Transcription Factor GmMYB29 Activates GmPP2C-37like Expression to Mediate Soybean Defense Against Heterodera glycines Race 3
Abstract
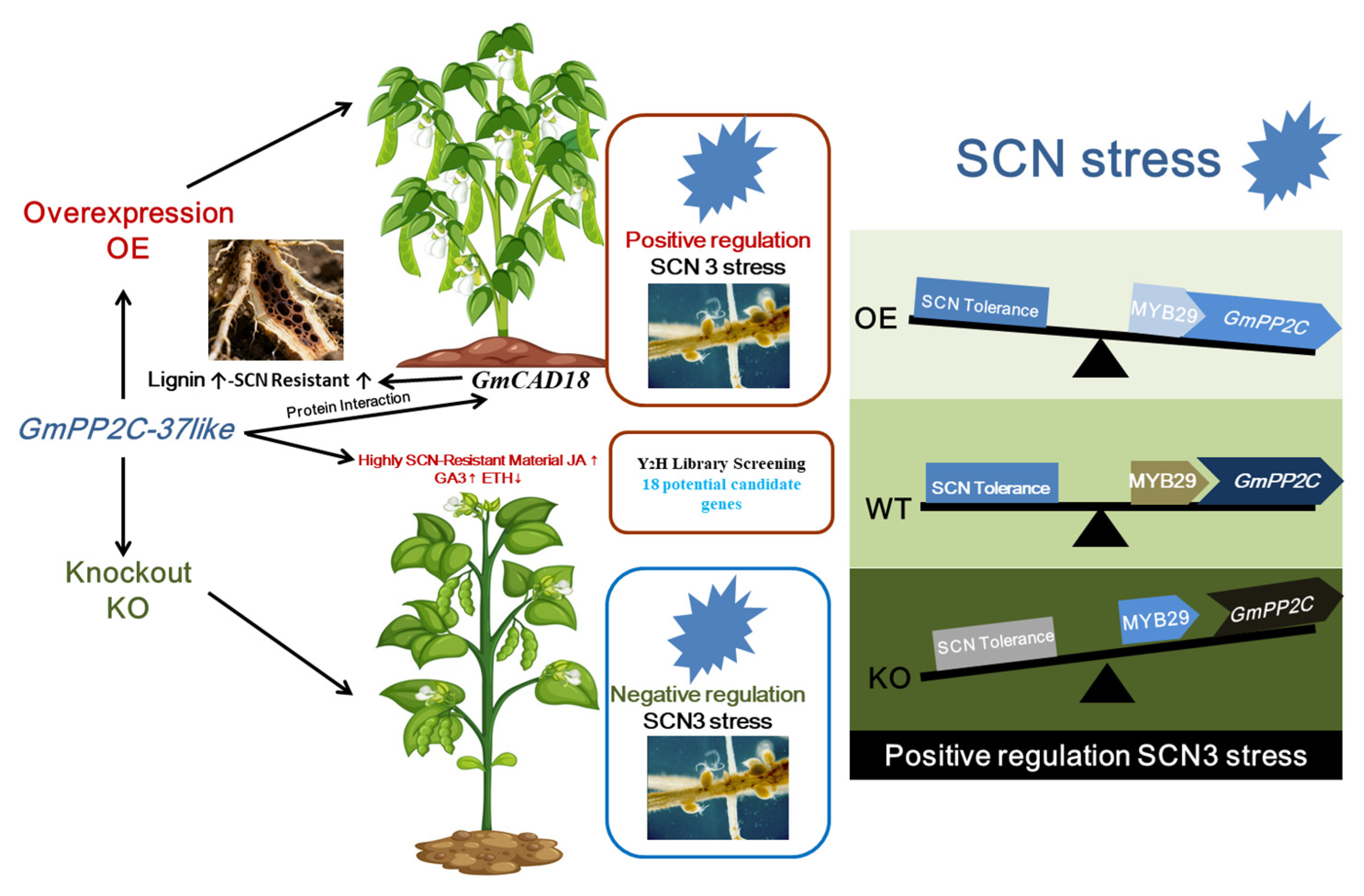
1. Introduction
2. Results
2.1. CHIP-Seq Sequencing Analysis of the GmMYB29 Strain
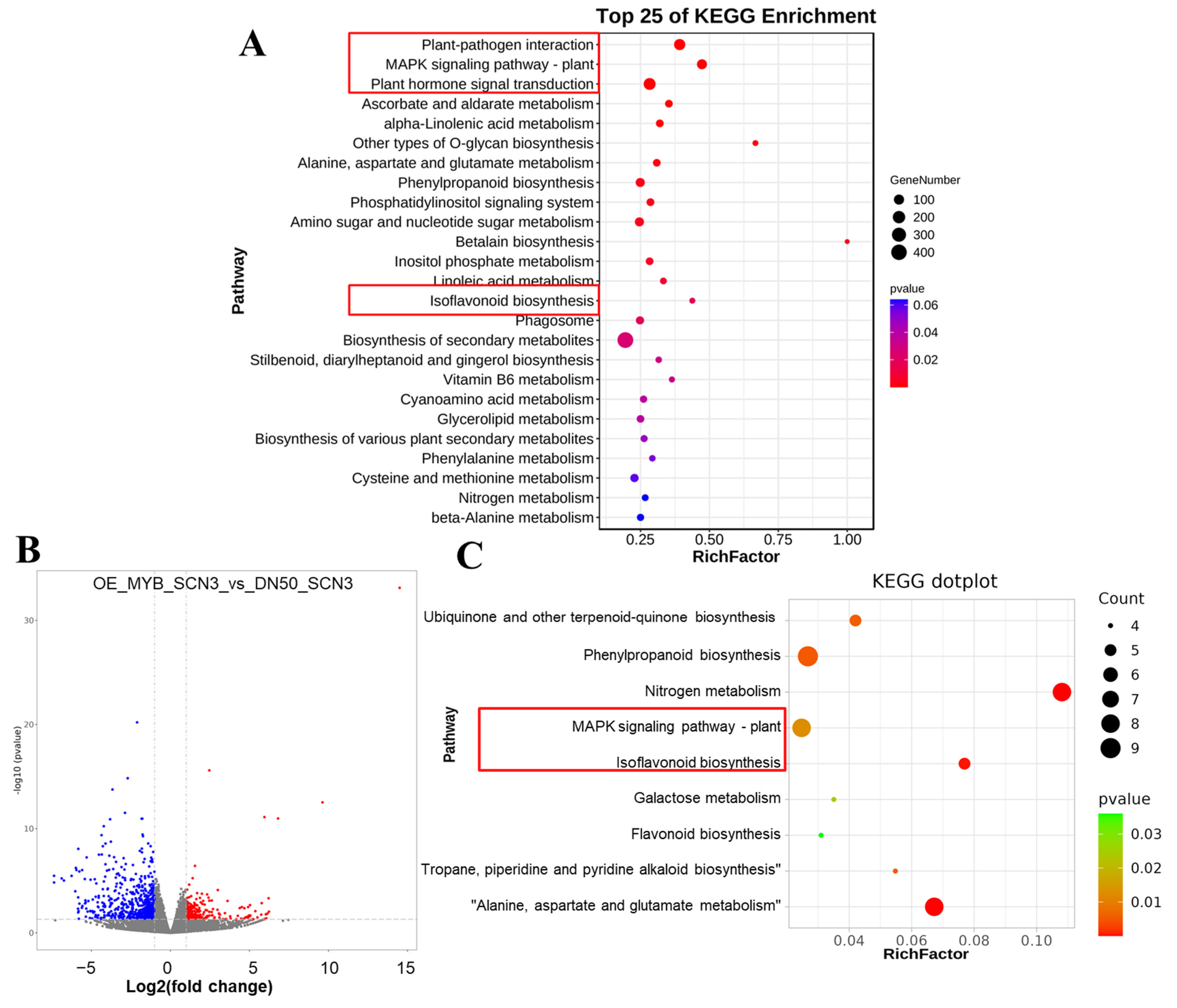
2.2. GO and KEGG Enrichment Analysis
2.3. RNA-Seq & ChIP-Seq Integrative Analysis
2.4. Enrichment Analysis to Validate Candidate Genes
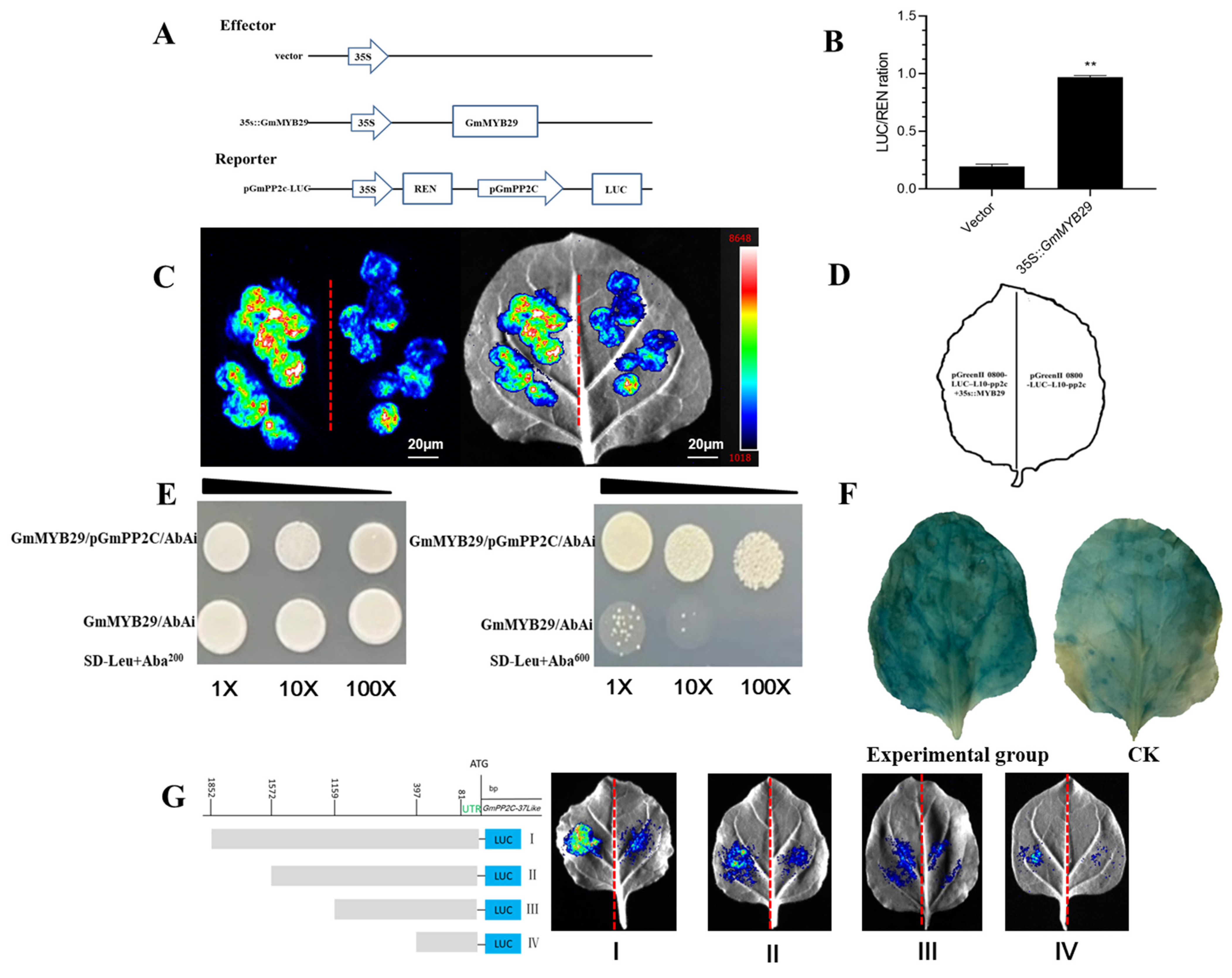
2.5. Luciferase Complementation Assay
2.6. Promoter Truncation Analysis Identifies the GmMYB29-Binding Motif in the LUC11 Promoter
2.7. Yeast One-Hybrid
2.8. GUS Staining Assay Confirms Specific Binding of GmMYB29 to the GmPP2C-37like Promoter
2.9. qRT-PCR Analysis Reveals Stress- and Hormone-Induced Expression of GmPP2C-37like in Soybean
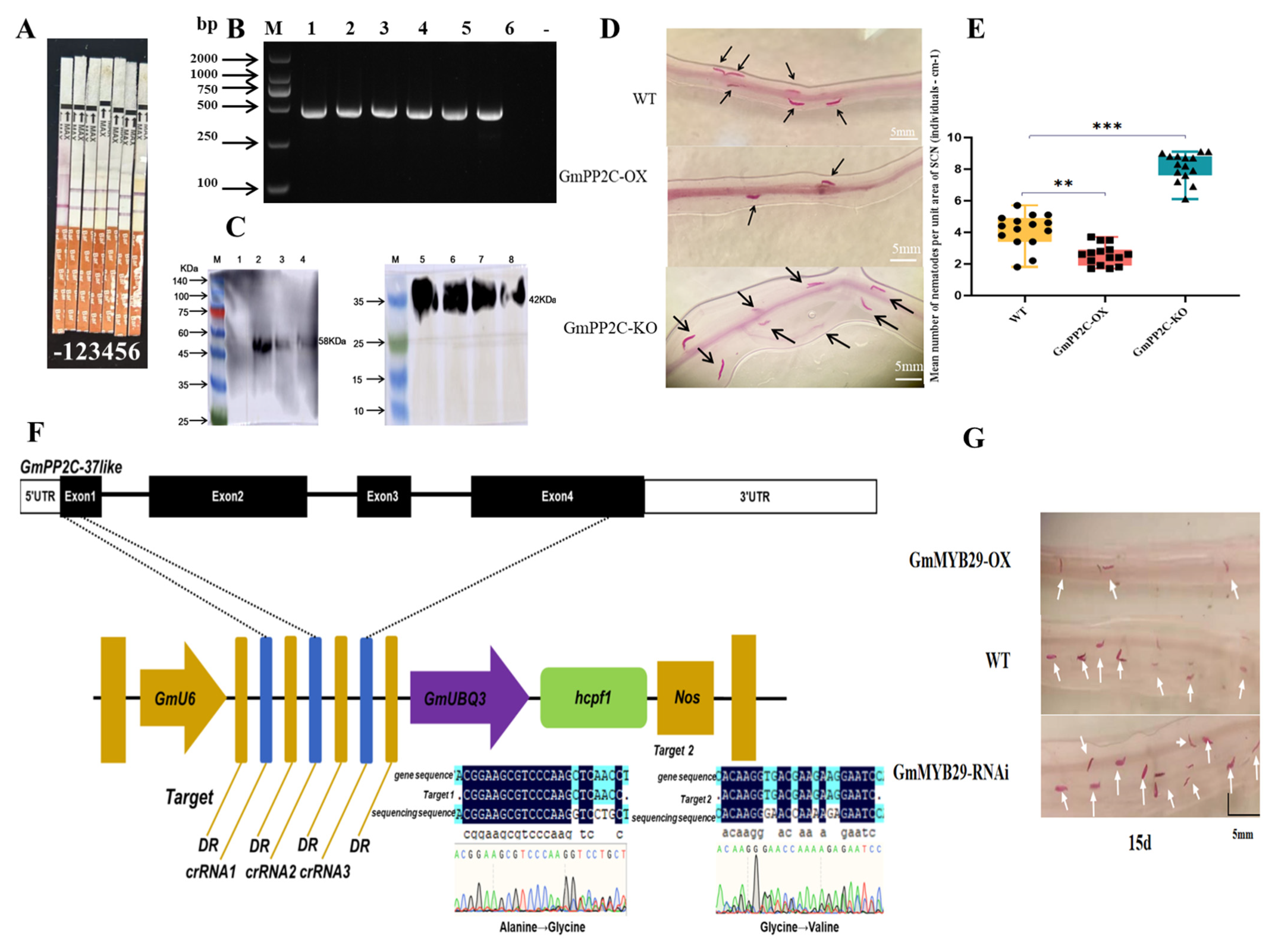
2.10. Bioinformatics Analysis and Phylogenetic Tree Analysis of GmPP2C-37like
2.11. Analysis of Resistance to Soybean Cyst Nematode (SCN)
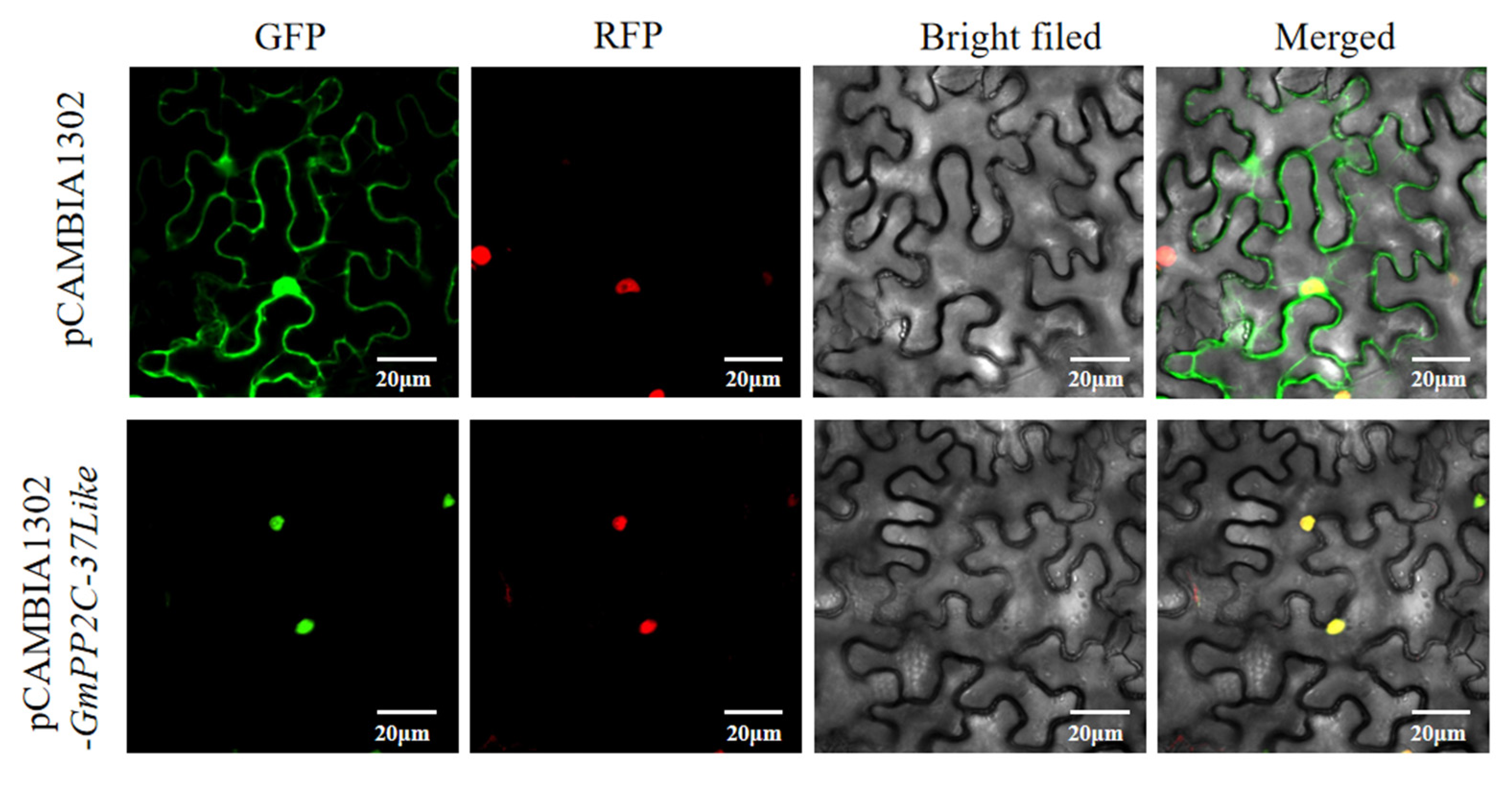
2.12. Subcellular Localization of GmPP2C-37like: Observation via Laser Confocal Microscopy in Agrobacterium-Infiltrated Plants
2.13. Yeast Two-Hybrid Library Screening Analysis
3. Discussion
4. Materials and Methods
4.1. Materials
4.1.1. Plant Material
4.1.2. Diseased Soil Material
4.2. Methods
4.2.1. CHIP-Seq and RNA-Seq Sequencing of GmMYB29 Strain
4.2.2. KEGG and GO Enrichment
4.2.3. RNA-Seq and CHIP-Seq Joint Analysis
4.2.4. Dual-Luciferase Assay
4.2.5. Promoter DNA Truncation Analysis
4.2.6. Yeast One-Hybrid Study
4.2.7. GUS Staining Analysis
4.2.8. Analysis of GmPP2C-37like Gene Expression Pattern
4.2.9. The Construction of Vectors Related to GmPP2C-37like and GmMYB29
4.2.10. Bioinformatics Analysis and Phylogenetic Tree of GmPP2C-37like
4.2.11. Identification of Agrobacterium K599 Transformed Soybean Hairy Roots and SCN
4.2.12. Subcellular Localization of GmPP2C-37like
4.2.13. Yeast Two-Hybrid Library Screen
