Potential of Trichoderma asperellum as a Growth Promoter in Hydroponic Lettuce Cultivated in a Floating-Root System
Abstract
1. Introduction
2. Results
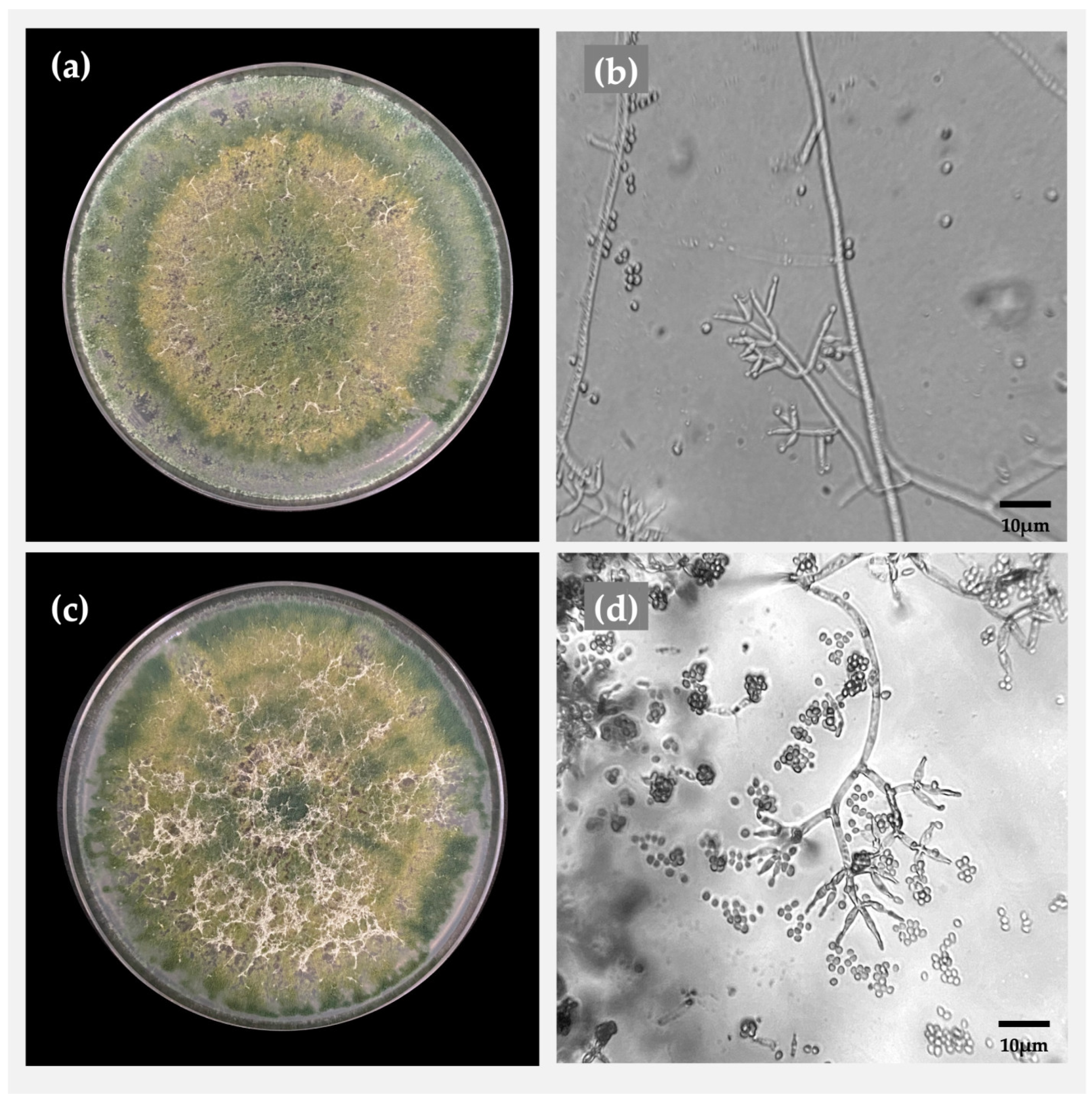
2.1. Morphological Identification of Trichoderma
2.2. Molecular Identification of Trichoderma
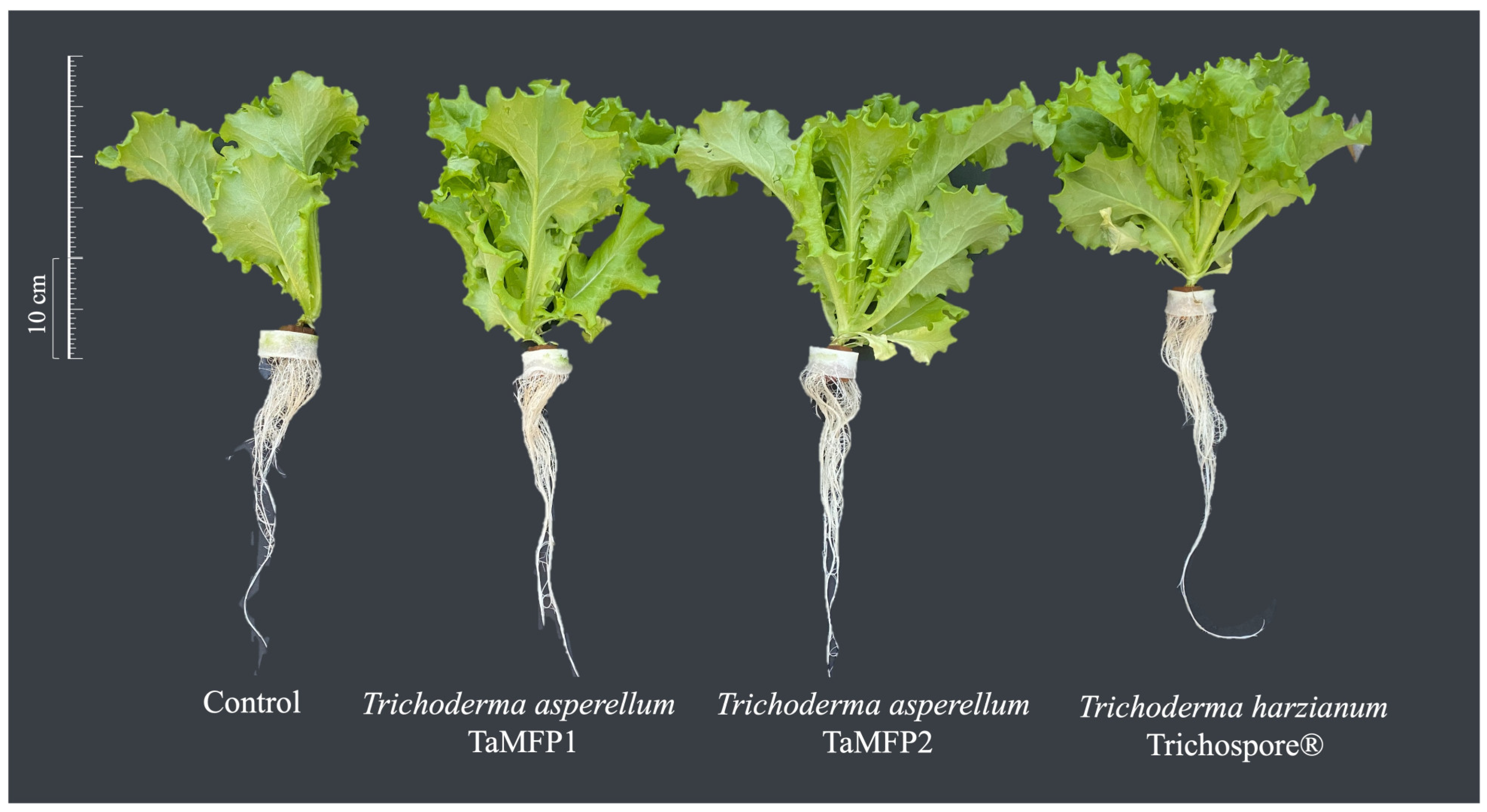
2.3. Trichoderma as Lettuce Plant-Growth-Promoting Fungi
2.4. Analysis Nutrimental
2.5. Lettuce Quality
2.6. Principal Component Analysis
3. Discussion
4. Materials and Methods
4.1. Localization
4.2. Samples Collection
4.3. Trichoderma Strain Isolation
4.4. Morphological Identification
4.5. Extraction, Amplification, and Sequencing of DNA
4.6. Phylogenetic Reconstruction
4.7. Seedling Production
4.8. Preparation of Inoculum
4.9. Trial Establishment
4.10. Plant Analysis
4.10.1. Morphological Variables
4.10.2. Photosynthetic Pigments
4.10.3. Foliar Nutrients
4.10.4. Nitrate Content
4.10.5. Crop Yield
4.10.6. Quality Parameters
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ray, P.; Lakshmanan, V.; Labbé, J.L.; Craven, K.D. Microbe to microbiome: A paradigm shifts in the application of microorganisms for sustainable agriculture. Front. Microbiol. 2020, 11, 622926. [Google Scholar] [CrossRef]
- Mącik, M.; Gryta, A.; Frąc, M. Biofertilizers in agriculture: An overview on concepts, strategies, and effects on soil microorganisms. Adv. Agron. 2020, 162, 31–87. [Google Scholar] [CrossRef]
- Ortiz, A.; Sansinenea, E. The role of beneficial microorganisms in soil quality and plant health. Sustainability 2022, 14, 5358. [Google Scholar] [CrossRef]
- Pirttilä, A.M.; Mohammad, P.T.H.; Baruah, N.; Koskimäki, J.J. Biofertilizers and biocontrol agents for agriculture: How to identify and develop new potent microbial strains and traits. Microorganisms 2021, 9, 817. [Google Scholar] [CrossRef] [PubMed]
- Elnahal, A.S.M.; El-Saadony, M.T.; Saad, A.M.; Desoky, E.S.M.; El-Tahan, A.M.; Rady, M.M.; AbuQamar, S.F.; El-Tarabily, K.A. The use of microbial inoculants for biological control, plant growth promotion, and sustainable agriculture: A review. Eur. J. Plant Pathol. 2022, 162, 759–792. [Google Scholar] [CrossRef]
- Zin, N.A.; Badaluddin, N.A. Biological functions of Trichoderma spp. for agriculture applications. Ann. Agric. Sci. 2020, 65, 168–178. [Google Scholar] [CrossRef]
- Bhandari, S.; Pandey, K.R.; Joshi, Y.R.; Lamichhane, S.K. An overview of multifaceted role of Trichoderma spp. for sustainable agriculture. Arch. Agric. Environ. Sci. 2021, 6, 72–79. [Google Scholar] [CrossRef]
- Woo, S.L.; Hermosa, R.; Lorito, M.; Monte, E. Trichoderma: A multipurpose, plant-beneficial microorganism for eco-sustainable agriculture. Nat. Rev. Microbiol. 2023, 21, 312–326. [Google Scholar] [CrossRef]
