Chlorophyll Fluorescence and Biochemical Biomarkers Reveal Plasticizer Di-n-Butyl Phthalate-Induced Stress in Azolla pinnata
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. DBP Treatment Preparation and Application
2.3. Morphological Measurement
2.4. Determination of Total Chlorophyll Content
2.5. Lipid Peroxidation (MDA) and Hydrogen Peroxide Quantification
2.6. Estimation of Antioxidant Enzyme Activities (SOD, CAT)
2.7. Fast Chlorophyll a Fluorescence Kinetics (ChlF)
2.8. Statistical Analysis
3. Result
3.1. Morphological Changes
3.2. Effect of DBP on Biochemical Parameters of A. pinnata
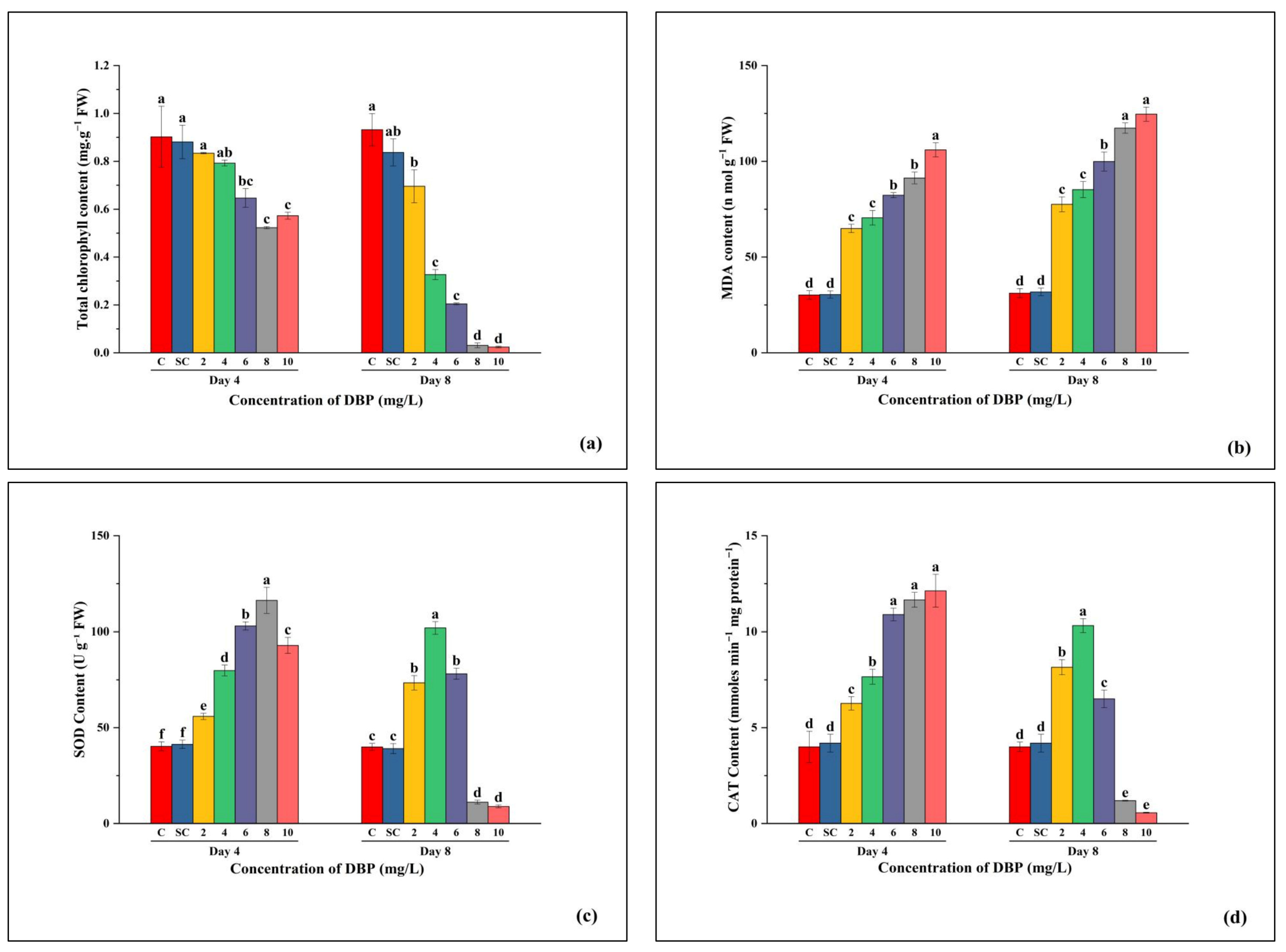
3.2.1. Total Chlorophyll Content
3.2.2. MDA Content
3.2.3. SOD Activity
3.2.4. CAT Activity
3.3. Photosynthetic Performance
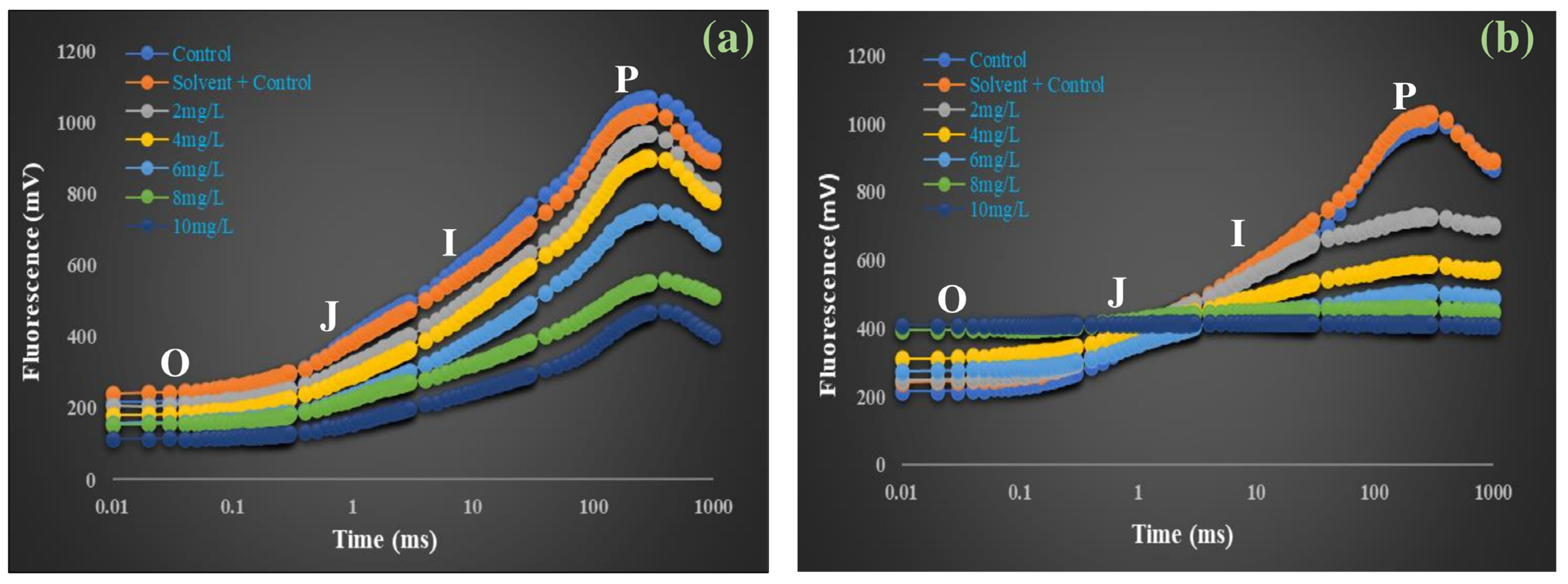
3.3.1. OJIP Chlorophyll Fluorescence Transients of A. pinnata Under DBP Exposure
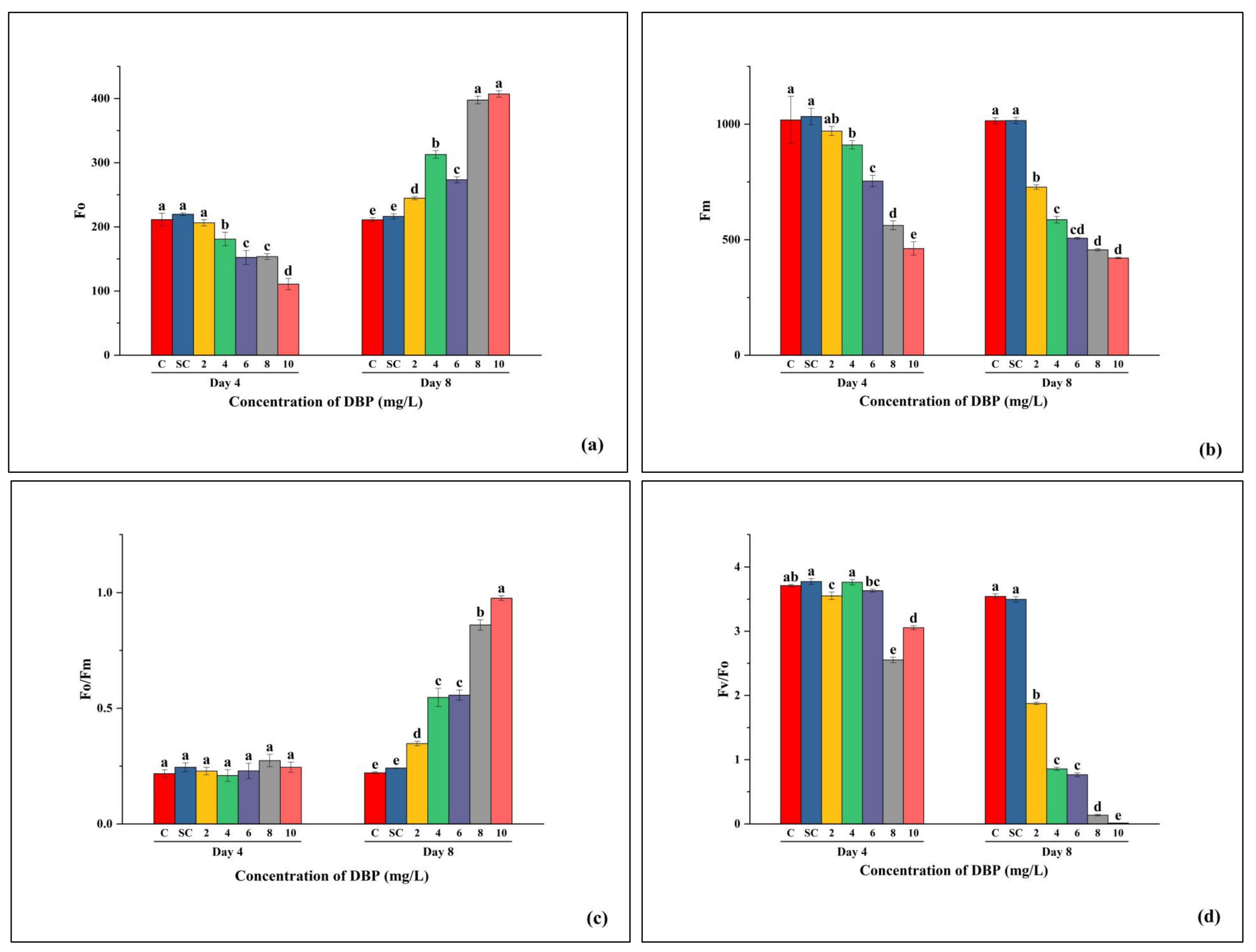
3.3.2. Effects of DBP on Biophysical Parameters
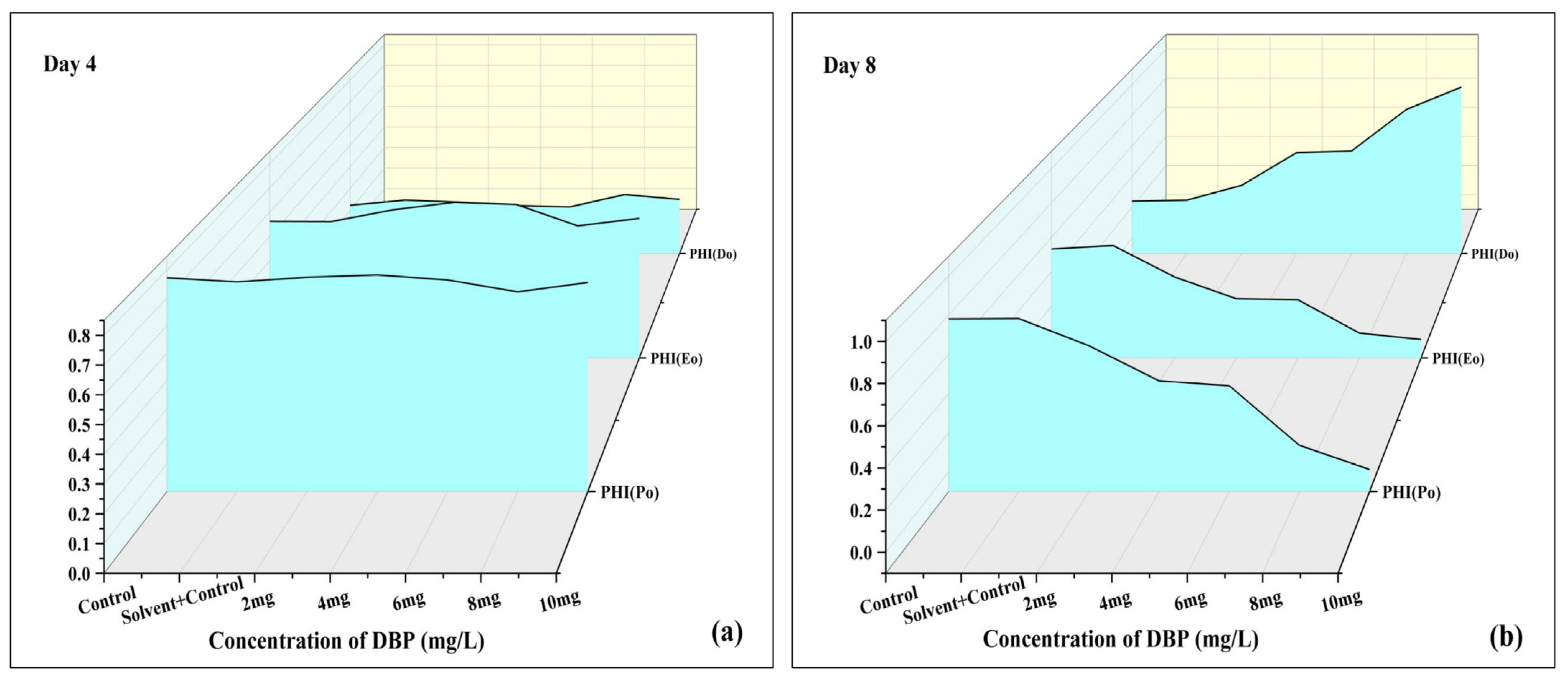
3.3.3. Leaf (Phenomenological) Model
3.3.4. Energy Flux Parameters per Cross Section
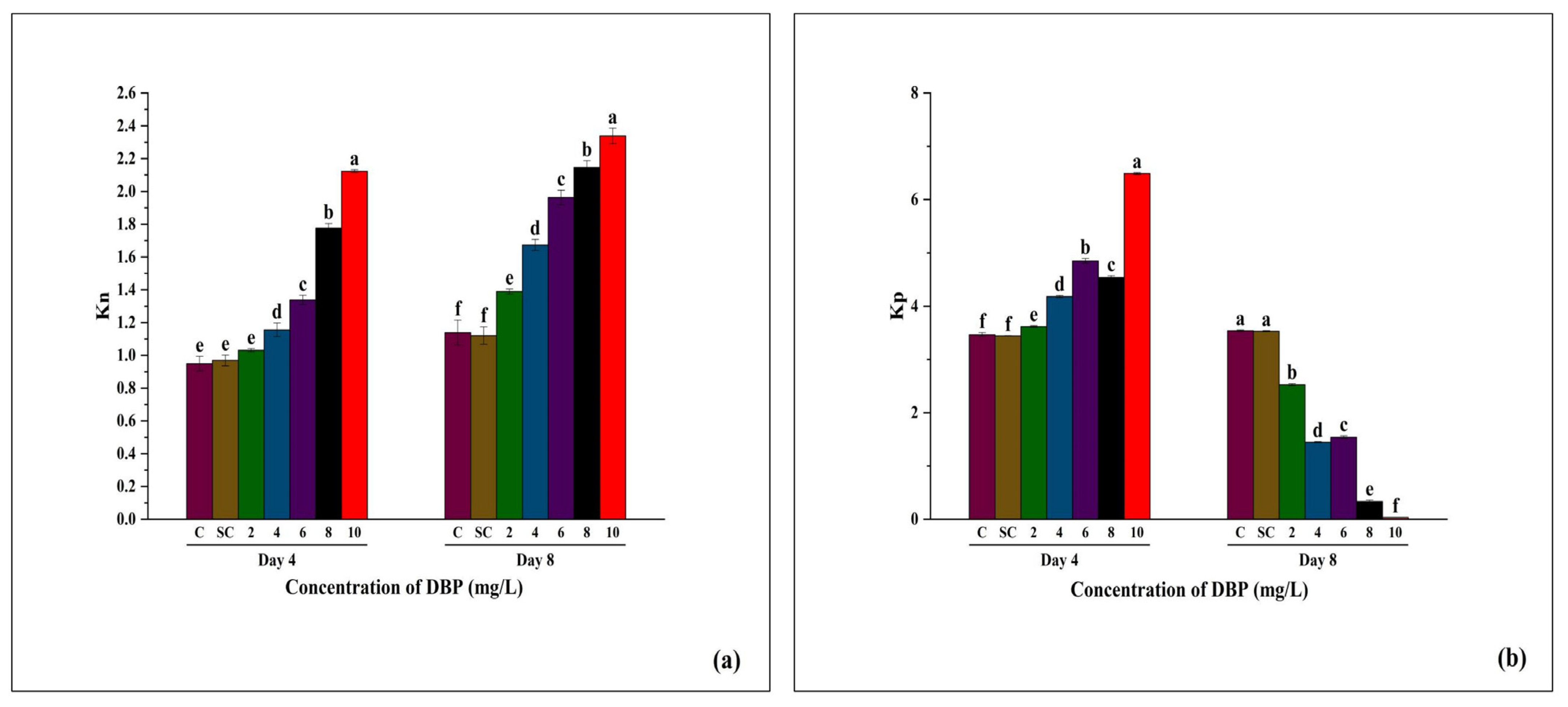
3.3.5. Changes in Kn and Kp of A. pinnata Under DBP Exposure
3.3.6. Quantum Yield of A. pinnata Under DBP Exposure
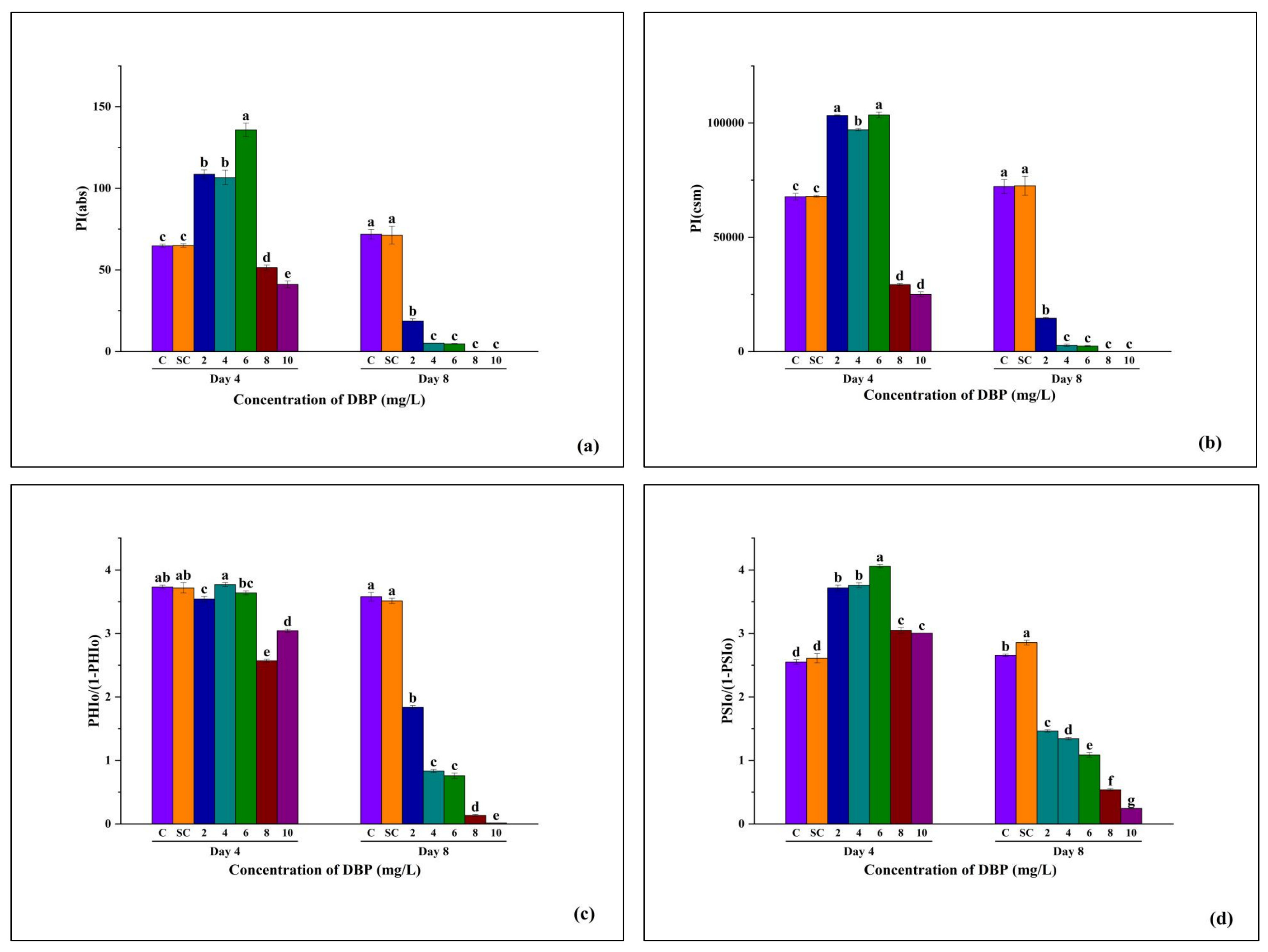
3.3.7. Photosynthetic Performance Indices and Primary and Secondary Photochemistry of A. pinnata Under DBP Exposure
- PI(abs):
- PI(cs):
- PHIo/(1–PHIo):
- PSIo/(1–PSIo):
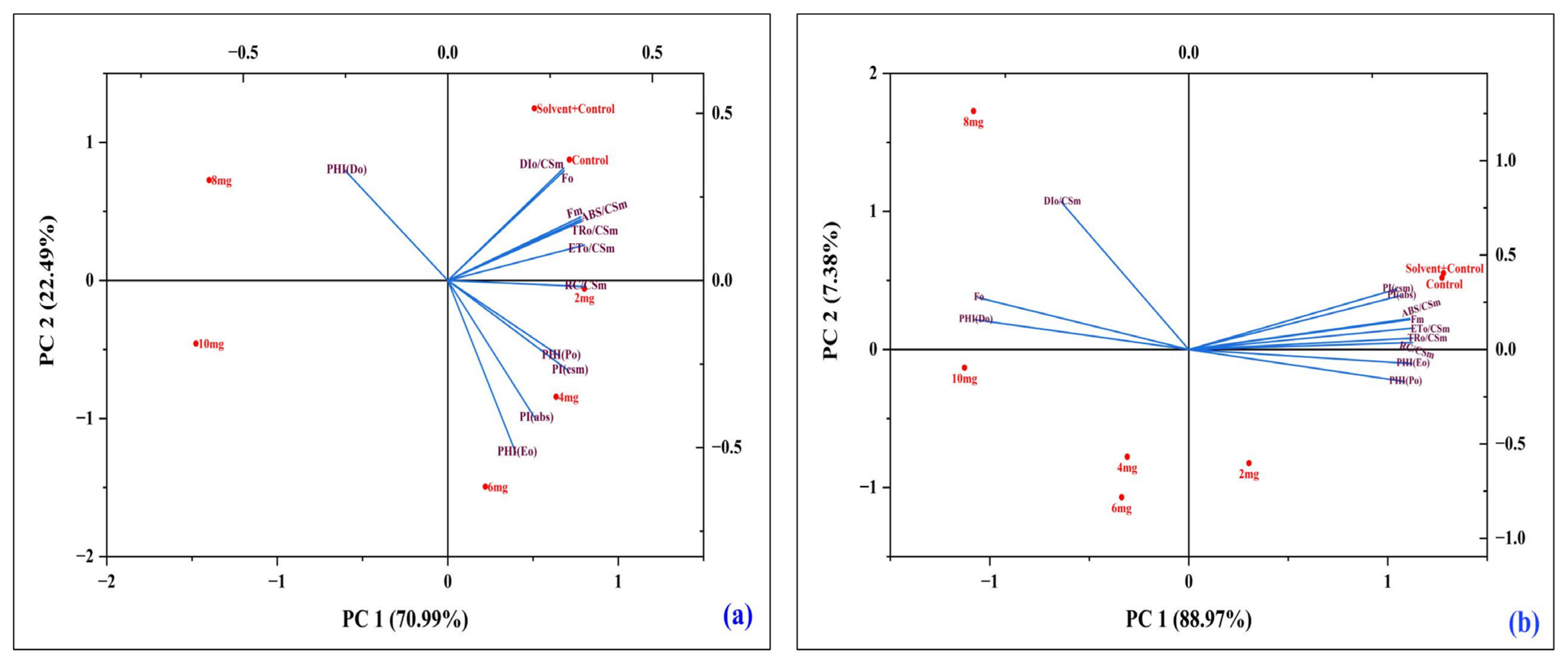
3.4. Multivariate Analysis
3.4.1. Principal Component Analysis (PCA) of Chlorophyll Fluorescence Parameters in A. pinnata Under DBP Stress
3.4.2. Correlation Analysis of Chlorophyll Fluorescence and Performance Parameters Under DBP Exposure
4. Discussion
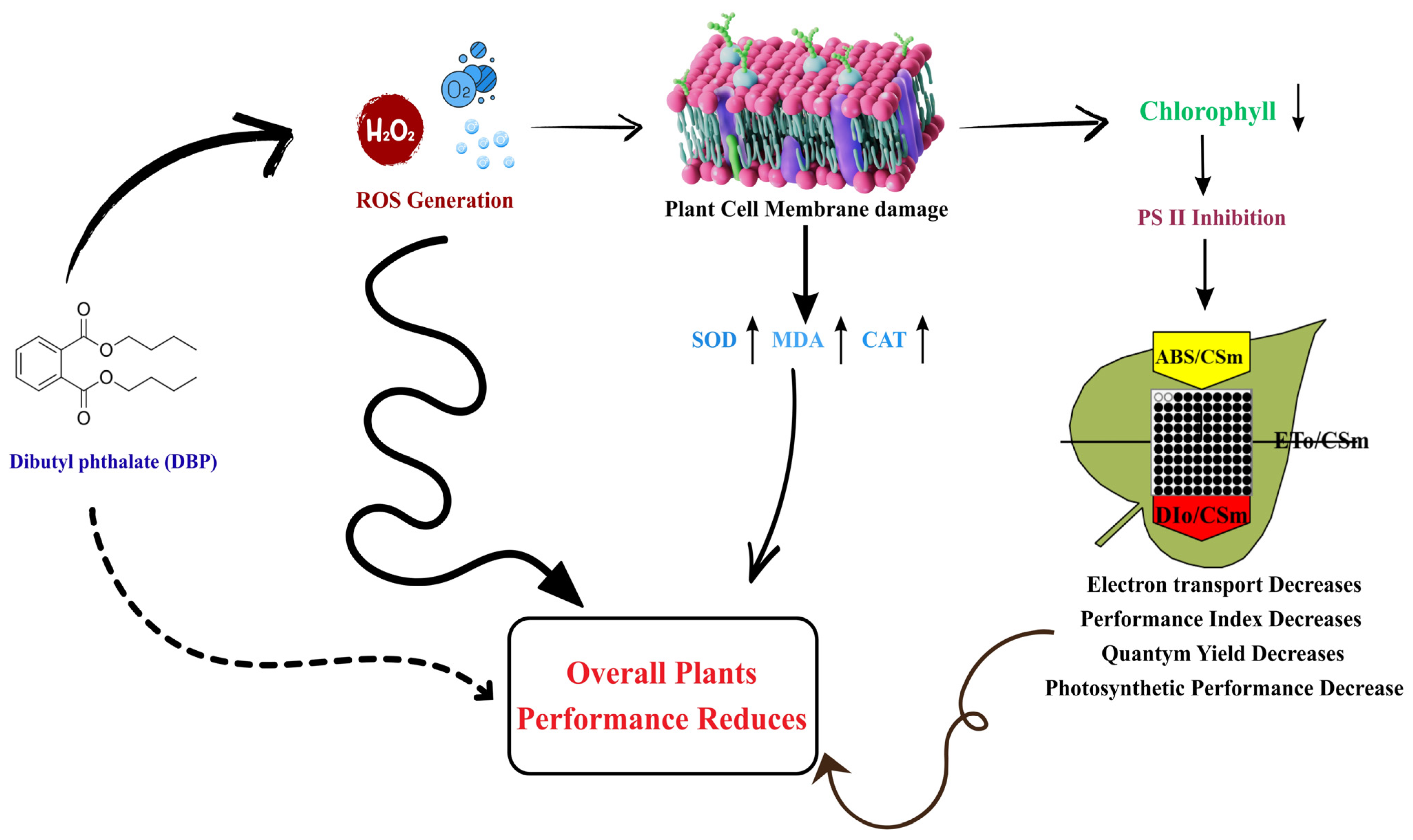
4.1. DBP-Induced Oxidative Stress and Antioxidant Enzyme Response
4.2. Distortion of OJIP Fluorescence Transients and PSII Photochemical Impairment
4.3. Biophysical Disruption of PSII Function and Energy Flux Imbalance
4.4. Quantum Yield Decline, Performance Indices, and Photochemical Inefficiency
4.5. Multivariate PCA and Correlation Analysis as Diagnostic Indicators of DBP Stress
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shende, N.; Singh, I.; Hippargi, G.; Ramesh Kumar, A. Occurrence and Health Risk Assessment of Phthalates in Municipal Drinking Water Supply of a Central Indian City. Arch. Environ. Contam. Toxicol. 2024, 86, 288–303. [Google Scholar] [CrossRef] [PubMed]
- Nikova, E.V.; Temkov, M.; Rocha, J.M. Occurrence of Meso/Micro/Nano Plastics and Plastic Additives in Food from Food Packaging. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2023; Volume 103, pp. 41–99. ISBN 1043-4526. [Google Scholar]
- Omidoyin, K.C.; Jho, E.H. Environmental Occurrence and Ecotoxicological Risks of Plastic Leachates in Aquatic and Terrestrial Environments. Sci. Total Environ. 2024, 954, 176728. [Google Scholar] [CrossRef]
- Pagoni, A.; Arvaniti, O.S.; Kalantzi, O.-I. Exposure to Phthalates from Personal Care Products: Urinary Levels and Predictors of Exposure. Environ. Res. 2022, 212, 113194. [Google Scholar] [CrossRef]
- Aldegunde-Louzao, N.; Lolo-Aira, M.; Herrero-Latorre, C. Phthalate Esters in Different Types of Cosmetic Products: A Five-Year Quality Control Survey. Molecules 2024, 29, 4823. [Google Scholar] [CrossRef]
- da Costa, J.M.; Kato, L.S.; Galvan, D.; Lelis, C.A.; Saraiva, T.; Conte-Junior, C.A. Occurrence of Phthalates in Different Food Matrices: A Systematic Review of the Main Sources of Contamination and Potential Risks. Compr. Rev. Food Sci. Food Saf. 2023, 22, 2043–2080. [Google Scholar] [CrossRef]
- Amritha, P.S.; Vinod, V.; Harathi, P.B. A Critical Review on Extraction and Analytical Methods of Phthalates in Water and Beverages. J. Chromatogr. A 2022, 1675, 463175. [Google Scholar] [CrossRef]
- Sokołowski, A.; Kończak, M.; Oleszczuk, P.; Gao, Y.; Czech, B. Environmental and Food Contamination by Phthalic Acid Esters (PAEs): Overview. Water Air Soil Pollut. 2024, 235, 313. [Google Scholar]
- Chen, H.; Mao, W.; Shen, Y.; Feng, W.; Mao, G.; Zhao, T.; Yang, L.; Yang, L.; Meng, C.; Li, Y. Distribution, Source, and Environmental Risk Assessment of Phthalate Esters (PAEs) in Water, Suspended Particulate Matter, and Sediment of a Typical Yangtze River Delta City, China. Environ. Sci. Pollut. Res. 2019, 26, 24609–24619. [Google Scholar] [CrossRef] [PubMed]