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qu, S.; Liu, F.; Song, G.C.; Hu, S.H.; Fang, Y.Y.; Zhao, X.; Han, Y.P. Soybean Cyst Nematode Resistant Zinc Finger Protein Gene GmC2H2-2like Cloning and Preliminary Functional Analysis. Acta Agric. Boreali-Sin. 2024, 39, 195–207. [Google Scholar] [CrossRef]
- Ma, H.; Bai, D.; Li, J.; Zhao, Y. Prediction and Analysis of China’s Soybean Consumption and Production in 2021–2025. Soybean Sci. 2022, 41, 358–362. [Google Scholar] [CrossRef]
- Cao, G.; Zhao, X.; Wang, Q.; Meng, X.; Wei, S.; Han, Y.; Wu, X.; Li, W. Resistance Evaluation of Soybean Germplasm to Races 1, 3 and 4 of Soybean Cyst Nematode (Heterodera giycines). Soybean Sci. 2014, 33, 563–565. [Google Scholar] [CrossRef]
- Dong, J.; Hudson, M.E. WI12Rhg1 interacts with DELLAs and mediates soybean cyst nematode resistance through hormone pathways. Plant Biotechnol. J. 2022, 20, 283–296. [Google Scholar] [CrossRef]
- Riggs, R.D.; Schmitt, D.P. Complete Characterization of the Race Scheme for Heterodera glycines. J. Nematol. 1988, 20, 392–395. [Google Scholar]
- Guo, Y.; Chen, L.; Jiang, H.; Zhan, Y. Cloning and expression analysis of soybean cyst nematode resistance candidate gene GmPEBP4-1. J. Northeast Agric. Univ. 2020, 51, 10–19. [Google Scholar] [CrossRef]
- Winter, S.M.; Shelp, B.J.; Anderson, T.R.; Welacky, T.W.; Rajcan, I. QTL associated with horizontal resistance to soybean cyst nematode in Glycine soja PI464925B. Theor. Appl. Genet. 2007, 114, 461–472. [Google Scholar] [CrossRef]
- Cook, D.E.; Lee, T.G.; Lee, X.; Melito, S.; Wang, K.; Bayless, A.M.; Wang, J.; Hughes, T.J.; Willis, D.K.; Clemente, T.E.; et al. Copy number variation of multiple genes at Rhg1 mediates nematode resistance in soybean. Science 2012, 338, 1206–1209. [Google Scholar] [CrossRef]
- Liu, S.; Kandoth, P.K.; Warren, S.D.; Yeckel, G.; Heinz, R.; Alden, J.; Yang, C.; Jamai, A.; El-Mellouki, T.; Juvale, P.S.; et al. A soybean cyst nematode resistance gene points to a new mechanism of plant resistance to pathogens. Nature 2012, 492, 256–260. [Google Scholar] [CrossRef]