- Abdullah, N.S.; Doni, F.; Mispan, M.S.; Saiman, M.Z.; Yusuf, Y.M.; Oke, M.A.; Suhaimi, N.S.M. Harnessing Trichoderma in agriculture for productivity and sustainability. Agronomy 2021, 11, 2559. [Google Scholar] [CrossRef]
- Tyskiewicz, R.; Nowak, A.; Ozimek, E.; Jaroszuk-Ściseł, J. Trichoderma: The current status of its application in agriculture for the biocontrol of fungal phytopathogens and stimulation of plant growth. Int. J. Mol. Sci. 2022, 23, 2329. [Google Scholar] [CrossRef] [PubMed]
- Andrzejak, R.; Janowska, B. Trichoderma spp. improves flowering, quality, and nutritional status of ornamental plants. Int. J. Mol. Sci. 2022, 23, 15662. [Google Scholar] [CrossRef] [PubMed]
- Kubiak, A.; Wolna-Maruwka, A.; Pilarska, A.A.; Niewiadomska, A.; Piotrowska-Cyplik, A. Fungi of the Trichoderma genus: Future perspectives of benefits in sustainable agriculture. Appl. Sci. 2023, 13, 6434. [Google Scholar] [CrossRef]
- Asghar, W.; Craven, K.D.; Kataoka, R.; Mahmood, A.; Asghar, N.; Raza, T.; Iftikhar, F. The application of Trichoderma spp., an old but new useful fungus, in sustainable soil health intensification: A comprehensive strategy for addressing challenges. Plant Stress 2024, 12, 100455. [Google Scholar] [CrossRef]
- Khatri, L.; Kunwar, A.; Bist, D.R. Hydroponics: Advantages and Challenges in Soilless Farming. Big Data Agric. (BDA) 2024, 6, 81–88. [Google Scholar] [CrossRef]
- Pomoni, D.I.; Koukou, M.K.; Vrachopoulos, M.G.; Vasiliadis, L. A review of hydroponics and conventional agriculture based on energy and water consumption, environmental impact, and land use. Energies 2023, 16, 1690. [Google Scholar] [CrossRef]
- Velazquez-Gonzalez, R.S.; Garcia-Garcia, A.L.; Ventura-Zapata, E.; Barceinas-Sanchez, J.D.O.; Sosa-Savedra, J.C. A review on hydroponics and the technologies associated for medium-and small-scale operations. Agriculture 2022, 12, 646. [Google Scholar] [CrossRef]
- Sousa, R.D.; Bragança, L.; da Silva, M.V.; Oliveira, R.S. Challenges, and solutions for sustainable food systems: The potential of home hydroponics. Sustainability 2024, 16, 817. [Google Scholar] [CrossRef]
- Sela, S.S.; Rodov, V.; Kenigsbuch, D.; Bar-Tal, A. Hydroponic agriculture, and microbial safety of vegetables: Promises, challenges, and solutions. Horticulturae 2023, 9, 51. [Google Scholar] [CrossRef]
- Chaparro, J.M.; Sheflin, A.M.; Manter, D.K.; Vivanco, J.M. Manipulating the soil microbiome to increase soil health and plant fertility. Biol. Fertil. Soils 2012, 48, 489–499. [Google Scholar] [CrossRef]
- De Corato, U. Soil microbiota manipulation and its role in suppressing soil-borne plant pathogens in organic farming systems under the light of microbiome-assisted strategies. Chem. Biol. Technol. Agric. 2020, 7, 17. [Google Scholar] [CrossRef]