- Qadeer, A.; Anis, M.; Warner, G.R.; Potts, C.; Giovanoulis, G.; Nasr, S.; Archundia, D.; Zhang, Q.; Ajmal, Z.; Tweedale, A.C. Global Environmental and Toxicological Data of Emerging Plasticizers: Current Knowledge, Regrettable Substitution Dilemma, Green Solution and Future Perspectives. Green Chem. 2024, 26, 5635–5683. [Google Scholar] [CrossRef]
- Godwin, A.D. Plasticizers. In Applied Plastics Engineering Handbook; Elsevier: Amsterdam, The Netherlands, 2024; pp. 595–618. [Google Scholar]
- Salikova, N.S.; Lovinskaya, A.V.; Kolumbayeva, S.Z.; Bektemissova, A.U.; Urazbayeva, S.E.; Rodrigo-Clavero, M.-E.; Rodrigo-Ilarri, J. Evaluation of Microplastic Toxicity in Drinking Water Using Different Test Systems. Water 2024, 16, 3250. [Google Scholar] [CrossRef]
- Chang, W.-H.; Herianto, S.; Lee, C.-C.; Hung, H.; Chen, H.-L. The Effects of Phthalate Ester Exposure on Human Health: A Review. Sci. Total Environ. 2021, 786, 147371. [Google Scholar] [CrossRef] [PubMed]
- Mariana, M.; Castelo-Branco, M.; Soares, A.M.; Cairrao, E. Phthalates’ Exposure Leads to an Increasing Concern on Cardiovascular Health. J. Hazard. Mater. 2023, 457, 131680. [Google Scholar] [CrossRef]
- Li, L.; Huang, L.; Lei, R.; Zhang, P.; Yang, Y.; Liu, H.; Zhang, Y. DEHP and DBP, Common Phthalates, Induce Glucose Metabolism Disorders in Rats via Oxidative Damage of PI3K/Akt/GLUT4 Signaling. Environ. Pollut. 2024, 341, 122948. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Gao, P.; Luo, Y.; Gou, X.; Xia, P.; Wang, P.; Yan, L.; Zhang, S.; Guo, J.; Zhang, X. Are New Phthalate Ester Substitutes Safer than Traditional DBP and DiBP? Comparative Endocrine-Disrupting Analyses on Zebrafish Using in Vivo, Transcriptome, and in Silico Approaches. Environ. Sci. Technol. 2023, 57, 13744–13756. [Google Scholar] [CrossRef]
- Souza, J.M.O.; Souza, M.C.O.; Rocha, B.A.; Nadal, M.; Domingo, J.L.; Barbosa Jr, F. Levels of Phthalates and Bisphenol in Toys from Brazilian Markets: Migration Rate into Children’s Saliva and Daily Exposure. Sci. Total Environ. 2022, 828, 154486. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yao, X.; Li, X.; Wang, Q.; Wang, J.; Zhu, L.; Wang, J. Effects of Dibutyl Phthalate on Microbial Community and the Carbon Cycle in Salinized Soil. J. Clean. Prod. 2023, 404, 136928. [Google Scholar] [CrossRef]
- Han, J.; Jiang, Z.; Li, P.; Wang, J.; Zhou, X. Contamination of Phthalic Acid Esters in China’s Agricultural Soils: Sources, Risk, and Control Strategies. Agronomy 2025, 15, 433. [Google Scholar] [CrossRef]
- Cao, J.; Gao, X.; Zhang, S.; Wei, Z.; Chen, X.; Ma, N.; Li, C.; Zhao, X. Migration Patterns of Phthalic Acid Esters from Mulch Plastic Film in the Soil-Plant-Atmosphere Continuum System. J. Hazard. Mater. 2024, 480, 136353. [Google Scholar] [CrossRef]
- Zeng, L.-J.; Huang, Y.-H.; Lü, H.; Geng, J.; Zhao, H.-M.; Xiang, L.; Li, H.; Li, Y.-W.; Mo, C.-H.; Cai, Q.-Y. Uptake Pathways of Phthalates (PAEs) into Chinese Flowering Cabbage Grown in Plastic Greenhouses and Lowering PAE Accumulation by Spraying PAE-Degrading Bacterial Strain. Sci. Total Environ. 2022, 815, 152854. [Google Scholar]
- Li, X.; Jiang, N.; Zhang, J.; Yao, X.; Liu, W.; Wang, Q.; Ding, J.; Hu, Z.; Zhu, L.; Wang, J. Soil Health Hazards of Di (2-Ethylhexyl) Phthalate: New Perspectives on Earthworms from Different Ecological Niches DNA Damage, Gut Microbial Disruption and Soil Enzyme Changes. J. Hazard. Mater. 2024, 467, 133700. [Google Scholar]
- Cao, J.; Wang, Q.; Lei, Y.; Jiang, X.; Li, M. Accumulation of Microplastics and Tcep Pollutants in Agricultural Soil: Exploring the Links between Metabolites and Gut Microbiota in Earthworm Homeostasis. Environ. Int. 2022, 170, 107590. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Guo, Z.; Deng, R.; Fan, T.; Dong, D.; Dai, Y.; Li, C. The Concentrations and Behavior of Classic Phthalates and Emerging Phthalate Alternatives in Different Environmental Matrices and Their Biological Health Risks. Environ. Sci. Pollut. Res. 2024, 31, 46790–46805. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.-J.; Mohan, H.; Lim, J.-M.; Lee, S.-W.; Park, J.-H.; Sathya, P.M.; Lee, G.-M.; Seralathan, K.-K.; Oh, B.-T. Enhanced Degradation of Dibutyl Phthalate Using a Synthetic Mixed Bacterial System and Its Impact on Environmental Toxicity. Sci. Total Environ. 2025, 967, 178796. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Yuan, B.; Liu, H.; Ke, Y.; Zhang, W.; Li, H.; Lu, H.; Liu, J.; Hong, H.; Yan, C. Microplastics Emerge as a Hotspot for Dibutyl Phthalate Sources in Rivers and Oceans: Leaching Behavior and Potential Risks. J. Hazard. Mater. 2024, 475, 134920. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, S.; Shiv, K.; Singh, A.; Kumar, P.; Prasad, L.B. Phytotoxic Impact of Di-Butyl Phthalate (DBP) on Physiological, Biochemical, and Oxidative Stress Parameters of Rice (Oryza sativa). Environ. Sci. Pollut. Res. 2025, 32, 4588–4602. [Google Scholar] [CrossRef]