- Fuchs, S.; Grill, E.; Meskiene, I.A. Schweighofer, Type 2C protein phosphatases in plants. FEBS J. 2013, 280, 681–693. [Google Scholar] [CrossRef]
- Xue, T.; Xue, D.; Zhang, S.; Ehlting, J.; Ni, F.; Jakab, S.; Zheng, C.; Zhong, Y. Genome-wide and expression analysis of protein phosphatase 2C in rice and Arabidopsis. BMC Genom. 2008, 9, 550. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Yuan, S.; Chen, J.; Chen, Y.; Li, Z.; Lin, W.; Zhang, Y.; Liu, J.; Lin, W. Molecular evolution and lineage-specific expansion of the PP2C family in Zea mays. Planta 2019, 250, 1521–1538. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Ke, H.; Hu, C.; Naseri, E.; Haider, M.S.; Ayaz, A.; Amjad, K.W.; Wang, J.; Hou, X. Genome-Wide Identification, Evolution, and Transcriptional Profiling of PP2C Gene Family in Brassica rapa. Biomed. Res. Int. 2019, 2019, 2965035. [Google Scholar] [CrossRef]
- Singh, A.; Pandey, A.; Srivastava, A.K.; Tran, L.S.; Pandey, G.K. Plant protein phosphatases 2C: From genomic diversity to functional multiplicity and importance in stress management. Crit. Rev. Biotechnol. 2016, 36, 1023–1035. [Google Scholar] [CrossRef]
- Ou, X.; Li, T.; Zhao, Y.; Chang, Y.; Wu, L.; Chen, G.; Day, B.; Jiang, K. Calcium-dependent ABA signaling functions in stomatal immunity by regulating rapid SA responses in guard cells. J. Plant Physiol. 2022, 268, 153585. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.W.; Baek, W.; Jung, J.; Kim, J.-H.; Sung, C.L. Function of ABA in Stomatal Defense against Biotic and Drought Stresses. Int. J. Mol. Sci. 2015, 16, 15251–15270. [Google Scholar] [CrossRef]
- Schweighofer, A.; Kazanaviciute, V.; Scheikl, E.; Teige, M.; Doczi, R.; Hirt, H.; Schwanninger, M.; Kant, M.; Schuurink, R.; Mauch, F.; et al. The PP2C-type phosphatase AP2C1, which negatively regulates MPK4 and MPK6, modulates innate immunity, jasmonic acid, and ethylene levels in Arabidopsis. Plant Cell 2007, 19, 2213–2224. [Google Scholar] [CrossRef]
