2,4-Epibrassinolide Enhances Drought Tolerance in Prunella vulgaris by Improving Photosynthesis, Redox Homeostasis, and Secondary Metabolism
Abstract
1. Introduction
2. Results
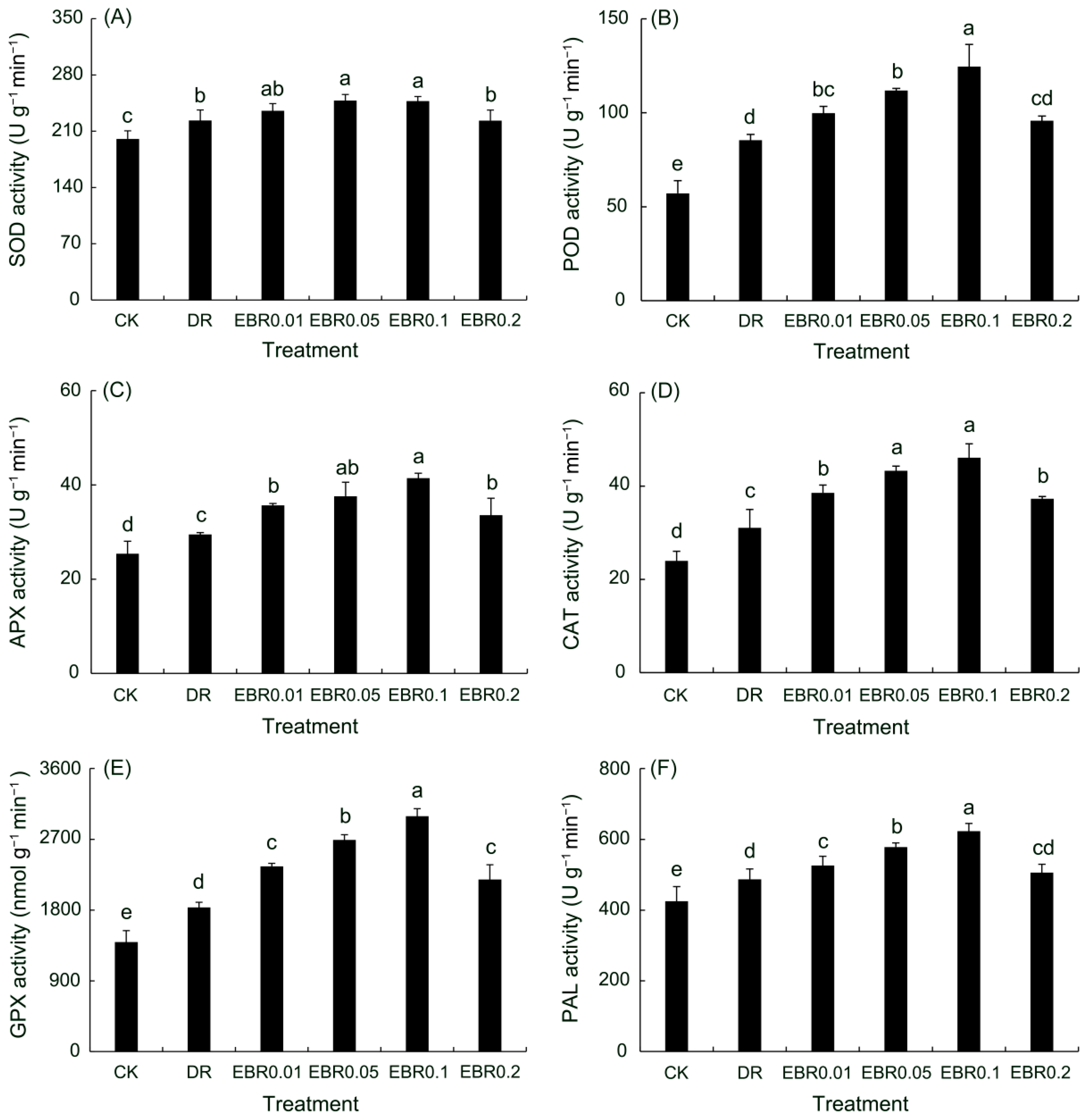
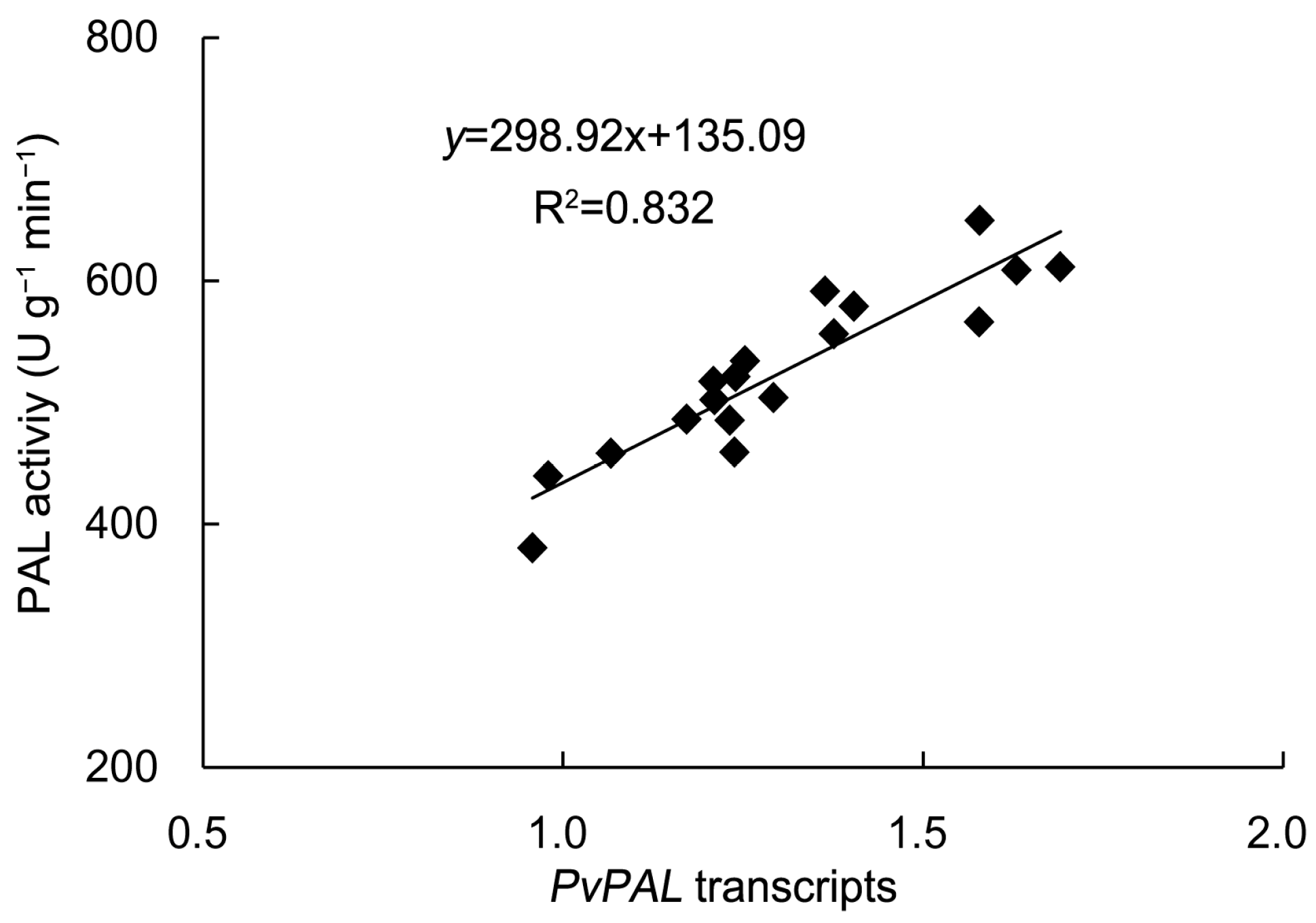
2.1. Antioxidant Enzyme Activities and Phenylalanine Ammonia-Lyase Activity
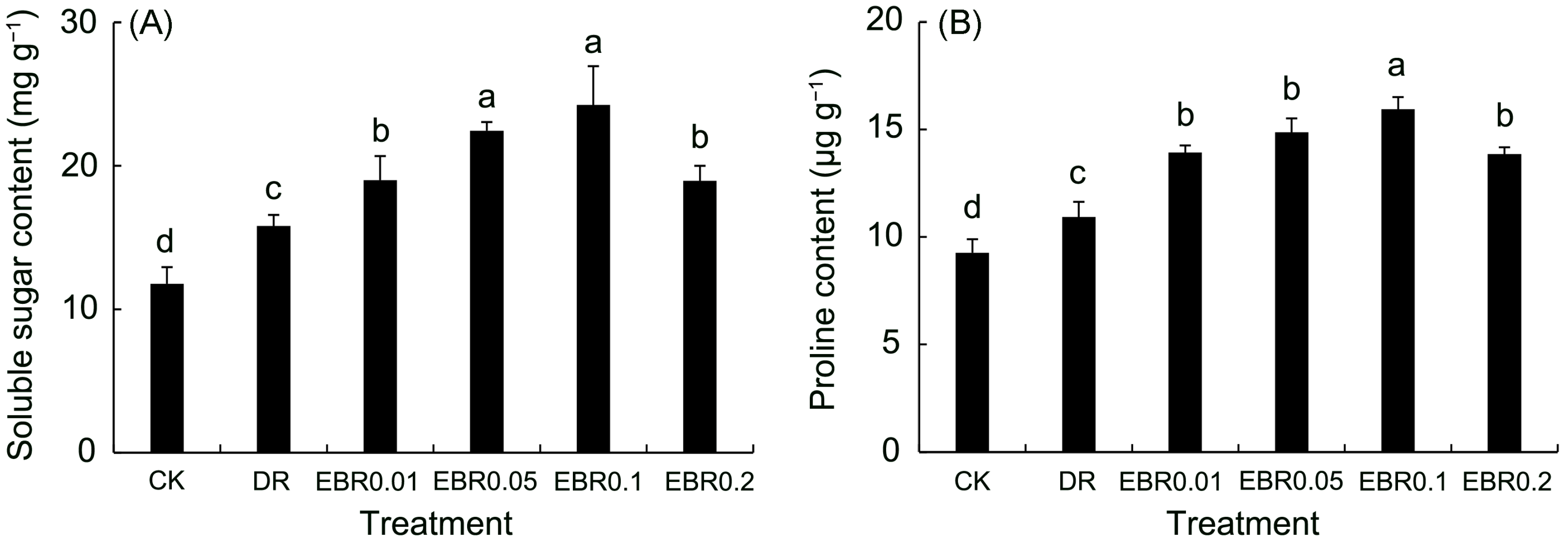
2.2. Osmotic Regulators
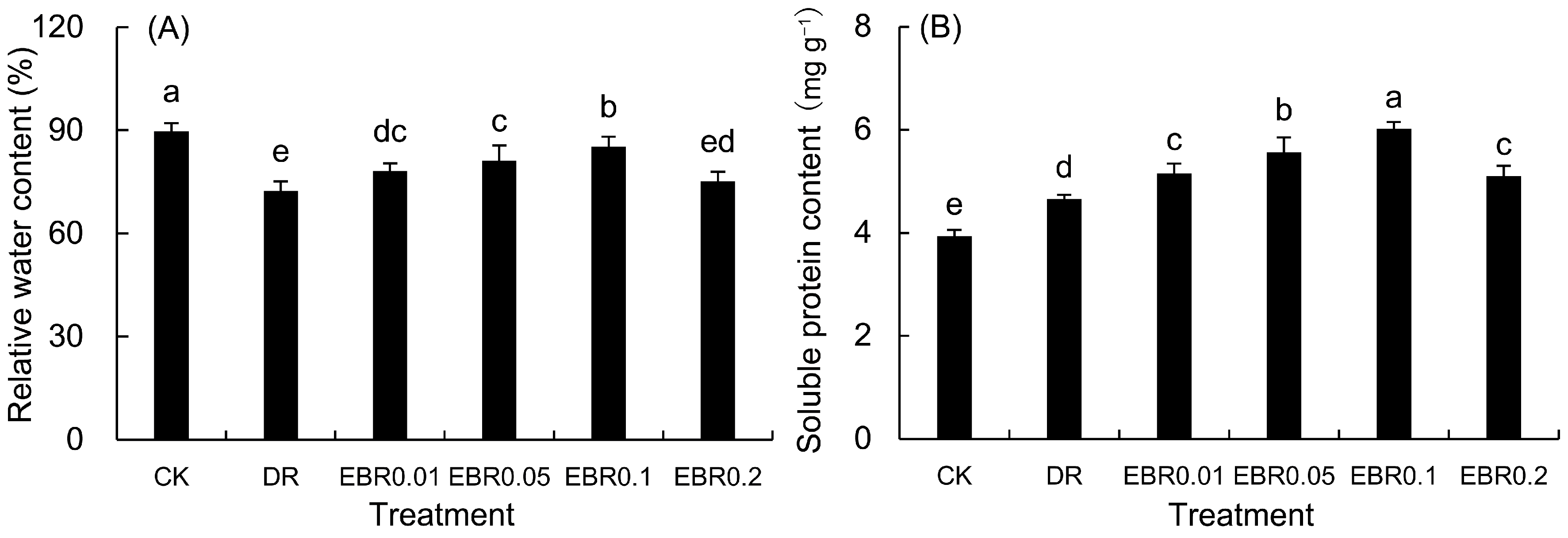
2.3. Leaf Relative Water Content and Soluble Protein
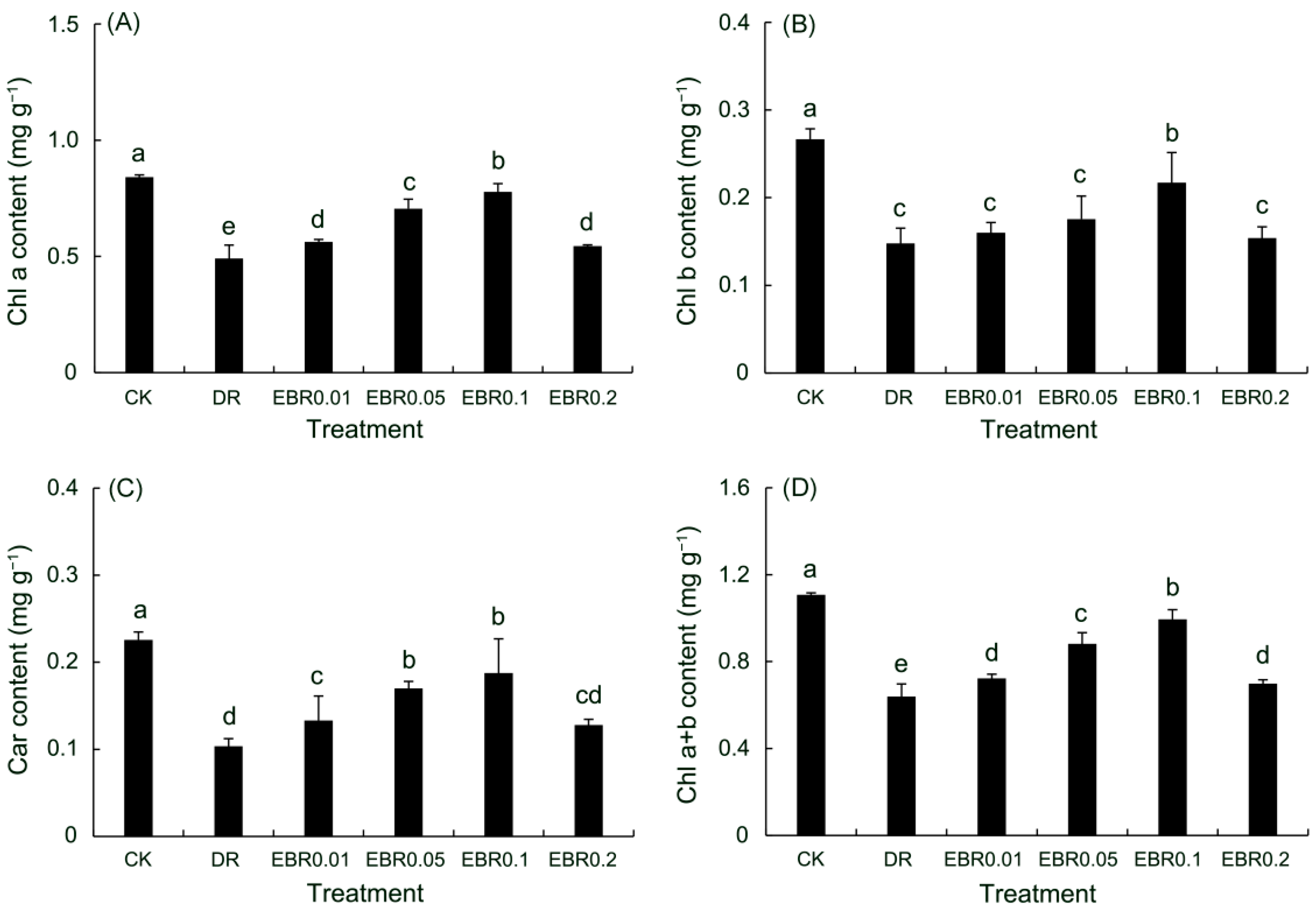
2.4. Chlorophyll Content
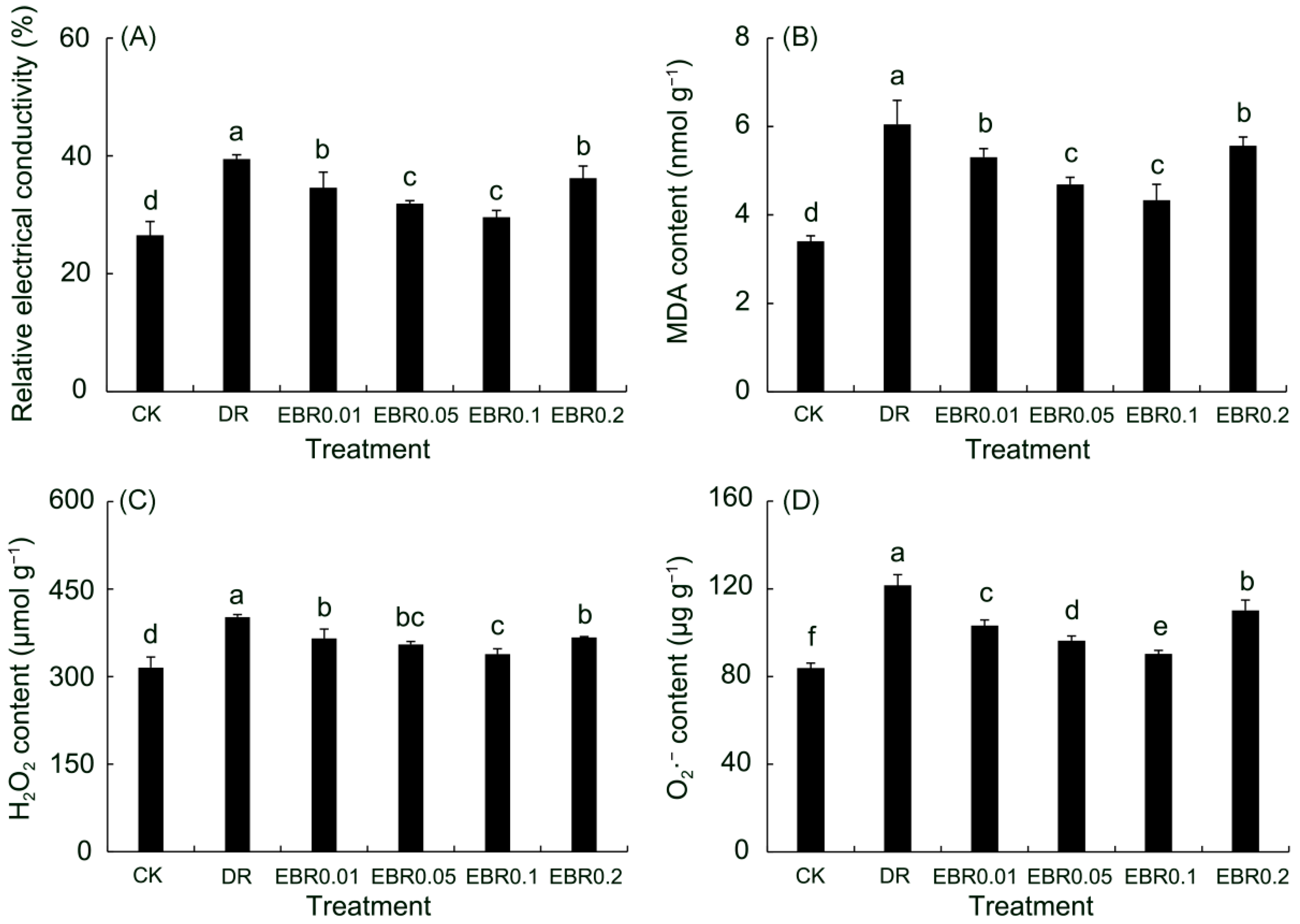
2.5. Oxidation Index
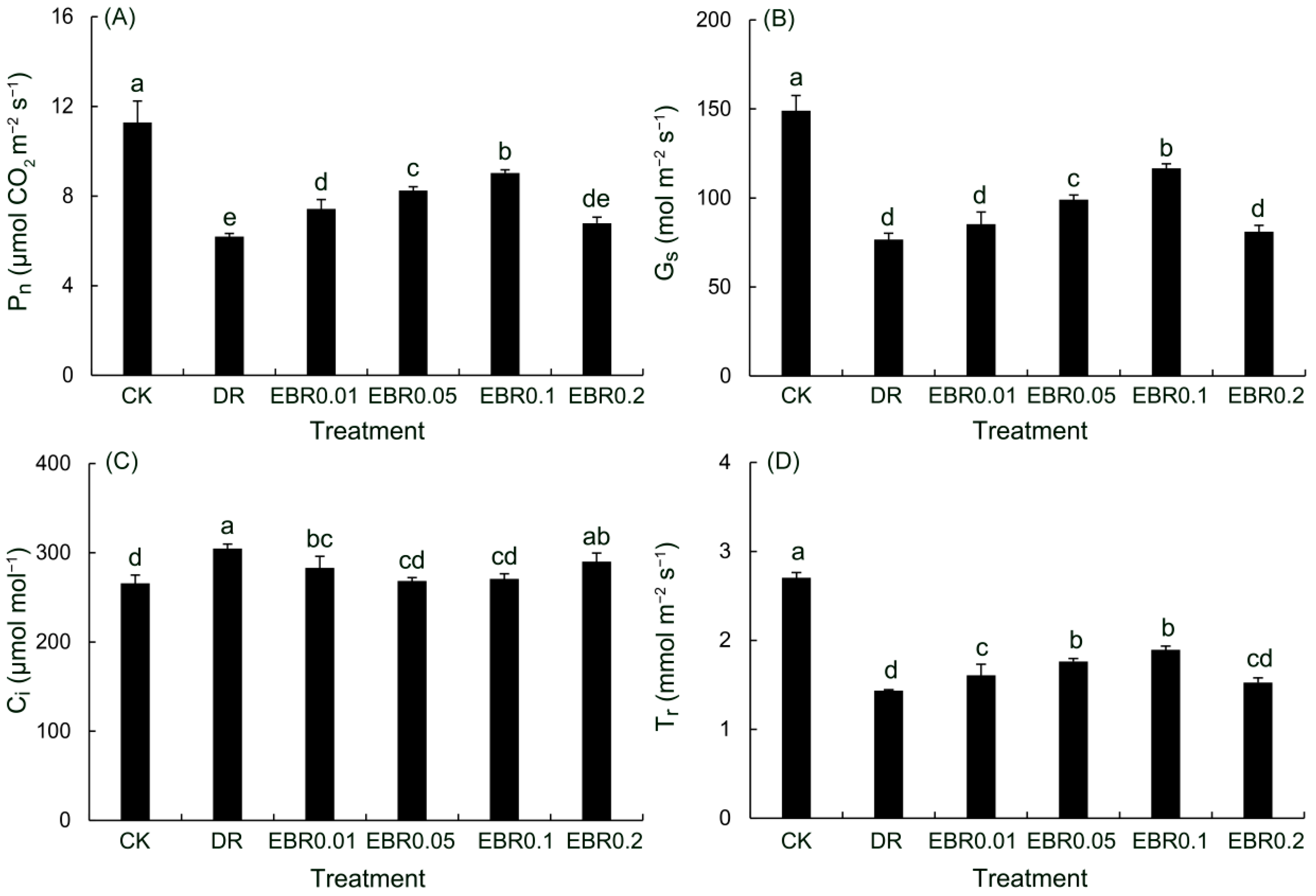
2.6. Gas Exchange Parameters
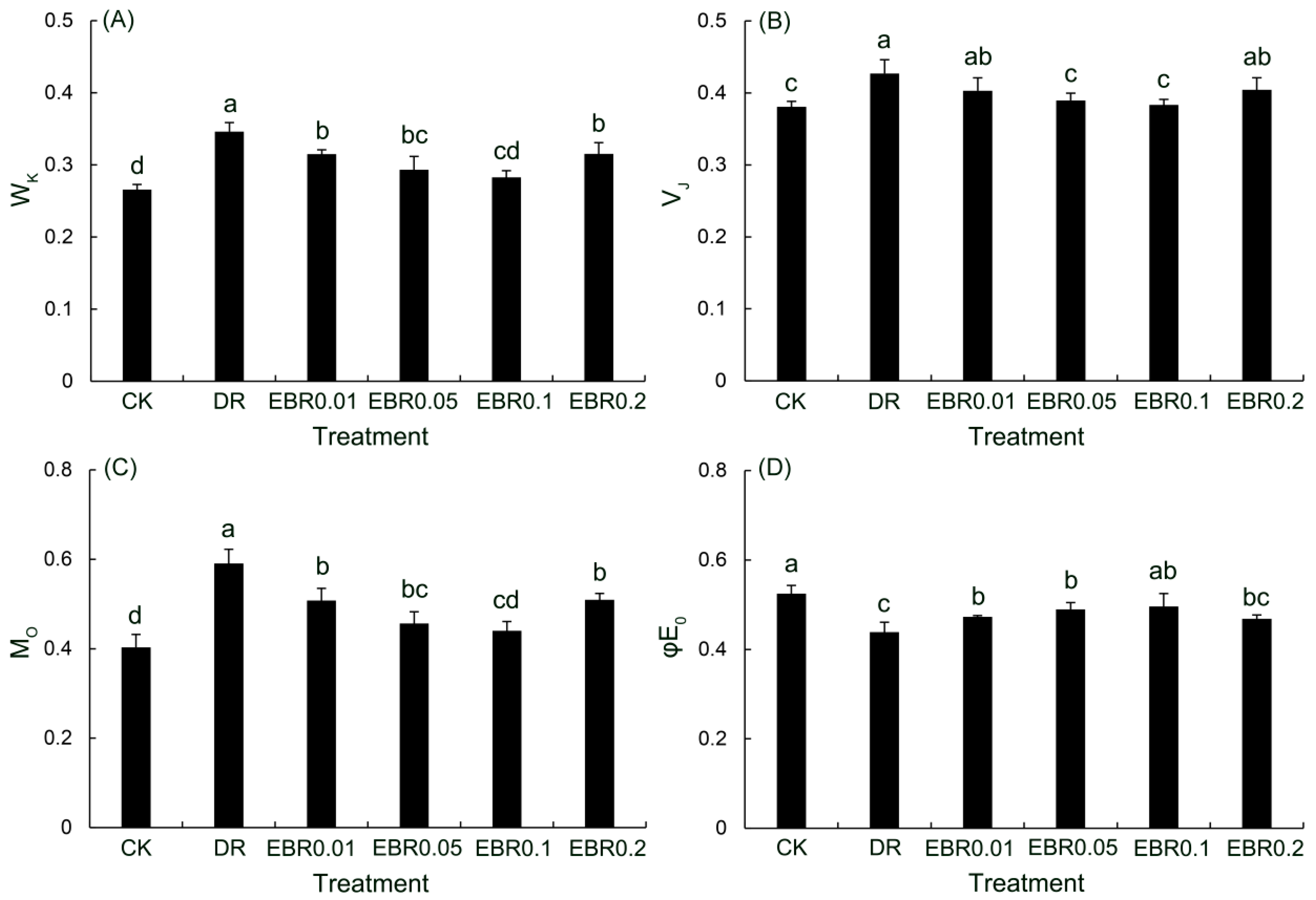
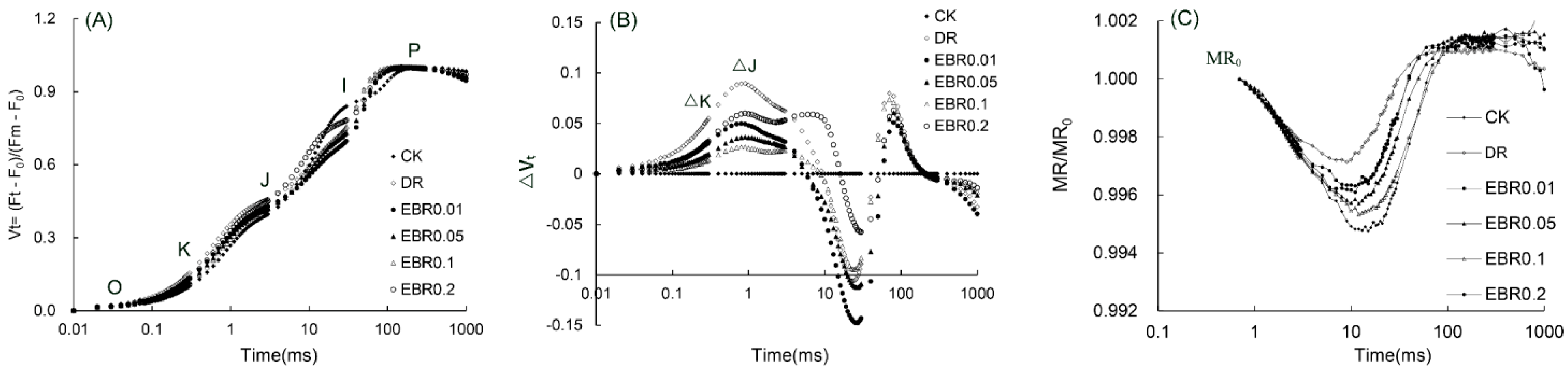
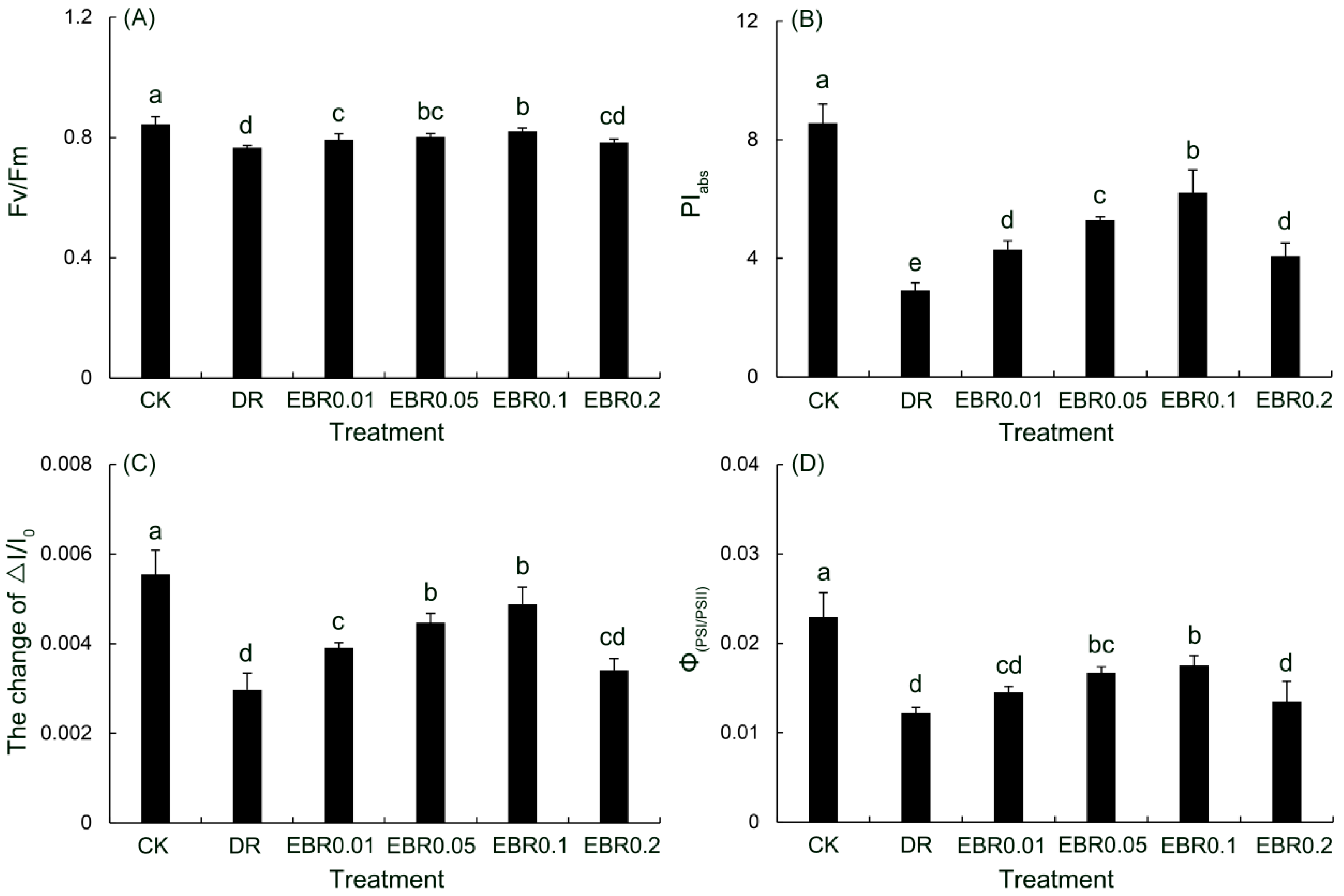
2.7. Rapid Chlorophyll Fluorescence Parameters
2.8. Functions and Coordination of Photosystem II (PSII) and Photosystem I (PSI)
2.9. Growth Characteristics
2.10. Secondary Metabolites
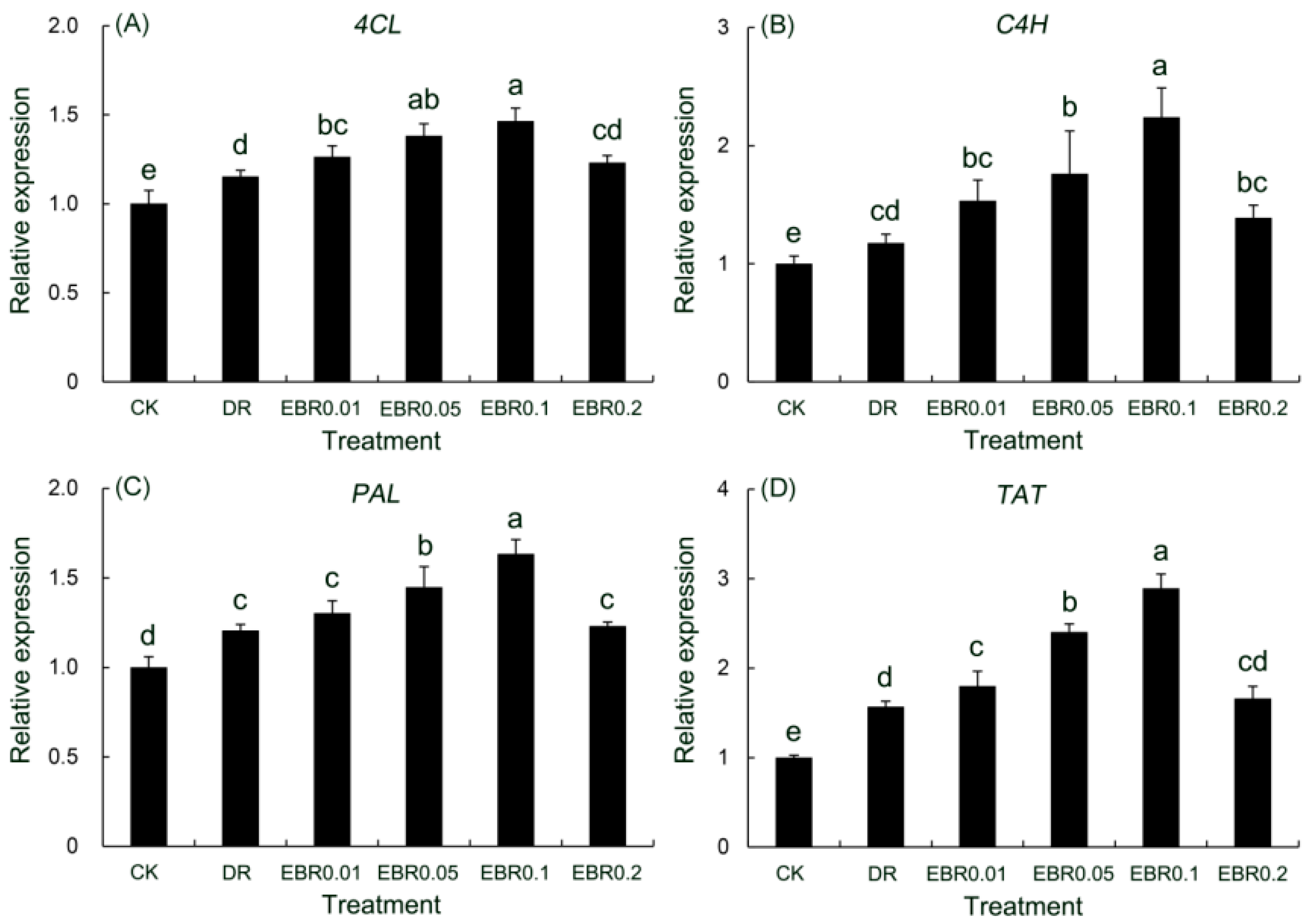
2.11. Gene Expression
3. Discussion
3.1. Effects of EBR on Physiological Parameters
3.2. Effects of EBR on Photosynthetic and Chlorophyll Fluorescence Parameters
3.3. Effects of EBR on the Growth of P. vulgaris
3.4. The Effects of EBR on Secondary Metabolites and Related Gene Expression
3.5. EBR Perception and Signaling in Transcriptional Regulation of Phenylpropanoid and Antioxidant Pathways
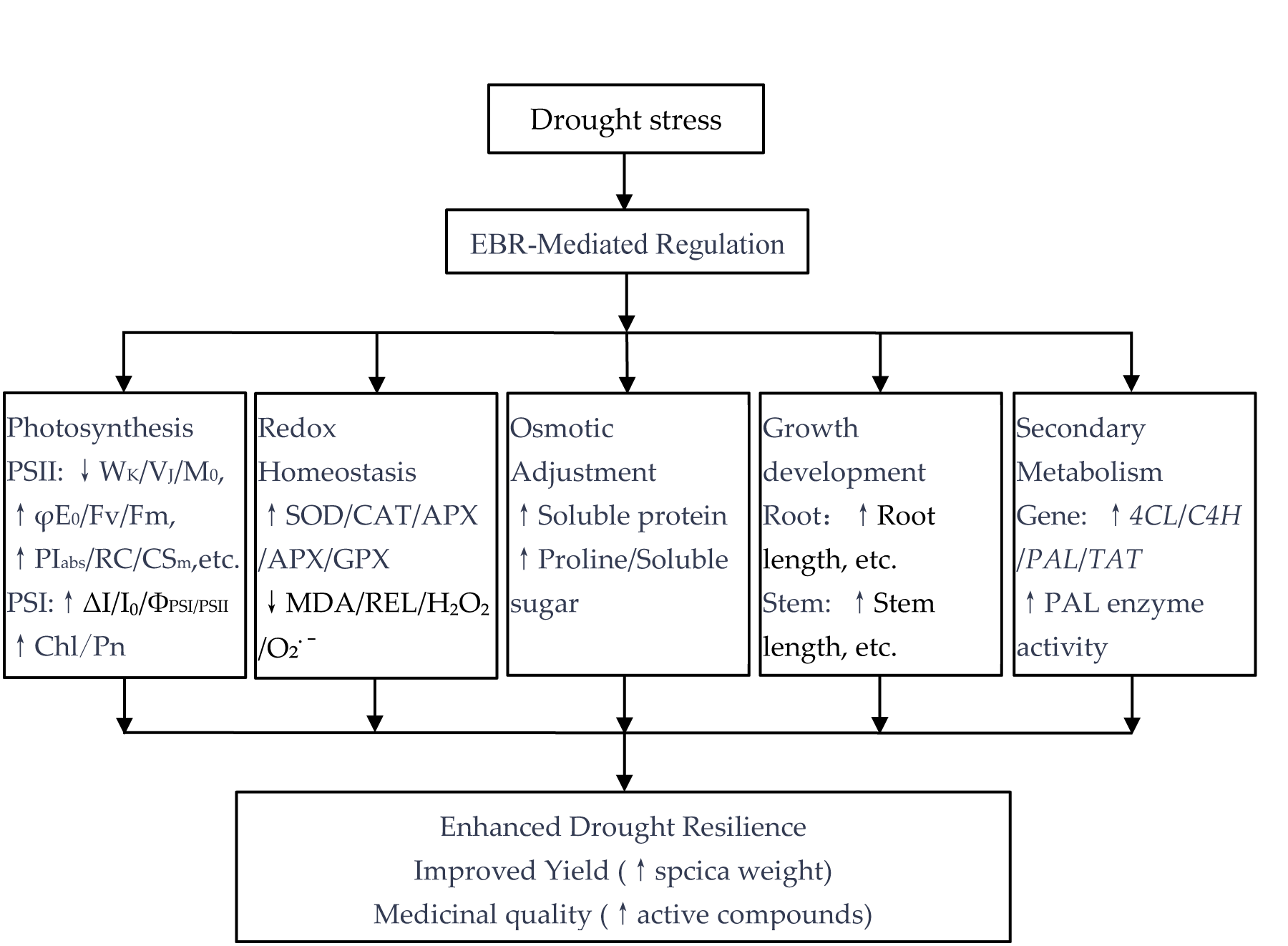
3.6. Novel Insights into EBR-Mediated Drought Resilience: Integrating Yield and Quality Enhancement
4. Materials and Methods
4.1. Plant Materials
4.2. Experimental Design
4.3. Determination of Antioxidant Enzyme Activity
4.4. Determination of Osmotic Adjustment Substances Content
4.5. Determination of Leaf Relative Water Content (RWC) and Soluble Protein
4.6. Determination of Photosynthetic Pigment Contents
4.7. Determination of Oxidative Stress Indicators
4.8. Measurement of Photosynthetic and Chlorophyll Fluorescence Parameters
4.9. Root Morphology
4.10. Plant Morphology and Biomass Measurement
4.11. Determination of Total Phenols and Secondary Metabolites
4.12. Gene Expression Analysis
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nadeem, M.; Li, J.; Yahya, M.; Sher, A.; Ma, C.; Wang, X.; Qiu, L. Research progress and perspective on drought stress in legumes: A review. Int. J. Mol. Sci. 2019, 20, 2541. [Google Scholar] [CrossRef]
- Kakamoukas, G.; Sarigiannidis, P.; Maropoulos, A.; Lagkas, T.; Zaralis, K.; Karaiskou, C. Towards climate smart farming—A reference architecture for integrated farming systems. Telecom 2021, 2, 52–74. [Google Scholar] [CrossRef]
- Chen, Y.; Yue, S.; Xia, W. Spatiotemporal variation characteristics of drought disaster in China and correlations with direct economic losses. J. Arid. Meteorol. 2024, 42, 485–497. [Google Scholar]
- Mukarram, M.; Choudhary, S.; Kurjak, D.; Petek, A.; Khan, M.M.A. Drought: Sensing, signalling, effects and tolerance in higher plants. Physiol. Plant 2021, 172, 1291–1300. [Google Scholar] [CrossRef]
- Razi, K.; Muneer, S. Drought stress-induced physiological mechanisms, signaling pathways and molecular response of chloroplasts in common vegetable crops. Crit. Rev. Biotechnol. 2021, 41, 669–691. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shi, Y.; Ao, Y.; Yu, D.; Wang, J.; Gao, S.; Knops, J.M.H.; Mu, C.; Li, Z. Summer drought decreases Leymus chinensis productivity through constraining the bud, tiller and shoot production. J. Agron. Crop Sci. 2019, 205, 554–561. [Google Scholar] [CrossRef]
- Song, Y.G.; Kang, L.; Tian, S.; Cui, L.L.; Li, Y.; Bai, M.; Fang, X.; Cao, L.; Coleman, K.; Miao, M. Study on the anti-hepatocarcinoma effect and molecular mechanism of Prunella vulgaris total flavonoids. J. Ethnopharmacol. 2021, 273, 113891. [Google Scholar] [CrossRef]
- Chen, Y.H.; Guo, Q.S.; Wang, C.Y. Textual research on the herbal medicine of Prunella vulgaris and changes in its medicinal parts. China J. Chin. Mater. Medica 2010, 35, 242–246. [Google Scholar]
- Ning, N.; Nan, Y.; Chen, G.; Huang, S.; Lu, D.; Yang, Y.; Meng, F.; Yuan, L. Anti-tumor effects and toxicity reduction mechanisms of Prunella vulgaris: A comprehensive review. Molecules 2024, 29, 1843. [Google Scholar] [CrossRef]
