Seed Survival in Silage: Reviewing 90 Years of Research
Abstract
1. Seeds and Silage Sustainability
2. Literature Search, Data Sets, and Data Analyses
2.1. Literature Search
2.2. Data Sets and Data Availability
2.2.1. Data on Seed Survival
| Study | Year | Study Author(s) | Plant Species Number | Replicate Number (Seeds per Replicate) | Seed Response | Substrate(s) Ensiled | Ensiling Duration(s) [days] 2 | Ensiling Modifications | Silo Scale | Further Treatments | Reference | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lab | Full | Animal | Biogas Reactor | Storage | |||||||||||
| A | 1934 | Shevkenek | 6 | 1 (100–1000) | g | n.r. | 105 | - | - | n.r. | - | - | manure | [8] | |
| B | 1937 | Tildesley | 19 | n.a. | v | n.a. | 21 | - | lab silo | - | - | - | - | [9] | |
| C | 1940 | Woodward | 26 | n.r. | g | alfalfa; maize; alfalfa + grass | 7–149 | molasses; DM | - | stack | - | - | - | [19] | |
| D | 1941 | Zahnley and Fitch | n.a. | n.a. | n.a. | maize; sorghum | n.a. | - | - | bunker | - | - | - | [31] | |
| E | 1959 | Anonymous | 14 | 1 (200–800) | g | sugar beet | 60 | - | - | bin | - | - | - | [20] | |
| F | 1991 | Blackshaw and Rode | 12 | 2 (150) | g + v | barley | 56 | - | - | bunker | bovine rumen | - | - | [7] | |
| G | 2000 | Mayer et al. | 6 | 1–10 (50) | g | grass | 90, 120, 270, 360 | - | - | bale | bovine rumen | - | manure, slurry | [21] | |
| H | 2002 | Overud | 1 | 4 (15–18) | g + v | clover + grass | 14, 28, 42, 56, 61, 70, 100, 141 | DM | glass jar | bale | - | - | - | [28] | |
| I | 2006 | van Eekeren et al. | 1 | 1 (n.r.) | v | grass | 14, 28, 42, 56 | DM | pot | - | - | - | - | [29] | |
| J | 2011 | James et al. | 1 | 5 (200) | g | grass; maize | 90 | - | - | stack; bale | - | - | effluent pond | [32] | |