- Li, L.; Xia, Y.; Chen, J.; Han, X.; Hao, L.; Li, D.; Liu, Y. DBP Exposure Induces Thyroid Inflammatory Impairment through Activating AKT/NF-ΚB/NLRP3 Signaling. Ecotoxicol. Environ. Saf. 2023, 264, 115385. [Google Scholar] [CrossRef]
- Das, A.M.; Gogia, A.; Garg, M.; Elaiyaraja, A.; Arambam, P.; Mathur, S.; Babu-Rajendran, R.; Deo, S.V.S.; Kumar, L.; Das, B.C. Urinary Concentration of Endocrine-Disrupting Phthalates and Breast Cancer Risk in Indian Women: A Case-Control Study with a Focus on Mutations in Phthalate-Responsive Genes. Cancer Epidemiol. 2022, 79, 102188. [Google Scholar]
- Zhang, M.; Deng, Y.-L.; Liu, C.; Lu, W.-Q.; Zeng, Q. Impacts of Disinfection Byproduct Exposures on Male Reproductive Health: Current Evidence, Possible Mechanisms and Future Needs. Chemosphere 2023, 331, 138808. [Google Scholar] [CrossRef]
- Adjei, J.K.; Ofori, A.; Megbenu, H.K.; Ahenguah, T.; Boateng, A.K.; Adjei, G.A.; Bentum, J.K.; Essumang, D.K. Health Risk and Source Assessment of Semi-Volatile Phenols, p-Chloroaniline and Plasticizers in Plastic Packaged (Sachet) Drinking Water. Sci. Total Environ. 2021, 797, 149008. [Google Scholar] [CrossRef]
- Zhou, N.; Sui, S.; Liu, H.; Yang, X.; Hong, H.; Patterson, T.A. Determining High Priority Disinfection Byproducts Based on Experimental Aquatic Toxicity Data and Predictive Models: Virtual Screening and In Vivo Study. Sci. Total Environ. 2024, 951, 175489. [Google Scholar] [CrossRef]
- Shahid, M.; Shafi, Z.; Ilyas, T.; Singh, U.B.; Pichtel, J. Crosstalk between Phytohormones and Pesticides: Insights into Unravelling the Crucial Roles of Plant Growth Regulators in Improving Crop Resilience to Pesticide Stress. Sci. Hortic. 2024, 338, 113663. [Google Scholar] [CrossRef]
- Saleem, M.H.; Zafar, S.; Javed, S.; Anas, M.; Ahmed, T.; Ali, S.; Mirmazloum, I.; Ahmad, A. Modulatory Effects of Glutamic Acid on Growth, Photosynthetic Pigments, and Stress Responses in Olive Plants Subjected to Cadmium Stress. J. King Saud. Univ. Sci. 2024, 36, 103540. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, Q.; Tan, W.; Song, G.; Lu, G.; Li, F. Biochemical Responses of Two Typical Duckweeds Exposed to Dibutyl Phthalate. J. Environ. Sci. Health Part A 2006, 41, 1615–1626. [Google Scholar] [CrossRef]
- Liao, C.-S.; Hong, Y.-H.; Nishikawa, Y.; Kage-Nakadai, E.; Chiou, T.-Y.; Wu, C.-C. Impacts of Endocrine Disruptor Di-n-Butyl Phthalate Ester on Microalga Chlorella vulgaris Verified by Approaches of Proteomics and Gene Ontology. Molecules 2020, 25, 4304. [Google Scholar] [CrossRef] [PubMed]
- Tasnim, N.; Das, E.J.; Tania, K.A.; Bhuiyan, M.A.R.; Swarna, F.A. Assessment of the Efficacy of Azolla Pinnata in Improving Textile Dye Wastewater Quality at Varying Concentrations. Bioremediat. J. 2025, 29, 274–287. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, Y.; Deng, S.; Ying, Z. Role of Azolla in Sustainable Agriculture and Climate Resilience: A Comprehensive Review. Front. Plant Sci. 2025, 16, 1661720. [Google Scholar] [CrossRef]
- Abu-Zahra, N.I.S.; Gouda, M.; Elseify, M.M.; Abass, M.E.; El-Gohary, M.S.; El-Sokary, E.T. Azolla Pinnata Mitigates Pendimethalin Induced Immunotoxicity, Oxidative Stress and Histopathological Changes in Oreochromis niloticus. Sci. Rep. 2025, 15, 16226. [Google Scholar] [CrossRef] [PubMed]
- Sweet, A.; Hills, L.V. A Study of Azolla Pinnata R. Brown. Am. Fern J. 1971, 61, 1–13. [Google Scholar] [CrossRef]
- Gamborg, O.L.; Wetter, L.R. Plant Tissue Culture Methods. Q. Rev. Biol. 1975, 51, 316. [Google Scholar]
- Gu, S.; Zheng, H.; Xu, Q.; Sun, C.; Shi, M.; Wang, Z.; Li, F. Comparative Toxicity of the Plasticizer Dibutyl Phthalate to Two Freshwater Algae. Aquat. Toxicol. 2017, 191, 122–130. [Google Scholar] [CrossRef]
- Santos, J.; Barreto, A.; Sousa, É.M.L.; Calisto, V.; Amorim, M.J.B.; Maria, V.L. The Role of Nanoplastics on the Toxicity of the Herbicide Phenmedipham, Using Danio Rerio Embryos as Model Organisms. Environ. Pollut. 2022, 303, 119166. [Google Scholar] [CrossRef]