- Feng, L.; Jia, X.B.; Chen, Y.; Li, X. Research progress on chemical constituents and anti-tumor mechanisms of Prunella vulgaris. Chin. J. Tradit. Chin. Med. Pharm. 2008, 23, 428–434. [Google Scholar]
- Board of Pharmacopoeia of China. Pharmacopoeia of the People’s Republic of China; China Medico-Pharmaceutical Science & Technology Puhlishig House: Beijing, China, 2025. [Google Scholar]
- Li, M.Q.; Shi, Y.; Yang, S.Y.; Li, J.Y.; Hu, Y.; Sun, W.X.; Li, L.J. Research progress in anti-tumor mechanisms of Prunellae Spica and its active components. Chin. J. Mod. Appl. Pharm. 2024, 41, 716–726. [Google Scholar]
- Zhang, L.; Qian, S.; Cao, Y.; He, L.; Zhao, X.F.; Zhao, Y.C. Characteristics of heat and drought events across southern China in 2022 and their impacts on vegetation net primary productivity. Chin. J. Ecol 2024, 43, 2182–2188. [Google Scholar]
- Zhang, L.X.; Chang, Q.S.; Hou, X.G.; Chen, S.D.; Dai, P.F. Effects of salicylic acid on seed germination of Prunella vulgaris under drought stress. J. Henan Univ. Sci. Technol. (Nat. Sci.) 2016, 37, 72–77. [Google Scholar]
- Ali, M.M.; Anwar, R.; Malik, A.U.; Khan, A.S.; Ahmad, S.; Hussain, Z.; Hasan, M.U.; Nasir, M.; Chen, F. Plant growth and fruit quality response of strawberry is improved after exogenous application of 24-epibrassinolide. J. Plant Growth Regul. 2022, 41, 1786–1799. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, S.; Chang, J.; Wei, H.; Li, H.; Li, C.; Yang, J.; Song, Z.; Wang, Z.; Lun, J.; et al. Foliar application of 24-epibrassinolide enhances leaf nicotine content under low temperature conditions during the mature stage of flue-cured tobacco by regulating cold stress tolerance. BMC Plant Biol. 2025, 25, 77. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Huang, S.; Xie, Y.; Wang, S.; Jin, N.; Jin, L.; Tie, J.; Meng, X.; Li, Z.; Lyu, J.; et al. Physiological responses of coriander (Coriandrum sativum L.) to exogenous 2,4-epibrassinolide at different concentrations. BMC Plant Biol. 2023, 23, 649. [Google Scholar] [CrossRef]
- Yao, H.R.; Chen, K.; Zhang, L.X.; Xie, M.Y.; Dong, H.Y.; Song, W.X.; Kang, Y.; Chang, Q.S.; Chen, G.Q. Effects of 2,4-epibrassinolide on seed germination and seedling physiological characteristics of Prunella vulgaris under drought stress. Chin. J. Grassl. 2025, 47, 48–60. [Google Scholar]
- Chang, Q.S.; Zhang, L.X.; Chen, S.C.; Gong, M.G.; Liu, L.C.; Hou, X.G.; Mi, Y.F.; Wang, X.H.; Wang, J.Z.; Zhang, Y.; et al. Exogenous melatonin enhances the yield and secondary metabolite contents of Prunella vulgaris by modulating antioxidant system, root architecture and photosynthetic capacity. Plants 2023, 12, 1129. [Google Scholar] [CrossRef]
- Liang, D.; Ni, Z.; Xia, H.; Xie, Y.; Lv, X.; Wang, J.; Lin, L.; Deng, Q.; Luo, X. Exogenous melatonin promotes biomass accumulation and photosynthesis of kiwifruit seedlings under drought stress. Sci. Hortic. 2019, 246, 34–43. [Google Scholar] [CrossRef]
- Cai, Y.F.; Peng, L.C.; Li, S.F.; Zhang, L.; Xie, W.J.; Song, J.; Wang, J.H. 24-epibrassionlide improves photosynthetic response of Rhododendron delavayi to drought. Nord. J. Bot. 2020, 38, e02900. [Google Scholar] [CrossRef]
- Hu, W.; Yan, X.; Li, X.; Cao, Z. Effects of 24-epibrassinolide on the chlorophyll fluorescence transient in leaves of pepper under drought stress. Bull. Bot. Res. 2021, 41, 53–59. [Google Scholar]
- Töpfer, V.; Melzer, M.; Snowdon, R.J.; Stahl, A.; Matros, A.; Wehner, G. PEG treatment is unsuitable to study root related traits as it alters root anatomy in barley (Hordeum vulgare L.). BMC Plant Biol. 2024, 24, 856. [Google Scholar] [CrossRef]
- Ranjan, A.; Sinha, R.; Singla-Pareek, S.L.; Pareek, A.; Singh, A.K. Shaping the root system architecture in plants for adaptation to drought stress. Physiol. Plant 2022, 174, e13651. [Google Scholar] [CrossRef] [PubMed]
- Hosseinpour, M.; Ebadi, A.; Habibi, H.; Nabizadeh, E.; Jahanbakhsh, S. Enhancing enzymatic and nonenzymatic response of Echinacea purpurea by exogenous 24-epibrassinolide under drought stress. Ind. Crops Prod. 2020, 146, 112045. [Google Scholar] [CrossRef]
- Pontes, C.V.S.; Dos Santos, A.H.A.; Lopes, L.K.C.; Barbosa, M.A.M.; Bajguz, A.; Da Silva Lobato, A.K. Exogenous serotonin and 24-epibrassinolide boost root protection and suppress oxidative damages occasioned by severe water deficit in soybean seedlings. J. Plant Growth Regul. 2024, 43, 1833–1843. [Google Scholar] [CrossRef]