| K | 2012 | Lück | 7 | 3 (100) | g + v | maize; rye | 103, 155 | - | glass jar | - | - | batch reactor | - | [22] | |
| L | 2012 | Stanton et al. | 14 | 4 (50) | g + v | cereals | 90 | - | plastic bag | - | bovine rumen | - | - | [23] | |
| M | 2012 | Westerman, Hildebrandt et al. | 17 | 3–10 (50–200) | g + v | maize; rye | 135, 207 | - | glass jar | - | - | batch reactor | - | [24] | |
| N | 2014 | Aper et al. | 1 | 4 (120) | g + v | maize | 28, 49, 112 | - | - | stack | bovine rumen | - | manure | [13] | |
| O | 2015 | Koarai et al. | 7 | n.a. | v | rice | 90, 180 | - | n.a. | n.a. | - | - | - | [25] | |
| P | 2015 | Trolove and Dowsett | 1 | n.a. (50) | g + v | n.a. | 1, 2, 3, 4, 5, 6, 7 | - | - | stack | - | - | - | [33] | |
| Q | 2016 | Simard and Lambert- Beaudet | 7 | 5 (100) | g + v | alfalfa; maize | 30, 90, 180 | - | lab silo | - | - | - | - | [11] | |
| R | 2016 | Weller et al. | 1 | 8 (25) | g + v | grass | 7, 14, 35, 42 | lactic acid bacteria | vacuum bag | - | - | - | - | [34] | |
| S | 2017 | Piltz et al. | 11 | 7 (50) | g + v | barley | 90 | - | plastic bag | - | bovine rumen | - | - | [10] | |
| T | 2021 | Hahn et al. | 10 | 2–9 (100–300) | g + v | maize; maize + wildflowers | 237 or 281 | soil suspension | glass jar | - | - | - | - | [12] | |
| U | 2021 | Piltz et al. | 16 | 3 (50) | g + v | alfalfa; clover + grass; sorghum; alfalfa chaff; cotton wool | 14–147 | organic acids; DM | plastic bag | - | bovine rumen | - | - | [26] | |
| V | 2022 | Asaduzzaman et al. | 2 | 3 (50) | g + v | alfalfa | 90 | - | plastic bag | - | bovine rumen | - | - | [27] | |
| W | 2023 | Asaduzzaman et al. | 1 | 3 (30) | g+ 1 | alfalfa | 90 | - | plastic bag | - | bovine rumen | - | - | [35] | |
2.2.2. Data on Ensiling Treatments
2.2.3. Data Analysis and Visualization