- Su, S.; Zhou, Y.; Qin, J.G.; Yao, W.; Ma, Z. Optimization of the Method for Chlorophyll Extraction in Aquatic Plants. J. Freshw. Ecol. 2010, 25, 531–538. [Google Scholar] [CrossRef]
- Zhang, J.; Han, C.; Liu, Z. Absorption Spectrum Estimating Rice Chlorophyll Concentration: Preliminary Investigations. J. Plant Breed. Crop Sci. 2009, 1, 223–229. [Google Scholar]
- Esterbauer, H.; Cheeseman, K.H. [42] Determination of Aldehydic Lipid Peroxidation Products: Malonaldehyde and 4-Hydroxynonenal. Methods Enzym. 1990, 186, 407–421. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative Stress and Some Antioxidant Systems in Acid Rain-Treated Bean Plants: Protective Role of Exogenous Polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Popova, A.V.; Borisova, P.; Mihailova, G.; Georgieva, K. Antioxidative Response of Arabidopsis Thaliana to Combined Action of Low Temperature and High Light Illumination When Lutein Is Missing. Acta Physiol. Plant 2022, 44, 10. [Google Scholar] [CrossRef]
- Aebi, H. [13] Catalase In Vitro. Methods Enzym. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Beyer, W.F.; Fridovich, I. Assaying for Superoxide Dismutase Activity: Some Large Consequences of Minor Changes in Conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. The Fluorescence Transient as a Tool to Characterize and Screen Photosynthetic Samples. Probing Photosynth. Mech. Regul. Adapt. 2000, 25, 445–483. [Google Scholar]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the Chlorophyll a Fluorescence Transient. In Chlorophyll a Fluorescence: A Signature of Photosynthesis; Springer: Berlin/Heidelberg, Germany, 2004; pp. 321–362. [Google Scholar]
- Kalaji, H.M.; Schansker, G.; Brestic, M.; Bussotti, F.; Calatayud, A.; Ferroni, L.; Goltsev, V.; Guidi, L.; Jajoo, A.; Li, P. Frequently Asked Questions about Chlorophyll Fluorescence, the Sequel. Photosynth. Res. 2017, 132, 13–66. [Google Scholar] [CrossRef] [PubMed]
- Stirbet, A. Govindjee on the Relation between the Kautsky Effect (Chlorophyll a Fluorescence Induction) and Photosystem II: Basics and Applications of the OJIP Fluorescence Transient. J. Photochem. Photobiol. B 2011, 104, 236–257. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Łukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a Fluorescence as a Tool to Monitor Physiological Status of Plants under Abiotic Stress Conditions. Acta Physiol. Plant 2016, 38, 102. [Google Scholar] [CrossRef]
- Tsimilli-Michael, M. Revisiting JIP-Test: An Educative Review on Concepts, Assumptions, Approximations, Definitions and Terminology. Photosynthetica 2020, 58, 275–292. [Google Scholar] [CrossRef]
- Tsimilli-Michael, M.; Eggenberg, P.; Biro, B.; Köves-Pechy, K.; Vörös, I.; Strasser, R.J. Synergistic and Antagonistic Effects of Arbuscular Mycorrhizal Fungi and Azospirillum and Rhizobium Nitrogen-Fixers on the Photosynthetic Activity of Alfalfa, Probed by the Polyphasic Chlorophyll a Fluorescence Transient OJIP. Appl. Soil Ecol. 2000, 15, 169–182. [Google Scholar] [CrossRef]
- Chaki, M.; Begara-Morales, J.C.; Barroso, J.B. Oxidative Stress in Plants. Antioxidants 2020, 9, 481. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Hossain, M.A.; Da Silva, J.A.T.; Fujita, M. Plant Response and Tolerance to Abiotic Oxidative Stress: Antioxidant Defense Is a Key Factor. In Crop Stress and Its Management: Perspectives and Strategies; Springer: Berlin/Heidelberg, Germany, 2012; pp. 261–315. [Google Scholar] [CrossRef]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M.; Hasanuzzaman, M. Abiotic Stress and Reactive Oxygen Species: Generation, Signaling, and Defense Mechanisms. Antioxidants 2021, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.B.; Hahn, E.-J.; Paek, K.-Y. Effects of Light Intensities on Antioxidant Enzymes and Malondialdehyde Content during Short-Term Acclimatization on Micropropagated Phalaenopsis Plantlet. Environ. Exp. Bot. 2005, 54, 109–120. [Google Scholar] [CrossRef]
- Hossain, M.A.; Bhattacharjee, S.; Armin, S.-M.; Qian, P.; Xin, W.; Li, H.-Y.; Burritt, D.J.; Fujita, M.; Tran, L.-S.P. Hydrogen Peroxide Priming Modulates Abiotic Oxidative Stress Tolerance: Insights from ROS Detoxification and Scavenging. Front. Plant Sci. 2015, 6, 420. [Google Scholar] [CrossRef]
