Recent Advances on the Individual Roles and Emerging Synergistic Effects of Plant Growth-Promoting Rhizobacteria and Silicon Nanoparticles in Mitigating Salinity Stress
Abstract
1. Introduction
2. Role of Si in Salinity Stress Tolerance
2.1. Si-Mediated Physiological and Biochemical Mechanisms Under Salinity Stress
2.2. Effects of SiNPs on Crops Under Salinity Stress
2.3. Silicon—Mediated Gene Expression
3. Role of PGPR in Salinity Stress Mitigation
3.1. General Mechanisms of PGPR
3.2. Physiological Mechanisms of PGPR
3.3. Mechanisms of Salt-Tolerant PGPR (ST-PGPR) in Enhancing Plant Salt Tolerance
3.4. PGPR- Mediated Gene Expression
4. Synergistic Effects of PGPR and SiNPs on Salinity Tolerance
5. Future Perspectives
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Roy, T.K.; Islam, M.S.; Mahiddin, N.A.; Hossain, S.A.; Biswas, T.; Antu, U.B.; Serity, S.A.; Miti, J.F.; Akter, S.; Roy, S. Application of Nanoparticles (NPs) to Ameliorate Abiotic Stress in Economically Important Crop Species: A Potential Review. J. Crop Health 2025, 77, 19. [Google Scholar] [CrossRef]
- Husen, A. Morpho-Anatomical, Physiological, Biochemical and molecular Responses of Plants to Air Pollution. In Harsh Environment and Plant Resilience: Molecular and Functional Aspects; Springer Nature: Berlin/Heidelberg, Germany, 2021; pp. 203–234. [Google Scholar]
- Husen, A. Plants and Their Interaction to Environmental Pollution: Damage Detection, Adaptation, Tolerance, Physiological and Molecular Responses; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Atta, K.; Mondal, S.; Gorai, S.; Singh, A.P.; Kumari, A.; Ghosh, T.; Roy, A.; Hembram, S.; Gaikwad, D.J.; Mondal, S. Impacts of salinity stress on crop plants: Improving salt tolerance through genetic and molecular dissection. Front. Plant Sci. 2023, 14, 1241736. [Google Scholar] [CrossRef]
- Gupta, B.; Huang, B. Mechanism of salinity tolerance in plants: Physiological, biochemical, and molecular characterization. Int. J. Genom. 2014, 2014, 701596. [Google Scholar] [CrossRef]
- Sarkar, B.; Bandyopadhyay, P.; Das, A.; Pal, S.; Hasanuzzaman, M.; Adak, M.K. Abscisic acid priming confers salt tolerance in maize seedlings by modulating osmotic adjustment, bond energies, ROS homeostasis, and organic acid metabolism. Plant Physiol. Biochem. 2023, 202, 107980. [Google Scholar] [CrossRef] [PubMed]
- Haghaninia, M.; Mashhouri, S.M.; Najafifar, A.; Soleimani, F.; Mirzaei, A. Impact of silicon nanoparticle priming on metabolic responses and seed quality of chia (Salvia hispanica L.) under salt stress. Food Biosci. 2025, 65, 106119. [Google Scholar] [CrossRef]
- Singh, A. Soil salinity: A global threat to sustainable development. Soil Use Manag. 2022, 38, 39–67. [Google Scholar] [CrossRef]
- Patel, P.; Sayyed, R.; Patel, H. PGPR: A sustainable Agricultural Mitigator for Stressed Agro-Environments. In Plant Growth Promoting Microorganisms of Arid Region; Springer: Berlin/Heidelberg, Germany, 2023; pp. 303–318. [Google Scholar]
- da Cunha Dias, T.A.; Lora, E.E.S.; Maya, D.M.Y.; del Olmo, O.A. Global potential assessment of available land for bioenergy projects in 2050 within food security limits. Land Use Policy 2021, 105, 105346. [Google Scholar] [CrossRef]
- Sagar, A.; Rai, S.; Ilyas, N.; Sayyed, R.; Al-Turki, A.I.; El Enshasy, H.A.; Simarmata, T. Halotolerant rhizobacteria for salinity-stress mitigation: Diversity, mechanisms and molecular approaches. Sustainability 2022, 14, 490. [Google Scholar] [CrossRef]
- Awaad, H.A. Salinity Resilience and Sustainable Crop Production Under Climate Change; Springer: Berlin/Heidelberg, Germany, 2023. [Google Scholar]
- Salim, N.; Raza, A. Nutrient use efficiency (NUE) for sustainable wheat production: A review. J. Plant Nutr. 2020, 43, 297–315. [Google Scholar] [CrossRef]
- Zahra, N.; Mahmood, S.; Raza, Z.A. Salinity stress on various physiological and biochemical attributes of two distinct maize (Zea mays L.) genotypes. J. Plant Nutr. 2018, 41, 1368–1380. [Google Scholar] [CrossRef]
- Javaid, T.; Farooq, M.A.; Akhtar, J.; Saqib, Z.A.; Anwar-ul-Haq, M. Silicon nutrition improves growth of salt-stressed wheat by modulating flows and partitioning of Na+, Cl− and mineral ions. Plant Physiol. Biochem. 2019, 141, 291–299. [Google Scholar] [CrossRef]
- El-Flaah, R.F.; El-Said, R.A.; Nassar, M.A.; Hassan, M.; Abdelaal, K. Effect of rhizobium, nano silica and ascorbic acid on morpho-physiological characters and gene expression of POX and PPO in faba bean (Vicia faba L.) under salinity stress conditions. Fresenius Environ. Bull 2021, 30, 5751–5764. [Google Scholar]
- Wang, S.; Shen, X.; Guan, X.; Sun, L.; Yang, Z.; Wang, D.; Chen, Y.; Li, P.; Xie, Z. Nano-silicon enhances tomato growth and antioxidant defense under salt stress. Environ. Sci. Nano 2025, 12, 315–324. [Google Scholar] [CrossRef]
- Jain, R. Role of Silicon in Sugarcane: A Review. Agric. Rev. 2025, 46, 288. [Google Scholar] [CrossRef]
- Gao, X.; Zou, C.; Wang, L.; Zhang, F. Silicon improves water use efficiency in maize plants. J. Plant Nutr. 2005, 27, 1457–1470. [Google Scholar] [CrossRef]
- Gao, X.; Zou, C.; Wang, L.; Zhang, F. Silicon decreases transpiration rate and conductance from stomata of maize plants. J. Plant Nutr. 2006, 29, 1637–1647. [Google Scholar] [CrossRef]
- Rai, M.; Ingle, A. Role of nanotechnology in agriculture with special reference to management of insect pests. Appl. Microbiol. Biotechnol. 2012, 94, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Zargar, S.M.; Mahajan, R.; Bhat, J.A.; Nazir, M.; Deshmukh, R. Role of silicon in plant stress tolerance: Opportunities to achieve a sustainable cropping system. 3 Biotech 2019, 9, 73. [Google Scholar] [CrossRef]
- Ali, R.; Bashir, Z.; Fayaz, U.; Nisa, M. Silicate Solubilizers: Role of Silicate Solubilizing Microorganisms in Organic Agriculture. In Organic Farming; CRC Press: Boca Raton, FL, USA, 2025; pp. 141–168. [Google Scholar]
- Liang, Y.; Sun, W.; Zhu, Y.-G.; Christie, P. Mechanisms of silicon-mediated alleviation of abiotic stresses in higher plants: A review. Environ. Pollut. 2007, 147, 422–428. [Google Scholar] [CrossRef]
- Debona, D.; Rodrigues, F.A.; Datnoff, L.E. Silicon’s role in abiotic and biotic plant stresses. Annu. Rev. Phytopathol. 2017, 55, 85–107. [Google Scholar] [CrossRef] [PubMed]
- Jeelani, P.G.; Mulay, P.; Venkat, R.; Ramalingam, C. Multifaceted application of silica nanoparticles. A review. Silicon 2020, 12, 1337–1354. [Google Scholar] [CrossRef]