3. Key Findings on Seed Survival in Silages
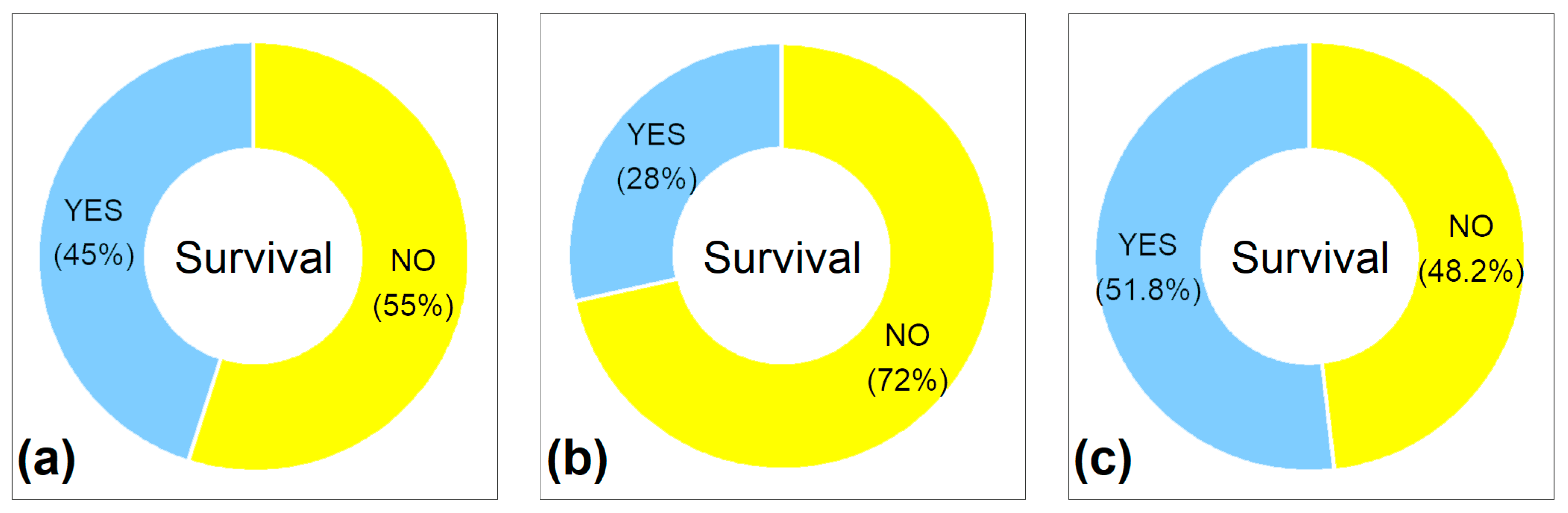
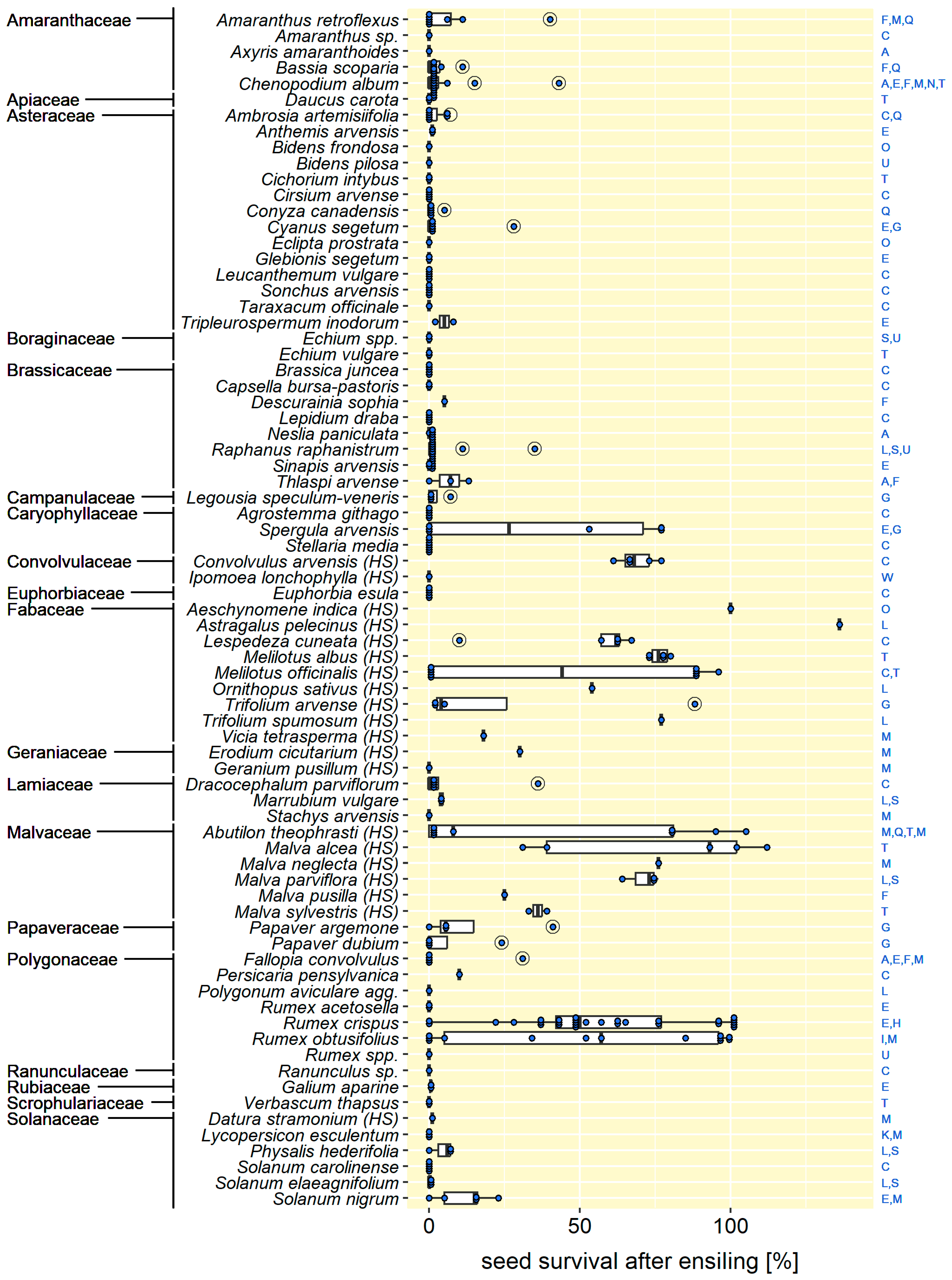
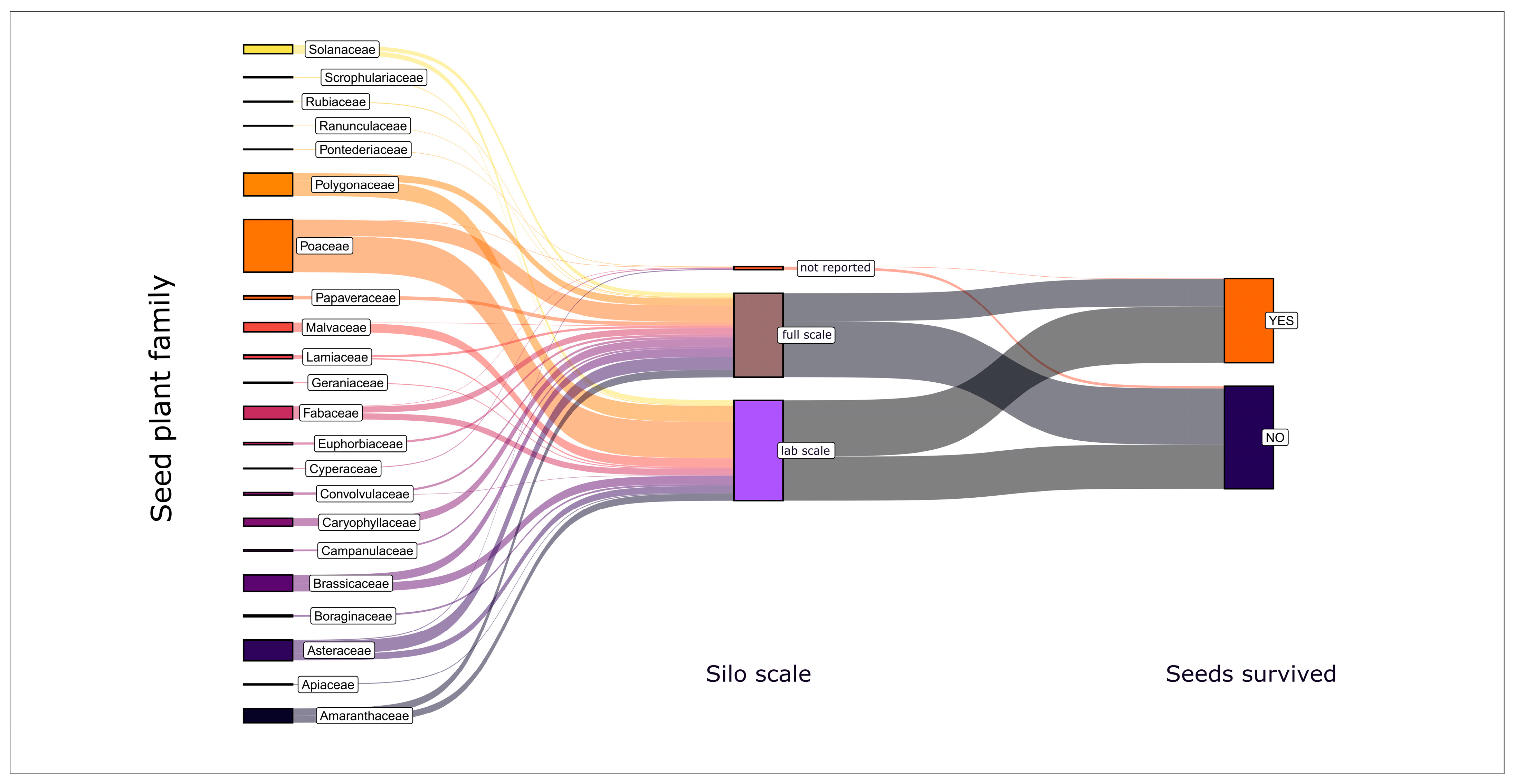
3.1. Seeds Can Survive Ensiling
3.2. Ensiling Reduced Seed Viability—In Most Cases
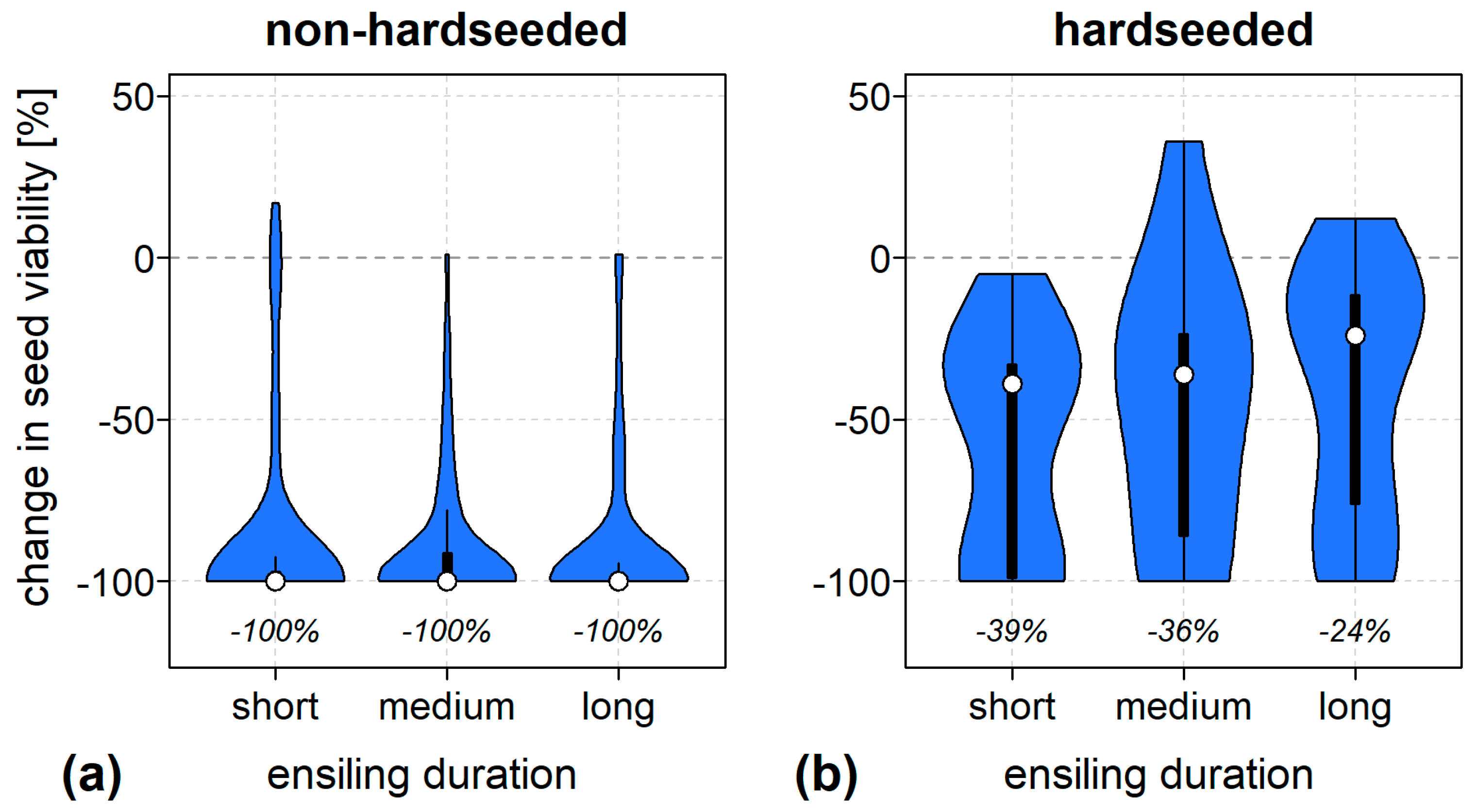
3.3. Some Seed Traits Promote Survival
3.4. The Vague Role of Ensiling Conditions
4. Implications for Sustainable Silage Production
5. Emerging Fields of Research
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Muck, R.E.; Shinners, K.J. Conserved forage (silage and hay): Progress and priorities. In Proceedings of the XIX International Grassland Congress, Sao Paulo, Brazil, 11–21 February 2001; pp. 1993–2023. [Google Scholar]
- Katoch, R. Conservation and Processing of Forages. In Techniques in Forage Quality Analysis, 1st ed.; Katoch, R., Ed.; Springer: Singapore, 2023; pp. 187–197. ISBN 978-981-19-6019-2. [Google Scholar]
- Pahlow, G. Basics and principles of ensiling. Ubers. Zur Tierernährung 2007, 35, 1–11. [Google Scholar]
- Wilkinson, J.M.; Rinne, M. Highlights of progress in silage conservation and future perspectives. Grass Forage Sci. 2018, 73, 40–52. [Google Scholar] [CrossRef]
- Borreani, G.; Tabacco, E.; Schmidt, R.J.; Holmes, B.J.; Muck, R.E. Silage review: Factors affecting dry matter and quality losses in silages. J. Dairy Sci. 2018, 101, 3952–3979. [Google Scholar] [CrossRef] [PubMed]
- de Meester, S.; Demeyer, J.; Velghe, F.; Peene, A.; van Langenhove, H.; Dewulf, J. The environmental sustainability of anaerobic digestion as a biomass valorization technology. Bioresour. Technol. 2012, 121, 396–403. [Google Scholar] [CrossRef]
- Blackshaw, R.E.; Rode, L.M. Effect of Ensiling and Rumen Digestion by Cattle on Weed Seed Viability. Weed Sci. 1991, 39, 104–108. [Google Scholar] [CrossRef]