- Park, C.J.; Peng, Y.; Chen, X.; Christopher, D.; DeLing, R.; Rebecca, B.; Patrick, E.C.; Pamela, C.R. Rice XB15, a protein phosphatase 2C, negatively regulates cell death and XA21-mediated innate immunity. PLoS Biol. 2008, 6, e281. [Google Scholar] [CrossRef]
- Jiang, H.; Qu, S.; Liu, F.; Sun, H.; Li, H.; Teng, W.; Zhan, Y.; Li, Y.; Han, Y.; Zhao, X. Multi-omics analysis identified the GmUGT88A1 gene, which coordinately regulates soybean resistance to cyst nematode and isoflavone content. Plant Biotechnol. J. 2025, 23, 1291–1307. [Google Scholar] [CrossRef]
- Fujita, K.; Miura, O. On the parasites of Heterodera schachtii Schmidt on beans. Trans. Sapporo Nat. Hist. Soc. 1934, 13, 359–364. [Google Scholar]
- Ross, J.P.; Brim, C.A. Resistance of soybeans to the soybean cyst nematode as determined by a double-row method. Plant Dis. Rep. 1957, 41, 923. [Google Scholar]
- Ross, J.P. Physiological strains Heterodera glycines. Plant Dis. Rep. 1962, 46, 766–779. [Google Scholar]
- Dia, K.A. Occurrence of Heterodera glycines from the Golden Island, Giza, UAR. Nematologica 1968, 14, 148. [Google Scholar] [CrossRef]
- Meng, F.L.; Yu, J.Y.; Li, C.J.; Huang, M.H.; Zhao, L.; Wang, X.; Jiang, Y.; Qin, R.F.; Wang, C.L. Research Progress on Occurrence and Control Technology of Soybean Cyst Nematode Disease in Northeast China. J. Northeast Agric. Univ. 2022, 53, 87–94. [Google Scholar] [CrossRef]
- Peng, D.; Peng, H.; Wu, D.; Huang, W.; Ye, W.; Cui, J. First report of soybean cyst nematode (Heterodera glycines) on soybean from Gansu and Ningxia China. Plant Dis. 2016, 100, 229. [Google Scholar] [CrossRef]
- Zhou, C.; Wu, Y.; Yu, J.; Liu, B.; Li, J.; Ma, L.; Chen, G. Evaluation of SCN 3 Resistance Phenotype of soybean Germplasm Resources in Northeast China. Heilongjiang Agric. Sci. 2023, 46, 47–51. [Google Scholar] [CrossRef]
- Kim, D.G.; Riggs, R.D.; Robbins, R.T.; Rakes, L. Distribution of Races of Heterodera glycines in the Central United States. J. Nematol. 1997, 29, 173–179. [Google Scholar] [PubMed]
