A Multidrug and Toxic Compound Extrusion Transporter, RgMATE6, Facilitates Vacuolar Transport of Acteoside in Rehmannia glutinosa
Abstract
1. Introduction
2. Results
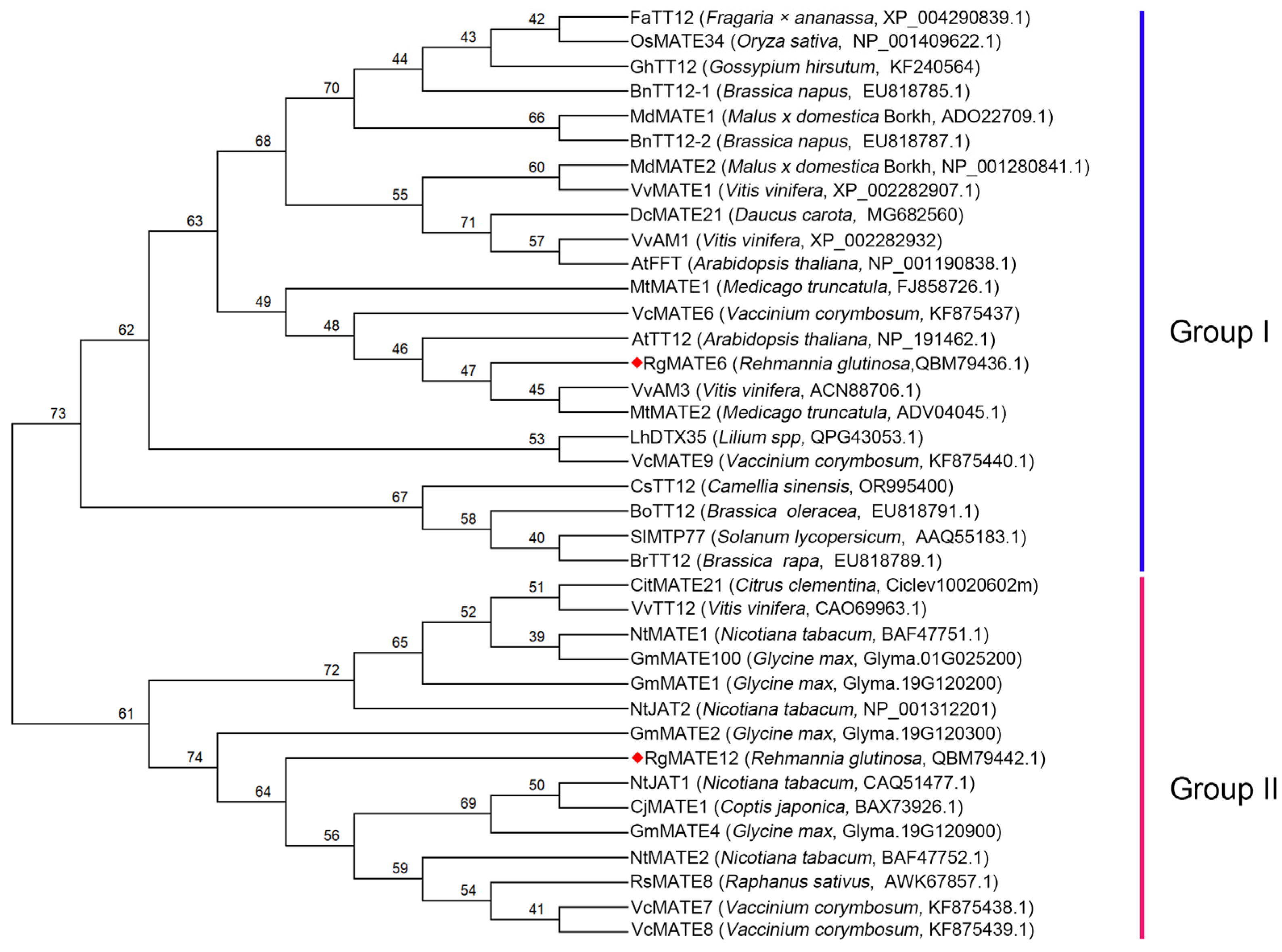
2.1. Sequence Analysis of the RgMATE Proteins
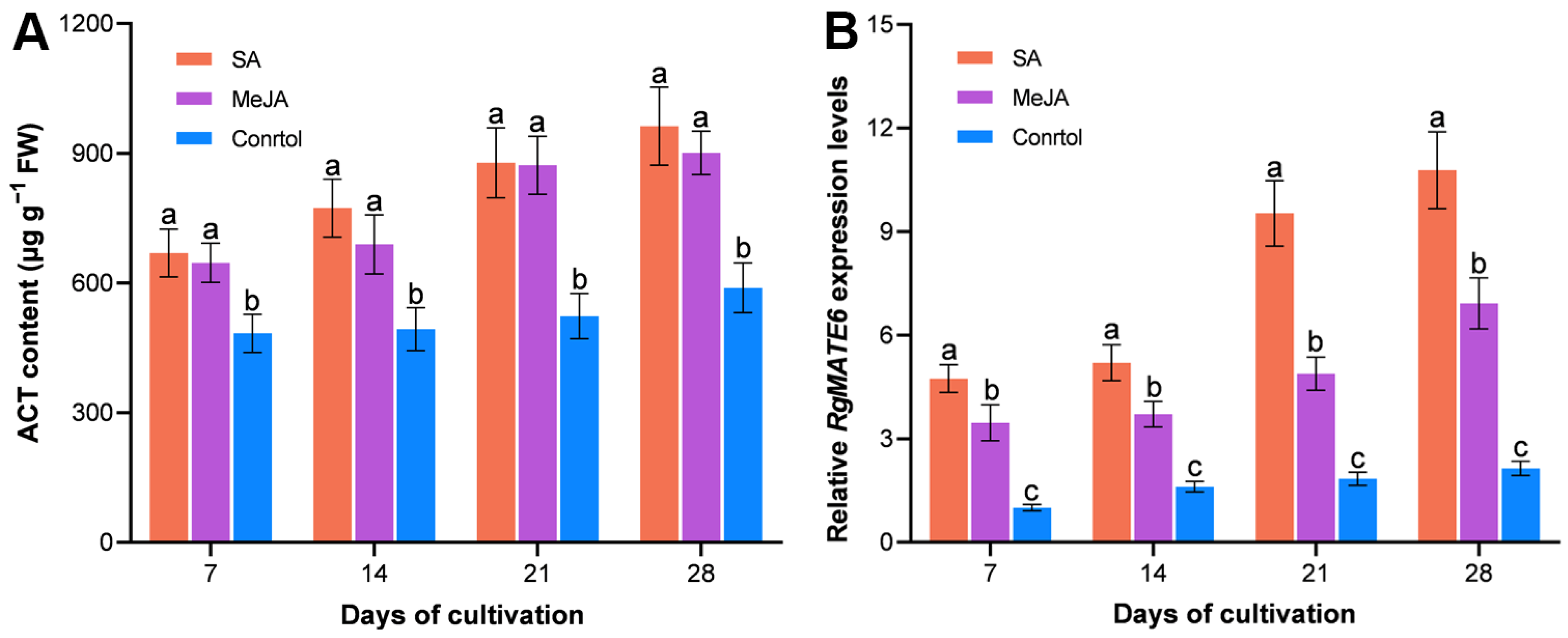
2.2. Correlation Analysis Between the HPG Accumulation and RgMATE Expression in R. glutinosa
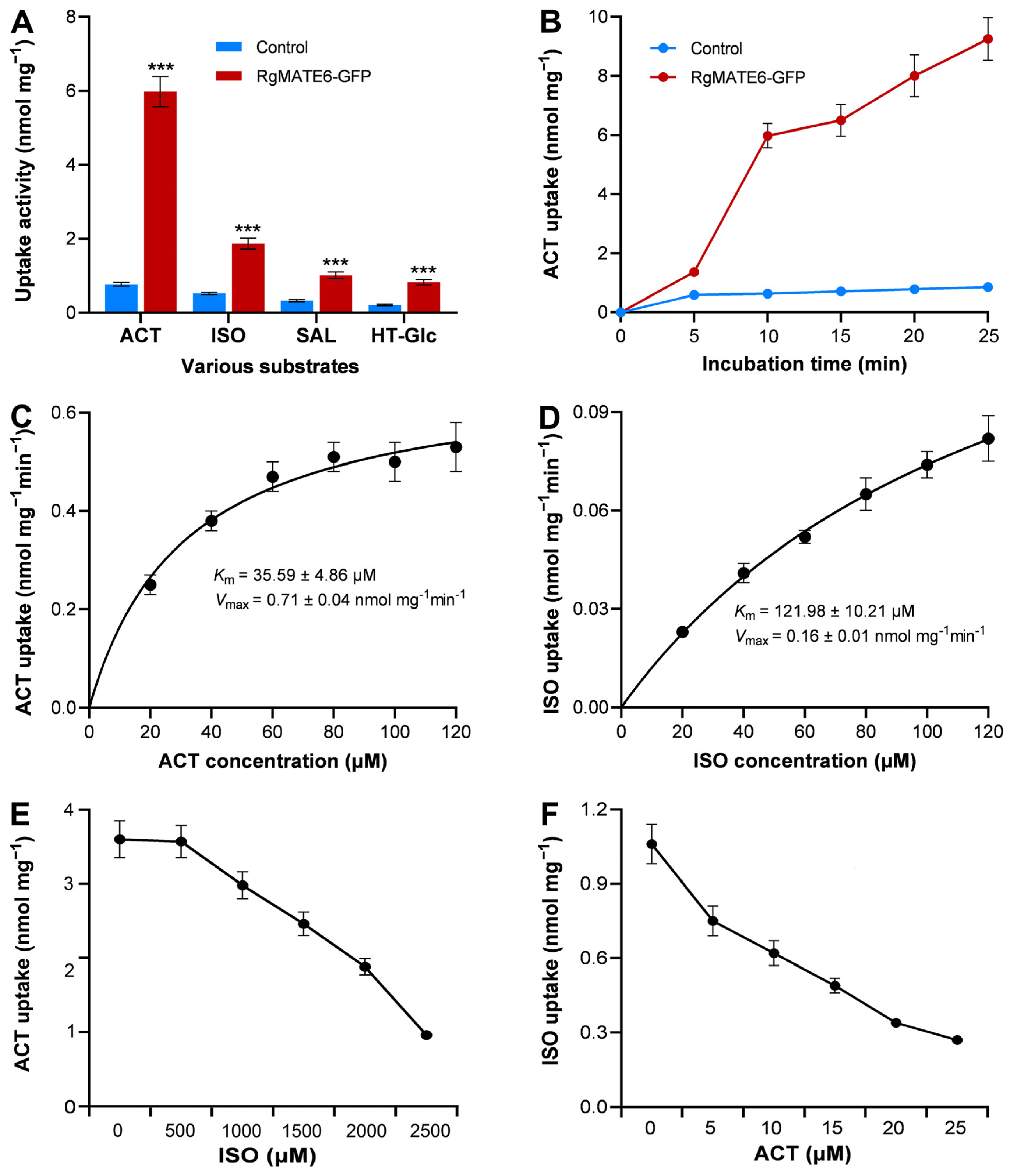
2.3. RgMATE6 Transports ACT More Efficiently than Other HPGs in the Nicotiana benthamiana System
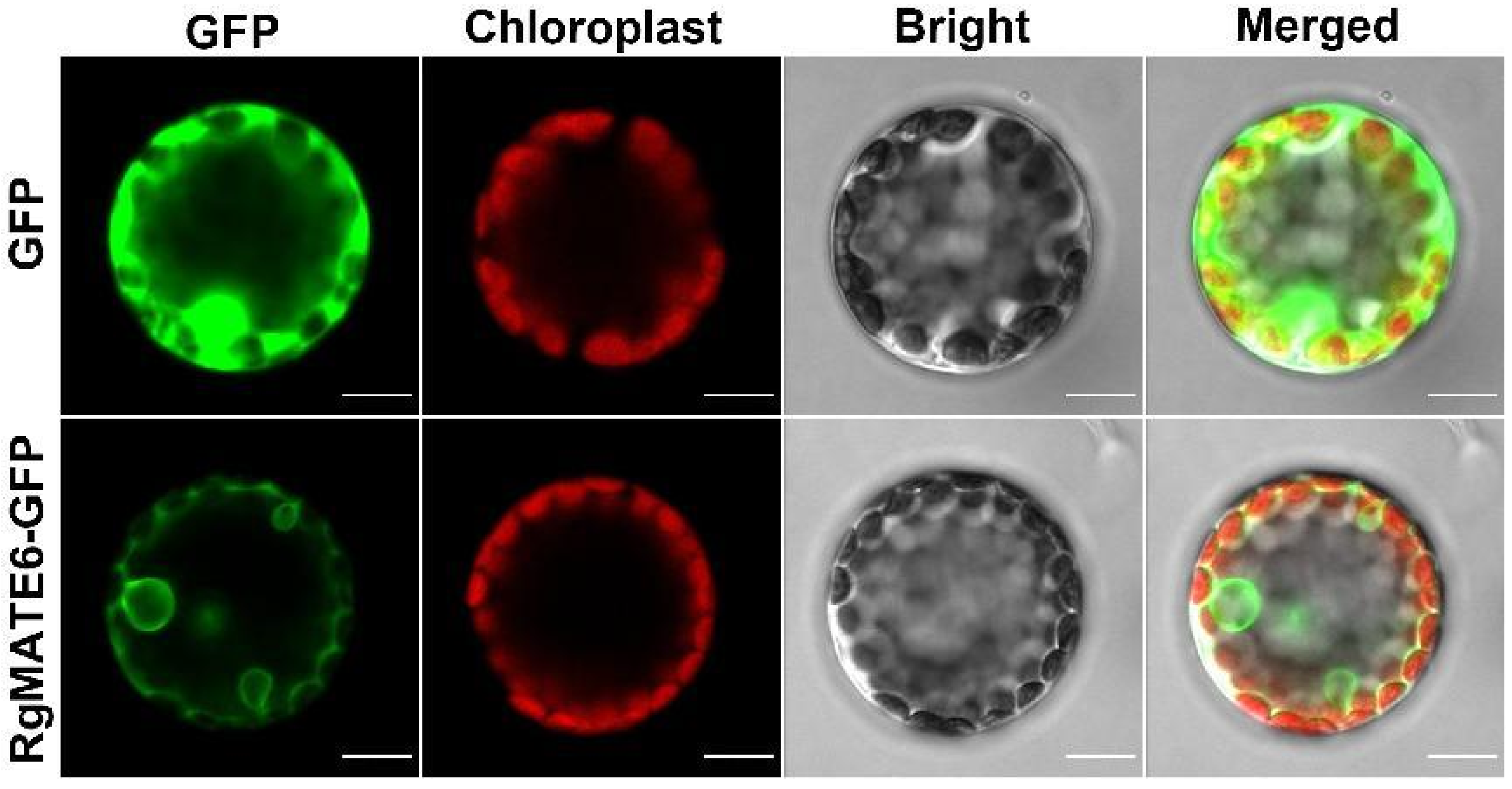
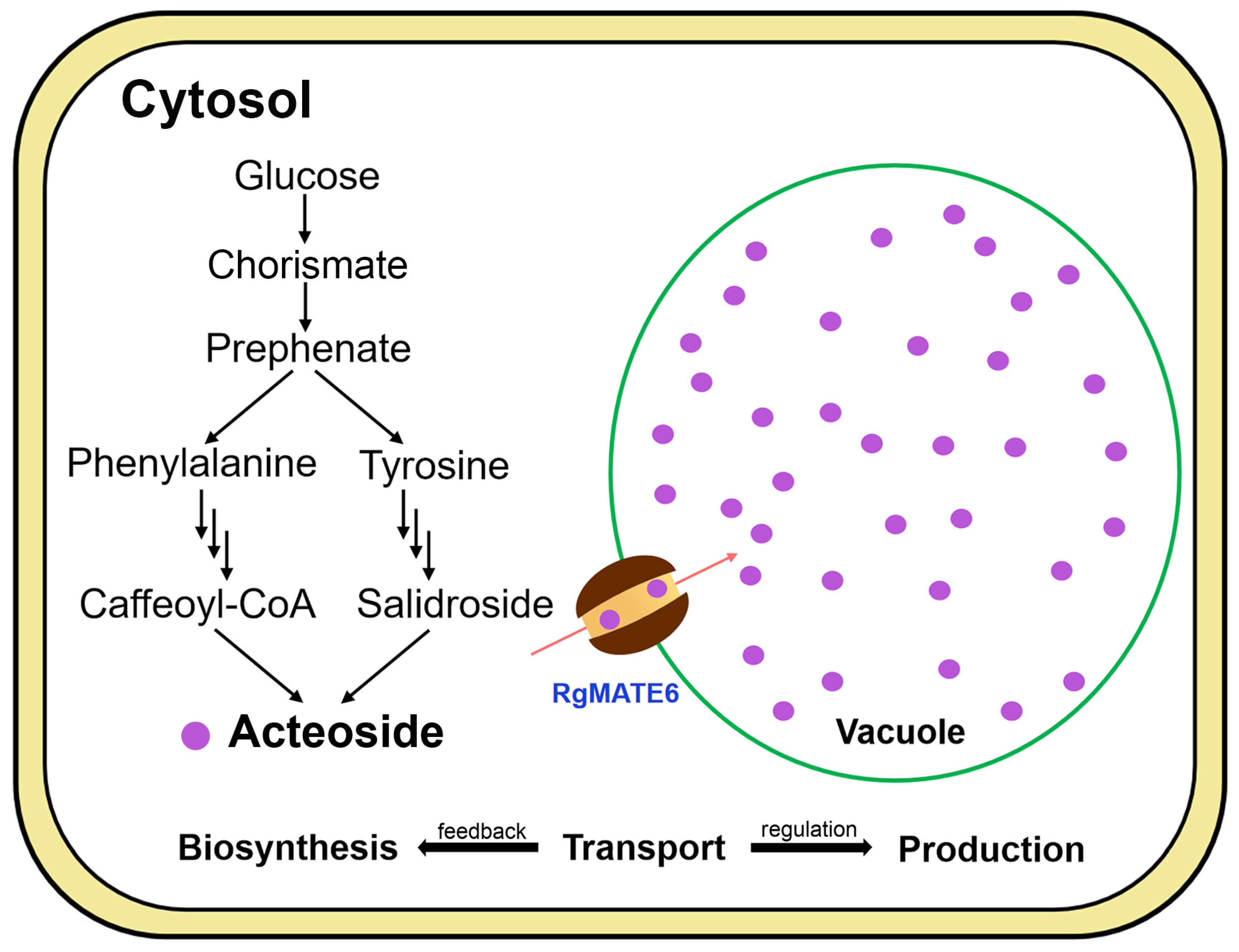
2.4. RgMATE6 Facilitates the Transport of ACT into Vacuoles in R. glutinosa
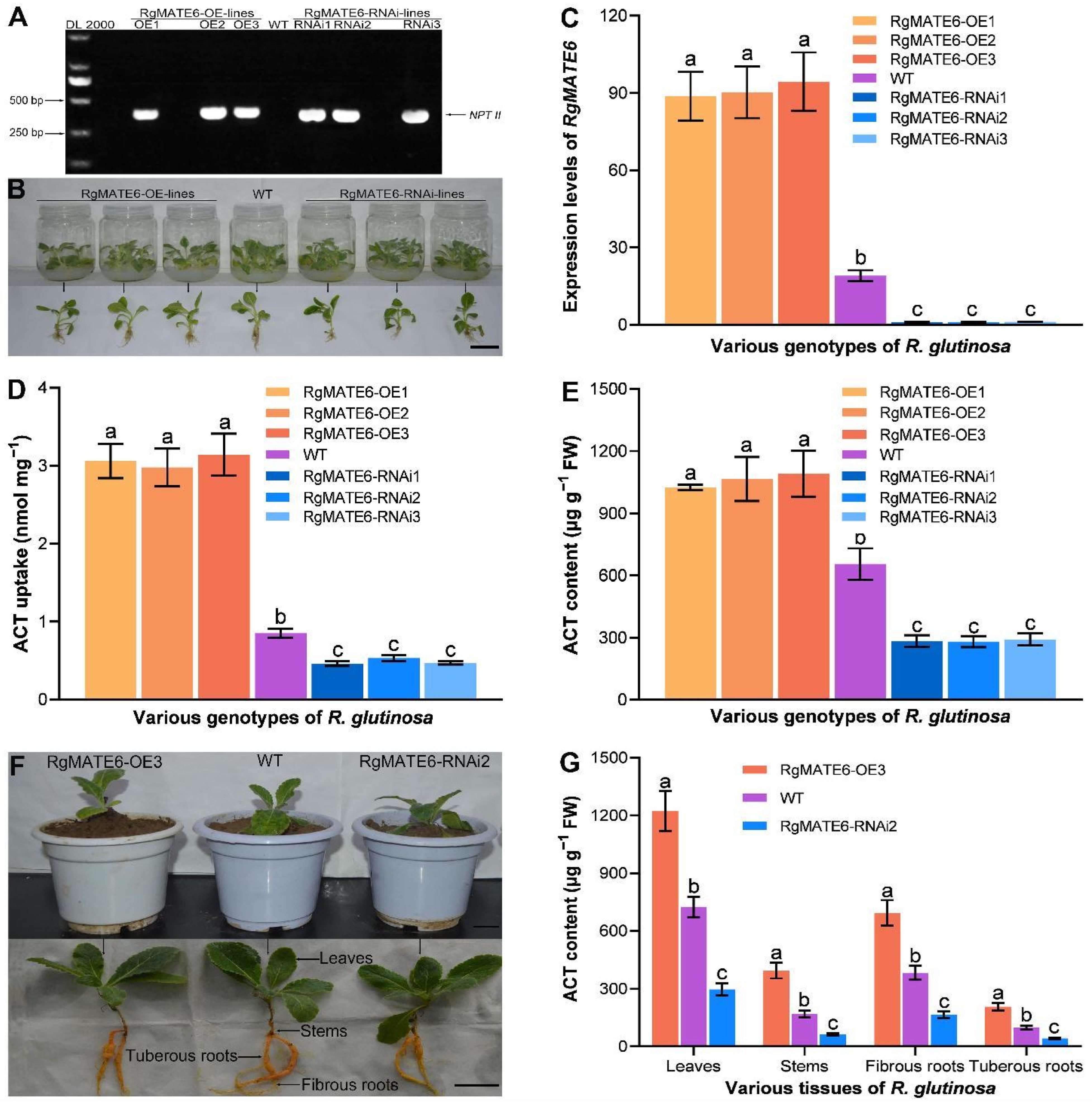
2.5. Genetic Manipulation of RgMATE6 Modifies ACT Production in R. glutinosa by Facilitating Vacuolar Import
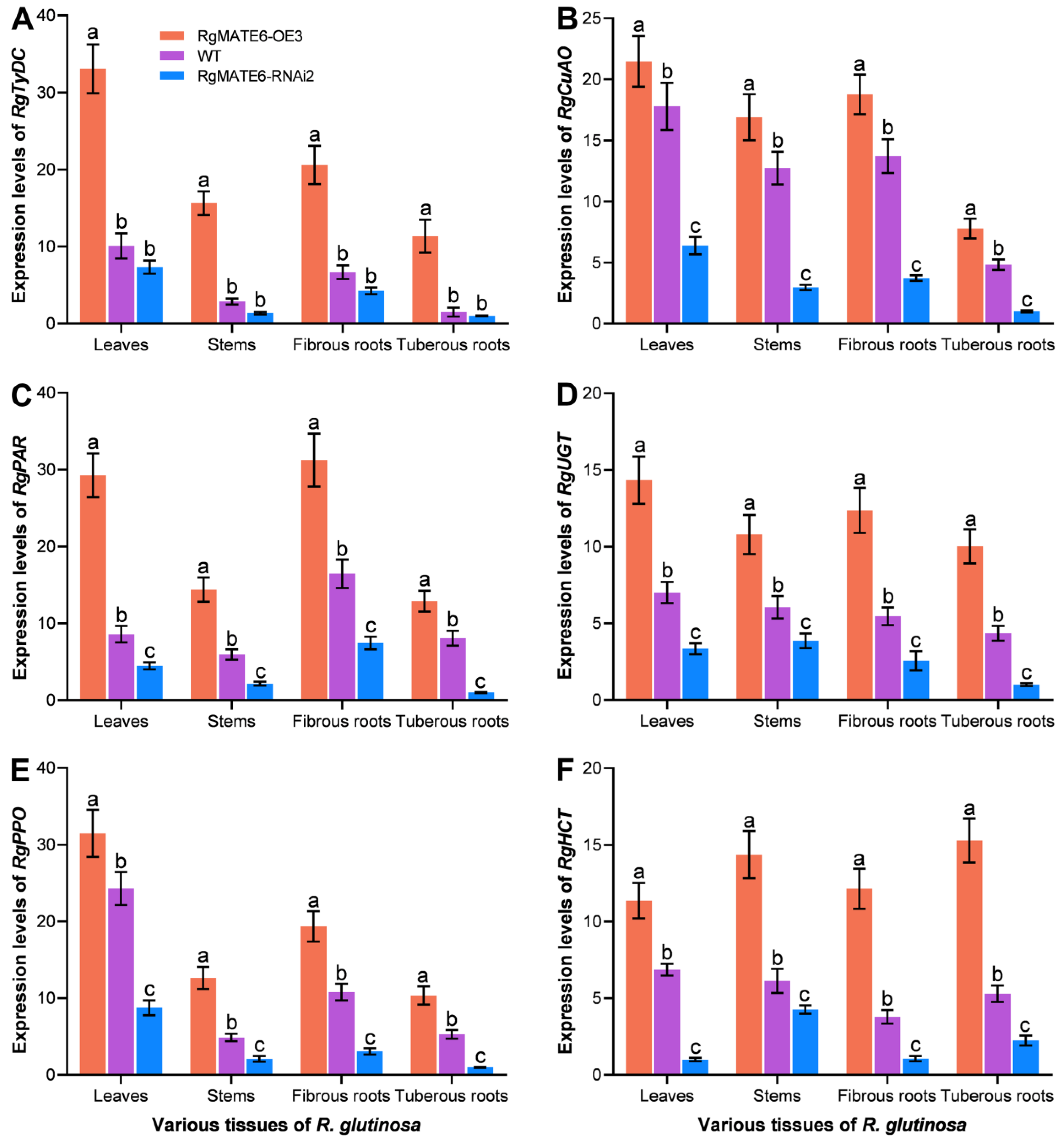
2.6. RgMATE6 Shows Positive Coordination with the Expression of ACT Biosynthetic Genes in R. glutinosa
3. Discussion
4. Materials and Methods
4.1. In Silico Analysis
4.2. Cultivation of R. glutinosa, RNA Extraction, and cDNA Synthesis
4.3. Quantitative Real-Time PCR Analysis
4.4. Measurement of the HPGs in R. glutinosa
4.5. Construction of Various Expression Vectors
4.6. N. benthamiana Transformation and Vacuolar Membrane Vesicle Preparation
4.7. Generation of RgMATE6 Transgenic Lines of R. glutinosa and the Isolation of Vacuolar Membrane Vesicles
4.8. Transport Activity Assays
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| HPG | Hydroxytyrosol-type phenylethanol glycoside |
| R. glutinosa | Rehmannia glutinosa |
| N. benthamiana | Nicotiana benthamiana |
| ORFs | Open reading frames |
| ACT | Acteoside |
| ISO | Isoacteoside |
| SAL | Salacteoside |
| HT-Glc | Hydroxytyrosol-O-glucoside |
| SA | Salicylic acid |
| MeJA | Methyl jasmonate |
| A. thaliana | Arabidopsis thaliana |
| RgTyDC | Tyrosine decarboxylase |
| RgCuAO | Copper amine oxidase |
| RgPAR | Phenylacetaldehyde reductase |
| RgPPO | Polyphenol oxidase |
| RgUGT | UDP-glucose glucosyltransferase |
| RgHCT | Shikimate O-hydroxycinnamoyltransferase |
References