- Zhou, X.H.; Li, J.S.; Wen, C.W.; Wu, X.C.; Yang, W.; Zeng, P.S.; Wu, X.Y. Correlations of polysaccharide and flavonoid contents in Prunella vulgaris L. with main environmental factors and high-quality provenance screen. J. South China Agric. Univ. 2021, 42, 96–101. [Google Scholar]
- Aghdasi, S.; Aghaalikhani, M.; Mohammad, M.S.; Kahrizi, D. Phytochemical responses of camelina to brassinolide and boron foliar spray under irrigation regimes. Heliyon 2025, 11, e42630. [Google Scholar] [CrossRef]
- Hasan, M.M.; Ali, M.A.; Soliman, M.H.; Alqarawi, A.A.; Allah, E.F.A.; Fang, X. Insights into 28-homobrassinolide (HBR)-mediated redox homeostasis, AsA-GSH cycle, and methylglyoxal detoxification in soybean under drought-induced oxidative stress. J. Plant Interact. 2020, 15, 371–385. [Google Scholar] [CrossRef]
- Chu, L.; Luo, S.; Chen, Q.; Chen, X.; Xu, N.; Sun, X. The Beneficial Effect of 24-Epibrassinolide against high-temperature stress in Gracilariopsis lemaneiformis revealed by physiological response and transcriptomic profiling. J. Plant Growth Regul. 2025, 44, 1948–1962. [Google Scholar] [CrossRef]
- Jiang, B.; Bai, W. Physiological and metabolic responses of sorghum seedlings to drought stress revealed by transcriptomic analysis. J. Plant Nutr. Fertil. 2024, 30, 1934–1951. [Google Scholar]
- Zhou, H.; Zhou, G.; He, Q.; Zhou, L.; Ji, Y.; Lv, X. Capability of leaf water content and its threshold values in reflection of soil–plant water status in maize during prolonged drought. Ecol. Indic. 2021, 124, 107395. [Google Scholar] [CrossRef]
- Desoky, E.M.; Mansour, E.; Ali, M.M.A.; Yasin, M.A.T.; Abdul-Hamid, M.I.E.; Rady, M.M.; Ali, E.F. Exogenously Used 24-Epibrassinolide Promotes Drought Tolerance in Maize Hybrids by Improving Plant and Water Productivity in an Arid Environment. Plants 2021, 10, 354. [Google Scholar] [CrossRef]
- Qian, Y.; Li, S.Y.; Rao, L.Y. Effects of saline-alkali stress on organic osmoregulatory substances and antioxidant enzyme systems of Helianthus tuberosus. Arid. Zone Res. 2023, 40, 1465–1471. [Google Scholar]
- Feng, C.J. Study on the Mechanisms Underlying the Effect of Brassinolide on Germination of Barley Seeds Under Drought Stress. Master’s Thesis, Shihezi University, Shihezi, China, 2022. [Google Scholar]
- Cui, Y.; Yan, S.; Zhang, Y.; Wang, R.; Song, L.; Ma, Y.; Guo, H.; Yang, P. Physiological, metabolome and gene expression analyses reveal the accumulation and biosynthesis pathways of soluble sugars and amino acids in sweet sorghum under osmotic stresses. Int. J. Mol. Sci. 2024, 25, 8942. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Fu, B.; Li, S.; Wang, X.; Song, W.X.; Ye, Y.N.; Hu, P.F.; Wang, T.R. Effects of exogenous melatonin on growth and physiological characteristics of Agropyron mongolicum seedlings under drought stress. Chin. J. Appl. Ecol. 2023, 34, 2947–2957. [Google Scholar]
- Yu, J.Q. A role for brassinosteroids in the regulation of photosynthesis in Cucumis sativus. J. Exp. Bot. 2004, 55, 1135–1143. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, B.; Guo, Y.; Wang, T.; Wei, Q.; Luo, Y.; Li, H.; Wu, H.; Wang, X.; Zhang, X. Identification of drought-resistant response in proso millet (Panicum miliaceum L.) root through physiological and transcriptomic analysis. Plants 2024, 13, 1693. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Vinocur, B.; Altman, A. Plant responses to drought, salinity and extreme temperatures: Towards genetic engineering for stress tolerance. Planta 2003, 218, 1–14. [Google Scholar] [CrossRef]
- Wang, L.R.; Zhao, P.S.; Zhao, X.; Wang, X.P.; Ma, X.F.; Li, Y. Physiological adaptations to osmotic stress and characterization of a polyethylene glycol—Responsive gene in Braya humilis. Acta Soc. Bot. Pol. 2016, 85, 3487. [Google Scholar]
- Wen, J.F.; Chi, B.W.; Peng, C.X.; Deng, M.H. 2,4-Epibrassinolide relieves the damage of drought stress on fresh-cut lilies by increasing antioxidant enzyme activities. Pak. J. Bot. 2022, 54, 2043–2049. [Google Scholar] [CrossRef]
- Verslues, P.E.; Agarwal, M.; Katiyar-Agarwal, S.; Zhu, J.; Zhu, J.K. Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stresses that affect plant water status. Plant J. 2006, 45, 523–539. [Google Scholar] [CrossRef] [PubMed]
- Kou, J.T. Mitigating effect of exogenous 2,4-epibrassinolide on the inhibition of oat seed germination under salt stress. Pratacult. Sci. 2020, 37, 916–925. [Google Scholar]
