Effect of Mineral Fertilization on Vegetation of HNV Pastures in the Apuseni Mountains (Romania)
Abstract
1. Introduction
2. Results
2.1. Cluster Analysis of Vegetation Under the Influence of Mineral Fertlization
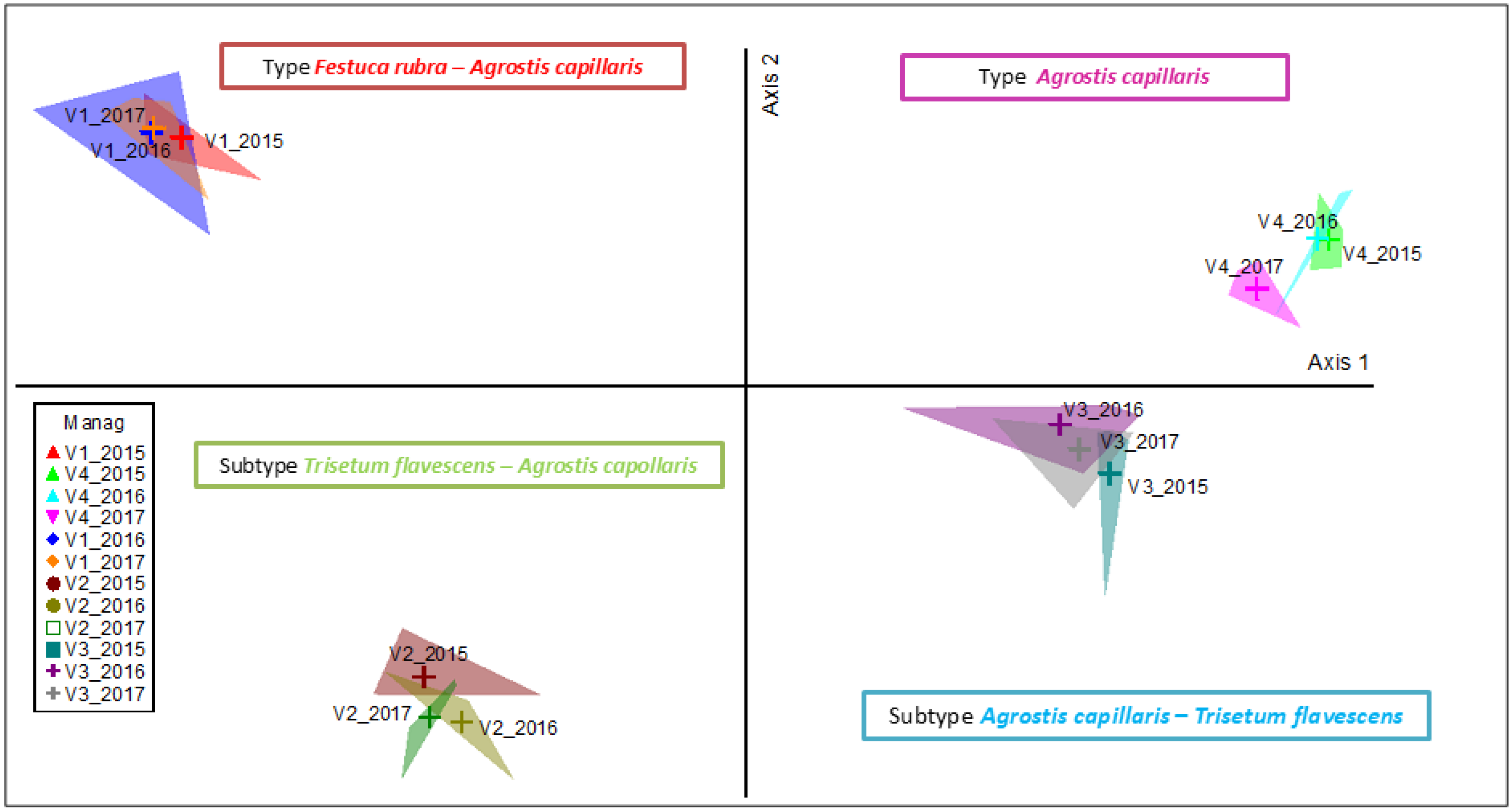
2.2. Spatial Distance in Plant Community Projection Due to Long-Term Fertilization
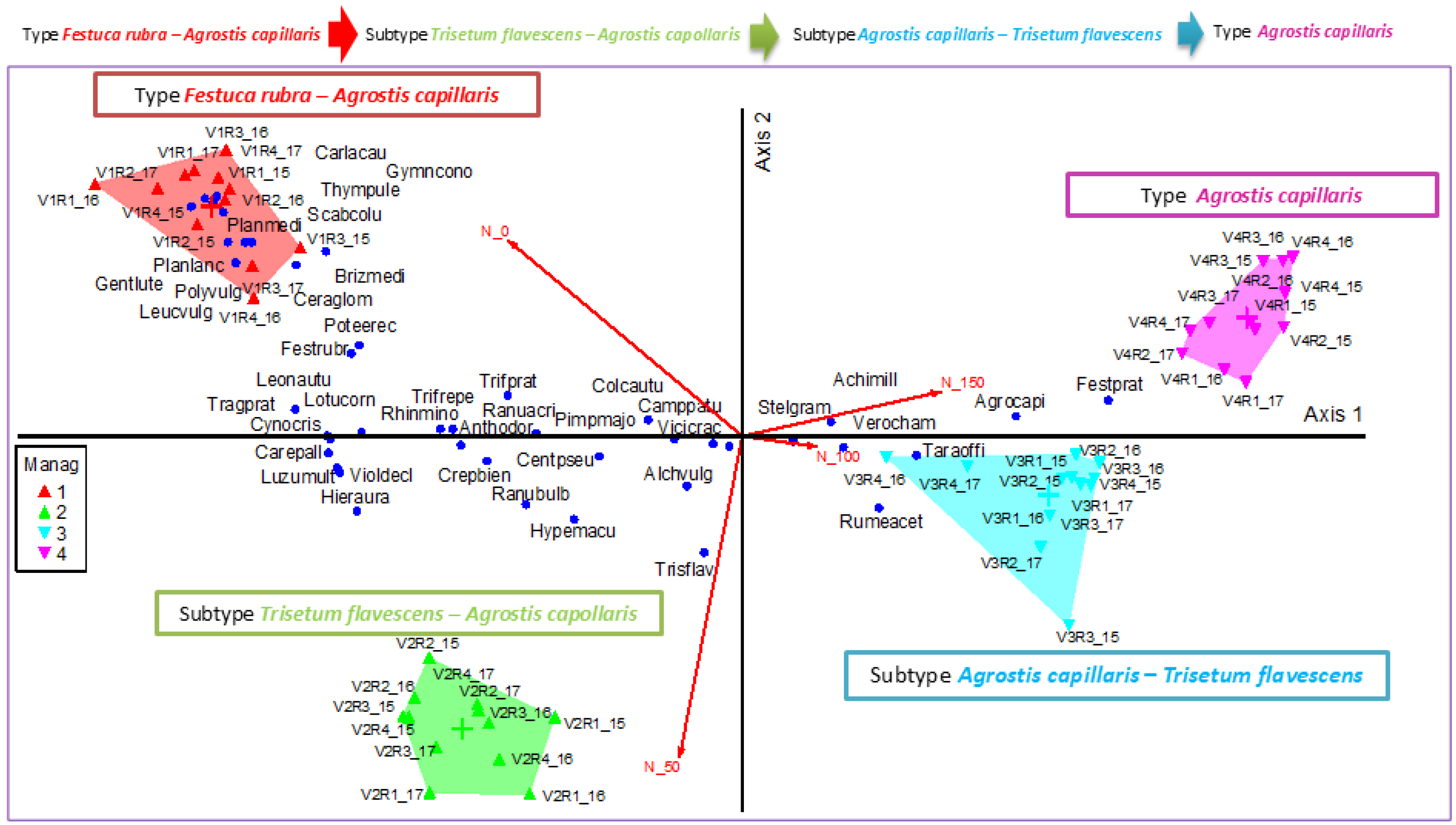
2.3. Species Reaction to the Gradient of Applied Inputs
2.4. The Influence of Mineral Fertilizer on Plant Diversity
3. Discussion
4. Materials and Methods
4.1. Study Area
4.2. Experimental Design
4.3. Floristic Composition
4.4. Data Analysis
4.5. Diversity Index Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Peeters, A. Environmental Impacts and Future Challenges of Grasslands and Grassland-Based Livestock Production Systems in Europe. In Grassland: A Global Resource Perspective; Ghosh, P.K., Mahanta, S.K., Singh, J.B., Pathak, P.S., Eds.; Wageningen Academic Pub: Wageningen, The Netherlands, 2015; pp. 365–390. [Google Scholar]
- Sima, N.; Păcurar, F. Quality of forage obtained from a mountain pasture as influenced by harvesting phenophase and management. Multi-function grasslands: Quality forages, animal products and landscapes. In Proceedings of the 19th General Meeting of the European Grassland Federation, La Rochelle, France, 27–30 May 2002. [Google Scholar]
- Varela, E.; Jay, M.; Flinzberger, L.; Mobarak, C.; Plieninger, T. A Review of High Nature Value Farming Systems in Europe: Biodiversity, Ecosystem Services, Drivers, Innovations and Future Prospects. People Nat. 2025, 00, 1–16. [Google Scholar] [CrossRef]
- Balázsi, Á.; Pacurar, F.; Mihu-Pintilie, A.; Konold, W. How Do Public Institutions on Nature Conservation and Agriculture Contribute to the Conservation of Species-Rich Hay Meadows? Int. J. Conserv. Sci. 2018, 9, 549–564. [Google Scholar]
- Reif, A.; Ruşdea, E.; Păcurar, F.; Rotar, I.; Brinkmann, K.; Auch, E.; Goia, A.; Bühler, J. A Traditional Cultural Landscape in Transformation. Mt. Res. Dev. 2008, 28, 18–22. [Google Scholar] [CrossRef]
- Torres-Miralles, M.; Jeanneret, P.; Lamminen, M.; Joly, F.; Dumont, B.; Tuomisto, H.; Herzon, I. High Nature Value Farming Systems in Europe: A Dataset Encompassing the Environmental Impact Assessment of Farms and Extensive Ruminant Food Products. Data Brief 2025, 58, 111164. [Google Scholar] [CrossRef]
- Veen, P.; Jefferson, R.; De Smidt, J.; Van der Straaten, J. Grasslands in Europe: Of High Nature Value; Brill: Leiden, The Netherlands, 2014. [Google Scholar]