- Schaller, J.; Brackhage, C.; Paasch, S.; Brunner, E.; Bäucker, E.; Dudel, E.G. Silica uptake from nanoparticles and silica condensation state in different tissues of Phragmites australis. Sci. Total Environ. 2013, 442, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, K.S.; Husen, A.; Zahra, N.; Moheman, A. Harnessing silicon nanoparticles and various forms of silicon for enhanced plant growth performance under salinity stress: Application and mechanism. Discov. Nano 2025, 20, 89. [Google Scholar] [CrossRef]
- Gómez-Godínez, L.J.; Aguirre-Noyola, J.L.; Martínez-Romero, E.; Arteaga-Garibay, R.I.; Ireta-Moreno, J.; Ruvalcaba-Gómez, J.M. A look at plant-growth-promoting bacteria. Plants 2023, 12, 1668. [Google Scholar] [CrossRef]
- Vishwakarma, K.; Singh, V.P.; Prasad, S.M.; Chauhan, D.K.; Tripathi, D.K.; Sharma, S. Silicon and plant growth promoting rhizobacteria differentially regulate AgNP-induced toxicity in Brassica juncea: Implication of nitric oxide. J. Hazard. Mater. 2020, 390, 121806. [Google Scholar] [CrossRef]
- Hasan, A.; Tabassum, B.; Hashim, M.; Khan, N. Role of plant growth promoting rhizobacteria (PGPR) as a plant growth enhancer for sustainable agriculture: A review. Bacteria 2024, 3, 59–75. [Google Scholar] [CrossRef]
- Aloo, B.; Dessureault-Rompré, J.; Tripathi, V.; Nyongesa, B.; Were, B. Signaling and crosstalk of rhizobacterial and plant hormones that mediate abiotic stress tolerance in plants. Front. Microbiol. 2023, 14, 1171104. [Google Scholar] [CrossRef] [PubMed]
- Joshi, H.; Bisht, N.; Mishra, S.K.; Prasad, V.; Chauhan, P.S. Bacillus amyloliquefaciens modulate carbohydrate metabolism in rice-PGPR cross-talk under abiotic stress and phytohormone treatments. J. Plant Growth Regul. 2023, 42, 4466–4483. [Google Scholar] [CrossRef]
- Ha-Tran, D.M.; Nguyen, T.T.M.; Hung, S.-H.; Huang, E.; Huang, C.-C. Roles of plant growth-promoting rhizobacteria (PGPR) in stimulating salinity stress defense in plants: A review. Int. J. Mol. Sci. 2021, 22, 3154. [Google Scholar] [CrossRef]
- Nguyen, T.T.M.; Ha-Tran, D.M.; Huang, C.-C. Plant-growth-promoting rhizobacteria for mitigating salinity stress in rice farming: A review of the Vietnamese Mekong Delta. Front. Plant Sci. 2025, 16, 1635193. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, P.; Wang, B.; Li, H.; Li, S.; Zhang, H.; Haider, F.U.; Li, X. Harnessing the role of rhizo-bacteria to mitigate salinity stress in rice (Orzya sativa); focus on antioxidant defense system, photosynthesis response, and rhizosphere microbial diversity. Rhizosphere 2025, 33, 101043. [Google Scholar] [CrossRef]
- Upadhyay, S.K.; Hidangmayum, A.; Jain, D.; Dwivedi, P. Mechanisms and applicability of nanotechnology-mediated beneficial microbes in mitigation of salinity stress in plants. Plant Physiol. Biochem. 2025, 228, 110306. [Google Scholar] [CrossRef]
- Bhattacharyya, P.N.; Jha, D.K. Plant growth-promoting rhizobacteria (PGPR): Emergence in agriculture. World J. Microbiol. Biotechnol. 2012, 28, 1327–1350. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, S.; Daur, I.; Yasir, M.; Waqas, M.; Hirt, H. Synergistic practicing of rhizobacteria and silicon improve salt tolerance: Implications from boosted oxidative metabolism, nutrient uptake, growth and grain yield in mung bean. Plants 2022, 11, 1980. [Google Scholar] [CrossRef]
- Suhail, S.; Zafar, A.; Zainab, D.E.; Khosa, S. From Soil to Sustenance: Organic Practices for agricultural sustainability. Plant Environ. 2021, 2, 18–35. [Google Scholar]
- Xu, F.; Liang, Y.; Wang, X.; Guo, Y.; Tang, K.; Feng, F. Synergic mitigation of saline-alkaline stress in wheat plant by silicon and Enterobacter sp. FN0603. Front. Microbiol. 2023, 13, 1100232. [Google Scholar] [CrossRef]
- Al-Garni, S.M.; Khan, M.M.A.; Bahieldin, A. Plant growth-promoting bacteria and silicon fertilizer enhance plant growth and salinity tolerance in Coriandrum sativum. J. Plant Interact. 2019, 14, 386–396. [Google Scholar] [CrossRef]
- Alsaeedi, A.; El-Ramady, H.; Alshaal, T.; El-Garawany, M.; Elhawat, N.; Al-Otaibi, A. Silica nanoparticles boost growth and productivity of cucumber under water deficit and salinity stresses by balancing nutrients uptake. Plant Physiol. Biochem. 2019, 139, 1–10. [Google Scholar] [CrossRef]
- Anwar, T.; Qureshi, H.; Fatimah, H.; Siddiqi, E.H.; Anwaar, S.; Moussa, I.M.; Adil, M.F. Improvement of physio-biochemical attributes and mitigation of salinity stress by combined application of melatonin and silicon nanoparticles in Brassica oleracea var. botrytis. Sci. Hortic. 2023, 322, 112456. [Google Scholar] [CrossRef]
- Rakesh, B.; Chitdeshwari, T.; Mohanapriya, G. Fascinating role of nanosilica in mitigating drought and nutrient stress–A review. Plant Stress 2024, 14, 100672. [Google Scholar] [CrossRef]
- Thorne, S.J.; Hartley, S.E.; Maathuis, F.J. Is silicon a panacea for alleviating drought and salt stress in crops? Front. Plant Sci. 2020, 11, 1221. [Google Scholar] [CrossRef]
- Alam, P.; Faizan, M.; Sultan, H.; Al Balawi, T. Silicon oxide nanoparticles boost rice resilience to salinity by enhancing antioxidant defenses and stress regulation. Plant Sci. 2025, 359, 112588. [Google Scholar] [CrossRef]
- Zhu, Y.-X.; Gong, H.-J.; Yin, J.-L. Role of silicon in mediating salt tolerance in plants: A review. Plants 2019, 8, 147. [Google Scholar] [CrossRef]
- Jinger, D.; Dhar, S.; Dass, A.; Sharma, V.; Shukla, L.; Paramesh, V.; Parihar, M.; Joshi, N.; Joshi, E.; Gupta, G. Residual silicon and phosphorus improved the growth, yield, nutrient uptake and soil enzyme activities of wheat. Silicon 2022, 14, 8949–8964. [Google Scholar] [CrossRef]
- Manimaran, G.; Duraisamy, S.; Subramanium, T.; Rangasamy, A.; Alagarsamy, S.; James, P.; Selvamani, S.; Perumal, D.; Veerappan, M.; Arunan, Y.E. Silicon-driven approaches to salinity stress tolerance: Mechanisms, uptake dynamics, and microbial transformations. Plant Stress 2025, 16, 100825. [Google Scholar] [CrossRef]
- Kumar, A.; Choudhary, A.; Kaur, H.; Singh, K.; Guha, S.; Choudhary, D.; Sonkar, A.; Mehta, S.; Husen, A. Exploring the role of silicon in enhancing sustainable plant growth, defense system, environmental stress mitigation and management. Discov. Appl. Sci. 2025, 7, 406. [Google Scholar] [CrossRef]