- Shevkenek, W. Viability of Weed Seeds in Manure and Silage. Master Thesis, University of Saskatchewan, Saskatoon SK, Canada, 1934. [Google Scholar]
- Tildesley, W.T. A Study of Some Ingredients Found in Ensilage Juice and its Effect on the Vitality of Certain Weed Seeds. Sci. Agric. 1937, 17, 492–501. [Google Scholar]
- Piltz, J.W.; Stanton, R.A.; Wu, H. Effect of ensiling and in sacco digestion on the viability of seeds of selected weed species. Weed Res. 2017, 57, 382–389. [Google Scholar] [CrossRef]
- Simard, M.-J.; Lambert-Beaudet, C. Weed seed survival in experimental mini-silos of corn and alfalfa. Can. J. Plant Sci. 2016, 96, 448–454. [Google Scholar] [CrossRef]
- Hahn, J.; de Mol, F.; Müller, J. Ensiling Reduces Seed Viability: Implications for Weed Management. Front. Agron. 2021, 3, 708851. [Google Scholar] [CrossRef]
- Aper, J.; de Cauwer, B.; de Roo, S.; Lourenço, M.; Fievez, V.; Bulcke, R.; Reheul, D. Seed germination and viability of herbicide resistant and susceptible Chenopodium album populations after ensiling, digestion by cattle and manure storage. Weed Res. 2014, 54, 169–177. [Google Scholar] [CrossRef]
- Leistra, M.; Boesten, J. Pesticide contamination of groundwater in western Europe. Agric. Ecosyst. Environ. 1989, 26, 369–389. [Google Scholar] [CrossRef]
- Kudsk, P. Advances in Integrated Weed Management; Burleigh Dodds Science Publishing: Cambridge, UK, 2022; ISBN 9781786767479. [Google Scholar]
- Clements, D.R.; Jones, V.L. Rapid Evolution of Invasive Weeds Under Climate Change: Present Evidence and Future Research Needs. Front. Agron. 2021, 3, 664034. [Google Scholar] [CrossRef]
- Westerman, P.R.; Heiermann, M.; Pottberg, U.; Rodemann, B.; Gerowitt, B. Weed seed survival during mesophilic anaerobic digestion in biogas plants. Weed Res. 2012, 52, 307–316. [Google Scholar] [CrossRef]