- Reif, A.; Michler, B.; Rusdea, E. Feldgraswirtschaft im Apuseni-Gebirge, Rumänien. Tuexenia: Mitteilungen der Floristisch-Soziologischen. Arbeitsgemeinschaft 2005, 25, 141–149. [Google Scholar]
- Reif, A.; Auch, E.; Bühler, J.; Brinkmann, K.; Goia, A.I.; Pacurar, F.; Rusdea, E. Landschaft Und Landnutzung Im Apusenigebirge Rumäniens. Carinth. II 2005, 195, 161–201. [Google Scholar]
- Cojocariu, L.; Copăcean, L.; Popescu, C. Conservation of grassland habitats biodiversity in the context of sustainable development of mountain area of Romania. Appl. Ecol. Environ. Res. 2019, 17, 8877–8894. [Google Scholar] [CrossRef]
- Păcurar, F.; Rotar, I.; Vidican, R.; Vaida, I. The Ecological and Agronomic Study of Some Grasslands Phytocenoses from the Site Natura 2000 Rosci 0238 Suatu-Cojocna-Crairît. Rom. J. Grassl. Forage Crops 2023, 27, 9–28. [Google Scholar]
- Dale, L.; Fernandez, J.A.; Vermeulen, P.; Lecler, B.; Bogdan, A.D.; Pacurar, F.; Rotar, I.; Thewis, A.; Baeten, V. Research on Crude Protein and Digestibility of Arnica montana L. Using Conventional NIR Spectrometry and Hyperspectral Imaging NIR. J. Food Agric. Environ. 2012, 10, 391–396. [Google Scholar]
- Rotar, I.; Vaida, I.; Păcurar, F. Species with Indicative Values for the Management of the Mountain Grasslands. Rom. Agric. Res. 2020, 37, 2067–5720. [Google Scholar] [CrossRef]
- Samuil, C.; Vintu, V.; Sirbu, C.; Saghin, G.; Muntianu, I.; Ciobanu, C. Low Input Management of Agrostis Capillaris+ Festuca Rubra Grasslands in Romania. Grassl. Sci. Eur. 2011, 16, 335–337. [Google Scholar]
- Greinwald, A.; Hartmann, M.; Heilmann, J.; Heinrich, M.; Luick, R.; Reif, A. Soil and vegetation drive sesquiterpene lactone content and profile in Arnica montana L. flower heads from apuseni-mountains, Romania. Front. Plant Sci. 2022, 62, 813939. [Google Scholar] [CrossRef] [PubMed]
- Kricsfalusy, V.V. Mountain grasslands of high conservation value in the Eastern Carpathians: Syntaxonomy, biodiversity, protection and management. Thaiszia 2013, 23, 67–112. [Google Scholar]
- Janišová, M.; Škodová, I.; Magnes, M.; Iuga, A.; Biro, A.-S.; Ivașcu, C.M.; Ďuricová, V.; Buzhdygan, O.Y. Role of Livestock and Traditional Management Practices in Maintaining High Nature Value Grasslands. Biol. Conserv. 2025, 309, 111301. [Google Scholar] [CrossRef]
- Păcurar, F.; Rotar, I.; Vidican, R.; Stoian, V.; Gärtner, S.M.; Allen, R.B. Impact of Climate on Vegetation Change in a Mountain Grassland-Succession and Fluctuation. Not. Bot. Horti Agrobot. Cluj-Napoca 2014, 42, 347–356. [Google Scholar] [CrossRef]