- Hurtado, A.C.; Chiconato, D.A.; de Mello Prado, R.; Junior, G.d.S.S.; Gratão, P.L.; Felisberto, G.; Viciedo, D.O.; Dos Santos, D.M.M. Different methods of silicon application attenuate salt stress in sorghum and sunflower by modifying the antioxidative defense mechanism. Ecotoxicol. Environ. Saf. 2020, 203, 110964. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, A.; Zia-ur-Rehman, M.; Rizwan, M.; Usman, M.; Anayatullah, S.; Alharby, H.F.; Bamagoos, A.A.; Alharbi, B.M.; Ali, S. Effects of silicon nanoparticles and conventional Si amendments on growth and nutrient accumulation by maize (Zea mays L.) grown in saline-sodic soil. Environ. Res. 2023, 227, 115740. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Afzal, S.; Parveen, A.; Kamran, M.; Javed, M.R.; Abbasi, G.H.; Malik, Z.; Riaz, M.; Ahmad, S.; Chattha, M.S. Silicon mediated improvement in the growth and ion homeostasis by decreasing Na+ uptake in maize (Zea mays L.) cultivars exposed to salinity stress. Plant Physiol. Biochem. 2021, 158, 208–218. [Google Scholar] [CrossRef]
- Al-Tabbal, J.; Al-Harahsheh, M.; Al-Zou’by, J.; Al-Zboon, K.; Al-Bakour Al-Rawashda, K. Silica nanoparticle: Eco-friendly waste having potential for seed germination of wheat (Triticum turgidum L. Var. Sham) under salt stress conditions. Waste Biomass Valorization 2024, 15, 2973–2987. [Google Scholar] [CrossRef]
- Alsamadany, H.; Alharby, H.F.; Ahmad, Z.; Al-Zahrani, H.S.; Alzahrani, Y.M.; Almaghamsi, A. Improving alkaline stress tolerance in maize through seed priming with silicon nanoparticles: A comprehensive investigation of growth, photosynthetic pigments, antioxidants, and ion balance. Silicon 2024, 16, 2233–2244. [Google Scholar] [CrossRef]
- Ullah, M.S.; Mahmood, A.; Ameen, M.; Nayab, A.; Ayub, A. Multidimensional role of silicon to mitigate biotic and abiotic stresses in plants: A comprehensive review. Silicon 2024, 16, 5471–5500. [Google Scholar] [CrossRef]
- Cooke, J.; Carey, J.C. Stress alters the role of silicon in controlling plant water movement. Funct. Ecol. 2023, 37, 2985–2999. [Google Scholar] [CrossRef]
- Ullah, M.S.; Mahmood, A.; Alawadi, H.F.N.; Seleiman, M.F.; Khan, B.A.; Javaid, M.M.; Wahid, A.; Abdullah, F.; Wasonga, D.O. Silicon-mediated modulation of maize growth, metabolic responses, and antioxidant mechanisms under saline conditions. BMC Plant Biol. 2025, 25, 3. [Google Scholar] [CrossRef]
- Dhiman, P.; Rajora, N.; Bhardwaj, S.; Sudhakaran, S.S.; Kumar, A.; Raturi, G.; Chakraborty, K.; Gupta, O.P.; Devanna, B.; Tripathi, D.K. Fascinating role of silicon to combat salinity stress in plants: An updated overview. Plant Physiol. Biochem. 2021, 162, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Souri, Z.; Khanna, K.; Karimi, N.; Ahmad, P. Silicon and plants: Current knowledge and future prospects. J. Plant Growth Regul. 2021, 40, 906–925. [Google Scholar] [CrossRef]
- Lai, M.; Ghouri, F.; Sarwar, S.; Alomrani, S.O.; Riaz, M.; Haider, F.U.; Liu, J.; Imran, M.; Ali, S.; Liu, X. Modulation of metal transporters, oxidative stress and cell abnormalities by synergistic application of silicon and titanium oxide nanoparticles: A strategy for cadmium tolerance in rice. Chemosphere 2023, 345, 140439. [Google Scholar] [CrossRef]
- Alam, P.; Arshad, M.; Al-Kheraif, A.A.; Azzam, M.A.; Al Balawi, T. Silicon nanoparticle-induced regulation of carbohydrate metabolism, photosynthesis, and ROS homeostasis in Solanum lycopersicum subjected to salinity stress. ACS Omega 2022, 7, 31834–31844. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Chen, Q.; Liu, Q.; Zhang, W.; Ding, R. Exogenous silicon (Si) increases antioxidant enzyme activity and reduces lipid peroxidation in roots of salt-stressed barley (Hordeum vulgare L.). J. Plant Physiol. 2003, 160, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Akbari, B.; Baghaei-Yazdi, N.; Bahmaie, M.; Mahdavi Abhari, F. The role of plant-derived natural antioxidants in reduction of oxidative stress. BioFactors 2022, 48, 611–633. [Google Scholar] [CrossRef]
- Laifa, I.; Ellouzi, H.; Idoudi, M.; Farhat, N.; Rabhi, M.; Mahmoudi, H.; Smaoui, A.; Debez, A.; Cabassa-Hourton, C.; Savouré, A. Silicon (Si) treatment has preferential beneficial effects on Photosystem I photochemistry in salt-treated Hordeum marinum (Huds.) plants. J. Soil Sci. Plant Nutr. 2023, 23, 3232–3248. [Google Scholar] [CrossRef]
- Chele, K.H.; Steenkamp, P.; Piater, L.A.; Dubery, I.A.; Huyser, J.; Tugizimana, F. A global metabolic map defines the effects of a Si-based biostimulant on tomato plants under normal and saline conditions. Metabolites 2021, 11, 820. [Google Scholar] [CrossRef]
- Manivannan, A.; Soundararajan, P.; Arum, L.S.; Ko, C.H.; Muneer, S.; Jeong, B.R. Silicon-mediated enhancement of physiological and biochemical characteristics of Zinnia elegans ‘Dreamland Yellow’grown under salinity stress. Hortic. Environ. Biotechnol. 2015, 56, 721–731. [Google Scholar] [CrossRef]
- Li, Y.; Hu, H.; Wang, R.; Yin, J.; Zhu, Y.; Chen, L. Silica nanoparticles promote the growth of pepper under salt stress by improving photosynthesis and modulating the water relationship. Sci. Hortic. 2025, 350, 114295. [Google Scholar] [CrossRef]
- Khan, I.; Awan, S.A.; Rizwan, M.; Huizhi, W.; Ulhassan, Z.; Xie, W. Silicon nanoparticles improved the osmolyte production, antioxidant defense system, and phytohormone regulation in Elymus sibiricus (L.) under drought and salt stress. Environ. Sci. Pollut. Res. 2024, 31, 8985–8999. [Google Scholar] [CrossRef] [PubMed]
- Almutairi, Z.M. Effect of nano-silicon application on the expression of salt tolerance genes in germinating tomato (‘Solanum lycopersicum’ L.) seedlings under salt stress. Plant Omics 2016, 9, 106–114. [Google Scholar]
- Kurt, Z.; Ateş, S. Application of Silicon Iron and Silver Nanoparticles Improve Vegetative Development and Physiological Characteristics of Boysenberry Plants Grown under Salinity Stress In Vitro Cultivation Conditions. Horticulturae 2024, 10, 1118. [Google Scholar] [CrossRef]
- Mahmoud, L.M.; Dutt, M.; Shalan, A.M.; El-Kady, M.E.; El-Boray, M.S.; Shabana, Y.M.; Grosser, J.W. Silicon nanoparticles mitigate oxidative stress of in vitro-derived banana (Musa acuminata ‘Grand Nain’) under simulated water deficit or salinity stress. S. Afr. J. Bot. 2020, 132, 155–163. [Google Scholar] [CrossRef]
- Badawy, S.A.; Zayed, B.A.; Bassiouni, S.M.; Mahdi, A.H.; Majrashi, A.; Ali, E.F.; Seleiman, M.F. Influence of nano silicon and nano selenium on root characters, growth, ion selectivity, yield, and yield components of rice (Oryza sativa L.) under salinity conditions. Plants 2021, 10, 1657. [Google Scholar] [CrossRef]