- Mourouzidou, S.; Ntinas, G.K.; Tsaballa, A.; Monokrousos, N. Introducing the power of plant growth promoting microorganisms in soilless systems: A promising alternative for sustainable agriculture. Sustainability 2023, 15, 5959. [Google Scholar] [CrossRef]
- Battaglia, M.E.; Martinez, S.I.; Covacevich, F.; Consolo, V.F. Trichoderma harzianum Enhances Root Biomass Production and Promotes Lateral Root Growth of Soybean and Common Bean under Drought Stress. Ann. Appl. Biol. 2024, 185, 36–48. [Google Scholar] [CrossRef]
- Fu, J.; Xiao, Y.; Wang, Y.; Liu, Z.; Yang, K. Saline–alkaline stress in growing maize seedlings is alleviated by Trichoderma asperellum through regulation of the soil environment. Sci. Rep. 2021, 11, 11152. [Google Scholar] [CrossRef] [PubMed]
- Moreira, V.D.A.; Oliveira, C.E.D.S.; Jalal, A.; Gato, I.M.B.; Oliveira, T.J.S.S.; Boleta, G.H.M.; Giolo, V.M.; Vitória, L.S.; Tamburi, K.V.; Filho, M.C.M.T. Inoculation with Trichoderma harzianum and Azospirillum brasilense increases nutrition and yield of hydroponic lettuce. Arch. Microbiol. 2022, 204, 440. [Google Scholar] [CrossRef]
- Campbell, R.C. Reference Sufficiency Ranges for Plant Analysis in the Southern Region of the United States; Southern Cooperative Series Bulletin #394; North Carolina Department of Agriculture and Consumer Services Agronomic Division: Raleigh, NC, USA, 2009; p. 11. [Google Scholar]
- Siddiquee, S. Morphology-Based Characterization of Trichoderma Species. In Practical Handbook of the Biology and Molecular Diversity of Trichoderma Species from Tropical Regions, Fungal Biology; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Contreras-Cornejo, H.A.; Schmoll, M.; Esquivel-Ayala, B.A.; González-Esquivel, C.E.; Rocha-Ramírez, V.; Larsen, J. Mechanisms for Plant Growth Promotion Activated by Trichoderma in Natural and Managed Terrestrial Ecosystem. Microbiol. Res. 2024, 281, 127621. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-García, B.M.; Espinosa-Huerta, E.; Villordo-Pineda, E.; Rodríguez-Guerra, R.; Mora-Avilés, M.A. Identificación Molecular y Evaluación Antagónica In Vitro de Cepas Nativas de Trichoderma spp. Sobre Hongos Fitopatógenos de Raíz en Frijol (Phaseolus vulgaris L.) cv. Montcalm. Agrociencia 2017, 51, 63–79. [Google Scholar]
- Zapata-Sarmiento, D.H.; Palacios-Pala, E.F.; Rodríguez-Hernández, A.A.; Melchor, D.L.M.; Rodríguez-Monroy, M.; Sepúlveda-Jiménez, G. Trichoderma asperellum, a Potential Biological Control Agent of Stemphylium vesicarium, on Onion (Allium cepa L.). Biol. Control 2020, 140, 104105. [Google Scholar] [CrossRef]