- Schulte, L.A.; Dale, B.E.; Bozzetto, S.; Liebman, M.; Souza, G.M.; Haddad, N.; Richard, T.L.; Basso, B.; Brown, R.C.; Hilbert, J.A.; et al. Meeting global challenges with regenerative agriculture producing food and energy. Nat. Sustain. 2022, 5, 384–388. [Google Scholar] [CrossRef]
- Woodward, T.E. The Viability of Seeds as Affected by the Siloing Process. J. Dairy Sci. 1940, 23, 267–271. [Google Scholar] [CrossRef]
- Anonymous. Weed-free germination after storage in silage (Ukrudtsfrøs spireevne efter opbevaring i ensilage). Forsøgsresultater. In Meddelelse 628–639 fra Statens Forsøgsvirksomhed i Plantekultur; Statens Forsøgsvirksomhed i Plantekultur: København, Denmark, 1959–1960; pp. 715–716. [Google Scholar]
- Mayer, F.; Albrecht, H.; Pfadenhauer, J. The influence of digestion and storage in silage and organic manure on the germinability of six weed species (Papaver argemone, P. dubium, Legousia speculum-veneris, Centaurea cyanus, Spergula arvensis, Trifolium arvense). In Ergebnisse der 20. Deutschen Arbeitsbesprechung über Fragen der Unkrautbiologie und -Bekämpfung vom 14. bis 16. März 2000 in Stuttgart-Hohenheim; Haas, H.U., Hurle, K., Eds.; JKI: Braunschweig, Germany, 2000; pp. 47–54. [Google Scholar]
- Lück, C. Überlebensfähigkeit von Gräsersamen in der Biogasprozesskette. Master Thesis, Universität Rostock, Rostock, Germany, 2012. [Google Scholar]
- Stanton, R.A.; Piltz, J.W.; Rodham, C.; Wu, H. Silage for managing weed seeds. In Proceedings of the 18th Australasian Weeds Conference 2012, Developing Solutions to Evolving Weed Problems, Melbourne, Victoria, Australia, 8–11 October 2012; Eldershaw, V., Ed.; Australasian Weeds Conference. CAWS: East Melbourne, Australia, 2012; pp. 219–221, ISBN 9781629932880. [Google Scholar]