- Epps, J.M. Soybean cyst nematode found in Tennessee. Plant Dis. Rep. 1956, 42, 594–595. [Google Scholar]
- Winstead, N.N.; Skotland, C.B.; Sasser, J.N. Soybean cyst nematode in North Carolina. Plant Dis. Rep. 1955, 39, 9–11. [Google Scholar]
- Sindermann, A.; Williams, G.; Sardanelli, S.; Krusbergl, L.R. Survey for Heterodera glycines in Maryland. J. Nematol. 1993, 25, 887–889. [Google Scholar]
- Sim, T.; Todd, T.C. First field observation of the soybean cyst nematode in Kansas. Plant Dis. 1986, 70, 603. [Google Scholar] [CrossRef]
- Tylka, G.; Marett, C.C. Distribution of the Soybean Cyst Nematode, Heterodera glycines, in the United States and Canada: 1954 to 2014. Plant Health Prog. 2014, 15, 85–87. [Google Scholar] [CrossRef]
- Powers, T.O.; Wysong, D.S. First report of soybean cyst nematode (Heterodera glycines) in Nebrask. Plant Dis. 1987, 71, 1146. [Google Scholar] [CrossRef]
- Hegee, A.H. Soybean cyst nematode, Heterodera glycines, in Missouri. Plant Dis. Rep. 1957, 41, 201. [Google Scholar]
- Hirschmann, H. Comparative morphological studies on the soybean cyst nematode, Heterodera glycines and the clover cyst nematode, H. trifolii (Nematoda: Heteroderidae). Proc. Helminthol. Soc. Wash. 1956, 23, 140–151. [Google Scholar]
- Gomez, T.J.; Medina, C. Heterodera glycines in soyabeans and dry beans in the Cauca Valley, Colombia. Nematropica 1983, 13, 229–237. [Google Scholar]
- Niblack, T.L. Soybean Cyst Nematode Management Reconsidered. Plant Dis. 2005, 89, 1020–1026. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, Q.; Wang, H.; Wang, C. Development of study and prospect of rectant breeding of SCN. J. Northeast Agric. Univ. 2002, 33, 384–390. [Google Scholar]
- Liu, B.; Li, Y.; Yu, B.; Wang, J.; Liu, Y.; Chang, R.; Qiu, L. Genetic Analysis of Immunity to Soybean Cyst Nematode Race 3 in Elite Line Zhongpin 03-5373. Acta Agron. Sin. 2015, 41, 15–21. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, R.; Wang, L.; Yan, Q.; Chen, P.; Li, Q. Study on Soybean Breeding for Resistance to Cyst Nematode. Sci. Agric. Sin. 2002, 35, 476–481. [Google Scholar]