- Chakraborty, B.N.; Chakraborty, U.; Dey, P.L.; Sunar, K. Phylogenetic Relationship of Trichoderma Isolates of North Bengal Based on Sequence Analysis of ITS Region of rDNA. J. Appl. Sci. Res. 2010, 6, 1477–1482. [Google Scholar]
- Leu, F.G.; Gilesky, N.; Petruzzi, L. Evaluación del efecto de Trichoderma atroviride cepa∝ cp8 y Bacillus velezensis en el cultivo de albahaca (Ocimum basilicum l.) en hidroponía en córdoba capital. Nexo Agropecu. 2023, 11, 46–53. [Google Scholar]
- Yedidia, I.; Srivastva, A.K.; Kapulnik, Y.; Chet, I. Effect of Trichoderma harzianum on microelement concentrations and increased growth of cucumber plants. Plant Soil 2001, 235, 235–242. [Google Scholar] [CrossRef]
- Pineda-Acosta, A.S.; Lara-Capistrán, L.; Hernández-Montiel, L.G.; Alafita-Vásquez, G.; Zulueta-Rodríguez, R. Efecto de microorganismos bioestimulantes en la morfometría de Lactuca sativa L. bajo un sistema hidropónico de raíz flotante. Rev. Int. Des. Reg. Sustentable 2021, 6, 27–37. [Google Scholar]
- Oliveira, C.E.d.S.; Jalal, A.; Oliveira, J.R.; Tamburi, K.V.; Teixeira Filho, M.C.M. Leaf Inoculation of Azospirillum brasilense and Trichoderma harzianum in Hydroponic Arugula Improve Productive Components and Plant Nutrition and Reduce Leaf Nitrate. Pesqui. Agropecu. Trop. 2022, 52, e72755. [Google Scholar] [CrossRef]
- Shoresh, M.; Harman, G.E. The Molecular Basis of Shoot Responses of Maize Seedlings to Trichoderma harzianum T22 Inoculation of the Root: A Proteomic Approach. Plant Physiol. 2008, 147, 2147–2163. [Google Scholar] [CrossRef]
- Shoresh, M.; Harman, G.E. The Relationship Between Increased Growth and Resistance Induced in Plants by Root-Colonizing Microbes. Plant Signal. Behav. 2008, 3, 737–739. [Google Scholar] [CrossRef]
- Garnica-Vergara, A.; Barrera-Ortiz, S.; Muñoz-Parra, E.; Raya-Gonzalez, J.; Mendez-Bravo, A.; Macías-Rodríguez, L.; Ruiz-Herrera, L.F.; López-Bucio, J. The Volatile 6-Pentyl-2H-Pyran-2-One from Trichoderma atroviride Regulates Arabidopsis thaliana Root Morphogenesis via Auxin Signaling and ethylene insensitive 2 Functioning. New Phytol. 2015, 209, 1496–1512. [Google Scholar] [CrossRef] [PubMed]
- Vargas, W.A.; Mandawe, J.C.; Kenerley, C.M. Plant-Derived Sucrose Is a Key Element in the Symbiotic Association Between Trichoderma virens and Maize Plants. Plant Physiol. 2009, 151, 792–808. [Google Scholar] [CrossRef]
- Goswami, D.; Thakker, J.N.; Dhandhukia, P.C. Portraying Mechanics of Plant Growth Promoting Rhizobacteria (PGPR): A Review. Cogent Food Agric. 2016, 2, 1127500. [Google Scholar] [CrossRef]
- Jalal, A.; da Silva Oliveira, C.E.; Freitas, L.A.; Galindo, F.S.; Lima, B.H.; Boleta, E.H.M.; da Silva, E.C.; Nascimento, V.D.; Nogueira, T.A.R.; Buzetti, S.; et al. Agronomic Biofortification and Productivity of Wheat with Soil Zinc and Diazotrophic Bacteria in Tropical Savannah. Crop Pasture Sci. 2022, 73, 817–830. [Google Scholar] [CrossRef]