- Liu, Y.; Yang, L.; Wang, J.; Chen, D. New lignans and phenylethanoid with antioxidant activity from aerial parts of Forsythia suspensa (Thunb.) Vahl. Nat. Prod. Res. 2023, 37, 725–733. [Google Scholar] [CrossRef]
- Chen, R.; Fan, J.; Wu, Y.; Huang, X.; Zhang, W.; Xu, Y.; Zhang, Y.; Li, L.; Wang, C.; Yu, M.; et al. Strobilanthes sarcorrhiza root phenolic extract prevent diabetic nephropathy in mice by regulating NF-κB/IL-1β signaling and glycerophospholipid metabolism. J. Pharmaceut. Biomed. 2025, 253, 116534. [Google Scholar] [CrossRef]
- Wu, L.; Georgiev, M.I.; Cao, H.; Nahar, L.; El-Seedi, H.R.; Sarker, S.D.; Xiao, J.; Lu, B. Therapeutic potential of phenylethanoid glycosides: A systematic review. Med. Res. Rev. 2020, 40, 2605–2649. [Google Scholar] [CrossRef] [PubMed]
- Pruccoli, L.; Nicolini, B.; Lianza, M.; Teti, G.; Falconi, M.; Tarozzi, A.; Antognoni, F. Antioxidant and anti-melanogenesis effects of Teucrium chamaedrys L. cell suspension extract and its main phenylethanoid glycoside in B16–F10 cells. Plants 2024, 13, 808. [Google Scholar] [CrossRef]
- Kurt-Celep, I.; Zheleva-Dimitrova, D.; Sinan, K.I.; Uba, A.I.; Nilofar; Mahomoodally, M.F.; Aumeeruddy, M.Z.; Cakilcioglu, U.; Dall’Acqua, S.; Zengin, G. Uncovering chemical profiles biological potentials and protection effect against ECM destruction in H2O2-treated HDF cells of the extracts of Stachys tundjeliensis. Arch. Pharm. 2024, 357, e2300528. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.Y.; Li, M.X.; Lin, T.; Qiu, Y.; Zhu, Y.T.; Li, X.L.; Tao, W.D.; Wang, P.; Ren, X.X.; Chen, L.P. A review on the structure and pharmacological activity of phenylethanoid glycosides. Eur. J. Med. Chem. 2021, 209, 112563. [Google Scholar] [CrossRef]
- Yang, Y.; Xi, D.; Wu, Y.; Liu, T. Complete biosynthesis of the phenylethanoid glycoside verbascoside. Plant Commun. 2023, 4, 100592. [Google Scholar] [CrossRef]
- Wang, F.; Zhi, J.; Zhang, Z.; Wang, L.; Suo, Y.; Xie, C.; Li, M.; Zhang, B.; Du, J.; Gu, L.; et al. Transcriptome analysis of salicylic acid treatment in Rehmannia glutinosa hairy roots using RNA-seq technique for identification of genes involved in acteoside biosynthesis. Front. Plant Sci. 2017, 8, 787. [Google Scholar] [CrossRef]