- Sezgin, A.; Altuntas, C.; Demiralay, M.; Cinemre, S.; Terzi, R. Exogenous alpha lipoic acid can stimulate photosystem II activity and the gene expressions of carbon fixation and chlorophyll metabolism enzymes in maize seedlings under drought stress. J. Plant Physiol. 2019, 232, 65–73. [Google Scholar] [CrossRef]
- Zhang, R.R.; Wang, Y.H.; Li, T.; Tan, G.F.; Tao, J.P.; Su, X.J.; Xu, Z.S.; Tian, Y.S.; Xiong, A.S. Effects of simulated drought stress on carotenoid contents and expression of related genes in carrot taproots. Protoplasma 2021, 258, 379–390. [Google Scholar] [CrossRef]
- Mafakheri, A.; Siosemardeh, A.; Bahramnejad, B.; Struik, P.C.; Sohrabi, E. Effect of drought stress on yield, proline and chlorophyll contents in three chickpea cultivars. Aust. J. Crop Sci. 2010, 4, 580–585. [Google Scholar]
- Wan, Q.W. Effects of Exogenous 24-Epibrassinolide on Photosynthetic Characteristics of Tea Plants Under Salt and Drought Stress. Master’s Thesis, Nanjing Agricultural University, Nanjing, China, 2021. [Google Scholar]
- Zhang, C.; Liang, Q.; Wang, Y.; Liang, S.; Huang, Z.; Li, H.; Escalona, V.H.; Yao, X.; Cheng, W.; Chen, Z.; et al. BoaBZR1.1 mediates brassinosteroid-induced carotenoid biosynthesis in Chinese kale. Hortic. Res. 2024, 11, uhae104. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.T.; Teodoro, G.S.; Da Silva, K.C.; Pereira Matos, Y.C.; Batista, B.L.; Lobato, A.K.S. 24-Epibrassinolide alleviates drought effects in young Carapa guianensis plants, improving the hydraulic safety margin, gas exchange and antioxidant defence. Plant Biol. 2023, 25, 924–934. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Yao, X.; Liu, X.; Qiao, Z.; Liu, Y.; Li, X.; Jiang, X. Brassinolide can improve drought tolerance of maize seedlings under drought stress: By inducing the photosynthetic performance, antioxidant capacity and ZmMYB gene expression of maize seedlings. J. Soil Sci. Plant Nutr. 2022, 22, 2092–2104. [Google Scholar] [CrossRef]
- Yousuf, W.; Bhat, S.A.; Bashir, S.; Rather, R.A.; Panigrahi, K.C.; John, R. Brassinosteroid improves light stress tolerance in tomato (Lycopersicon esculentum) by regulating redox status, photosynthesis and photosystem II. Funct. Plant Biol. 2024, 51, FP24170. [Google Scholar] [CrossRef]
- Martins, J.P.R.; Moreira, S.W.; Braga, P.C.S.; Conde, L.T.; Cipriano, R.; Falqueto, A.R.; Gontijo, A.B.P.L. Photosynthetic apparatus performance and anatomical modulations of Alcantarea imperialis (Bromeliaceae) exposed to selenium during in vitro growth. Photosynthetica 2021, 59, 529–537. [Google Scholar] [CrossRef]
- Chalanika De Silva, H.C.; Asaeda, T. Effects of heat stress on growth, photosynthetic pigments, oxidative damage and competitive capacity of three submerged macrophytes. J. Plant Interact. 2017, 12, 228–236. [Google Scholar] [CrossRef]
- Mathur, S.; Jajoo, A.; Mehta, P.; Bharti, S. Analysis of elevated temperature-induced inhibition of photosystem II using chlorophyll a fluorescence induction kinetics in wheat leaves (Triticum aestivum). Plant Biol. 2011, 13, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhang, J.T.; Zhang, H.W.; Xu, Y.; An, Y.Y.; Wang, L.J. Effect of 5-Aminolevulinic acid (5-ALA) on leaf chlorophyll fast fluorescence characteristics and mineral element content of Buxus megistophylla grown along urban roadsides. Horticulturae 2021, 7, 95. [Google Scholar] [CrossRef]
- Fang, P.; Yan, M.; Chi, C.; Wang, M.; Zhou, Y.; Zhou, J.; Shi, K.; Xia, X.; Foyer, C.H.; Yu, J. Brassinosteroids act as a positive regulator of photoprotection in response to chilling stress. Plant Physiol. 2019, 180, 2061–2076. [Google Scholar] [CrossRef]
- Guo, Q.S.; Zhou, L.J.; Gong, W.H.; Wu, X.S. Effect of different water treatments on quality and yield of spadix in Prunella vulgaris. Chin. J. Chin. Mater. Med. 2010, 35, 1795–1798. [Google Scholar]
- Chang, L.; Fan, Z.; Liang, H.; Li, Z.; Zhang, S.; Li, Y. Effects of drought stress on photosynthetic characteristics and root water absorption of Glycyrrhiza uralensis. Pratacult. Sci. 2024, 41, 382–393. [Google Scholar]
- Wei, Z.; Li, J. Brassinosteroids regulate root growth, development, and symbiosis. Mol. Plant 2016, 9, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, D.; Tang, Z.; Zhang, Y.; Zhang, K.; Dong, J.; Wang, F. Exogenous brassinosteroids promotes root growth, enhances stress tolerance, and increases yield in maize. Plant Signal. Behav. 2022, 17, 2095139. [Google Scholar] [CrossRef]