- Spaepen, S.; Vanderleyden, J.; Remans, R. Indole-3-Acetic Acid in Microbial and Microorganism-Plant Signaling. FEMS Microbiol. Rev. 2007, 31, 425–448. [Google Scholar] [CrossRef]
- Vega-Celedón, P.; Canchignia, H.M.; González, M.; Seeger, M. Biosíntesis de Ácido Indol-3-Acético y Promoción del Crecimiento de Plantas por Bacterias. Cult. Trop. 2016, 37, 31–37. [Google Scholar] [CrossRef]
- Patloková, K.; Pokluda, R. Optimization of Plant Nutrition in Aquaponics: The Impact of Trichoderma harzianum and Bacillus mojavensis on Lettuce and Basil Yield and Mineral Status. Plants 2024, 13, 291. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.N.; Dwivedi, P.; Sarma, B.K.; Singh, G.S.; Singh, H.B. Trichoderma asperellum T42 Reprograms Tobacco for Enhanced Nitrogen Utilization Efficiency and Plant Growth When Fed with N Nutrients. Front. Plant Sci. 2018, 9, 163. [Google Scholar] [CrossRef]
- Pereira, F.T.; Oliveira, J.B.D.; Muniz, P.H.P.; Peixoto, G.H.S.; Guimarães, R.R.; Carvalho, D.D.C. Growth promotion and productivity of lettuce using Trichoderma spp. commercial strains. Hortic. Bras. 2019, 37, 69–74. [Google Scholar] [CrossRef]
- Senger, M.; Moresco, E.; Briega, A.H.; Harakava, R.; Lucon, C.M.M. The Agronomic efficiency of the inoculant FT10 (Trichoderma asperelloides) on four lettuce varieties. Comun. Sci. 2022, 13, e3750. [Google Scholar] [CrossRef]
- Agüero, M.V.; Barg, M.V.; Yommi, A.; Camelo, A.; Roura, S.I. Postharvest changes in water status and chlorophyll content of lettuce (Lactuca sativa L.) and their relationship with overall visual quality. J. Food Sci. 2008, 73, S47–S55. [Google Scholar] [CrossRef]
- Ha, T.M. Production efficiency and quality of mustard green (Brassica juncea (L.) Czern) cultivated according to the Vietnamese good agricultural practice (VietGAP) guideline in Thai Nguyen city. Asian J. Agric. Food Sci. 2014, 2, 329–335. Available online: https://www.ajouronline.com/index.php/AJAFS/article/view/1602 (accessed on 3 January 2025).
- Werres, S. PROTOCOL 01-09.1: Preparation of Hyphal Tip Phytophthora Cultures. In Laboratory Protocols for Phytophthora Species; Werres, S., Ed.; The American Phytopathological Society: St. Paul, MN, USA, 2015; pp. 1–2. [Google Scholar]
- Samuels, G.J.; Hebbar, P.K. Trichoderma: Identification and Agricultural Applications; APS Press: St. Paul, MN, USA, 2015; Volume 602. [Google Scholar]