- Faizan, M.; Sultan, H.; Alam, P.; Karabulut, F.; Cheng, S.-H.; Rajput, V.D.; Minkina, T.; Hayat, S.; Khan, M.N.; Nie, L. Melatonin and its cross-talk with other signaling molecules under abiotic stress. Plant Stress 2024, 11, 100410. [Google Scholar] [CrossRef]
- Abd-El-Aty, M.S.; Kamara, M.M.; Elgamal, W.H.; Mesbah, M.I.; Abomarzoka, E.A.; Alwutayd, K.M.; Mansour, E.; Abdelmalek, I.B.; Behiry, S.I.; Almoshadak, A.S. Exogenous application of nano-silicon, potassium sulfate, or proline enhances physiological parameters, antioxidant enzyme activities, and agronomic traits of diverse rice genotypes under water deficit conditions. Heliyon 2024, 10, e26077. [Google Scholar] [CrossRef]
- Mahawar, L.; Ramasamy, K.P.; Suhel, M.; Prasad, S.M.; Živčák, M.; Brestic, M.; Rastogi, A.; Skalický, M. Silicon nanoparticles: Comprehensive review on biogenic synthesis and applications in agriculture. Environ. Res. 2023, 232, 116292. [Google Scholar] [CrossRef]
- Mukarram, M.; Petrik, P.; Mushtaq, Z.; Khan, M.M.A.; Gulfishan, M.; Lux, A. Silicon nanoparticles in higher plants: Uptake, action, stress tolerance, and crosstalk with phytohormones, antioxidants, and other signalling molecules. Environ. Pollut. 2022, 310, 119855. [Google Scholar] [CrossRef]
- Hameed, A.; Farooq, T.; Hameed, A.; Sheikh, M.A. Silicon-Mediated Priming Induces Acclimation to Mild Water-Deficit Stress by Altering Physio-Biochemical Attributes in Wheat Plants. Front. Plant Sci. 2021, 12, 625541. [Google Scholar] [CrossRef]
- Hasanaklou, N.T.; Mohagheghi, V.; Hasanaklou, H.T.; Ma’mani, L.; Malekmohammadi, M.; Moradi, F.; Dalvand, Y. Seed nano-priming using silica nanoparticles: Effects in seed germination and physiological properties of Stevia Rebaudiana Bertoni. Chem. Biol. Technol. Agric. 2023, 10, 96. [Google Scholar] [CrossRef]
- Dhingra, P.; Sharma, S.; Singh, K.H.; Kushwaha, H.S.; Barupal, J.K.; Haq, S.; Kothari, S.; Kachhwaha, S. Seed priming with carbon nanotubes and silicon dioxide nanoparticles influence agronomic traits of Indian mustard (Brassica juncea) in field experiments. J. King Saud Univ. Sci. 2022, 34, 102067. [Google Scholar] [CrossRef]
- Ahmadi-Nouraldinvand, F.; Sharifi, R.S.; Siadat, S.A.; Khalilzadeh, R. Reduction of salinity stress in wheat through seed bio-priming with mycorrhiza and growth-promoting bacteria and its effect on physiological traits and plant antioxidant activity with silicon nanoparticles application. Silicon 2023, 15, 6813–6824. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, P.; Zhao, S.; Sun, Y.; Liu, Y.; Chen, S.; Chen, W.; Zhao, G.; Wei, G.; Chen, C. Functionalized Silica Nanoparticles Mitigate Salt Stress in Soybean: Comprehensive Insights of Physiological, Metabolomic, and Microbiome Responses. J. Agric. Food Chem. 2025, 73, 10814–10825. [Google Scholar] [CrossRef]
- Alam, P.; Yalcin, M.; Faizan, M.; Albalawi, T. Response of tomato to silicon dioxide nanoparticles under salinity: Impact on photosynthesis, antioxidant enzymes activity, stress biomarkers and osmoregulatory substances. Plant Nano Biol. 2025, 13, 100171. [Google Scholar] [CrossRef]
- Al-Tabbal, J.; Al-Harahsheh, M.; Al-Zou’by, J.Y.; Alrawashdeh, K.A.B.; Al-Zboon, K.K. Converting waste into opportunity: Silica nanoparticles for mitigating salinity stress in wheat “Triticum turgidum”. Int. J. Phytoremediat. 2025, 27, 1741–1753. [Google Scholar] [CrossRef]
- Abd-Elzaher, M.A.; El-Desoky, M.A.; Khalil, F.A.; Eissa, M.A.; Amin, A.E.-E.A. Exogenously applied proline with silicon and zinc nanoparticles to mitigate salt stress in wheat plants grown on saline soil. J. Plant Nutr. 2025, 48, 1559–1576. [Google Scholar] [CrossRef]
- Sharma, S. Mechanisms of silicon for abiotic stress tolerance in higher plants: A review. Pharma Innov. J. 2022, 11, 1647–1657. [Google Scholar]
- Deshmukh, R.K.; Vivancos, J.; Guérin, V.; Sonah, H.; Labbé, C.; Belzile, F.; Bélanger, R.R. Identification and functional characterization of silicon transporters in soybean using comparative genomics of major intrinsic proteins in Arabidopsis and rice. Plant Mol. Biol. 2013, 83, 303–315. [Google Scholar] [CrossRef]
- Van Bockhaven, J.; De Vleesschauwer, D.; Höfte, M. Towards establishing broad-spectrum disease resistance in plants: Silicon leads the way. J. Exp. Bot. 2013, 64, 1281–1293. [Google Scholar] [CrossRef]
- Bosnic, P.; Bosnic, D.; Jasnic, J.; Nikolic, M. Silicon mediates sodium transport and partitioning in maize under moderate salt stress. Environ. Exp. Bot. 2018, 155, 681–687. [Google Scholar] [CrossRef]
- Arora, N.K.; Fatima, T.; Mishra, J.; Mishra, I.; Verma, S.; Verma, R.; Verma, M.; Bhattacharya, A.; Verma, P.; Mishra, P. Halo-tolerant plant growth promoting rhizobacteria for improving productivity and remediation of saline soils. J. Adv. Res. 2020, 26, 69–82. [Google Scholar] [CrossRef]
- Pacifico, D.; Squartini, A.; Crucitti, D.; Barizza, E.; Lo Schiavo, F.; Muresu, R.; Carimi, F.; Zottini, M. The role of the endophytic microbiome in the grapevine response to environmental triggers. Front. Plant Sci. 2019, 10, 1256. [Google Scholar] [CrossRef]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant growth-promoting rhizobacteria: Context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9, 1473. [Google Scholar] [CrossRef] [PubMed]
- Habib, S.H.; Kausar, H.; Saud, H.M. Plant growth-promoting rhizobacteria enhance salinity stress tolerance in okra through ROS-scavenging enzymes. BioMed Res. Int. 2016, 2016, 6284547. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, Y.; Wu, G.; Veronican Njeri, K.; Shen, Q.; Zhang, N.; Zhang, R. Induced maize salt tolerance by rhizosphere inoculation of Bacillus amyloliquefaciens SQR9. Physiol. Plant. 2016, 158, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Win, K.T.; Wasai-Hara, S.; Tanaka, F.; Oo, A.Z.; Minamisawa, K.; Shimoda, Y.; Imaizumi-Anraku, H. Synergistic N2-fixation and salt stress mitigation in soybean through dual inoculation of ACC deaminase-producing Pseudomonas and Bradyrhizobium. Sci. Rep. 2023, 13, 17050. [Google Scholar] [CrossRef]
- Nadeem, S.M.; Zahir, Z.A.; Naveed, M.; Nawaz, S. Mitigation of salinity-induced negative impact on the growth and yield of wheat by plant growth-promoting rhizobacteria in naturally saline conditions. Ann. Microbiol. 2013, 63, 225–232. [Google Scholar] [CrossRef]