- Batool, R.; Umer, M.J.; Hussain, B.; Anees, M.; Wang, Z. Molecular Mechanisms of Superoxide Dismutase (SODs)-Mediated Defense in Controlling Oxidative Stress in Plants. In Antioxidant Defense in Plants: Molecular Basis of Regulation; Springer: Berlin/Heidelberg, Germany, 2022; pp. 157–179. [Google Scholar]
- Mondal, S.; Bandyopadhyay, A. Antioxidants in Mitigating Phthalate-Induced Male Reproductive Toxicity: A Comprehensive Review. Chemosphere 2024, 364, 143297. [Google Scholar] [CrossRef] [PubMed]
- Oluranti, O.I.; Alabi, B.A.; Michael, O.S.; Ojo, A.O.; Fatokun, B.P. Rutin Prevents Cardiac Oxidative Stress and Inflammation Induced by Bisphenol A and Dibutyl Phthalate Exposure via NRF-2/NF-ΚB Pathway. Life Sci. 2021, 284, 119878. [Google Scholar] [CrossRef]
- Qi, D.U.; Le, X.I.A.; Xiao-guang, W.; Yi, H.A.N.; Jing, W.; Hai-qiu, Y.U. Effects of Potassium Deficiency on Photosynthesis, Chloroplast Ultrastructure, ROS, and Antioxidant Activities in Maize (Zea mays L.). J. Integr. Agric. 2019, 18, 395–406. [Google Scholar] [CrossRef]
- Garcia-Caparros, P.; De Filippis, L.; Gul, A.; Hasanuzzaman, M.; Ozturk, M.; Altay, V.; Lao, M.T. Oxidative Stress and Antioxidant Metabolism under Adverse Environmental Conditions: A Review. Bot. Rev. 2021, 87, 421–466. [Google Scholar] [CrossRef]
- Anjum, S.A.; Tanveer, M.; Ashraf, U.; Hussain, S.; Shahzad, B.; Khan, I.; Wang, L. Effect of Progressive Drought Stress on Growth, Leaf Gas Exchange, and Antioxidant Production in Two Maize Cultivars. Environ. Sci. Pollut. Res. 2016, 23, 17132–17141. [Google Scholar] [CrossRef]
- Nawaz, T.; Fahad, S.; Gu, L.; Xu, L.; Zhou, R. Harnessing Nitrogen-Fixing Cyanobacteria for Sustainable Agriculture: Opportunities, Challenges, and Implications for Food Security. Nitrogen 2025, 6, 16. [Google Scholar] [CrossRef]
- Nawaz, T.; Gu, L.; Fahad, S.; Saud, S.; Bleakley, B.; Zhou, R. Exploring Sustainable Agriculture with Nitrogen-Fixing Cyanobacteria and Nanotechnology. Molecules 2024, 29, 2534. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Schansker, G.; Ladle, R.J.; Goltsev, V.; Bosa, K.; Allakhverdiev, S.I.; Brestic, M.; Bussotti, F.; Calatayud, A.; Dąbrowski, P. Frequently Asked Questions about in Vivo Chlorophyll Fluorescence: Practical Issues. Photosynth. Res. 2014, 122, 121–158. [Google Scholar] [CrossRef] [PubMed]
- Joly, D.; Carpentier, R. The Oxidation/Reduction Kinetics of the Plastoquinone Pool Controls the Appearance of the I-Peak in the O–J–I–P Chlorophyll Fluorescence Rise: Effects of Various Electron Acceptors. J. Photochem. Photobiol. B 2007, 88, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Ripoll, J.; Bertin, N.; Bidel, L.P.R.; Urban, L. A User’s View of the Parameters Derived from the Induction Curves of Maximal Chlorophyll a Fluorescence: Perspectives for Analyzing Stress. Front. Plant Sci. 2016, 7, 232160. [Google Scholar] [CrossRef]
- Tóth, S.Z.; Schansker, G.; Strasser, R.J. A Non-Invasive Assay of the Plastoquinone Pool Redox State Based on the OJIP-Transient. Photosynth. Res. 2007, 93, 193–203. [Google Scholar] [CrossRef]
- Sharma, J.; Shah, G.; Strasser, R.J.; Soni, V. Effects of Malachite Green on Biochemistry and Photosystem II Photochemistry of Eichhornia Crassipes. Funct. Plant Biol. 2023, 50, 663–675. [Google Scholar] [CrossRef]
- Shah, G.; Bhatt, U.; Singh, H.; Chaudhary, H.D.; Soni, V. Phytotoxic Effects of Cigarette Smoke on Indoor Plant Epipremnum Aureum: In Vivo Analysis Using Chlorophyll a Fluorescence Transients. Front. Plant Sci. 2025, 16, 1595713. [Google Scholar] [CrossRef]
- Singh, H.; Kumar, D.; Soni, V. Copper and Mercury Induced Oxidative Stresses and Antioxidant Responses of Spirodela polyrhiza (L.) Schleid. Biochem. Biophys. Rep. 2020, 23, 100781. [Google Scholar] [CrossRef]
- Kumar, D.; Singh, H.; Raj, S.; Soni, V. Chlorophyll a Fluorescence Kinetics of Mung Bean (Vigna radiata L.) Grown under Artificial Continuous Light. Biochem. Biophys. Rep. 2020, 24, 100813. [Google Scholar] [CrossRef]
- Sharma, J.; Singh, H.; Sharma, S.; Kumar, D.; Bhatt, U.; Soni, V. Rhodamine B Induced Alteration in Antioxidant Enzymes and Photosynthetic Performance of Eichhornia Crassipes. Plant Physiol. Rep. 2022, 27, 603–617. [Google Scholar] [CrossRef]
- Anaya, F.; Fghire, R.; Wahbi, S.; Carvalho, I.S.; Loutfi, K. Multifaceted Impact of Exogenous Salicylic Acid on Vicia faba L. Under Salt Stress: Plant Growth, Water Status, and Photosynthetic Performance (OJIP Fluorescence). J. Soil Sci. Plant Nutr. 2025, 25, 6756–6772. [Google Scholar] [CrossRef]