- Martínez-González, C.R.; Ramírez-Mendoza, R.; Jiménez-Ramírez, J.; Gallegos-Vázquez, C.; Luna-Vega, I. Improved Method for Genomic DNA Extraction for Opuntia Mill. (Cactaceae). Plant Methods 2017, 13, 82. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification, and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Hall, T. A User-Friendly Biological Sequence Alignment Editor and Analysis Program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Zhang, Z.; Schwartz, S.; Wagner, L.; Miller, W. A Greedy Algorithm for Aligning DNA Sequences. J. Comput. Biol. 2000, 7, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis Across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Gu, X.; Wang, R.; Sun, Q.; Wu, B.; Sun, J.Z. Four New Species of Trichoderma in the Harzianum Clade from Northern China. MycoKeys 2020, 73, 109–132. [Google Scholar] [CrossRef] [PubMed]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and high-performance computing. Nature Methods 2012, 9, 772. [Google Scholar] [CrossRef] [PubMed]
- Huelsenbeck, J.P.; Ronquist, F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef] [PubMed]
- Morgan, L. The pH factor in hydroponics. Grow. Edge 1998, 9, 25–33. [Google Scholar]
- Gao, Y.; Zeng, X.D.; Ren, B.; Zeng, J.R.; Xu, T.; Yang, Y.Z.; Hu, X.C.; Zhu, Z.Y.; Shi, L.M.; Zhou, G.Y.; et al. Antagonistic activity against rice blast disease and elicitation of host-defence response capability of an endophytic Streptomyces albidoflavus OsiLf-2. Plant Pathol. 2019, 69, 259–271. [Google Scholar] [CrossRef]
- Patrignani, A.; Ochsner, T.E. Canopeo: A powerful new tool for measuring fractional green canopy cover. Agron. J. 2015, 107, 2312–2320. [Google Scholar] [CrossRef]
- Lichtenthaler, H.; Wellburn, A.R. Determinations of Total Carotenoids and Chlorophylls a and b of Leaf Extracts in Different Solvents; Portland Press Limited: London, UK, 1983. [Google Scholar] [CrossRef]
- Hernández, A.; Castillo, H.; Ojeda, D.; Arras, A.; López, J.; Sánchez, E. Effect of Vermicompost and Compost on Lettuce Production. Chilean J. Agric. Res. 2010, 70, 583–589. [Google Scholar] [CrossRef]
- Smith, M.W.; Cheary, B.; Carroll, B. Response of Pecan to Nitrogen Rate and Nitrogen Application Time. HortScience 2004, 39, 1412–1415. [Google Scholar] [CrossRef]
- Estefan, G.; Sommer, R.; Ryan, J. Methods of soil, plant, and water analysis. A Man. West Asia N. Afr. Reg. 2013, 3, 65–119. [Google Scholar]
- Cataldo, D.A.; Maroon, M.; Schrader, L.E.; Youngs, V.L. Rapid Colorimetric Determination of Nitrate in Plant Tissue by Nitration of Salicylic Acid. Commun. Soil Sci. Plant Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]
- Kader, A.A.; Lipton, W.J.; Morris, L.L. Systems for Scoring Quality of Harvested Lettuce. HortSci 1973, 8, 408–409. [Google Scholar] [CrossRef]
- Tabachnick, B.G.; Fidell, L.S. Using Multivariate Statistics, 5th ed.; Pearson Education, Inc.: Boston, MA, USA, 2007. Available online: https://lccn.loc.gov/2017040173 (accessed on 29 December 2024).