- Salami, M.; Heidari, B.; Batley, J.; Wang, J.; Tan, X.L.; Richards, C.; Tan, H. Integration of genome-wide association studies, metabolomics, and transcriptomics reveals phenolic acid- and flavonoid-associated genes and their regulatory elements under drought stress in rapeseed flowers. Front. Plant Sci. 2023, 14, 1249142. [Google Scholar] [CrossRef]
- Wang, H.; Dong, Q.; Duan, D.; Zhao, S.; Li, M.; van Nocker, S.; Ma, F.; Mao, K. Comprehensive genomic analysis of the tyrosine aminotransferase (TAT) genes in apple (Malus domestica) allows the identification of MdTAT2 conferring tolerance to drought and osmotic stresses in plants. Plant Physiol. Biochem. 2018, 133, 81–91. [Google Scholar] [CrossRef]
- Albertos, P.; Dündar, G.; Schenk, P.; Carrera, S.; Cavelius, P.; Sieberer, T.; Poppenberger, B. Transcription factor BES1 interacts with HSFA1 to promote heat stress resistance of plants. EMBO J. 2022, 41, e108664. [Google Scholar] [CrossRef]
- Hodaei, M.; Rahimmalek, M.; Arzani, A.; Talebi, M. The effect of water stress on phytochemical accumulation, bioactive compounds and expression of key genes involved in flavonoid biosynthesis in Chrysanthemum morifolium L. Ind. Crops Prod. 2018, 120, 295–304. [Google Scholar] [CrossRef]
- Chen, G.; Li, D.; Yao, P.; Chen, F.; Yuan, J.; Ma, B.; Yang, Z.; Ding, B.; He, N. Metabolic and transcriptional analysis reveals flavonoid involvement in the drought stress response of mulberry leaves. Int. J. Mol. Sci. 2024, 25, 7417. [Google Scholar] [CrossRef] [PubMed]
- Olsen, K.M.; Lea, U.S.; Slimestad, R.; Verheul, M.; Lillo, C. Differential expression of four Arabidopsis PAL genes; PAL1 and PAL2 have functional specialization in abiotic environmental-triggered flavonoid synthesis. J. Plant Physiol. 2008, 165, 1491–1499. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhu, Q.; Liu, W.; Zhang, J. Exogenous brassinosteroid enhances zinc tolerance by activating the phenylpropanoid biosynthesis pathway in Citrullus lanatus L. Plant Signal. Behav. 2023, 18, 2186640. [Google Scholar]
- Liang, T.S.; Zhao, X.Q.; Gao, J.P. Effect of EBR on seed germination, seedling growth and key enzyme activities for flavonoid biosynthesis in Astragalus membranaceus var. mongholicus under PEG-induced drought stress. Chin. J. Exp. Tradit. Med. Formulae 2021, 27, 139–145. [Google Scholar]
- Zhang, L.X.; Chang, Q.S.; Zhao, X.L.; Guo, Q.; Chen, S.S.; Zhang, Q.M.; He, Y.L.; Chen, S.D.; Chen, K.; Ban, R.G.; et al. Selenium improves yield and quality in Prunella Vulgaris by regulating antioxidant defense, photosynthesis, growth, secondary metabolites, and gene expression under acid stress. Plants 2025, 14, 920. [Google Scholar] [CrossRef]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.; Ruan, X.; Zhao, Y.; Wei, F.; Wang, Q. Response of plant secondary metabolites to environmental factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef] [PubMed]
- Khatib, A.; Shaari, K.; Abas, F.; Shitan, M.; Kneer, R.; Neto, V.; Lajis, N. Discrimination of three pegaga (Centella) varieties and determination of growth-lighting effects on metabolites content based on the chemometry of 1h nuclear magnetic resonance spectroscop. J. Agric. Food Chem. 2012, 60, 410–417. [Google Scholar]
- Wu, N.B.; Li, L.L.; Yang, W.X.; Zhou, Y.T. Effects of light intensity on carbon-nitrogen metabolism and secondary metabolite of Catharanthus roseus leaves. Pratacult. Sci. 2014, 31, 1508–1514. [Google Scholar]
- Yamawo, A.; Tomlinson, K.W. Defence plasticity in the spiny plant Aralia elata (Miq.) Seem. in response to light and soil fertility. Ann. Bot. 2023, 131, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Zhu, Z.B.; Guo, Q.S.; Zhang, L.X.; Zhang, X.M. Variation in concentrations of major bioactive compounds in Prunella vulgaris L. related to plant parts and phenological stages. Biol. Res. 2012, 45, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, T.; Cano-Delgado, A.; Seto, H.; Hiranuma, S.; Fujioka, S.; Yoshida, S.; Chory, J. Binding of brassinosteroids to the extracellular domain of plant receptor kinase BRI1. Nature 2005, 433, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.H.; Li, J. BRI1/BAK1, a receptor kinase pair mediating brassinosteroid signaling. Cell 2002, 110, 203–212. [Google Scholar] [CrossRef]