- Yang, Y.H.; Yang, M.R.; Zhu, J.Y.; Dong, K.W.; Yi, Y.J.; Li, R.F.; Zeng, L.; Zhang, C.F. Functional characterization of tyrosine decarboxylase genes that contribute to acteoside biosynthesis in Rehmannia glutinosa. Planta 2022, 255, 64. [Google Scholar] [CrossRef]
- Cui, Q.; Pan, Y.; Xu, X.; Zhang, W.; Wu, X.; Qu, S.; Liu, X. The metabolic profile of acteoside produced by human or rat intestinal bacteria or intestinal enzyme in vitro employed UPLC-Q-TOF-MS. Fitoterapia 2016, 109, 67–74. [Google Scholar] [CrossRef]
- Fuji, Y.; Ohtsuki, T.; Matsufuji, H. Accumulation and subcellular localization of acteoside in sesame plants (Sesamum indicum L.). Acs Omega 2018, 3, 17287–17294. [Google Scholar] [CrossRef]
- Alipieva, K.; Korkina, L.; Orhan, I.E.; Georgiev, M.I. Verbascoside—A review of its occurrence (bio)synthesis and pharmacological significance. Biotechnol. Adv. 2014, 32, 1065–1076. [Google Scholar] [CrossRef] [PubMed]
- Sciandra, F.; Bottoni, P.; De Leo, M.; Braca, A.; Brancaccio, A.; Bozzi, M. Verbascoside elicits its beneficial effects by enhancing mitochondrial spare respiratory capacity and the Nrf2/HO-1 mediated antioxidant system in a murine skeletal muscle cell line. Int. J. Mol. Sci. 2023, 24, 15276. [Google Scholar] [CrossRef]
- Pongkitwitoon, B.; Putalun, W.; Triwitayakorn, K.; Kitisripanya, T.; Kanchanapoom, T.; Boonsnongcheep, P. Anti-inflammatory activity of verbascoside- and isoverbascoside-rich Lamiales medicinal plants. Heliyon 2023, 10, e23644. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Gao, S.; Zhang, Y.; Wang, M.; Liu, Y.; Li, T.; Gao, C.; Zhou, Y.; Bian, B.; Wang, H.; et al. Altered metabolic profiles and targets relevant to the protective effect of acteoside on diabetic nephropathy in db/db mice based on metabolomics and network pharmacology studies. J. Ethnopharmacol. 2024, 318, 117073. [Google Scholar] [CrossRef]
- Bai, P.; Yang, Y.; Tang, J.; Xi, D.; Hao, Y.; Jiang, L.; Yin, H.; Liu, T. High-level sustainable production of complex phenylethanoid glycosides from glucose through engineered yeast cell factories. Metab. Eng. 2025, 87, 95–108. [Google Scholar] [CrossRef]