- Jan, M.F.; Li, M.; Liaqat, W.; Altaf, M.T.; Liu, C.; Ahmad, H.; Khan, E.H.; Ali, Z.; Barutçular, C.; Mohamed, H.I. Chlorophyll Fluorescence: A Smart Tool for Maize Improvement. Cereal Res. Commun. 2024, 53, 617–648. [Google Scholar] [CrossRef]
- Ahmad, N.; Krzesinski, W.; Spychalski, M.; Kukawka, R.; Smiglak, M. Effect of BTHWA Biostimulation on Lettuce Using Chlorophyll Fluorescence, Gas Exchange, and Thermography. Agronomy 2024, 14, 2559. [Google Scholar] [CrossRef]
- Garab, G.; Magyar, M.; Sipka, G.; Lambrev, P.H. New Foundations for the Physical Mechanism of Variable Chlorophyll a Fluorescence. Quantum Efficiency versus the Light-Adapted State of Photosystem II. J. Exp. Bot. 2023, 74, 5458–5471. [Google Scholar] [CrossRef]
- Janeeshma, E.; Johnson, R.; Amritha, M.S.; Noble, L.; Raj Aswathi, K.P.; Telesiński, A.; Kalaji, H.M.; Auriga, A.; Puthur, J.T. Modulations in Chlorophyll a Fluorescence Based on Intensity and Spectral Variations of Light. Int. J. Mol. Sci. 2022, 23, 5599. [Google Scholar] [CrossRef] [PubMed]
- Hassannejad, S.; Lotfi, R.; Ghafarbi, S.P.; Oukarroum, A.; Abbasi, A.; Kalaji, H.M.; Rastogi, A. Early Identification of Herbicide Modes of Action by the Use of Chlorophyll Fluorescence Measurements. Plants 2020, 9, 529. [Google Scholar] [CrossRef]
- Antal, T.K.; Volgusheva, A.A.; Drozdenko, T.V.; Konyukhov, I.V.; Khruschev, S.S.; Chervitsov, R.N.; Plyusnina, T.Y.; Riznichenko, G.Y.; Rubin, A.B. Analysis of Chlorophyll Fluorescence Induction Curves (OJIP Transients) of Phytoplankton under Conditions of High Photosynthetic Activity. J. Appl. Phycol. 2025, 37, 873–884. [Google Scholar] [CrossRef]
- Xia, Q.; Tang, H.; Tan, J.L.; Allakhverdiev, S.I.; Guo, Y. Determination of Rice (Oryza sativa L.) Drought Stress Levels Based on Chlorophyll a Fluorescence through Independent Component Analysis. Photosynthetica 2025, 63, 73. [Google Scholar] [CrossRef]
- Chen, X.; Liu, X.; Cong, Y.; Jiang, Y.; Zhang, J.; Yang, Q.; Liu, H. Melatonin Alleviates Photosynthetic Injury in Tomato Seedlings Subjected to Salt Stress via OJIP Chlorophyll Fluorescence Kinetics. Plants 2025, 14, 824. [Google Scholar] [CrossRef]
- Tomaškinová, J.; Tomaškin, J.; Drimal, M.; Bellido, J. The Impact of Abiotic Environmental Stressors on Fluorescence and Chlorophyll Content in Glycine max (L.) Merrill. Agronomy 2025, 15, 263. [Google Scholar] [CrossRef]
- Gan, T.; Yin, G.; Zhao, N.; Tan, X.; Wang, Y. A Sensitive Response Index Selection for Rapid Assessment of Heavy Metals Toxicity to the Photosynthesis of Chlorella Pyrenoidosa Based on Rapid Chlorophyll Fluorescence Induction Kinetics. Toxics 2023, 11, 468. [Google Scholar] [CrossRef] [PubMed]
- Pietrini, F.; Iannilli, V.; Passatore, L.; Carloni, S.; Sciacca, G.; Cerasa, M.; Zacchini, M. Ecotoxicological and Genotoxic Effects of Dimethyl Phthalate (DMP) on Lemna minor L. and Spirodela polyrhiza (L.) Schleid. Plants under a Short-Term Laboratory Assay. Sci. Total Environ. 2022, 806, 150972. [Google Scholar] [CrossRef]
- Sharma, S.; Bhatt, U.; Sharma, J.; Darkalt, A.; Mojski, J.; Soni, V. Effect of Different Waterlogging Periods on Biochemistry, Growth, and Chlorophyll a Fluorescence of Arachis hypogaea L. Front. Plant Sci. 2022, 13, 1006258. [Google Scholar]
- Goltsev, V.N.; Kalaji, H.M.; Paunov, M.; Bąba, W.; Horaczek, T.; Mojski, J.; Kociel, H.; Allakhverdiev, S.I. Variable Chlorophyll Fluorescence and Its Use for Assessing Physiological Condition of Plant Photosynthetic Apparatus. Russ. J. Plant Physiol. 2016, 63, 869–893. [Google Scholar] [CrossRef]
- Mosadegh, H.; Trivellini, A.; Lucchesini, M.; Ferrante, A.; Maggini, R.; Vernieri, P.; Mensuali Sodi, A. UV-B Physiological Changes under Conditions of Distress and Eustress in Sweet Basil. Plants 2019, 8, 396. [Google Scholar] [CrossRef] [PubMed]
- Zuo, G. Non-photochemical Quenching (NPQ) in Photoprotection: Insights into NPQ Levels Required to Avoid Photoinactivation and Photoinhibition. New Phytol. 2025, 246, 1967–1974. [Google Scholar] [CrossRef]
- Cetner, M.D.; Kalaji, H.M.; Borucki, W.; Kowalczyk, K. Phosphorus Deficiency Affects the I-Step of Chlorophyll a Fluorescence Induction Curve of Radish. Photosynthetica 2020, 58, 671–681. [Google Scholar] [CrossRef]