- Li, Q.; Lu, J.; Yu, J.; Zhang, C.; He, J.; Liu, Q. The brassinosteroid-regulated transcription factors BZR1/BES1 function as a coordinator in multisignal-regulated plant growth. Biochim. et Biophys. Acta Gene Regul. Mech. 2018, 1861, 561–571. [Google Scholar] [CrossRef]
- Li, L.; Deng, X.W. It runs in the family: Regulation of brassinosteroid signaling by the BZR1–BES1 class of transcription factors. Trends Plant Sci. 2005, 10, 266–268. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, Z.; Shu, S.; Li, X.; Li, Y.; Liu, L.; Liu, L.; Wang, X.; Li, F.; Qanmber, G.; et al. GhEXL3 participates in brassinosteroids regulation of fiber elongation in Gossypium hirsutum. Plant J. 2024, 120, 491–504. [Google Scholar] [CrossRef]
- Zheng, B.; Su, Y.; Chen, C.; Cao, B.; Zhou, X.; Hu, S.; Liu, L.; Li, X.; Che, L.; Bin, T.; et al. Brassinosteroid-related transcription factor BZR1 regulates vegetative development and flavonoids biosynthesis in Scutellaria baicalensis. Int. J. Biol. Macromol. 2025, 308, 142383. [Google Scholar] [CrossRef]
- Oh, M.; Wang, X.; Kota, U.; Goshe, M.B.; Clouse, S.D.; Huber, S.C. Tyrosine phosphorylation of the BRI1 receptor kinase emerges as a component of brassinosteroid signaling in Arabidopsis. Proc. Natl. Acad. Sci. USA 2009, 106, 658–663. [Google Scholar] [CrossRef]
- Nolan, T.M.; Vukasinovic, N.; Liu, D.; Russinova, E.; Yin, Y. Brassinosteroids: Multidimensional regulators of plant growth, development, and stress responses. Plant Cell 2020, 32, 295–318. [Google Scholar] [CrossRef]
- Divi, U.K.; Rahman, T.; Krishna, P. Brassinosteroid-mediated stress tolerance in Arabidopsis shows interactions with abscisic acid, ethylene and salicylic acid pathways. BMC Plant Biol. 2010, 10, 151. [Google Scholar] [CrossRef] [PubMed]
- Divi, U.K.; Rahman, T.; Krishna, P. Gene expression and functional analyses in brassinosteroid-mediated stress tolerance. Plant Biotechnol. J. 2016, 14, 419–432. [Google Scholar] [CrossRef]
- Alam, P.; Albalawi, T.H.; Altalayan, F.H.; Bakht, M.A.; Ahanger, M.A.; Raja, V.; Ashraf, M.; Ahmad, P. 24-Epibrassinolide (EBR) confers tolerance against NaCl stress in soybean plants by up-regulating antioxidant system, ascorbate-glutathione cycle, and glyoxalase system. Biomolecules 2019, 9, 640. [Google Scholar] [CrossRef]
- Ahammed, G.J.; He, B.; Qian, X.; Zhou, Y.; Shi, K.; Zhou, J.; Yu, J.; Xia, X. 24-Epibrassinolide alleviates organic pollutants-retarded root elongation by promoting redox homeostasis and secondary metabolism in Cucumis sativus L. Environ. Pollut. 2017, 229, 922–931. [Google Scholar] [CrossRef]
- Chang, Q.; Zhang, L.; Mi, Y.; Sha, C.; Wang, Q.; Huang, Y.; Zhao, Y.; Guan, J.; Yu, Y.; Han, X. Effects of exogenous ALA on antioxidant capacities and photosynthetic characteristics in Prunella vulgaris seedlings under salt stress. J. Nucl. Agric. Sci. 2017, 31, 2055–2062. [Google Scholar]
- Rezayian, M.; Niknam, V.; Ebrahimzadeh, H. Effects of drought stress on the seedling growth, development, and metabolic activity in different cultivars of canola. Soil. Sci. Plant Nutr. 2018, 64, 360–369. [Google Scholar] [CrossRef]
- Ghobadi, M.; Taherabadi, S.; Ghobadi, M.; Mohammadi, G.; Jalali-Honarmand, S. Antioxidant capacity, photosynthetic characteristics and water relations of sunflower (Helianthus annuus L.) cultivars in response to drought stress. Ind. Crops Prod. 2013, 50, 29–38. [Google Scholar] [CrossRef]
- Zhang, L.X.; Chang, Q.S.; Hou, X.G.; Chen, S.D.; Zhang, Q.M.; Wang, J.Z.; Liu, S.S.; Li, S. Biochemical and photosystem characteristics of wild-type and Chl b-deficient mutant in tree peony (Paeonia suffruticosa). Photosynthetica 2021, 59, 256–265. [Google Scholar] [CrossRef]
- Wang, X.; Gao, Y.; Wang, Q.; Chen, M.; Ye, X.; Li, D.; Chen, X.; Li, L.; Gao, D. 24-Epibrassinolide-alleviated drought stress damage influences antioxidant enzymes and autophagy changes in peach (Prunus persicae L.) leaves. Plant Physiol. Biochem. 2019, 135, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Luo, X.; Wang, Z.; Liu, J. Mitigating effect of exogenous melatonin on salt and drought stress in Cyperus esculentus L. during the tillering stage. Agronomy 2024, 14, 1009. [Google Scholar] [CrossRef]