- Tirumalaraju, S.V.; Jain, M.; Gallo, M. Differential gene expression in roots of nematode-resistant and -susceptible peanut (Arachis hypogaea) cultivars in response to early stages of peanut root-knot nematode (Meloidogyne arenaria) parasitization. J. Plant Physiol. 2010, 168, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Duan, Y.; Wang, J.; Li, J.; Chen, L. Rotation Crop Evaluation for Management of the Soybean Cyst Nematode. Soybean Sci. 2009, 2, 256–259. [Google Scholar]
- Xu, Y.; Chen, Y.; Si, Z.; Li, Z.; Li, C.; Wen, G. The effects of the root diffusate of different crops from different rotation systems on the egg hatch of soybean cyst nematode Heterodera glycines. Acta Phytopathol. Sin. 2004, 34, 526–529. [Google Scholar] [CrossRef]
- Jackson, T.A.; Smith, G.S.; Niblack, T.L. Heterodera glycines Infectivity and Egg Viability Following Nonhost Crops and During Overwintering. J. Nematol. 2005, 37, 259–264. [Google Scholar] [PubMed Central]
- Si, Z. The Effect of Eniroment Situation on Soybean Cyst Nematode Hatch and the Mechanism of Soybean Resisting Soybean Cyst Nematode. Master’s Thesis, Northeast Agricultural University, Harbin, China, 2004. [Google Scholar]
- Hu, W.; Kidane, E.; Neher, D.A.; Chen, S. Field and greenhouse evaluations of soil suppressiveness to Heterodera glycines in the Midwest corn-soybean production systems. J. Nematol. 2019, 51, e2019-32. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, D.; Wu, X. Preliminary studies on the parasitic from the syst of Heteroderaa glycines. J. China Agric. Univ. 1991, 17, 87–91. [Google Scholar]
- Liu, Q.; Gao, Y.; Ning, D.; Du, J.; Zhang, W.; Han, Y. The Fungi Isolated from Cyst Its Effect on Soybean Cyst. J. Heilongjiang Bayi Agric. Univ. 2010, 22, 25–27. [Google Scholar]