- Zhang, X.; Li, C.; Wang, L.; Fei, Y.; Qin, W. Analysis of Centranthera grandiflora Benth transcriptome explores genes of catalpol acteoside and azafrin biosynthesis. Int. J. Mol. Sci. 2019, 20, 6034. [Google Scholar] [CrossRef]
- Saimaru, H.; Orihara, Y. Biosynthesis of acteoside in cultured cells of Olea europaea. J. Nat. Med. 2010, 64, 139–145. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhu, J.; Shao, L.; Guo, M. Current advances in acteoside biosynthesis pathway elucidation and biosynthesis. Fitoterapia 2020, 142, 104495. [Google Scholar] [CrossRef]
- Yang, Y.; Cao, Y.; Zhu, C.; Jin, Y.; Sun, H.; Wang, R.; Li, M.; Zhang, Z. Functional activities of three Rehmannia glutinosa enzymes: Elucidation of the Rehmannia glutinosa salidroside biosynthesis pathway in Saccharomyces cerevisiae. Gene 2024, 928, 148815. [Google Scholar] [CrossRef]
- Luaces, P.; Sánche, R.; Expósito, J.; Pérez-Pulido, A.J.; Pérez, A.G.; Sanz, C. Functional and physiological characterization of tyrosine decarboxylases from Olea europaea L. involved in the synthesis of the main phenolics in Olive fruit and Virgin Olive Oil. Int. J. Mol. Sci. 2024, 25, 10892. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Han, X.; Zhao, M.; Liu, L.; Deng, Z.; Zhao, Q.; Yu, Y. Functional characterization of polyphenol oxidase of PPO2 supports its involvement in parallel biosynthetic pathways of acteoside. Plant J. 2024, 119, 927–941. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, F.G.; Tang, R.J.; Yu, Y.; Song, J.; Wang, Y.; Li, L.; Luan, S. Two tonoplast MATE proteins function as turgor-regulating chloride channels in Arabidopsis. Proc. Natl. Acad. Sci. USA 2017, 114, E2036–E2045. [Google Scholar] [CrossRef]
- Shao, D.; Li, Y.; Zhu, Q.; Zhang, X.; Liu, F.; Xue, F.; Sun, J. GhGSTF12 a glutathione S-transferase gene is essential for anthocyanin accumulation in cotton (Gossypium hirsutum L.). Plant Sci. 2021, 305, 110827. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Yuan, J.; Qin, H.; Zhang, M.; Zhang, F.; Yu, F.; Tian, Z.; Wang, G. GmMATE100 is involved in the import of Soyasaponins A and B into vacuoles in soybean plants (Glycine max L.). J. Agric. Food Chem. 2024, 72, 9994–10004. [Google Scholar] [CrossRef]