- Sângeorzan, D.D.; Păcurar, F.; Reif, A.; Weinacker, H.; Rușdea, E.; Vaida, I.; Rotar, I. Detection and Quantification of Arnica Montana l. Inflorescences in Grassland Ecosystems Using Convolutional Neural Networks and Drone-Based Remote Sensing. Remote Sens. 2024, 16, 2012. [Google Scholar] [CrossRef]
- Pergola, M.; De Falco, E.; Cerrato, M. Grassland ecosystem services: Their economic evaluation through a systematic review. Land 2024, 13, 1143. [Google Scholar] [CrossRef]
- Păcurar, F.; Morea, A.; Gârda, N. Productivity and biodiversity evolution of Festuca rubra grasslands during a seven years period. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Agric. 2008, 65, 1843–5246. [Google Scholar]
- Maruşca, T.; Păcurar, F.S.; Taulescu, E.; Vaida, I.; Nicola, N.; Scrob, N.; Dragoș, M.M. Indicative Species for the Agrochemical Properties of Mountain Grasslands Soil from the Apuseni Natural Park (Rosci 0002). Rom. J. Grassl. Forage Crops 2022, 25, 31. [Google Scholar]
- Pellaton, R.; Lellei-Kovács, E.; Báldi, A. Cultural Ecosystem Services in European Grasslands: A Systematic Review of Threats. Ambio 2022, 51, 2462–2477. [Google Scholar] [CrossRef]
- Pardo, I.; Zabalza, S.; Berastegi, A.; Ripoll-Bosch, R.; Astrain, C. Assessment of Determinants of High Nature Value (HNV) Farmland at Plot Scale in Western Pyrenees. J. Environ. Manag. 2024, 349, 119516. [Google Scholar] [CrossRef]
- Vaida, I.; Rotar, I.; Pacurar, F. The Cumulative Effect of Manure on a Festuca rubra Grasslands for 15 Years. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Agric. 2017, 74, 126. [Google Scholar] [CrossRef] [PubMed]
- Păcurar, F.; Nagy, M. Study on DM production and floristic composition of a complex forage mixture. Rom. J. Grassl. Forage Crops 2014, 10, 25. [Google Scholar]
- Păcurar, F.; Rotar, I.; Pleșa, A.; Balázsi, Á.; Vidican, R. Study of the Floristic Composition of Certain Secondary Grasslands in Different Successional Stages as a Result of Abandonment. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Agric. 2015, 72, 193–201. [Google Scholar] [CrossRef]
- Jelinčić, A.; Perčin, A.; Zgorelec, Ž.; Papković, D. Local-scale changes in plant community composition following succession of oak-hornbeam forest after grassland abandonment. Acta Bot. Croat. 2023, 82, 147–150. [Google Scholar] [CrossRef]