- Zhou, Y.Y.; Chen, J.S.; Feng, Y.X.; Xiang, P.; Li, J.; Chen, L.J.; Guo, Y.X. Biocontrol Potential of Bacillus strains against soybean cyst nematode (Heterodera glycines) and for promotion of soybean growth. BMC Microbiol. 2024, 24, 371. [Google Scholar] [CrossRef]
- Daniele, R.; Bert, D.R.; Valérie, D.T.; Alexandre, P.; Julien, A.; Joop, E.M.V.; Misako, Y.; York-Dieter, S.; Tom, B.; Niko, G. A novel protein family mediates Casparian strip formation in the endodermis. Nature 2011, 473, 380–383. [Google Scholar] [CrossRef]
- Hu, Y.F.; You, J.; Pan, F.J. Crosstalk between ethylene and salicylic acid in a compatible soybean-Heterodera glycines interaction. Soils Crops 2020, 9, 240–248. [Google Scholar] [CrossRef]
- Bernabé-Antonio, A.; Castro-Rubio, C.; Rodríguez-Anda, R.; Silva-Guzmán, J.A.; Manríquez-González, R.; Hurtado-Díaz, I.; Sánchez-Ramos, M.; Hinojosa-Ventura, G.; Romero-Estrada, A. Jasmonic and Salicylic Acids Enhance Biomass, Total Phenolic Content, and Antioxidant Activity of Adventitious Roots of Acmella radicans (Jacq.) R.K. Jansen Cultured in Shake Flasks. Biomolecules 2023, 13, 746. [Google Scholar] [CrossRef]



| Gene ID | Annotation | Start | End | Functional Annotation |
|---|---|---|---|---|
| Glyma.13G128900 | AT4G28730.1 | 24,209,750 | 24,212,616 | Glutaredoxin family protein |
| Glyma.05G114900 | AT1G07930.1 | 30,481,777 | 30,484,482 | GTP binding Elongation factor Tu family protein |
| Glyma.14G117700 | / | 15,530,056 | 15,530,697 | / |
| Glyma.20G090100 | AT5G26710.1 | 33,247,170 | 33,252,176 | Glutamyl/glutaminyl-tRNA synthetase, class Ic |
| Glyma.08G112300 | AT1G55260.1 | 8,648,286 | 8,649,896 | Bifunctional inhibitor/lipid-transfer protein/seed storage 2S albumin superfamily protein |
| Glyma.15G136100 | AT1G30630.1 | 10,998,218 | 11,001,584 | Coatomer epsilon subunit |
| Glyma.16G155500 | AT2G28370.1 | 31,572,611 | 31,575,650 | Uncharacterized protein family (UPF0497) |
| Glyma.01G221600 | AT5G41460.1 | 55,067,816 | 55,073,251 | Protein of unknown function (DUF604) |
| Glyma.20G148400 | AT3G22640.1 | 38,684,625 | 38,687,183 | Cupin family protein |