- Gani, U.; Nautiyal, A.K.; Kundan, M.; Rout, B.; Pandey, A.; Misra, P. Two homeologous MATE transporter genes NtMATE21 and NtMATE22 are involved in the modulation of plant growth and flavonol transport in Nicotiana tabacum. J. Exp. Bot. 2022, 73, 6186–6206. [Google Scholar] [CrossRef]
- Li, F.; Shahsavarani, M.; Handy-Hart, C.J.; Côté, A.; Brasseur-Trottier, X.; Montgomery, V.; Beech, R.N.; Liu, L.; Bayen, S.; Qu, Y. Characterization of a vacuolar importer of secologanin in Catharanthus roseus. Commu. Biol. 2024, 7, 939. [Google Scholar] [CrossRef]
- Gani, U.; Vishwakarma, R.A.; Misra, P. Membrane transporters: The key drivers of transport of secondary metabolites in plants. Plant Cell Rep. 2021, 40, 1–18. [Google Scholar] [CrossRef]
- Ma, Y.; Li, D.; Zhong, Y.; Wang, X.; Li, L.; Osbourn, A.; Lucas, W.J.; Huang, S.; Shang, Y. Vacuolar MATE/DTX protein-mediated cucurbitacin C transport is co-regulated with bitterness biosynthesis in cucumber. New Phyto. 2023, 238, 995–1003. [Google Scholar] [CrossRef]
- Saad, K.R.; Kumar, G.; Puthusseri, B.; Srinivasa, S.M.; Giridhar, P.; Shetty, N.P. Genome-wide identification of MATE functional analysis and molecular dynamics of DcMATE21 involved in anthocyanin accumulation in Daucus carota. Phytochemistry 2023, 210, 113676. [Google Scholar] [CrossRef]
- Zhao, J.; Dixon, R.A. MATE transporters facilitate vacuolar uptake of epicatechin 3’-O-glucoside for proanthocyanidin biosynthesis in Medicago truncatula and Arabidopsis. Plant Cell 2009, 21, 2323–2340. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Huhman, D.; Shadle, G.; He, X.Z.; Sumner, L.W.; Tang, Y.; Dixon, R.A. MATE2 mediates vacuolar sequestration of flavonoid glycosides and glycoside malonates in Medicago truncatula. Plant Cell 2011, 23, 1536–1555. [Google Scholar] [CrossRef] [PubMed]