- Westerman, P.R.; Hildebrandt, F.; Gerowitt, B. Weed seed survival following ensiling and mesophilic anaerobic digestion in batch reactors. Weed Res. 2012, 52, 286–295. [Google Scholar] [CrossRef]
- Koarai, A.; Hattori, I.; Suzuki, T.; Sumiyoshi, T.; Ohdan, H.; Sato, K.; Kato, N.; Yasuda, K. Seed viability of paddy weeds ensiled by forage rice. J. Weed Sci. Technol. 2015, 60, 93–100. [Google Scholar] [CrossRef][Green Version]
- Piltz, J.W.; Bailes, K.L.; Boschma, S.P.; Weston, L.A. The Impact of Ensiling at Different Moisture Contents on Germinability and Viability of Selected Weed Species’ Seeds. Agronomy 2021, 11, 1639. [Google Scholar] [CrossRef]
- Asaduzzaman, M.; Piltz, J.W.; Koetz, E.; Hopwood, M.; Shephard, A.; Wu, H. Seed viability of feathertop Rhodes grass (Chloris virgata Sw.) reduced by silage, digestion, and sheep rumen digestion. Front. Agron. 2022, 4, 954153. [Google Scholar] [CrossRef]
- Overud, S. Effects of ensiling on seed germinability and viability in Rumex crispus L. Master Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2002. [Google Scholar]
- van Eekeren, N.; Fehér, L.; Smeding, F.; Prins, U.; Jansonius, P.J. Controlling broad-leaved dock (Rumex obtusifolius) in grass clover mixtures. In Sustainable Grassland Productivity, Proceedings of 21st General Meeting of the European Grassland Federation, Badajoz, Spain, 3–6 April 2006; Lloveras, J., Gonzáles-Rodríguez, A., Vázquez-Yánez, O., Pineiro, J., Santamaría, O., Olea, L., Poblaciones, M.J., Eds.; EGF: Madrid, Spain, 2006; pp. 396–398. ISBN 84 689 6711 4. [Google Scholar]
- GBIF: The Global Biodiversity Information Facility: What is GBIF? Available online: https://www.gbif.org/ (accessed on 10 January 2025).