- Shipley, J.R.; Frei, E.R.; Bergamini, A.; Boch, S.; Schulz, T.; Ginzler, C.; Barandun, M.; Bebi, P.; Bolliger, J.; Bollmann, K. Agricultural Practices and Biodiversity: Conservation Policies for Semi-Natural Grasslands in Europe. Curr. Biol. 2024, 34, R753–R761. [Google Scholar] [CrossRef]
- Vaida, I.; Rotar, I.; Păcurar, F.; Vidican, R.; Pleşa, A.; Mălinaş, A.; Stoian, V. Impact on the Abandonment of Semi-Natural Grasslands from Apuseni Mountains. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Agric. 2016, 73, 323. [Google Scholar] [CrossRef]
- Elliott, T.; Thompson, A.; Klein, A.; Albert, C.; Eisenhauer, N.; Jansen, F.; Schneider, A.; Sommer, M.; Straka, T.; Settele, J.; et al. Abandoning Grassland Management Negatively Influences Plant but Not Bird or Insect Biodiversity in Europe. Conserv. Sci. Pract. 2023, 5, e13008. [Google Scholar] [CrossRef]
- Meyer, M.; Schaub, S.; Bonev, P. Woody Plant Encroachment, Grassland Loss, and Farm Subsidies. J Agric. Econ. 2025, 76, 570–581. [Google Scholar] [CrossRef]
- Bellini, E.; Moriondo, M.; Dibari, C.; Leolini, L.; Staglianò, N.; Stendardi, L.; Filippa, G.; Galvagno, M.; Argenti, G. Impacts of Climate Change on European Grassland Phenology: A 20-Year Analysis of MODIS Satellite Data. Remote Sens. 2022, 15, 218. [Google Scholar] [CrossRef]
- Straffelini, E.; Luo, J.; Tarolli, P. Climate Change Is Threatening Mountain Grasslands and Their Cultural Ecosystem Services. Catena 2024, 237, 107802. [Google Scholar] [CrossRef]
- Bucała-Hrabia, A. Reflections on Land Use and Land Cover Change under Different Socio-Economic Regimes in the Polish Western Carpathians. Reg. Environ. Change 2024, 24, 28. [Google Scholar] [CrossRef]
- Vârban, R.; Vârban, D.I.; Mihăiescu, T.; Păcurar, F. Research on the optimisation of Arnica montana L seedling production. Analele Univ. Din Oradea Fasc. Protecția Mediu. 2014, 5, 157–162. [Google Scholar]
- Zieliński, M.; Łopatka, A.; Koza, P.; Gołębiewska, B. The Carpathian Agriculture in Poland in Relation to Other EU Countries, Ukraine and the Environmental Goals of the EU CAP 2023–2027. Agriculture 2024, 14, 1325. [Google Scholar] [CrossRef]
- Balfour, N.J.; Harris, C.; Storkey, J.; Ratnieks, F.L. Trade-off between Pollinator-Wildflower Diversity & Grassland Yields. npj Biodivers. 2025, 4, 1. [Google Scholar] [CrossRef]
- Piton, G.; Foulquier, A.; Bernard, L.; Bonin, A.; Pommier, T.; Lavorel, S.; Geremia, R.; Clement, J.C. Mineral Fertilization Reduces the Drought Resistance of Soil Multifunctionality in a Mountain Grassland System through Plant-Soil Interactions. Peer Community J. 2025, 5, e31. [Google Scholar] [CrossRef]