- Vejan, P.; Abdullah, R.; Khadiran, T.; Ismail, S.; Nasrulhaq Boyce, A. Role of plant growth promoting rhizobacteria in agricultural sustainability—A review. Molecules 2016, 21, 573. [Google Scholar] [CrossRef]
- Zaheer, M.S.; Raza, M.A.S.; Saleem, M.F.; Khan, I.H.; Ahmad, S.; Iqbal, R.; Manevski, K. Investigating the effect of Azospirillum brasilense and Rhizobium pisi on agronomic traits of wheat (Triticum aestivum L.). Arch. Agron. Soil Sci. 2019, 65, 1154–1564. [Google Scholar] [CrossRef]
- Chen, D.; Saeed, M.; Ali, M.N.H.A.; Raheel, M.; Ashraf, W.; Hassan, Z.; Hassan, M.Z.; Farooq, U.; Hakim, M.F.; Rao, M.J. Plant growth promoting rhizobacteria (PGPR) and arbuscular mycorrhizal fungi combined application reveals enhanced soil fertility and rice production. Agronomy 2023, 13, 550. [Google Scholar] [CrossRef]
- Ahmad, M.; Zahir, Z.A.; Asghar, H.N.; Asghar, M. Inducing salt tolerance in mung bean through coinoculation with rhizobia and plant-growth-promoting rhizobacteria containing 1-aminocyclopropane-1-carboxylate deaminase. Can. J. Microbiol. 2011, 57, 578–589. [Google Scholar] [CrossRef]
- Grover, M.; Bodhankar, S.; Sharma, A.; Sharma, P.; Singh, J.; Nain, L. PGPR mediated alterations in root traits: Way toward sustainable crop production. Front. Sustain. Food Syst. 2021, 4, 618230. [Google Scholar] [CrossRef]
- Han, H.; Lee, K. Physiological responses of soybean-inoculation of Bradyrhizobium japonicum with PGPR in saline soil conditions. Res. J. Agric. Biol. Sci. 2005, 1, 216–221. [Google Scholar]
- Ahmad, H.T.; Hussain, A.; Aimen, A.; Jamshaid, M.U.; Ditta, A.; Asghar, H.N.; Zahir, Z.A. Improving Resilience Against Drought Stress Among Crop Plants Through Inoculation Of Plant Growth-Promoting Rhizobacteria. In Harsh Environment and Plant Resilience: Molecular and Functional Aspects; Springer: Cham, Switzerland, 2021; pp. 387–408. [Google Scholar]
- Azadikhah, M.; Jamali, F.; Nooryazdan, H.-R.; Bayat, F. Growth promotion and yield enhancement of barley cultivars using ACC deaminase producing Pseudomonas fluorescens strains under salt stress. Span. J. Agric. Res. 2019, 17, e0801. [Google Scholar] [CrossRef]
- Li, X.; Sun, P.; Zhang, Y.; Jin, C.; Guan, C. A novel PGPR strain Kocuria rhizophila Y1 enhances salt stress tolerance in maize by regulating phytohormone levels, nutrient acquisition, redox potential, ion homeostasis, photosynthetic capacity and stress-responsive genes expression. Environ. Exp. Bot. 2020, 174, 104023. [Google Scholar] [CrossRef]
- Vandenkoornhuyse, P.; Quaiser, A.; Duhamel, M.; Le Van, A.; Dufresne, A. The importance of the microbiome of the plant holobiont. New Phytol. 2015, 206, 1196–1206. [Google Scholar] [CrossRef]
- Dastogeer, K.M.; Zahan, M.I.; Tahjib-Ul-Arif, M.; Akter, M.A.; Okazaki, S. Plant salinity tolerance conferred by arbuscular mycorrhizal fungi and associated mechanisms: A meta-analysis. Front. Plant Sci. 2020, 11, 588550. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Wirth, S.; Bellingrath-Kimura, S.D.; Mishra, J.; Arora, N.K. Salt-tolerant plant growth promoting rhizobacteria for enhancing crop productivity of saline soils. Front. Microbiol. 2019, 10, 2791. [Google Scholar] [CrossRef]
- Sarkar, A.; Ghosh, P.K.; Pramanik, K.; Mitra, S.; Soren, T.; Pandey, S.; Mondal, M.H.; Maiti, T.K. A halotolerant Enterobacter sp. displaying ACC deaminase activity promotes rice seedling growth under salt stress. Res. Microbiol. 2018, 169, 20–32. [Google Scholar] [CrossRef]
- Win, K.T.; Tanaka, F.; Okazaki, K.; Ohwaki, Y. The ACC deaminase expressing endophyte Pseudomonas spp. enhances NaCl stress tolerance by reducing stress-related ethylene production, resulting in improved growth, photosynthetic performance, and ionic balance in tomato plants. Plant Physiol. Biochem. 2018, 127, 599–607. [Google Scholar] [CrossRef] [PubMed]
- El-Esawi, M.A.; Al-Ghamdi, A.A.; Ali, H.M.; Alayafi, A.A. Azospirillum lipoferum FK1 confers improved salt tolerance in chickpea (Cicer arietinum L.) by modulating osmolytes, antioxidant machinery and stress-related genes expression. Environ. Exp. Bot. 2019, 159, 55–65. [Google Scholar] [CrossRef]
- Andrés-Barrao, C.; Alzubaidy, H.; Jalal, R.; Mariappan, K.G.; de Zélicourt, A.; Bokhari, A.; Artyukh, O.; Alwutayd, K.; Rawat, A.; Shekhawat, K. Coordinated bacterial and plant sulfur metabolism in Enterobacter sp. SA187–induced plant salt stress tolerance. Proc. Natl. Acad. Sci. USA 2021, 118, e2107417118. [Google Scholar] [CrossRef]
- Li, Y.; You, X.; Tang, Z.; Zhu, T.; Liu, B.; Chen, M.X.; Xu, Y.; Liu, T.Y. Isolation and identification of plant growth-promoting rhizobacteria from tall fescue rhizosphere and their functions under salt stress. Physiol. Plant. 2022, 174, e13817. [Google Scholar] [CrossRef]
- Numan, M.; Bashir, S.; Khan, Y.; Mumtaz, R.; Shinwari, Z.K.; Khan, A.L.; Khan, A.; Al-Harrasi, A. Plant growth promoting bacteria as an alternative strategy for salt tolerance in plants: A review. Microbiol. Res. 2018, 209, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol. Res. 2014, 169, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Barquero, M.; Poveda, J.; Laureano-Marín, A.M.; Ortiz-Liébana, N.; Brañas, J.; González-Andrés, F. Mechanisms involved in drought stress tolerance triggered by rhizobia strains in wheat. Front. Plant Sci. 2022, 13, 1036973. [Google Scholar] [CrossRef] [PubMed]
- Ruzzi, M.; Aroca, R. Plant growth-promoting rhizobacteria act as biostimulants in horticulture. Sci. Hortic. 2015, 196, 124–134. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, S.; Gaurav, A.K.; Srivastava, S.; Verma, J.P. Plant growth-promoting bacteria: Biological tools for the mitigation of salinity stress in plants. Front. Microbiol. 2020, 11, 1216. [Google Scholar] [CrossRef]
- Xiong, Y.-W.; Gong, Y.; Li, X.-W.; Chen, P.; Ju, X.-Y.; Zhang, C.-M.; Yuan, B.; Lv, Z.-P.; Xing, K.; Qin, S. Enhancement of growth and salt tolerance of tomato seedlings by a natural halotolerant actinobacterium Glutamicibacter halophytocola KLBMP 5180 isolated from a coastal halophyte. Plant Soil 2019, 445, 307–322. [Google Scholar] [CrossRef]
- Khan, A.; Sayyed, R.; Seifi, S. Rhizobacteria: Legendary Soil Guards in Abiotic Stress Management. In Plant Growth Promoting Rhizobacteria for Sustainable Stress Management: Volume 1: Rhizobacteria in Abiotic Stress Management; Springer: Cham, Switzerland, 2019; pp. 327–343. [Google Scholar]