| Parameter 1 | Treatments | |||
|---|---|---|---|---|
| Control | T. asperellum TaMFP1 | T. asperellum TaMFP2 | Trichospore® | |
| PH (cm) | 20.41 ± 2.90 b | 23.58 ± 2.35 a | 24.95 ± 2.90 a | 24.34 ± 2.53 a |
| SL (cm) | 6.19 ± 0.46 b | 7.42 ± 1.01 a | 6.90 ± 1.38 a | 7.17 ± 0.56 ab |
| RL (cm) | 29.39 ± 5.13 b | 36.56 ± 4.78 a | 37.77 ± 8.67 a | 36.48 ± 7.96 a |
| LA (cm2 plant−1) * | 814.19 ± 133.76 b | 932.55 ± 162.81 ab | 1076.58 ± 299.21 a | 1098.96 ± 309.07 a |
| NL (plant−1) ** | 13.31 ± 2.33 b | 15.50 ± 2.31 a | 15.75 ± 2.77 a | 15.94 ± 1.29 a |
| Treatment 1 | Control | T. asperellum TaMFP1 | T. asperellum TaMFP2 | Trichospore® |
|---|---|---|---|---|
| Fresh biomass (g plant−1) | ||||
| Leaves | 22.99 ± 4.75 b | 36.79 ± 5.96 a | 42.39 ± 14.29 a | 45.33 ± 15.09 a |
| Stem | 1.76 ± 0.43 b | 2.23 ± 0.45 a | 2.74 ± 1.02 a | 2.88 ± 0.94 a |
| Root * | 2.64 ± 0.98 b | 4.04 ± 1.44 ab | 3.93 ± 1.81 ab | 4.63 ± 1.96 a |
| Total | 27.39 ± 5.32 b | 43.06 ± 6.98 a | 49.06 ± 16.76 a | 52.83 ± 17.09 a |
| Dry biomass (g plant−1) | ||||
| Leaves | 1.04 ± 0.31 b | 1.77 ± 0.40 a | 1.87 ± 0.65 a | 2.18 ± 0.63 a |
| Stem | 0.09 ± 0.03 b | 0.10 ± 0.02 b | 0.12 ± 0.05 ab | 0.15 ± 0.05 a |
| Root * | 0.07 ± 0.03 b | 0.10 ± 0.04 ab | 0.09 ± 0.04 ab | 0.13 ± 0.03 a |
| Total | 1.19 ± 0.34 b | 1.98 ± 0.44 a | 2.08 ± 0.71 a | 2.46 ± 0.68 a |
| Treatment 1 | Control | T. asperellum TaMFP1 | T. asperellum TaMFP2 | Trichospore® |
|---|---|---|---|---|
| Photosynthetic pigments (mg/g g FW−1) | ||||
| Chlorophyll a | 1.80 ± 0.18 a | 1.67 ± 0.27 a | 1.58 ± 0.27 a | 1.66 ± 0.29 a |
| Chlorophyll b | 0.74 ± 0.08 a | 0.68 ± 0.11 a | 0.66 ± 0.10 a | 0.67 ± 0.12 a |
| Carotenoids | 1.73 ± 0.15 a | 1.58 ± 0.26 a | 1.54 ± 0.21 a | 1.59 ± 0.28 a |
| Treatment 1 | Control | Trichospore® | TaMFP1 | TaMFP2 | Sufficiency Range 2 |
|---|---|---|---|---|---|
| Macronutrients (%) | |||||
| N | 3.31 ± 0.08 ab | 3.70 ± 0.13 a | 3.08 ± 0.27 b | 3.75 ± 0.29 a | 4.5–6.5 |
| P | 1.27 ± 0.21 a | 1.24 ± 0.10 a | 0.91 ± 0.02 b | 0.90 ± 0.09 b | 0.3–0.8 |
| K | 4.43 ± 0.76 a | 4.67 ± 0.39 a | 4.98 ± 0.36 a | 4.05 ± 0.22 a | 6–10 |
| Ca * | 1.52 ± 0.26 a | 1.32 ± 0.06 ab | 1.38 ± 0.02 a | 1.24 ± 0.08 a | 1–2 |
| Mg | 0.66 ± 0.01 a | 0.52 ± 0.03 b | 0.53 ± 0.03 b | 0.52 ± 0.04 b | 0.35–0.75 |
| Micronutrients (ppm) | |||||
| Fe | 287.0 ± 32.35 a | 167.87 ± 5.20 b | 180.87 ± 18.95 b | 184.87 ± 11.62 b | 50–200 |
| Mn | 103.75 ± 10.13 a | 67.37 ± 6.90 c | 80.75 ± 8.79 bc | 90.75 ± 8.45 ab | 20–200 |
| Cu | 11.50 ± 1.77 a | 10.12 ± 2.78 a | 10.75 ± 1.70 a | 11.00 ± 0.91 a | 5–15 |