- Ng, M.S.; Ku, Y.S.; Yung, W.S.; Cheng, S.S.; Man, C.K.; Yang, L.; Song, S.; Chung, G.; Lam, H.M. MATE-Type Proteins are responsible for isoflavone transportation and accumulation in soybean seeds. Int. J. Mol. Sci. 2021, 22, 12017. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.H.; Yang, H.; Li, M.J.; Zhang, Z.Y.; Yi, Y.J.; Li, R.F.; Hong, C.; Wang, C.J. Identification subcellular localization and expression analysis of the RgMATEs potentially involved in phenolics release in Rehmannia glutinosa. Int. J. Agric. Biol. 2020, 25, 1196–1206. [Google Scholar]
- Upadhyay, N.; Kar, D.; Deepak Mahajan, B.; Nanda, S.; Rahiman, R.; Panchakshari, N.; Bhagavatula, L.; Datta, S. The multitasking abilities of MATE transporters in plants. J. Exp. Bot. 2019, 70, 4643–4656. [Google Scholar] [CrossRef]
- Zhu, Y.; Hu, X.; Dong, L.; Yang, H.; Zhou, D.; Liu, X.; Dong, C.; Yue, X.; Zhao, L. Green synthesized silver nanoparticles induced accumulation of biomass and secondary metabolites in hairy roots of Rehmannia glutinosa. Int. J. Mol. Sci. 2024, 25, 13088. [Google Scholar] [CrossRef]
- Gomez, C.; Terrier, N.; Torregrosa, L.; Vialet, S.; Fournier-Level, A.; Verriès, C.; Souquet, J.M.; Mazauric, J.P.; Klein, M.; Cheynier, V.; et al. Grapevine MATE-type proteins act as vacuolar H+-dependent acylated anthocyanin transporters. Plant Physiol. 2009, 150, 402–415. [Google Scholar] [CrossRef]
- Shitan, N.; Minami, S.; Morita, M.; Hayashida, M.; Ito, S.; Takanashi, K.; Omote, H.; Moriyama, Y.; Sugiyama, A.; Goossens, A.; et al. Involvement of the leaf-specific multidrug and toxic compound extrusion (MATE) transporter Nt-JAT2 in vacuolar sequestration of nicotine in Nicotiana tabacum. PLoS ONE 2014, 9, e108789. [Google Scholar] [CrossRef]
- Takanashi, K.; Yamada, Y.; Sasaki, T.; Yamamoto, Y.; Sato, F.; Yazaki, K. A multidrug and toxic compound extrusion transporter mediates berberine accumulation into vacuoles in Coptis japonica. Phytochemistry 2017, 138, 76–82. [Google Scholar] [CrossRef]
- Kikowska, M.; Kędziora, I.; Krawczyk, A.; Thiem, B. Methyl jasmonate yeast extract and sucrose stimulate phenolic acids accumulation in Eryngium planum L. shoot cultures. Acta Biochim. Pol. 2015, 62, 197–200. [Google Scholar] [CrossRef]