- Dale, A.; Rotar, I.; Boudry, C.; Păcurar, F.S.; Lecler, B.; Agneessens, R.; Dardenne, P.; Baeten, V. Fertilization Effects on the Chemical Composition and in Vitro Organic Matter Digestibility of Semi-Natural Meadows as Predicted by NIR Spectrometry. Not. Bot. Horti Agrobot. Cluj-Napoca 2013, 41, 58–64. [Google Scholar] [CrossRef]
- Culicov, O.A.; Tarcau, D.; Zinicovscaia, I.; Duliu, O.G.; Stavarache, M.; Vintu, V. Fertilizers’ Impact on Grassland in Northeastern Romania. Separations 2024, 11, 139. [Google Scholar] [CrossRef]
- Mălinas, A.; Rotar, I.; Vidican, R.; Iuga, V.; Păcurar, F.; Mălinas, C.; Moldovan, C. Designing a Sustainable Temporary Grassland System by Monitoring Nitrogen Use Efficiency. Agronomy 2020, 10, 149. [Google Scholar] [CrossRef]
- Vaida, I.; Păcurar, F.; Rotar, I.; Tomoș, L.; Stoian, V. Changes in Diversity Due to Long-Term Management in a High Natural Value Grassland. Plants 2021, 10, 739. [Google Scholar] [CrossRef]
- Dullau, S.; Kirmer, A.; Tischew, S.; Holz, F.; Meyer, M.H.; Schmidt, A. Effects of Fertilizer Levels and Drought Conditions on Species Assembly and Biomass Production in the Restoration of a Mesic Temperate Grassland on Ex-Arable Land. Glob. Ecol. Conserv. 2023, 48, e02730. [Google Scholar] [CrossRef]
- Gaga, I.; Pacurar, F.; Vaida, I.; Plesa, A.; Rotar, I. Responses of Diversity and Productivity to Organo-Mineral Fertilizer Inputs in a High-Natural-Value Grassland, Transylvanian Plain, Romania. Plants 2022, 11, 1975. [Google Scholar] [CrossRef]
- Jetter, K.; Jani, K.; Wilhelm, K.; Stehle, U.; Chamedjeu, R.; Riedel, C.; Wilfert, L.; Schäfer, P.; Sommer, S. Fertilization impacts microbiomes along the grassland trophic chain. ISME Commun. 2025, 5, ycaf162. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Luo, Z.; Li, L.; Nian, L.; Li, L.; Niu, Y.; He, R.; Liu, J. Nitrogen Fertilization Shapes Soil Microbial Diversity and Ecosystem Multifunctionality by Modulating Soil Nutrients. Microorganisms 2025, 13, 540. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Qu, Y.; Jiang, T.; Zhang, X.; Lyu, J.; Su, X. Ecosystems Resilience Assessment of Forest and Grassland Subjected to Ecological Drought. Ecol. Indic. 2025, 173, 113437. [Google Scholar] [CrossRef]
- Păcurar, F. Cercetări privind dezvoltarea sustenabilă (durabilă) a satului Gheţari, comuna Gârda prin îmbunătăţirea pajiştilor naturale şi a unor culturi agricole. Teză de doctorat-USAMV Cluj-Napoca 2005, 71, 10362. [Google Scholar]