| Zn | 66.25 ± 6.30 a | 54.37 ± 2.49 a | 60.87 ± 8.01 a | 56.50 ± 4.77 a | 20–75 |
| Trait | Score | Description |
|---|---|---|
| Firmness description | 1 | Soft, easily compressed, or spongy |
| 2 | Fairly firm, neither soft nor firm, good head formation | |
| 3 | Firm, compact but may yield slight to moderate pressure | |
| 4 | Hard, compact, and solid | |
| 5 | Extra hard, over-mature, may have cracked mid ribs | |
| Visual quality | 1 | Extremely poor, disposable |
| 3 | Poor, many defects, limit of salability | |
| 5 | Fair, slightly to moderately defects, lower limit of sales appeal | |
| 7 | Good, minor defects | |
| 9 | Excellent, essentially free from defects | |
| Decay | 1 | Extreme, disposable |
| 3 | Severe, salvageable but usually not salable | |
| 5 | Moderate, objectionable, definitely impairs salability | |
| 7 | Slight, slightly objectionable, may impair salability | |
| 9 | None | |
| Wilting | 1 | Extreme, not acceptable under normal conditions |
| 3 | Severe, definitely objectionable | |
| 5 | Moderate, becoming objectionable | |
| 7 | Slight, not objectionable | |
| 9 | None, fresh cut appearance |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez-Chávez, A.; Robles-Hernández, L.; Guerrero, B.I.; González-Franco, A.C.; Medina-Pérez, G.; Acevedo-Barrera, A.A.; Hernández-Huerta, J. Potential of Trichoderma asperellum as a Growth Promoter in Hydroponic Lettuce Cultivated in a Floating-Root System. Plants 2025, 14, 382. https://doi.org/10.3390/plants14030382
Gutiérrez-Chávez A, Robles-Hernández L, Guerrero BI, González-Franco AC, Medina-Pérez G, Acevedo-Barrera AA, Hernández-Huerta J. Potential of Trichoderma asperellum as a Growth Promoter in Hydroponic Lettuce Cultivated in a Floating-Root System. Plants. 2025; 14(3):382. https://doi.org/10.3390/plants14030382
Chicago/Turabian StyleGutiérrez-Chávez, Aldo, Loreto Robles-Hernández, Brenda I. Guerrero, Ana Cecilia González-Franco, Gabriela Medina-Pérez, Angélica Anahí Acevedo-Barrera, and Jared Hernández-Huerta. 2025. "Potential of Trichoderma asperellum as a Growth Promoter in Hydroponic Lettuce Cultivated in a Floating-Root System" Plants 14, no. 3: 382. https://doi.org/10.3390/plants14030382
APA StyleGutiérrez-Chávez, A., Robles-Hernández, L., Guerrero, B. I., González-Franco, A. C., Medina-Pérez, G., Acevedo-Barrera, A. A., & Hernández-Huerta, J. (2025). Potential of Trichoderma asperellum as a Growth Promoter in Hydroponic Lettuce Cultivated in a Floating-Root System. Plants, 14(3), 382. https://doi.org/10.3390/plants14030382