- Liu, J.; Yang, L.; Luan, M.; Wang, Y.; Zhang, C.; Zhang, B.; Shi, J.; Zhao, F.G.; Lan, W.; Luan, S. A vacuolar phosphate transporter essential for phosphate homeostasis in Arabidopsis. Proc. Natl. Acad. Sci. USA 2015, 112, E6571–E6578. [Google Scholar] [CrossRef]
- Selwal, N.; Rahayu, F.; Herwati, A.; Latifah, E.; Supriyono; Suhara, C.; Kade Suastika, I.B.; Mahayu, W.M.; Wani, A.K. Enhancing secondary metabolite production in plants: Exploring traditional and modern strategies. J. Agric. Food Res. 2023, 14, 100702. [Google Scholar] [CrossRef]
- Fu, L.; Niu, B.; Zhu, Z.; Wu, S.; Li, W. CD-HIT: Accelerated for clustering the next-generation sequencing data. Bioinformatics 2012, 28, 3150–3152. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Suleski, M.; Sanderford, M.; Sharma, S.; Tamura, K. MEGA12: Molecular evolutionary genetic analysis version 12 for adaptive and green computing. Mol. Biol. Evol. 2024, 41, msae263. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kumar, S. Discovering Fragile Clades and Causal Sequences in Phylogenomics by Evolutionary Sparse Learning. Mol. Biol. Evol. 2024, 41, msae131. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, B.; Jin, Y.; Li, M.; Wang, Z.; Zhao, J.; Ma, H.; Wu, T.; Zhang, Z. Rehmannia glutinosa RgMATE35 participates in the root secretion of phenolic acids and modulates the development of plant replant disease. Plants 2024, 13, 3007. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using Real-Time Quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Yoo, S.D.; Cho, Y.H.; Sheen, J. Arabidopsis mesophyll protoplasts: A versatile cell system for transient gene expression analysis. Nat. Protoc. 2007, 2, 1565–1572. [Google Scholar] [CrossRef]
- Yamanishi, H.; Kasamo, K. Binding of 7-Chloro-4-nitrobenzo-2-oxa-13-diazole to an essential Cysteine Residue(s) in the tonoplast H-ATPase from Mung Bean (Vigna radiata L.) hypocotyls. Plant Physiol. 1992, 99, 652–658. [Google Scholar] [CrossRef]