- Zhang, L.X.; Chang, Q.S.; He, Y.L.; Zhao, X.L.; Liu, W.; Guo, Q.; Chen, K.; Hou, X.G. Selenite foliar application increased the accumulation of medicinal components in Paeonia ostii by promoting antioxidant capacity, reducing oxidative stress, and improving photosynthetic capacity. Photosynthetica 2024, 62, 168–179. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Qiang, S.; Goltsev, V. Simultaneous in vivo recording of prompt and delayed fluorescence and 820-nm reflection changes during drying and after rehydration of the resurrection plant Haberlea rhodopensis. BBA Bioenerg. 2010, 1797, 1313–1326. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.T.; Xu, W.W.; Ren, B.Z.; Zhao, B.; Zhang, J.; Liu, P.; Zhang, Z.S. High temperature reduces photosynthesis in maize leaves by damaging chloroplast ultrastructure and photosystem II. J. Agron. Crop Sci. 2020, 206, 548–564. [Google Scholar] [CrossRef]
- Susamcı, E.; Tuncay, Ö.; Bayraktar, H.; önal, S. Phenol content and β-glucosidase activity during the ripening period of olive fruits (Erkence cv.) from different locations. Grasas Aceites 2024, 75, 2017. [Google Scholar] [CrossRef]
- Tang, H.; Hu, J.; Zhao, M.; Cao, L.; Chen, Y. Comparative study of the physiological responses, secondary metabolites, and gene expression of medicinal plant Prunella vulgaris L. treated with exogenous methyl jasmonate and salicylic acid. Acta Physiol. Plant 2023, 45, 20. [Google Scholar] [CrossRef]
- Chen, Y.H.; Zhang, X.R.; Guo, Q.S.; Cao, L.P.; Qin, Q.; Li, C.; Zhao, M.; Wang, W.M. Plant morphology, physiological characteristics, accumulation of secondary metabolites and antioxidant activities of Prunella vulgaris L. under UV solar exclusion. Biol. Res. 2019, 52, 17. [Google Scholar] [CrossRef]
| Parameter | CK | DR | EBR0.01 | EBR0.05 | EBR0.1 | EBR0.2 |
|---|---|---|---|---|---|---|
| ABS/RC | 1.05 ± 0.04 e | 1.51 ± 0.07 a | 1.32 ± 0.03 bc | 1.22 ± 0.08 cd | 1.16 ± 0.03 d | 1.34 ± 0.1 b |
| DI0/RC | 0.16 ± 0.01 d | 0.35 ± 0.03 a | 0.28 ± 0.03 b | 0.24 ± 0.02 bc | 0.22 ± 0.003 c | 0.29 ± 0.04 b |
| Tr0/RC | 0.89 ± 0.02 d | 1.15 ± 0.04 a | 1.05 ± 0.02 b | 0.98 ± 0.06 bc | 0.94 ± 0.03 cd | 1.05 ± 0.05 b |
| ET0/RC | 0.55 ± 0.01 b | 0.66 ± 0.06 a | 0.63 ± 0.02 ab | 0.60 ± 0.05 ab | 0.58 ± 0.05 ab | 0.63 ± 0.05 ab |
| RC/CSm | 26,792.95 ± 1092.63 a | 13,806.68 ± 1040.97 d | 16,366.11 ± 1285.26 c | 17,900.29 ± 620.56 bc | 18,868.83 ± 1040.11 b | 16,214.68 ± 1810.20 c |
| φD0 | 0.155 ± 0.007 d | 0.234 ± 0.007 a | 0.208 ± 0.019 b | 0.198 ± 0.01 bc | 0.187 ± 0.006 c | 0.214 ± 0.018 ab |
| ABS/CSm | 33,554.50 ± 614.48 a | 24,888.50 ± 1184.02 b | 25,992.50 ± 1597.54 b | 26,102.50 ± 495.59 b | 26,606.67 ± 513.84 b | 25,922.50 ± 1030.25 b |
| DI0/CSm | 5211.50 ± 321.73 b | 5823.00 ± 106.39 a | 5371.25 ± 216.57 b | 5160.25 ± 324.27 b | 4978.67 ± 158.19 b | 5192.00 ± 239.00 b |
| Tr0/CSm | 28,343.00 ± 292.74 a | 19,065.50 ± 1084.32 c | 20,621.25 ± 1768.44 bc | 20,942.25 ± 370.42 bc | 21,628.00 ± 152.42 b | 19,230.50 ± 2912.57 bc |
| ET0/CSm | 17,598.00 ± 296.98 a | 10,903.25 ± 461.48 d | 12,285.50 ± 684.25 bc | 12,784.50 ± 600.07 b | 13,199.33 ± 764.41 b | 11,433.50 ± 1412.09 cd |
| Treatment | Root Length (cm) | Root Surface Area (cm2) | Root Volume (cm3) | The Number of Root Tip | Branch Number |
|---|---|---|---|---|---|
| CK | 2427.68 ± 65.75 a | 372.44 ± 8.52 a | 4.55 ± 0.09 a | 3991.67 ± 87.56 a | 2266.67 ± 97.45 a |
| DR | 1170.32 ± 50.18 f | 171.69 ± 8.53 f | 2.01 ± 0.13 e | 2694.00 ± 302.23 d | 750.60 ± 48.40 e |
| EBR0.01 | 1665.44 ± 28.74 d | 253.65 ± 5.62 d | 3.08 ± 0.18 d | 3219.75 ± 324.79 bc | 1094.25 ± 19.87 d |
| EBR0.05 | 1853.48 ± 115.18 c | 287.75 ± 16.71 c | 3.58 ± 0.15 c | 3433.00 ± 360.62 b | 1555.33 ± 229.52 bc |
| EBR0.1 | 2013.44 ± 139.33 b | 314.79 ± 6.02 b | 3.92 ± 0.15 b | 3655.67 ± 142.66 ab | 1740.33 ± 183.56 b |
| EBR0.2 | 1544.69 ± 37.06 e | 239.08 ± 20.52 e | 2.93 ± 0.42 d | 3038.33 ± 325.59 cd | 1376.33 ± 180.03 c |
| Treatment | Stem Length (cm) | Spica Length (cm) | Number of Branches per Plant(cm) | Number of Spica per Plant | The Weight of Spica per Plant (g) | The Total Weight per Plant (g) |
|---|---|---|---|---|---|---|
| CK | 14.77 ± 0.93 a | 3.89 ± 0.26 a | 6.63 ± 0.52 a | 11.88 ± 0.64 a | 0.64 ± 0.02 a | 5.52 ± 0.15 a |
| DR | 12.63 ± 0.34 c | 2.67 ± 0.25 c | 3.88 ± 0.64 d | 7.25 ± 0.89 d | 0.40 ± 0.03 e | 3.61 ± 0.16 e |