- Apahidean, A.S.; Apahidean, M.; Pacurar, F. Experimental results on the possibilities of vegetable growing in the area of western Carpathian Mountains from Romania. Not. Bot. Horti Agrobot. Cluj-Napoca 2004, 32, 30. [Google Scholar]
- Su, J.; Zhao, Y.; Xu, F.; Bai, Y. Multiple Global Changes Drive Grassland Productivity and Stability: A Meta-analysis. J. Ecol. 2022, 110, 2850–2869. [Google Scholar] [CrossRef]
- Cojocariu, L.L.; Copăcean, L.; Ursu, A.; Sărăţeanu, V.; Popescu, C.A.; Horablaga, M.N.; Bordean, D.-M.; Horablaga, A.; Bostan, C. Assessment of the Impact of Population Reduction on Grasslands with a New “Tool”: A Case Study on the “Mountainous Banat” Area of Romania. Land 2024, 13, 134. [Google Scholar] [CrossRef]
- González-Moreno, P.; Schmitt, E.; Moreno-Ortiz, J.; Pinto-Correia, T.; Guiomar, N.; Delgado-Serrano, M.D.M. Assessing the Vulnerability of Mountain Value Chains to Environmental and Social Drivers in Europe: A Land-Use and Stakeholder-Based Approach. Ambio 2025, 54, 1386–1403. [Google Scholar] [CrossRef]
- Luick, R.; Schrode, S.; Schnyder, H.; Isselstein, J.; Taube, F. Extensive grasslands beyond the year 2013—Present situation and options for the future. Grassl. Sci. Eur. 2010, 15, 97–99. [Google Scholar]
- Ghione, F.; Hauser, S.; Reidy, B. Floristic diversity, yield and forage quality: Recent snapshots from a long-term alpine grassland fertilisation trial. Wagening. Acad. EGF Symp. Proc. 2025, 245–247. [Google Scholar] [CrossRef]
- Richter, F.J.; Suter, M.; Lüscher, A.; Buchmann, N.; El Benni, N.; Feola Conz, R.; Hartmann, M.; Jan, P.; Klaus, V.H. Effects of Management Practices on the Ecosystem-Service Multifunctionality of Temperate Grasslands. Nat. Commun. 2024, 15, 3829. [Google Scholar] [CrossRef] [PubMed]
- Świerszcz, S.; Czarniecka-Wiera, M.; Szymura, T.H.; Szymura, M. From Invasive Species Stand to Species-Rich Grassland: Long-Term Changes in Plant Species Composition during Solidago Invaded Site Restoration. J. Environ. Manag. 2024, 353, 120216. [Google Scholar] [CrossRef]
- Dalle Fratte, M.; Montagnoli, A.; Anelli, S.; Armiraglio, S.; Beatrice, P.; Ceriani, A.; Lipreri, E.; Miali, A.; Nastasio, P.; Cerabolini, B.E.L. Mulching in Lowland Hay Meadows Drives an Adaptive Convergence of Above-and below-Ground Traits Reducing Plasticity and Improving Biomass: A Possible Tool for Enhancing Phytoremediation. Front. Plant Sci. 2022, 13, 1062911. [Google Scholar] [CrossRef]