- Stirbet, A.; Lazár, D.; Kromdijk, J. Govindjee Chlorophyll a Fluorescence Induction: Can Just a One-Second Measurement Be Used to Quantify Abiotic Stress Responses? Photosynthetica 2018, 56, 86–104. [Google Scholar] [CrossRef]
- Bhattacharya, A. Effect of Low Temperature Stress on Photosynthesis and Allied Traits: A Review. Physiol. Process. Plants Under Low. Temp. Stress. 2022, 199–297. [Google Scholar]
- Strasserf, R.J.; Srivastava, A. Govindjee Polyphasic Chlorophyll a Fluorescence Transient in Plants and Cyanobacteria. Photochem. Photobiol. 1995, 61, 32–42. [Google Scholar] [CrossRef]
- Gupta, R. The Oxygen-Evolving Complex: A Super Catalyst for Life on Earth, in Response to Abiotic Stresses. Plant Signal Behav. 2020, 15, 1824721. [Google Scholar] [CrossRef]
- Ali, N.A.; Dewez, D.; Didur, O.; Popovic, R. Inhibition of Photosystem II Photochemistry by Cr Is Caused by the Alteration of Both D1 Protein and Oxygen Evolving Complex. Photosynth. Res. 2006, 89, 81–87. [Google Scholar] [CrossRef]
- Gupta, S.K.; Sharma, M.; Deeba, F.; Pandey, V. Role of Reactive Oxygen Species in Photophosphorylation and Damage to D1 Protein: Past and Present. React. Oxyg. Species Plants Boon Or. Bane-Revisit Role ROS 2017, 165–186. [Google Scholar]
- Bhatt, U.; Sharma, S.; Kalaji, H.M.; Strasser, R.J.; Chomontowski, C.; Soni, V. Sunlight-Induced Repair of Photosystem II in Moss Semibarbula Orientalis under Submergence Stress. Funct. Plant Biol. 2023, 50, 777–791. [Google Scholar] [CrossRef]
- Shah, G.; Bhatt, U.; Singh, H.; Kumar, D.; Sharma, J.; Strasser, R.J.; Soni, V. Ecotoxicological Assessment of Cigarette Butts on Morphology and Photosynthetic Potential of Azolla pinnata. BMC Plant Biol. 2024, 24, 300. [Google Scholar] [CrossRef]
- Liu, H.; Zheng, D.; Liu, X.; Hou, J.; Wu, Q.; Li, Y. Environmental Microplastic and Phthalate Esters Co-Contamination, Interrelationships, Co-Toxicity and Mechanisms. A Review. Environ. Geochem. Health 2024, 46, 525. [Google Scholar] [CrossRef]
- Yuan, L.; Liu, J.; Huang, Y.; Shen, G.; Pang, S.; Wang, C.; Li, Y.; Mu, X. Integrated Toxicity Assessment of DEHP and DBP toward Aquatic Ecosystem Based on Multiple Trophic Model Assays. Environ. Sci. Pollut. Res. 2022, 29, 87402–87412. [Google Scholar] [CrossRef]
- Li, W.-Y.; Chen, Y.; Wang, W.-L.; Chen, Y.-L.; Wu, Q.-Y. The Impact of Dissolved Organic Matter in Natural Receiving Systems on the Formation Potential and Toxicity of Disinfection By-Products: Insights from Origins, Chemical Properties, and Transformations. Curr. Pollut. Rep. 2025, 11, 29. [Google Scholar] [CrossRef]
- Strasser, R.J.; Krüger, G.H.J.; Berner, J.M.; Scheepers, C.C.W. Differential Response of Photosynthetic Electron Transport and CO2 Assimilation in Sensitive (S156) and Resistant (R123) Phaseolus vulgaris L. (Bush Bean) Genotypes to Chronic Ozone Exposure. Suid-Afr. Tydskr. Vir. Natuurwetenskap En. Tegnol. 2018, 37, 1–12. [Google Scholar]
- Singh, H.; Kumar, D.; Soni, V. Performance of Chlorophyll a Fluorescence Parameters in Lemna minor under Heavy Metal Stress Induced by Various Concentration of Copper. Sci. Rep. 2022, 12, 10620. [Google Scholar] [CrossRef]
- Sarkar, A.; Gogoi, N.; Roy, S. Bisphenol-A Incite Dose-Dependent Dissimilitude in the Growth Pattern, Physiology, Oxidative Status, and Metabolite Profile of Azolla Filiculoides. Environ. Sci. Pollut. Res. 2022, 29, 91325–91344. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, U.; Sharma, S.; Kumar, D.; Soni, V. Impact of Streetlights on Physiology, Biochemistry and Diversity of Urban Bryophyte: A Case Study on Moss Semibarbula Orientalis. J. Urban Ecol. 2022, 8, juac019. [Google Scholar] [CrossRef]
- Soni, V.; Bhatt, U.; Tailor, P.; Strasser, R.J. Impact of Synthetic and Herbal Dyes on Photosynthesis and ROS Scavenging Enzyme Activities in Spirodela Polyrhiza. Sci. Rep. 2025, 15, 24775. [Google Scholar] [CrossRef]
- Strasser, R. On the OJIP Fluorescence Transient in Leaves and D1 Mutants of Chlamydomonas Reinhardtii. Research in Photosynthesis. In Proceedings of the IXth International Congress on Photosynthesis, Nagoya, Japan, 30 August–5 September 1992; Volume 2. [Google Scholar]

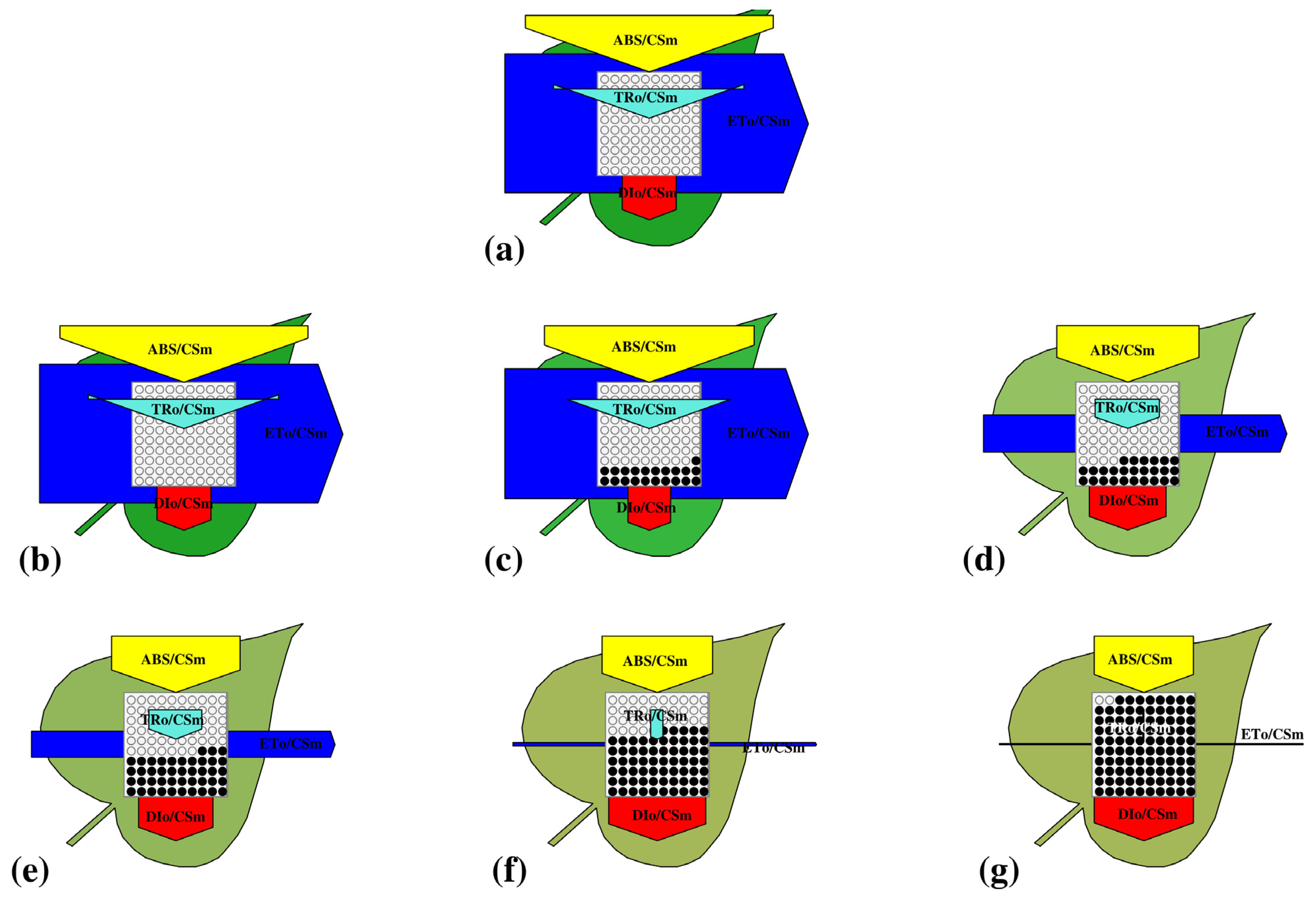
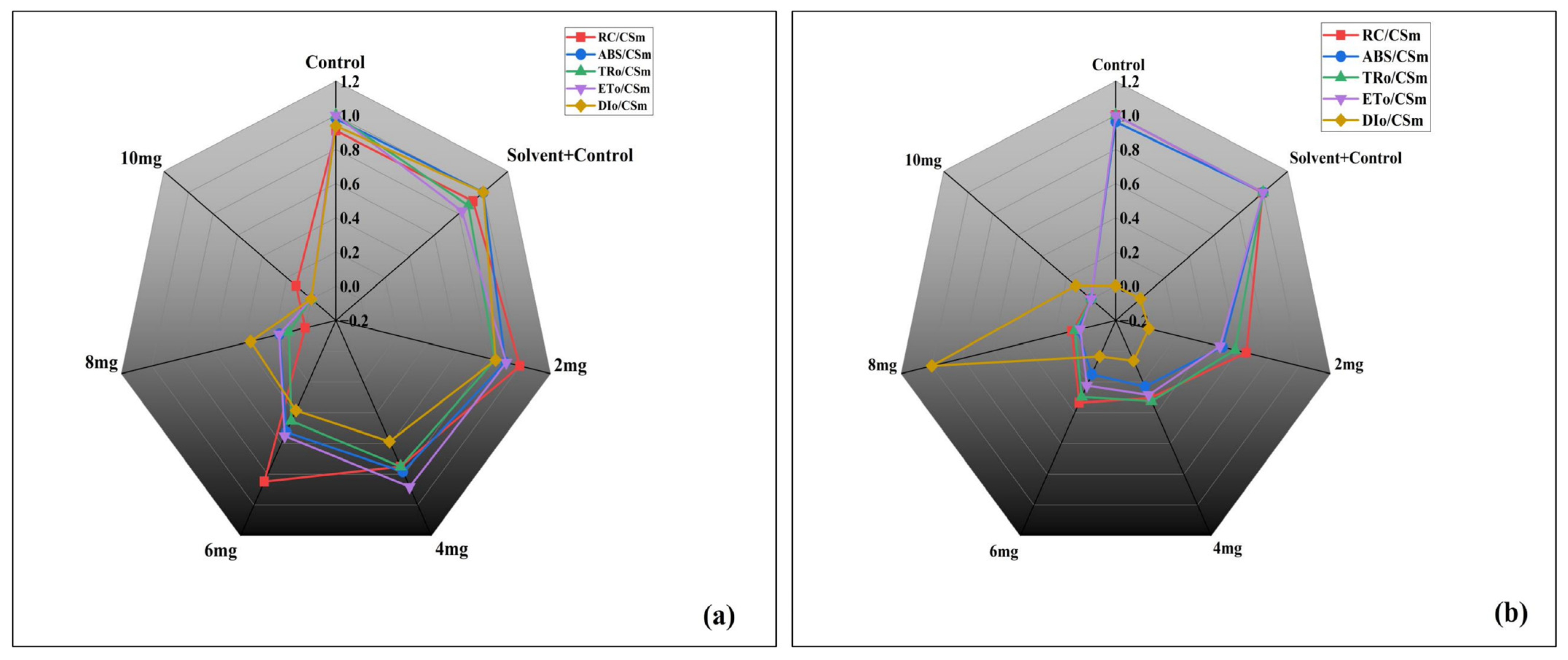

| S.N. | Dynamics | Symbol | Formula | Definitions |
|---|---|---|---|---|
| Fluorescence Parameters | ||||
| 1. | Minimal fluorescence | Fo | F0 ≅ F50μs | The minimum fluorescence signal was recorded at nearly 50 μs with the PEA fluorimeter, whereas the Handy-PEA instrument detected it earlier, at around 20 μs. |