- Zahnley, J.W.; Fitch, J.B. Effect of Ensiling on the Viability of Weed Seeds. Agron. J. 1941, 33, 816–822. [Google Scholar] [CrossRef]
- James, T.K.; Rahman, A.; McGill, C.R.; Trivedi, P.D. Biology and survival of broom corn millet (Panicum miliaceum) seed. New Zealand Plant Prot. 2011, 64, 142–148. [Google Scholar] [CrossRef]
- Trolove, M.R.; Dowsett, C.A. Yellow bristle grass seed killed in maize silage. N. Z. Plant Prot. 2015, 68, 442. [Google Scholar] [CrossRef]
- Weller, S.L.; Florentine, S.K.; Sillitoe, J.F.; Grech, C.J.; McLaren, D.A. An investigation of the effects of stage of ensilage on Nassella neesiana seeds, for reducing seed viability and injury to livestock. Sci. Rep. 2016, 6, 22345. [Google Scholar] [CrossRef] [PubMed]
- Asaduzzaman, M.; Koetz, E.; Wu, H.; Piltz, J.W.; Charles, G. Germination ecology and growth phenology of cowvine (Ipomoea lonchophylla) as influenced by environmental parameters. Weed Sci. 2023, 71, 378–386. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2022. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis, 2nd ed.; Springer: Cham, Switzerland, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Wickham, H.; François, R.; Henry, L.; Müller, K.; Vaughan, D. dplyr: A Grammar of Data Manipulation: R package. Available online: https://CRAN.R-project.org/package=dplyr (accessed on 11 January 2025).
- Wickham, H. forcats: Tools for Working with Categorical Variables (Factors): R-package. Available online: https://CRAN.R-project.org/package=forcats (accessed on 11 January 2025).
- Moon, K.-W. webr: Data and Functions for Web-Based Analysis: R package. Available online: https://CRAN.R-project.org/package=webr (accessed on 11 January 2025).
- Adler, D.; Kelly, S.T.; Elliott, T.; Adamson; Jordan. vioplot: violin plot: R package. Available online: https://github.com/TomKellyGenetics/vioplot (accessed on 11 January 2025).
- Sjoberg, D. ggsankey: Sankey, Alluvial and Sankey Bump Plots: R package. Available online: https://github.com/davidsjoberg/ggsankey (accessed on 11 January 2025).
- Traveset, A. Effect of seed passage through vertebrate frugivores’ guts on germination: A review. Perspect. Plant Ecol. Evol. System. 1998, 1, 151–190. [Google Scholar] [CrossRef]
- Zhou, L.; Hülsemann, B.; Merkle, W.; Guo, J.; Dong, R.; Piepho, H.-P.; Gerhards, R.; Müller, J.; Oechsner, H. Influence of Anaerobic Digestion Processes on the Germination of Weed Seeds. Gesunde Pflanzen. 2020, 72, 181–194. [Google Scholar] [CrossRef]
- Hahn, J.; Westerman, P.R.; de Mol, F.; Heiermann, M.; Gerowitt, B. Viability of Wildflower Seeds After Mesophilic Anaerobic Digestion in Lab-Scale Biogas Reactors. Front. Plant Sci. 2022, 13, 942346. [Google Scholar] [CrossRef] [PubMed]
- Baskin, J.M.; Baskin, C.C.; Li, X. Taxonomy, anatomy and evolution of physical dormancy in seeds. Plant Spec. Biol. 2000, 15, 139–152. [Google Scholar] [CrossRef]
- Westerman, P.R.; Gerowitt, B. Weed Seed Survival during Anaerobic Digestion in Biogas Plants. Bot. Rev. 2013, 79, 281–316. [Google Scholar] [CrossRef]
- Starfinger, U.; Sölter, U. Recommendations on safety of composting or use as biogas fuel of common ragweed seed contaminated material. In HALT Ambrosia—Final Project Report and General Publication of Project Findings; Sölter, U., Starfinger, U., Verschwele, A., Eds.; Julius-Kühn-Archiv: Quedlinburg, Germany, 2016; pp. 50–57. [Google Scholar]
- Tanke, A.; Müller, J.; de Mol, F. Seed Viability of Heracleum mantegazzianum (Apiaceae) Is Quickly Reduced at Temperatures Prevailing in Biogas Plants. Agronomy 2019, 9, 332. [Google Scholar] [CrossRef]
- Rolston, M.P. Water impermeable seed dormancy. Bot. Rev. 1978, 44, 365–396. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination; Academic Press: San Diego, CA, USA, 1998; ISBN 0-12-080260-0. [Google Scholar]