- Gaisler, J.; Pavlů, V.; Pavlů, L.; Hejcman, M. Long-term effects of different mulching and cutting regimes on plant species composition of Festuca rubra grassland. Agric. Ecosyst. Environ. 2013, 178, 10–17. [Google Scholar] [CrossRef]
- Zarzycki, J.; Józefowska, A.; Kopeć, M. Can Mulching or Composting Be Applied to Maintain Semi-Natural Grassland Managed for Biodiversity? J. Nat. Conserv. 2024, 78, 126584. [Google Scholar] [CrossRef]
- Brinkmann, K.; Pacurar, F.; Reif, A. Secondary succession and fluctuations in meadows of the Apuseni Mountains (Transylvania, Romania) under different fertilisation regimes. Transylv. Rev. Syst. Ecol. Res. 2009, 7, 41–58. [Google Scholar]
- Păcurar, F.; Reif, A.; Rusḑea, E. Conservation of Oligotrophic Grassland of High Nature Value (HNV) through Sustainable Use of Arnica Montana in the Apuseni Mountains, Romania. In Medicinal Agroecology; Taylor & Francis: Abingdon, UK, 2023. [Google Scholar]
- Samuil, C.; Vintu, V.; Sirbu, C.; Stavarache, M. Influence of Fertilizers on the Biodiversity of Semi-Natural Grassland in the Eastern Carpathians. Not. Bot. Horti Agrobot. Cluj-Napoca 2013, 41, 195–200. [Google Scholar] [CrossRef]
- Bellocchi, G.; Chabbi, A. Grassland Management for Sustainable Agroecosystems. Agronomy 2020, 10, 78. [Google Scholar] [CrossRef]
- Scotton, M.; Ziliotto, U. Long-Term Patterns of Grassland Vegetation and Species Richness in a Full-Factorial NPK Fertilization Experiment. Sci. Total Environ. 2024, 906, 167555. [Google Scholar] [CrossRef]
- Nazare, A.-I.; Stavarache, M.; Samuil, C.; Vîntu, V. The improvement of Nardus stricta L. permanent meadow from the Dorna Depression through mineral and organic fertilization. Sci. Papers Ser. A Agron. 2024, 67, 297–301. [Google Scholar]
- Pavlů, V.; Gaisler, J.; Pavlů, L.; Hejcman, M.; Ludvíková, V. Effect of Fertiliser Application and Abandonment on Plant Species Composition of Festuca Rubra Grassland. Acta Oecologica 2012, 45, 42–49. [Google Scholar] [CrossRef]
- Heinsoo, K.; Sammul, M.; Kukk, T.; Kull, T.; Melts, I. The Long-Term Recovery of a Moderately Fertilised Semi-Natural Grassland. Agric. Ecosyst. Environ. 2020, 289, 106744. [Google Scholar] [CrossRef]
- Lepš, J.; Lisner, A. Resistance and Resilience of Species Composition: Thirty Years of Experimental Mismanagement and Subsequent Restoration in a Species Rich Meadow. Ecol. Evol. 2025, 15, e70923. [Google Scholar] [CrossRef]
- Onete, M.; Chiriac, L.S.; Nicoară, R.G.; Bodescu, F.P.; Manu, M. Agrostis capillaris L.-A review of the distribution, characteristics, ecological and agronomic aspects, and usage. Sci. Papers Ser. A Agron. 2023, 66, 505–513. [Google Scholar]