| Glyma.19G170100 | AT5G43940.1 | 43,081,671 | 43,084,907 | GroES-like zinc-binding dehydrogenase family protein |
| Glyma.08G027600 | AT5G35360.1 | 2,200,021 | 2,207,704 | Acetyl Co-enzyme a carboxylase biotin carboxylase subunit |
| Glyma.11G211900 | AT1G28520.1 | 30,435,707 | 30,438,967 | Vascular plant one zinc finger protein |
| Glyma.08G021100 | / | 1,709,026 | 1,709,853 | / |
| Glyma.08G206000 | AT1G15270.1 | 16,669,079 | 16,671,058 | Translation machinery associated TMA7 |
| Glyma.01G230200 | AT3G03150.1 | 55,772,244 | 55,776,690 | / |
| Glyma.11G013200 | AT3G03150.1 | 916,689 | 922,046 | / |
| Glyma.18G177000 | AT4G37990.1 | 42,202,681 | 42,208,498 | Elicitor-activated gene 3-2 |
| Glyma.01G230200 | AT3G03150.1 | 55,772,244 | 55,776,690 | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, S.; Hu, S.; Song, G.; Zhang, M.; Han, Y.; Teng, W.; Li, Y.; Wang, H.; Li, H.; Zhao, X. Transcription Factor GmMYB29 Activates GmPP2C-37like Expression to Mediate Soybean Defense Against Heterodera glycines Race 3. Plants 2025, 14, 3612. https://doi.org/10.3390/plants14233612
Qu S, Hu S, Song G, Zhang M, Han Y, Teng W, Li Y, Wang H, Li H, Zhao X. Transcription Factor GmMYB29 Activates GmPP2C-37like Expression to Mediate Soybean Defense Against Heterodera glycines Race 3. Plants. 2025; 14(23):3612. https://doi.org/10.3390/plants14233612
Chicago/Turabian StyleQu, Shuo, Shihao Hu, Gengchen Song, Miaoli Zhang, Yingpeng Han, Weili Teng, Yongguang Li, Hui Wang, Haiyan Li, and Xue Zhao. 2025. "Transcription Factor GmMYB29 Activates GmPP2C-37like Expression to Mediate Soybean Defense Against Heterodera glycines Race 3" Plants 14, no. 23: 3612. https://doi.org/10.3390/plants14233612
APA StyleQu, S., Hu, S., Song, G., Zhang, M., Han, Y., Teng, W., Li, Y., Wang, H., Li, H., & Zhao, X. (2025). Transcription Factor GmMYB29 Activates GmPP2C-37like Expression to Mediate Soybean Defense Against Heterodera glycines Race 3. Plants, 14(23), 3612. https://doi.org/10.3390/plants14233612