| EBR0.01 | 13.76 ± 0.62 b | 3.04 ± 0.20 b | 4.50 ± 0.53 bc | 8.38 ± 0.52 c | 0.44 ± 0.03 d | 4.08 ± 0.09 d |
| EBR0.05 | 14.18 ± 0.69 ab | 3.09 ± 0.24 b | 4.75 ± 0.46 b | 8.88 ± 0.83 bc | 0.48 ± 0.02 c | 4.80 ± 0.12 c |
| EBR0.1 | 14.27 ± 0.61 ab | 3.22 ± 0.15 b | 4.88 ± 0.64 b | 9.38 ± 0.92 b | 0.54 ± 0.03 b | 5.13 ± 0.20 b |
| EBR0.2 | 12.98 ± 0.25 c | 2.76 ± 0.24 c | 4.00 ± 0.53 cd | 7.50 ± 0.93 d | 0.41 ± 0.04 de | 3.91 ± 0.10 d |
| Treatment | Total Phenols | Caffeic Acid | Ferulic Acid | Rosmarinic Acid | Hyperoside |
|---|---|---|---|---|---|
| CK | 4.467 ± 0.161 e | 0.026 ± 0.003 e | 0.275 ± 0.018 d | 1.341 ± 0.064 e | 0.120 ± 0.010 e |
| DR | 5.384 ± 0.194 d | 0.037 ± 0.005 d | 0.311 ± 0.023 cd | 1.606 ± 0.109 d | 0.148 ± 0.011 d |
| EBR0.01 | 5.976 ± 0.309 bc | 0.047 ± 0.005 c | 0.443 ± 0.051 b | 1.886 ± 0.134 c | 0.182 ± 0.013 c |
| EBR0.05 | 6.471 ± 0.521 b | 0.057 ± 0.003 b | 0.572 ± 0.015 a | 2.182 ± 0.062 b | 0.219 ± 0.012 b |
| EBR0.1 | 7.347 ± 0.379 a | 0.081 ± 0.006 a | 0.623 ± 0.055 a | 2.769 ± 0.146 a | 0.267 ± 0.011 a |
| EBR0.2 | 5.659 ± 0.438 cd | 0.032 ± 0.002 de | 0.350 ± 0.019 c | 1.656 ± 0.047 d | 0.159 ± 0.007 d |
| Fluorescence Parameters | Description |
|---|---|
| WK = (FK − F0)/(FJ − F0) | Normalized relative variable fluorescence |
| VJ = (FJ − F0)/(Fm − F0) | Relative variable fluorescence intensity at the J step |
| M0 = 4(F300μs − F0)/(Fm − F0) | Initial slope of the relative variable fluorescence of the relative rate at which QA is reduced |
| φE0 = ET0/ABS = [1 − (F0/Fm)] ψ0 | Quantum yield for electron transport |
| ABS/RC = M0(1/VJ) (1/φP0) | Absorption flux per reaction center |
| TR0/RC = M0(1/VJ) | Trapped energy flux per RC |
| ET0/RC = M0 (1/VJ) ψE0 | Electron transport flux per RC |
| DI0/RC = (ABS/RC) − (TR0/RC) | Dissipated energy flux per RC |
| RC/CSm = φP0 (VJ/M0) (ABS/CSm) | Density of RCs per excited cross-section (CS) |
| ABS/CSm ≈ Fm | Absorbed energy flux per CS |
| TR0/CSm = φP0(ABS/CSm) | Trapped energy flux per CS |
| ET0/CSm = φE0(ABS/CSm) | Electron transport flux per CS |
| DI0/CSm = ABS/CSm−TR0/CSm | Dissipated energy flux per CS |
| Fv/Fm | Maximal quantum yield of PSII photochemistry |
| PIABS = (RC/ABS) [φP0/(1 − φP0)] [ψ0/(1 − ψ0)] | Performance index on absorption basis |
| φDo | Quantum ratio for dissipated energy |
| Gene | Genbank Accession Number | Primer Name | Primer Sequence(5′ → 3′) | PCR Product (bp) |
|---|---|---|---|---|
| Pv4CL | KJ010817.1 | Pv4CL forward | CCACCATGGCCAATCCCTATT | 114 |
| Pv4CL reverse | CATAGTCCCGCACCTTGTCG | |||
| PvC4H | KJ010816 | PvC4H forward | ATCGTTGTCGCCGCCGTTGTGT | 136 |
| PvC4H reverse | CGTAGTCGGTGAGGTTTCGGTGGTTC | |||
| PvPAL | KJ010815.1 | PvPAL forward | TCCGTGCTTGTGTGTTTGTGCCTGTC | 203 |
| PvPAL reverse | GGCTTCCTGAACTCCTCCACCATCCT | |||
| PvTAT | KM053278 | PvTAT forward | CGTCTACTCGCATCAGCATCTCAGGA | 194 |
| PvTAT reverse | GCCAACCAGGGATCAACCACCTCTTC | |||
| β-actin | KJ010818 | β-actin forward | GCAGTTCTCTCCCTATACGCCAGTGG | 205 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Q.; Sun, Y.; Yao, H.; Zhang, B.; Zhang, L.; Wang, Z.; Zhang, Q.; Chen, S.; Liu, R.; Chang, W.; et al. 2,4-Epibrassinolide Enhances Drought Tolerance in Prunella vulgaris by Improving Photosynthesis, Redox Homeostasis, and Secondary Metabolism. Plants 2025, 14, 3587. https://doi.org/10.3390/plants14233587
Chang Q, Sun Y, Yao H, Zhang B, Zhang L, Wang Z, Zhang Q, Chen S, Liu R, Chang W, et al. 2,4-Epibrassinolide Enhances Drought Tolerance in Prunella vulgaris by Improving Photosynthesis, Redox Homeostasis, and Secondary Metabolism. Plants. 2025; 14(23):3587. https://doi.org/10.3390/plants14233587
Chicago/Turabian StyleChang, Qingshan, Yiming Sun, Hairui Yao, Biao Zhang, Lixia Zhang, Zi Wang, Qiaoming Zhang, Sudan Chen, Rongrong Liu, Wenxin Chang, and et al. 2025. "2,4-Epibrassinolide Enhances Drought Tolerance in Prunella vulgaris by Improving Photosynthesis, Redox Homeostasis, and Secondary Metabolism" Plants 14, no. 23: 3587. https://doi.org/10.3390/plants14233587
APA StyleChang, Q., Sun, Y., Yao, H., Zhang, B., Zhang, L., Wang, Z., Zhang, Q., Chen, S., Liu, R., Chang, W., Wang, X., Zheng, Y., & Hou, X. (2025). 2,4-Epibrassinolide Enhances Drought Tolerance in Prunella vulgaris by Improving Photosynthesis, Redox Homeostasis, and Secondary Metabolism. Plants, 14(23), 3587. https://doi.org/10.3390/plants14233587