- Wang, X.; Xu, L.; Qi, X.; Huang, J.; Han, M.; Wang, C.; Li, X.; Jiang, H. Microbial assembly and stress-tolerance mechanisms in salt-adapted plants along the shore of a Salt Lake: Implications for saline–alkaline soil remediation. Microorganisms 2025, 13, 1942. [Google Scholar] [CrossRef]
- Kim, K.; Jang, Y.-J.; Lee, S.-M.; Oh, B.-T.; Chae, J.-C.; Lee, K.-J. Alleviation of salt stress by Enterobacter sp. EJ01 in tomato and Arabidopsis is accompanied by up-regulation of conserved salinity responsive factors in plants. Mol. Cells 2014, 37, 109–117. [Google Scholar] [CrossRef]
- Vaishnav, A.; Kumari, S.; Jain, S.; Varma, A.; Tuteja, N.; Choudhary, D.K. PGPR-mediated expression of salt tolerance gene in soybean through volatiles under sodium nitroprusside. J. Basic Microbiol. 2016, 56, 1274–1288. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; Sun, H.; Han, X.; Peng, Y.; Liu, J.; Liu, K.; Ding, Y.; Wang, C.; Du, B. Transcriptome profiles reveal the growth-promoting mechanisms of Paenibacillus polymyxa YC0136 on tobacco (Nicotiana tabacum L.). Front. Microbiol. 2020, 11, 584174. [Google Scholar] [CrossRef]
- Li, J.; Han, G.; Sun, C.; Sui, N. Research advances of MYB transcription factors in plant stress resistance and breeding. Plant Signal. Behav. 2019, 14, 1613131. [Google Scholar] [CrossRef] [PubMed]
- Finatto, T.; Viana, V.E.; Woyann, L.G.; Busanello, C.; Maia, L.C.d.; Oliveira, A.C.d. Can WRKY transcription factors help plants to overcome environmental challenges? Genet. Mol. Biol. 2018, 41, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.; Kim, H.R.; Rahmatallah, Y.; Wiggins, G.; Yang, Q.; Singh, R.; Glazko, G.; Mukherjee, A. RNA-seq reveals differentially expressed genes in rice (Oryza sativa) roots during interactions with plant-growth promoting bacteria, Azospirillum brasilense. PLoS ONE 2019, 14, e0217309. [Google Scholar] [CrossRef] [PubMed]
- Bharti, N.; Pandey, S.S.; Barnawal, D.; Patel, V.K.; Kalra, A. Plant growth promoting rhizobacteria Dietzia natronolimnaea modulates the expression of stress responsive genes providing protection of wheat from salinity stress. Sci. Rep. 2016, 6, 34768. [Google Scholar] [CrossRef]
- Malviya, M.K.; Li, C.-N.; Solanki, M.K.; Singh, R.K.; Htun, R.; Singh, P.; Verma, K.K.; Yang, L.-T.; Li, Y.-R. Comparative analysis of sugarcane root transcriptome in response to the plant growth-promoting Burkholderia anthina MYSP113. PLoS ONE 2020, 15, e0231206. [Google Scholar] [CrossRef]
- Ho, W.W.H.; Hill, C.B.; Doblin, M.S.; Shelden, M.C.; van de Meene, A.; Rupasinghe, T.; Bacic, A.; Roessner, U. Integrative multi-omics analyses of barley rootzones under salinity stress reveal two distinctive salt tolerance mechanisms. Plant Commun. 2020, 1, 100031. [Google Scholar] [CrossRef]
- Gomez, M.Y.; Schroeder, M.M.; Chieb, M.; McLain, N.K.; Gachomo, E.W. Bradyrhizobium japonicum IRAT FA3 promotes salt tolerance through jasmonic acid priming in Arab. thaliana. BMC Plant Biol. 2023, 23, 60. [Google Scholar] [CrossRef]
- Feng, L.; Li, Q.; Zhou, D.; Jia, M.; Liu, Z.; Hou, Z.; Ren, Q.; Ji, S.; Sang, S.; Lu, S. B. subtilis CNBG-PGPR-1 induces methionine to regulate ethylene pathway and ROS scavenging for improving salt tolerance of tomato. Plant J. 2024, 117, 193–211. [Google Scholar] [CrossRef] [PubMed]
- Zaib, S.; Shakeel, S.N.; Ali, A.; Akhtar, K. Quantitative RT-PCR Analysis of Plant Growth-Promoting Rhizobacteria-Induced Transcriptional Responses in Barley (Hordeum vulgare L.) under High Salt and Drought Stress Conditions. ACS Agric. Sci. Technol. 2025, 5, 687–700. [Google Scholar] [CrossRef]
- Ali, A.; Maggio, A.; Bressan, R.A.; Yun, D.-J. Role and functional differences of HKT1-type transporters in plants under salt stress. Int. J. Mol. Sci. 2019, 20, 1059. [Google Scholar] [CrossRef]
- Wani, S.H.; Nataraj, V.; Singh, G.P. Transcription Factors for Abiotic Stress Tolerance in Plants; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Naidu, S.; Pandey, J.; Mishra, L.C.; Chakraborty, A.; Roy, A.; Singh, I.K.; Singh, A. Silicon nanoparticles: Synthesis, uptake and their role in mitigation of biotic stress. Ecotoxicol. Environ. Saf. 2023, 255, 114783. [Google Scholar] [CrossRef]
- Oleńska, E.; Małek, W.; Wójcik, M.; Swiecicka, I.; Thijs, S.; Vangronsveld, J. Beneficial features of plant growth-promoting rhizobacteria for improving plant growth and health in challenging conditions: A methodical review. Sci. Total Environ. 2020, 743, 140682. [Google Scholar] [CrossRef]
- Khoshru, B.; Fallah Nosratabad, A.; Khosravi, H.; Asgharzadeh, A.; Faridian, L. Enhancing agricultural productivity using PGPR and nanoparticles: Mechanisms, challenges, and future directions. J. Sol Biol. 2025, 12, 279–313. [Google Scholar]
- Islam, W.; Tayyab, M.; Khalil, F.; Hua, Z.; Huang, Z.; Chen, H.Y. Silicon-mediated plant defense against pathogens and insect pests. Pestic. Biochem. Physiol. 2020, 168, 104641. [Google Scholar] [CrossRef]
- Tayyab, M.; Islam, W.; Zhang, H. Promising role of silicon to enhance drought resistance in wheat. Commun. Soil Sci. Plant Anal. 2018, 49, 2932–2941. [Google Scholar] [CrossRef]
- Ranjan, A.; Rajput, V.D.; Prazdnova, E.V.; Gurnani, M.; Sharma, S.; Bhardwaj, P.; Shende, S.S.; Mandzhieva, S.S.; Sushkova, S.; Minkina, T. Augmenting abiotic stress tolerance and root architecture: The function of phytohormone-producing PGPR and their interaction with nanoparticles. South Afr. J. Bot. 2024, 167, 612–629. [Google Scholar] [CrossRef]
- Kubi, H.A.A.; Khan, M.A.; Adhikari, A.; Imran, M.; Kang, S.-M.; Hamayun, M.; Lee, I.-J. Silicon and plant growth-promoting rhizobacteria Pseudomonas psychrotolerans CS51 mitigates salt stress in Zea mays L. Agriculture 2021, 11, 272. Agriculture 2021, 11, 272. [Google Scholar] [CrossRef]
- Alharbi, K.; Osman, H.S.; Rashwan, E.; Hafez, E.M.; Omara, A.E.-D. Stimulating the growth, anabolism, antioxidants, and yield of rice plants grown under salt stress by combined application of bacterial inoculants and nano-silicon. Plants 2022, 11, 3431. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, K.; Rashwan, E.; Mohamed, H.H.; Awadalla, A.; Omara, A.E.-D.; Hafez, E.M.; Alshaal, T. Application of silica nanoparticles in combination with two bacterial strains improves the growth, antioxidant capacity and production of barley irrigated with saline water in salt-affected soil. Plants 2022, 11, 2026. [Google Scholar] [CrossRef]