- Hilhorst, H.W.M. The regulation of secondary dormancy. The membrane hypothesis revisited. Seed Sci. Res. 1998, 8, 77–90. [Google Scholar] [CrossRef]
- Jaganathan, G.K.; Yule, K.; Liu, B. On the evolutionary and ecological value of breaking physical dormancy by endozoochory. Perspect. Plant Ecol. Evol. Syst. 2016, 22, 11–22. [Google Scholar] [CrossRef]
- Bomgardner, M.M. Chasing cheap feedstocks: Biobased chemical companies seek RAW MATERIALS that can beat corn and natural gas. Chem. Eng. News 2013, 91, 11–15. [Google Scholar] [CrossRef]
- Sharma, V.; Pant, S. Weed as Underutilized Bio-resource and Management Tool: A Comprehensive Review. Waste Biomass Valor. 2019, 10, 1795–1810. [Google Scholar] [CrossRef]
- Papamatthaiakis, N.; Laine, A.; Haapala, A.; Ikonen, R.; Kuittinen, S.; Pappinen, A.; Kolström, M.; Mola-Yudego, B. New energy crop alternatives for Northern Europe: Yield, chemical and physical properties of Giant knotweed (Fallopia sachalinensis var. ‘Igniscum’) and Virginia mallow (Sida hermaphrodita). Fuel 2021, 304, 121349. [Google Scholar] [CrossRef]
- von Cossel, M. Renewable Energy from Wildflowers—Perennial Wild Plant Mixtures as a Social-Ecologically Sustainable Biomass Supply System. Adv. Sustain. Syst. 2020, 4, 2000037. [Google Scholar] [CrossRef]
- Råberg, T.; Carlsson, G.; Jensen, E.S. Nitrogen balance in a stockless organic cropping system with different strategies for internal N cycling via residual biomass. Nutr. Cycl. Agroecosystems 2018, 112, 165–178. [Google Scholar] [CrossRef]
- Wilkinson, J.M.; Muck, R.E. Ensiling in 2050: Some challenges and opportunities. Grass Forage Sci. 2019, 74, 178–187. [Google Scholar] [CrossRef]
- Baute, K.A.; Robinson, D.E.; van Eerd, L.L.; Edson, M.; Sikkema, P.H.; Gilroyed, B.H. Survival of seeds from perennial biomass species during commercial-scale anaerobic digestion. Weed Res. 2016, 56, 258–266. [Google Scholar] [CrossRef]
- Walck, J.L.; Hidayati, S.N.; Dixon, K.W.; Thompson, K.; Poschlod, P. Climate change and plant regeneration from seed. Glob. Chang. Biol. 2011, 17, 2145–2161. [Google Scholar] [CrossRef]
- Varanasi, A.; Prasad, P.V.; Jugulam, M. Impact of Climate Change Factors on Weeds and Herbicide Efficacy. In Advances in Agronomy, 1st ed.; Sparks, D.L., Ed.; Academic Press: London, UK, 2016; pp. 107–146. ISBN 978-0-12-804693-7. [Google Scholar]
- Ramesh, K.; Matloob, A.; Aslam, F.; Florentine, S.K.; Chauhan, B.S. Weeds in a Changing Climate: Vulnerabilities, Consequences, and Implications for Future Weed Management. Front. Plant Sci. 2017, 8, 95. [Google Scholar] [CrossRef] [PubMed]
- Cosyns, E.; Delporte, A.; Lens, L.; Hoffmann, M. Germination success of temperate grassland species after passage through ungulate and rabbit guts. J. Ecol. 2005, 93, 353–361. [Google Scholar] [CrossRef]
- Milotić, T.; Hoffmann, M. How does gut passage impact endozoochorous seed dispersal success? Evidence from a gut environment simulation experiment. Basic Appl. Ecol. 2016, 17, 165–176. [Google Scholar] [CrossRef]
- Hahn, J.; Plogsties, V.; Gerowitt, B.; Heiermann, M. Survival of plant seeds in digestate storage—With and without prior anaerobic digestion. Front. Energy Res. 2024, 12. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hahn, J.; Müller, J.; Heiermann, M. Seed Survival in Silage: Reviewing 90 Years of Research. Plants 2025, 14, 351. https://doi.org/10.3390/plants14030351
Hahn J, Müller J, Heiermann M. Seed Survival in Silage: Reviewing 90 Years of Research. Plants. 2025; 14(3):351. https://doi.org/10.3390/plants14030351
Chicago/Turabian StyleHahn, Juliane, Jürgen Müller, and Monika Heiermann. 2025. "Seed Survival in Silage: Reviewing 90 Years of Research" Plants 14, no. 3: 351. https://doi.org/10.3390/plants14030351
APA StyleHahn, J., Müller, J., & Heiermann, M. (2025). Seed Survival in Silage: Reviewing 90 Years of Research. Plants, 14(3), 351. https://doi.org/10.3390/plants14030351







