. Inermis Seed Oil in the Protection and the Healing of Experimentally Induced Gastric Mucosa Ulcer. Oxid. Med. Cell. Longev. 2019, 2019, 1568720. [Google Scholar] [CrossRef]
- Shoukat, R.; Cappai, M.; Pia, G.; Pilia, L. An Updated Review: Opuntia Ficus Indica (OFI) Chemistry and Its Diverse Applications. Appl. Sci. 2023, 13, 7724. [Google Scholar] [CrossRef]
- Matthäus, B.; Özcan, M.M. Habitat Effects on Yield, Fatty Acid Composition and Tocopherol Contents of Prickly Pear (Opuntia ficus-indica L.) Seed Oils. Sci. Hortic. 2011, 131, 95–98. [Google Scholar] [CrossRef]
- Chougui, N.; Tamendjari, A.; Hamidj, W.; Hallal, S.; Barras, A.; Richard, T.; Larbat, R. Oil Composition and Characterisation of Phenolic Compounds of Opuntia ficus-indica Seeds. Food Chem. 2013, 139, 796–803. [Google Scholar] [CrossRef]
- Hassanien, M.; Moersel, J.-T. Oil Cactus Pear (Opuntia ficus-indica L.). Food Chem. 2003, 82, 339–345. [Google Scholar] [CrossRef]
- Al-Naqeb, G.; Cafarella, C.; Aprea, E.; Ferrentino, G.; Gasparini, A.; Buzzanca, C.; Micalizzi, G.; Dugo, P.; Mondello, L.; Rigano, F. Supercritical Fluid Extraction of Oils from Cactus Opuntia ficus-indica L. and Opuntia dillenii Seeds. Foods 2023, 12, 618. [Google Scholar] [CrossRef] [PubMed]



| Sample | Moisture * | Ash | Lipids | Protein | Total Fiber | Carbohydrates ** | |
|---|---|---|---|---|---|---|---|
| Juice | Summer | 88.44 ± 0.06 a | 0.35 ± 0.02b | 0.36 ± 0.02 c | 3.26 ± 0.06 d | 2.50 ± 0.11 | 93.53 ± 0.27 |
| Winter | 88.80 ± 0.16 a | 0.37 ± 0.01 b | 0.40 ± 0.02 c | 3.39 ± 0.29 d | 3.74 ± 0.06 | 92.10± 0.38 | |
| Pulp | Summer | 82.26 ± 0.63 | 0.27 ± 0.03 | 0.66 ± 0.05 e | 3.48 ± 0.04f | 32.19 ± 2.02 g | 63.40 ± 2.10 |
| Winter | 84.95 ± 0.91 | 0.41 ± 0.03 | 0.53 ± 0.04 e | 3.96 ± 0.40 f | 32.68 ± 1.06 g | 62.42± 1.09 | |
| Peel | Summer | 84.56 ± 0.43 | 1.51 ± 0.07 h | 0.94 ± 0.01 i | 2.92 ± 0.15j | 26.18 ± 0.48 | 68.45 ± 0.71 |
| Winter | 86.48 ± 0.29 | 1.76 ± 0.11 h | 0.81 ± 0.01 i | 2.96 ± 0.20 j | 18.31 ± 0.24 | 76.16 ± 0.32 | |
| Seed | Summer | 49.52 ± 1.41 | 1.77 ± 0.11 | 6.14 ± 0.34 k | 6.83 ± 0.25 l | 80.20 ± 0.16 | 5.06 ± 0.86 |
| Winter | 54.96 ± 1.01 | 2.22 ± 0.01 | 7.07 ± 0.10 k | 6.72 ± 0.00 l | 73.98 ± 1.56 | 10.01 ± 1.67 | |
| Vitamin E (mg·kg−1 of Dry Weight) | Samples | |
|---|---|---|
| Summer | Winter | |
| α-tocopherol | 5.17 ± 0.59 | 11.69 ± 1.16 |
| β-tocopherol | 1.16 ± 0.08 a | 1.43 ± 0.03 a |
| γ-tocopherol | 39.02 ± 2.82 | 33.15 ± 3.29 |
| δ-tocopherol | 0.82 ± 0.03 b | 0.82 ± 0.02 b |
| Total | 46.17 ± 3.04 c | 47.09 ± 4.26 c |
| Compound Name | Summer | Winter |
|---|---|---|
| Myristic Acid (C14:0) | 0.06 ± 0.01 a | 0.10 ± 0.00 a |
| Palmitic Acid (C16:0) | 12.24 ± 0.06 b | 12.86 ± 0.34 b |
| Palmitoleic Acid (C16:1) | 0.67 ± 0.03 c | 0.81 ± 0.02 c |
| Stearic Acid (C18:0) | 3.59 ± 0.19 d | 3.77 ± 0.23 d |
| Oleic Acid (C18:1n9c) | 17.61 ± 0.40 e | 18.09 ± 1.80 e |
| Vaccenic Acid (C18:1n7) * | 5.71 ± 0.13 f | 5.90 ± 0.23 f |
| Linoleic acid (C18:2n6c) | 59.26 ± 0.21 g | 57.39 ± 1.73 g |
| Arachidic Acid (C20:0) | 0.23 ± 0.01 h | 0.25 ± 0.02 h |
| α-Linolenic Acid (C18:3n3) | 0.17 ± 0.01 | 0.24 ± 0.02 |
| Eicosanoic Acid (C20:1n9) | 0.14 ± 0.01 | 0.17 ± 0.00 |
| Behenic Acid (C22:0) | 0.18 ± 0.01 i | 0.18 ± 0.01 i |
| Lignoceric Acid (C24:0) | 0.11 ± 0.01 j | 0.25 ± 0.02 j |
| Total SFA | 16.43 ± 0.28 | 17.41 ± 0.63 |
| Total MUFA | 24.14 ± 0.57 | 24.97 ± 2.06 |
| Total PUFA | 59.44 ± 0.22 | 57.62 ± 1.75 |
| Total UFA | 83.58 ± 0.79 | 82.59 ± 3.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jorge, A.O.S.; Costa, A.S.G.; Ferreira, D.M.; Oliveira, M.B.P.P. Seasonal Variation in Nutritional and Chemical Profiles of Wild Opuntia ficus-indica Fruits. Plants 2025, 14, 409. https://doi.org/10.3390/plants14030409
Jorge AOS, Costa ASG, Ferreira DM, Oliveira MBPP. Seasonal Variation in Nutritional and Chemical Profiles of Wild Opuntia ficus-indica Fruits. Plants. 2025; 14(3):409. https://doi.org/10.3390/plants14030409
Chicago/Turabian StyleJorge, Ana O. S., Anabela S. G. Costa, Diana Melo Ferreira, and Maria Beatriz P. P. Oliveira. 2025. "Seasonal Variation in Nutritional and Chemical Profiles of Wild Opuntia ficus-indica Fruits" Plants 14, no. 3: 409. https://doi.org/10.3390/plants14030409
APA StyleJorge, A. O. S., Costa, A. S. G., Ferreira, D. M., & Oliveira, M. B. P. P. (2025). Seasonal Variation in Nutritional and Chemical Profiles of Wild Opuntia ficus-indica Fruits. Plants, 14(3), 409. https://doi.org/10.3390/plants14030409