- Zhao, J.; Han, Q.; Cui, B.; Wang, J.; Hu, C.; Li, R.; Lin, Y.; Xu, Y.; Liu, C. Synergistic Effects of Salt-Tolerant PGPR and Foliar Silicon on Pak Choi Antioxidant Defense Under Salt Stress. Plants 2025, 14, 2065. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Guo, H.; Liu, Y.; Tao, Y.; Liang, Y.; Gao, Z.; Tang, K.; Feng, F. Combination of silicon and plant growth promoting rhizobacteria consortia promoted the growth of melon seedlings under salt stress. Hortic. Environ. Biotechnol. 2024, 65, 1069–1078. [Google Scholar] [CrossRef]
- Alharbi, K.; Hafez, E.; Omara, A.E.-D.; Awadalla, A.; Nehela, Y. Plant growth promoting rhizobacteria and silica nanoparticles stimulate sugar beet resilience to irrigation with saline water in salt-affected soils. Plants 2022, 11, 3117. [Google Scholar] [CrossRef]
- Adhikari, A.; Khan, M.A.; Lee, K.-E.; Kang, S.-M.; Dhungana, S.K.; Bhusal, N.; Lee, I.-J. The halotolerant rhizobacterium—Pseudomonas koreensis MU2 enhances inorganic silicon and phosphorus use efficiency and augments salt stress tolerance in soybean (Glycine max L.). Microorganisms 2020, 8, 1256. [Google Scholar] [CrossRef]
- Mahmood, S.; Daur, I.; Hussain, M.B.; Nazir, Q.; Al-Solaimani, S.G.; Ahmad, S.; Bakhashwain, A.A.; Elsafor, A.K. Silicon application and rhizobacterial inoculation regulate mung bean response to saline water irrigation. Clean–Soil Air Water 2017, 45, 1600436. [Google Scholar] [CrossRef]
- Hafez, E.M.; Osman, H.S.; Gowayed, S.M.; Okasha, S.A.; Omara, A.E.-D.; Sami, R.; Abd El-Monem, A.M.; Abd El-Razek, U.A. Minimizing the adversely impacts of water deficit and soil salinity on maize growth and productivity in response to the application of plant growth-promoting rhizobacteria and silica nanoparticles. Agronomy 2021, 11, 676. [Google Scholar] [CrossRef]
- ALKahtani, M.; Hafez, Y.; Attia, K.; Al-Ateeq, T.; Ali, M.A.; Hasanuzzaman, M.; Abdelaal, K. Bacillus thuringiensis and silicon modulate antioxidant metabolism and improve the physiological traits to confer salt tolerance in lettuce. Plants 2021, 10, 1025. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi-Nouraldinvand, F.; Sharifi, R.S.; Siadat, S.A.; Khalilzadeh, R. Fascinating role of silicon dioxide nanoparticles and co-inoculation of mycorrhiza and rhizobacteria to combat NaCl stress: Changes in physiological characteristics, uptake of nutrient elements, and enhancing photosystem ii activities in wheat. J. Soil Sci. Plant Nutr. 2024, 24, 277–294. [Google Scholar] [CrossRef]
- Hosseini-Nasr, F.; Etesami, H.; Alikhani, H.A. Silicon improves plant growth-promoting effect of nodule non-rhizobial bacterium on nitrogen concentration of alfalfa under salinity stress. J. Soil Sci. Plant Nutr. 2023, 23, 496–513. [Google Scholar] [CrossRef]
- Hafez, E.M.; Osman, H.S.; El-Razek, U.A.A.; Elbagory, M.; Omara, A.E.-D.; Eid, M.A.; Gowayed, S.M. Foliar-applied potassium silicate coupled with plant growth-promoting rhizobacteria improves growth, physiology, nutrient uptake and productivity of faba bean (Vicia faba L.) irrigated with saline water in salt-affected soil. Plants 2021, 10, 894. [Google Scholar] [CrossRef]
- Ferrusquía-Jiménez, N.I.; González-Arias, B.; Rosales, A.; Esquivel, K.; Escamilla-Silva, E.M.; Ortega-Torres, A.E.; Guevara-González, R.G. Elicitation of Bacillus cereus-Amazcala (Bc-A) with SiO2 nanoparticles improves its role as a plant growth-promoting bacteria (PGPB) in chili pepper plants. Plants 2022, 11, 3445. [Google Scholar] [CrossRef]
- Ayman, M.; Fahmy, M.A.; Elnahal, A.S.; Alfassam, H.E.; Rudayni, H.A.; Allam, A.A.; Farahat, E.M. Enhancing wheat tolerance to salinity using nanomaterials, proline, and biochar-inoculated with Bacillus subtilis. Heliyon 2024, 10, e37160. [Google Scholar] [CrossRef]
- Mahmood, S.; Daur, I.; Al-Solaimani, S.G.; Ahmad, S.; Madkour, M.H.; Yasir, M.; Hirt, H.; Ali, S.; Ali, Z. Plant growth promoting rhizobacteria and silicon synergistically enhance salinity tolerance of mung bean. Front. Plant Sci. 2016, 7, 876. [Google Scholar] [CrossRef]
- Ahmed, N.; Ahsen, S.; Ali, M.A.; Hussain, M.B.; Hussain, S.B.; Rasheed, M.K.; Butt, B.; Irshad, I.; Danish, S. Rhizobacteria and silicon synergy modulates the growth, nutrition and yield of mungbean under saline soil. Pak. J. Bot. 2020, 52, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Kumar, P.; Khan, A. Optimization of PGPR and silicon fertilization using response surface methodology for enhanced growth, yield and biochemical parameters of French bean (Phaseolus vulgaris L.) under saline stress. Biocatal. Agric. Biotechnol. 2020, 23, 101463. [Google Scholar] [CrossRef]
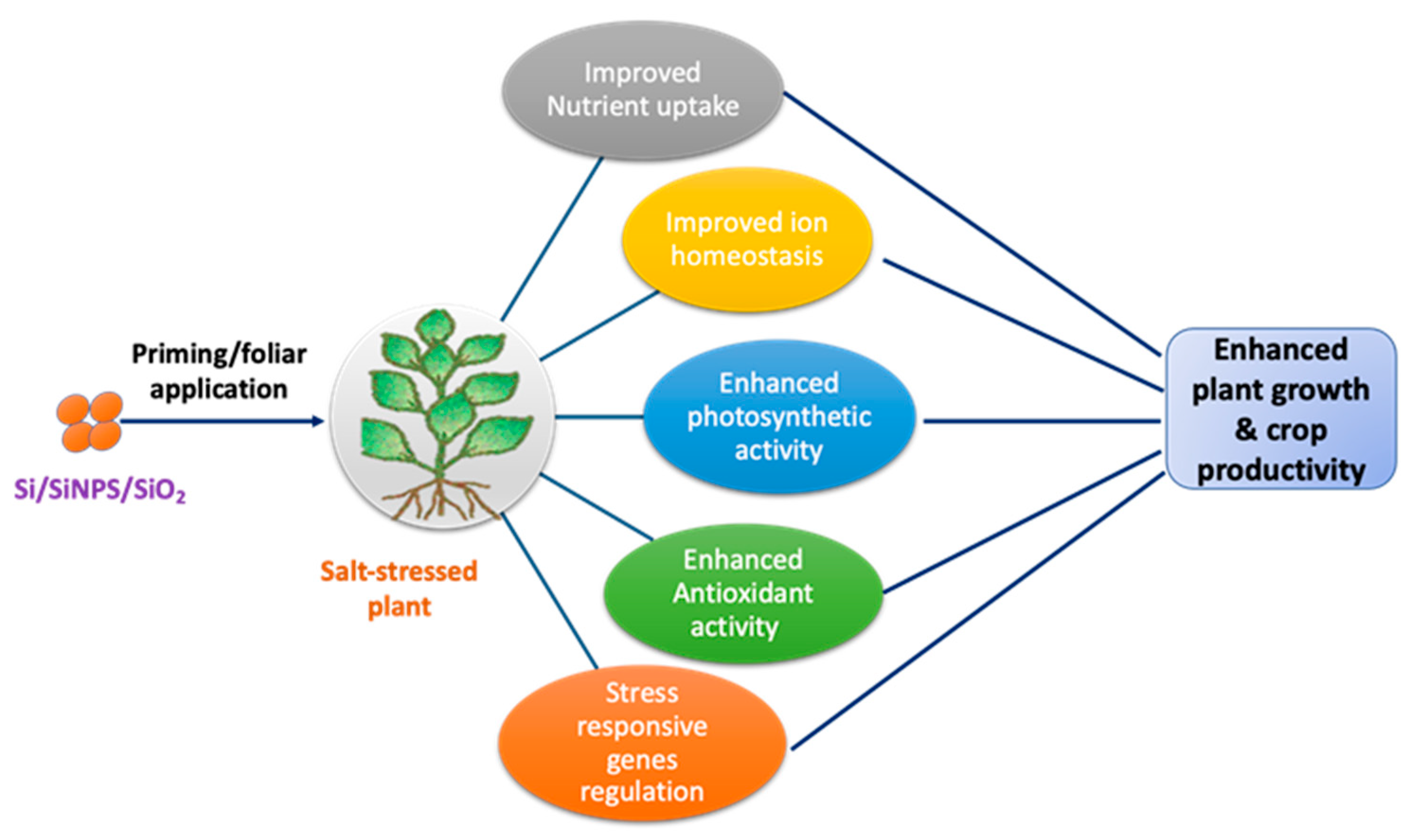

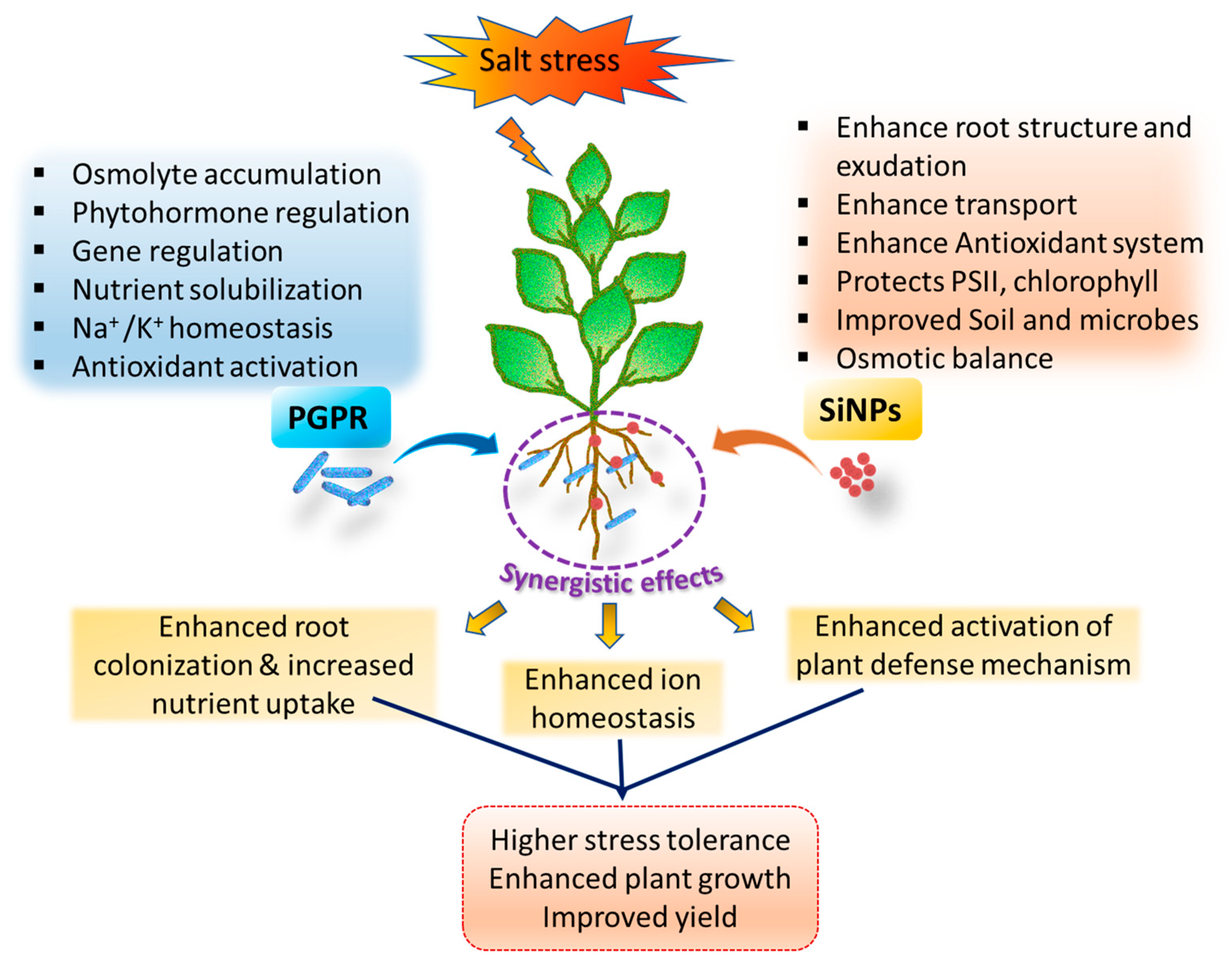
| Application Type | Crop | Si Form | Concentration | Observed Effect | Ref. |
|---|---|---|---|---|---|
| Exogenous application | Rice | SiNPs | Not specified | Enhanced water retention, improved ion homeostasis, and contributed to improved plant vigor under salinity stress | [74] |
| Seed Priming | Stevia | SiO2NPs | Moderate | Increase germination, increase root/shoot dry weight | [80] |
| Hydroponic Addition | Sea barley | Na2SiO3 | 0.5 mM | Increase chlorophyll, decrease oxidative stress | [66] |
| Seed Priming | B. juncea | SiNPs | Optimal | improving plant stress tolerance | [81] |
| Foliar Spray | Wheat | SiNPs | 60 mg/L | Mitigating the salinity stress by enhancing antioxidant activity, improved physiological parameters by stomatal conductance, electrical conductivity, electrolytic leakage, and proline | [82] |
| Mixed in Soil | Soybean | SiO2NPs | 1 g/kg of dry soil | Promoted root development, altered root exudation patterns, stimulated the recruitment and colonization of beneficial microbes, and enriched the rhizosphere microbiome | [83] |
| Seed Priming | chia | SiNPs | 200 mg/L | Alleviated salt stress by enhancing antioxidant enzyme activity, maintaining ionic equilibrium, and improving seed yield and oil content | [7] |
| Mixed in Soil | Tomato | SiO2NPs | 50 ppm | enhancing antioxidant enzyme activity, Improved proline metabolism, increased osmoregulatory substances, and decreased H2O2 levels. | [84] |
| Mixed in Soil | Wheat | SiNPs | 10%(optimal) | improved chlorophyll content, proline accumulation, soluble sugars, nutrient content, and water retention, and reduced electrolyte leakage | [85] |
| Hydroponic Addition | Tomato | SiNPs | 200 mg /L | Improved growth, photosynthesis, and chlorophyll content, increased antioxidant enzymatic activity, and reduced H2O2 levels. Increased K+ and Si content and decreased Na+ absorption. | [17] |