| Maximal fluorescence | Fm | Fm ≅ FP | The maximum fluorescence level associated with the P-step of the OJIP transient. | |
| Variable Fluorescence | Fv | Fm − Fo | The potential or peak variable fluorescence component that indicates the photochemical capacity of PSII. | |
| Density of Active PSII RCs per Cross-Section | ||||
| 2. | Density of active PSII reaction centres per cross-section | RC/CSm | Density of operational PSII centres relative to a unit of excited cross-section. | |
| Quantum Efficiency Parameters | ||||
| 3. | Quantum yield for primary photochemistry | PHI(Po) | The highest quantum yield of primary photochemical reactions. | |
| 4. | Quantum yield of electron transfer | PHI(Eo) | Quantum efficiency of electron transport beyond QA− | |
| 5. | Quantum yield of dissipation | PHI(Do) | Quantum yield of excitation energy dissipated as heat and fluorescence | |
| Specific energy fluxes | ||||
| 6. | Absorption per reaction centre | ABS/CSm | Effective absorption load borne by each active PSII reaction centre | |
| 7. | Trapping per reaction centre | TRo/RC | Flux of absorbed photons that are effectively trapped and drive primary charge separation at one RC | |
| 8. | Electron transfer per reaction centre) | ETo/RC | Electrons captured through photochemistry and transferred beyond QA, expressed per active PSII reaction centre. | |
| 9. | Dissipation per reaction centre | DIo/RC | Non-photochemical dissipation of energy (as heat or fluorescence) expressed per reaction centre. | |
| Phenomenological energy fluxes | ||||
| 10. | Absorption per cross-section | ABS/CSm | Photon absorption per unit illuminated cross-section, representing the total excitonic input to PSII antenna pigments. | |
| 11. | Trapping per cross-section | TRo/CSm | Excitation energy successfully captured and used for primary photochemistry, expressed per unit cross-section. | |
| 12. | Electron transfer per cross-section | ETo/CSm | Electron flux beyond QA− normalized to a cross-section, indicating PSII’s areal electron transport capacity. | |
| 13. | Dissipation per cross-section | DIo/CSm | Proportion of absorbed energy dissipated as heat or fluorescence per cross-section, representing non-photochemical energy loss on an areal basis. | |
| De-excitation rate constants of PSII antenna | ||||
| 14. | Non-photochemical de-excitation rate constant | Kn | The rate constant for non-photochemical energy dissipation, with Kf representing the corresponding constant for photon re-emission as fluorescence. | |
| 15. | photochemical de-excitation rate constant | Kp | Photochemical quenching (Kp) refers to the process by which absorbed light energy in chlorophyll is used for photochemistry in photosystem II, mainly driving electron transport. It reflects the efficiency of open PSII reaction centers in utilizing excitation energy for photosynthesis. | |
| De-excitation rate constants of PSII antenna | ||||
| 16. | Performance index on an absorption basis | PIabs | An integrated parameter reflecting the potential efficiency of excitonic energy conversion into electron flow past QA into the intersystem electron transport pathway. | |
| 17. | Performance index on cross section basis | PIcsm | Performance index per cross-section, combining light absorption, excitation trapping, and electron transport efficiency. | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaudhary, H.D.; Bhatt, U.; Soni, V. Chlorophyll Fluorescence and Biochemical Biomarkers Reveal Plasticizer Di-n-Butyl Phthalate-Induced Stress in Azolla pinnata. Plants 2025, 14, 3629. https://doi.org/10.3390/plants14233629
Chaudhary HD, Bhatt U, Soni V. Chlorophyll Fluorescence and Biochemical Biomarkers Reveal Plasticizer Di-n-Butyl Phthalate-Induced Stress in Azolla pinnata. Plants. 2025; 14(23):3629. https://doi.org/10.3390/plants14233629
Chicago/Turabian StyleChaudhary, Hari Dev, Upma Bhatt, and Vineet Soni. 2025. "Chlorophyll Fluorescence and Biochemical Biomarkers Reveal Plasticizer Di-n-Butyl Phthalate-Induced Stress in Azolla pinnata" Plants 14, no. 23: 3629. https://doi.org/10.3390/plants14233629
APA StyleChaudhary, H. D., Bhatt, U., & Soni, V. (2025). Chlorophyll Fluorescence and Biochemical Biomarkers Reveal Plasticizer Di-n-Butyl Phthalate-Induced Stress in Azolla pinnata. Plants, 14(23), 3629. https://doi.org/10.3390/plants14233629