- Wise, A.A.; Liu, Z.; Binns, A.N. Three methods for the introduction of foreign DNA into Agrobacterium. Methods Mol. Biol. 2006, 343, 43–53. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Z.; Li, R.; Yi, Y.; Yang, H.; Wang, C.; Wang, Z.; Liu, Y. RgC3H involves in the biosynthesis of allelopathic phenolic acids and alters their release amount in Rehmannia glutinosa Roots. Plants 2020, 9, 567. [Google Scholar] [CrossRef]
- Murray, M.G.; Thompson, W.F. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980, 8, 4321–4325. [Google Scholar] [CrossRef]
- Bartholomew, D.M.; Van Dyk, D.E.; Lau, S.M.; O’Keefe, D.P.; Rea, P.A.; Viitanen, P.V. Alternate energy-dependent pathways for the vacuolar uptake of glucose and glutathione conjugates. Plant Physiol. 2002, 130, 1562–1572. [Google Scholar] [CrossRef]

| Expression Levels | RgMATE6 | RgMATE12 | |
|---|---|---|---|
| Content of HPGs | |||
| ACT | 0.861 * | 0.459 | |
| ISO | 0.859 * | 0.508 | |
| SAL | 0.854 | 0.575 | |
| HT-Glc | 0.839 | 0.546 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Li, Y.; Wang, Y.; Li, M.; Zhang, Z.; Li, R.; Wang, W.; Shen, F.; Yan, M. A Multidrug and Toxic Compound Extrusion Transporter, RgMATE6, Facilitates Vacuolar Transport of Acteoside in Rehmannia glutinosa. Plants 2025, 14, 3608. https://doi.org/10.3390/plants14233608
Yang Y, Li Y, Wang Y, Li M, Zhang Z, Li R, Wang W, Shen F, Yan M. A Multidrug and Toxic Compound Extrusion Transporter, RgMATE6, Facilitates Vacuolar Transport of Acteoside in Rehmannia glutinosa. Plants. 2025; 14(23):3608. https://doi.org/10.3390/plants14233608
Chicago/Turabian StyleYang, Yanhui, Yuying Li, Yuxuan Wang, Mingjie Li, Zhongyi Zhang, Ruifang Li, Weiwei Wang, Fuxi Shen, and Mengman Yan. 2025. "A Multidrug and Toxic Compound Extrusion Transporter, RgMATE6, Facilitates Vacuolar Transport of Acteoside in Rehmannia glutinosa" Plants 14, no. 23: 3608. https://doi.org/10.3390/plants14233608
APA StyleYang, Y., Li, Y., Wang, Y., Li, M., Zhang, Z., Li, R., Wang, W., Shen, F., & Yan, M. (2025). A Multidrug and Toxic Compound Extrusion Transporter, RgMATE6, Facilitates Vacuolar Transport of Acteoside in Rehmannia glutinosa. Plants, 14(23), 3608. https://doi.org/10.3390/plants14233608