| Exogenous application | Rice | SiNPs | 200 mM | Alleviated salt stress by enhancing antioxidant enzyme activity, maintaining ionic equilibrium. | [47] |
| Exogenous application | Wheat | SiO2-ZnO NPs | - | Alliviated salt stress, improved growth, yield, and nutrient content. | [86] |
| Gene | Plant Source | Function/Role | Localization |
|---|---|---|---|
| Lsi1 | Rice, Maize | Influx transporter; uptake of Si into root cells | Distal exodermis |
| Lsi2 | Rice, Barley | Efflux transporter; exports Si toward xylem | Proximal exodermis |
| Lsi3 | Rice | Efflux transporter; assists Si movement within stem nodes | Stem nodes |
| Lsi6 | Rice, Wheat | Distributes Si from xylem to aerial tissues | Leaf nodes, shoots |
| GmNIP2-1, GmNIP2-2 | Soybean | Aquaporin family; function as Si transporters | Root tissues |
| Gene | Function/Role | Plant System/PGPR | Ref. |
|---|---|---|---|
| iaaM | IAA secretion | PGPR strains | [120] |
| nifU | Nitrogen fixation | PGPR strains | [120] |
| phzCEF | Phenazine biosynthesis | PGPR strains | [120] |
| sbnA | Siderophore production | PGPR strains | [120] |
| speB | Spermidine biosynthesis salt tolerance gene | PGPR strains | [120] |
| GmST1 | Salt tolerance gene | Soybean | [121] |
| GmLAX3 | Auxin transport gene | Soybean | [121] |
| htpX | Heat resistant | PGPR strains | [122] |
| otsA | Osmoprotection | PGPR strains | [122] |
| katE | Antioxidant gene | PGPR strains | [122] |
| uvrA | UV radiation resistant | PGPR strains | [122] |
| Gene | Function/Role | Plant/PGPR Strain | Ref. |
|---|---|---|---|
| P5CS1 | Proline biosynthesis | Arabidopsis | [123] |
| RD29A/RD29B | Dehydration responsive genes (ABA dependent/independent) | Arabidopsis | [123] |
| DREB2b | Dehydration-responsive element binding TF | Arabidopsis | [123] |
| RAB18 | ABA-responsive gene | Arabidopsis | [123] |
| HKT1 | High affinity K+ transporter (Na+ exclusion) | Rice, Wheat | [135] |
| WRKY TFs | Transcription factors regulating stress tolerance | Multiple crops | [136] |
| DAHAR, MSD1, MYC2, RD22 | Salt stress-responsive genes (shoots) | Arabidopsis under B. japonicum | [132] |
| ADc2, ANACO55, DHAR, GTR1, RD20, RD29B, VSP1, V2p2 | Salt stress-responsive genes (roots) | Arabidopsis under B. japonicum | [132] |
| Glutathione and Peroxidase pathways genes | ROS detoxification, linked to methionine metabolism | Arabidopsis with CNBG-PGPR-1 | [133] |
| FAD3, LOX1, AOS, AOC | Jasmonic acid pathway genes | Barley with P. putida KT2440 | [134] |
| MAPKK | MAPK signaling under salt stress | Barley with P. putida KT2440 | [134] |
| NHX1 | Na+/H+ antiporter | Barley with P. putida KT2440 | [134] |
| NRT2.2 | Nitrate transporter | Barley with P. fluorescens SBW25 | [134] |
| CAT2 | CAT (ROS detoxification) | Barley with P. fluorescens | [134] |
| GR | Glutathione reductase | Barley with P. putida | [134] |
| S.N. | PGPR spps. | Si Source | Crops | Co-Inoculation Effects | Ref. |
|---|---|---|---|---|---|
| 1 | B. drentensis | K2SiO3 | Mung bean | Enhanced K+, Si, and Ca2+ concentrations in shoots, lowered Na+ content compared to the control, and higher pod yield. | [150] |
| 2. | P. pseudoalcaligenes | K2SiO3 | Coriander | Enhanced photosynthetic pigment levels, higher RWC, increased POD activity, and an improved root system contributed to enhanced plant growth. | [42] |
| 3. | A. lipoferum and Bacillus circulance | SiO2 | Maize | Significant improvements in soil health and plant growth accompanied by enhanced nutrient uptake, yield-related traits, and maize productivity. | [151] |
| 4. | Enterobacter sp. | Na2SiO3 | Wheat seeds | Increased stress tolerance indices, resulting in a drastic improvement in plant growth compared to the individual application of Si or bacteria. | [41] |
| 5. | Bacillus thuringiensis | K2SiO3 | Lettuce | Higher head weight, yield, and antioxidant enzyme activities (CAT, SOD, POD, and polyphenol oxidase) with increased proline accumulation in lettuce leaves. | [152] |
| 6. | Flavobacterium and Pseudomonas, along with arbuscular mycorrhizal fungi | SiO2 | Wheat | Improved Si, P, and K+ levels and lowered Na+ uptake, thereby increasing grain yield. | [153] |
| 7. | Advenella incenata and Ensifer meliloti | K2SiO3 | Alfalfa | Enhanced both morphological and physiological traits of alfalfa plants, lowered Na+, and increased K+ content. | [154] |
| 8. | Rhizobium leguminosarum and Bacillus circulans | K2SiO3 | Faba bean | Lowered exchangeable sodium percentage and promoted urease and dehydrogenase activities, with values similar to the control (fresh water), contributing to soil quality restoration and ultimately improving plant growth. | [155] |
| 9. | Bacillus cereus-Amazcala | SiO2 | Chilli pepper | SiO2-NPs enhanced PGPB’s phosphate solubilization capacity and GA7 production. While upregulating CAT and SOD activities, indicating that SiO2-NPs function as a eustressor. | [156] |
| 10. | Bacillus subtilis | Nano-SiO2 | Wheat | Enhanced nutrient content and wheat growth, mitigating the detrimental effects of salinity stress. | [157] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sajida; Kashtoh, H.; Lama Tamang, T.; Baek, K.-H. Recent Advances on the Individual Roles and Emerging Synergistic Effects of Plant Growth-Promoting Rhizobacteria and Silicon Nanoparticles in Mitigating Salinity Stress. Plants 2025, 14, 3632. https://doi.org/10.3390/plants14233632
Sajida, Kashtoh H, Lama Tamang T, Baek K-H. Recent Advances on the Individual Roles and Emerging Synergistic Effects of Plant Growth-Promoting Rhizobacteria and Silicon Nanoparticles in Mitigating Salinity Stress. Plants. 2025; 14(23):3632. https://doi.org/10.3390/plants14233632
Chicago/Turabian StyleSajida, Hamdy Kashtoh, Tensangmu Lama Tamang, and Kwang-Hyun Baek. 2025. "Recent Advances on the Individual Roles and Emerging Synergistic Effects of Plant Growth-Promoting Rhizobacteria and Silicon Nanoparticles in Mitigating Salinity Stress" Plants 14, no. 23: 3632. https://doi.org/10.3390/plants14233632
APA StyleSajida, Kashtoh, H., Lama Tamang, T., & Baek, K.-H. (2025). Recent Advances on the Individual Roles and Emerging Synergistic Effects of Plant Growth-Promoting Rhizobacteria and Silicon Nanoparticles in Mitigating Salinity Stress. Plants, 14(23), 3632. https://doi.org/10.3390/plants14233632