- Ludvíková, V.; Pavlů, V.V.; Gaisler, J.; Hejcman, M.; Pavlů, L. Long term defoliation by cattle grazing with and without trampling differently affects soil penetration resistance and plant species composition in Agrostis capillaris grassland. Agric. Ecosyst. Environ. 2014, 197, 204–211. [Google Scholar] [CrossRef]
- Sackl, T.P.; Kaligarič, M.; Ivajnšič, D.; Škornik, S. Plant communities with yellow oat grass (Trisetum flavescens (L.) PB.) in the submontane and montane regions of Slovenia. Hacquetia 2012, 11, 179–207. [Google Scholar] [CrossRef]
- Mosquera-Losada, M.R.; Rigueiro-Rodríguez, A. Agroforestry Systems: An Option for Mitigation and Adaptation to Overcome Global Climate Change. Future Eur. Grassl. 2014, 19, 148. [Google Scholar]
- Broadbent, A.; Stevens, C.J.; Peltzer, D.A.; Ostle, N.J.; Orwin, K.H. Belowground Competition Drives Invasive Plant Impact on Native Species Regardless of Nitrogen Availability. Oecologia 2018, 186, 577–587. [Google Scholar] [CrossRef]
- Kozhouharov, Y.; Lingorski, V. Influence of Mineral Fertilization on Some Biological and Productive Indicators of Natural Meadow of Agrostis Capillaris- Festuca Fallax Type in the Rhodope Mountains (Southern Bulgaria). Biotechnol. Anim. Husb. 2012, 28, 613–622. [Google Scholar] [CrossRef]
- Păcurar, F.S.; Rotar, I.; Vidican, R.; Vaida, I.; Pleşa, A. Ecological and Agronomical Value of Agrostis Capillaris GRASSLANDS. Rom. J. Grassl. Forage Crops 2021, 23, 49. [Google Scholar]
- Hopkins, A.; Holz, B. Grassland for agriculture and nature conservation: Production, quality and multi-functionality. Agron. Res. 2006, 4, 3–20. [Google Scholar]
- Edwards, P.J.; Ekins, J.R.; Hollis, S. Long-Term Effects of Free-Ranging Cattle and Ponies on the Soil and Vegetation of Reseeded Grasslands in the New Forest, England. Perspectives in Plant Ecology, Evol. Syst. 2025, 67, 125875. [Google Scholar] [CrossRef]
- Pacurar, F.; Rotar, I.; Bogdan, A.D.; Vidican, R.M.; Dale, L. The Influence of Mineral and Organic Long-Term Fertilization upon the Floristic Composition of Festuca Rubra L.-Agrostis Capillaris L. Grassland in Apuseni Mountains, Romania. J. Food Agric. Environ. 2012, 10, 866–879. [Google Scholar]
- Francksen, R.M.; Turnbull, S.; Rhymer, C.M.; Hiron, M.; Bufe, C.; Klaus, V.H.; Newell-Price, P.; Stewart, G.; Whittingham, M.J. The Effects of Nitrogen Fertilisation on Plant Species Richness in European Permanent Grasslands: A Systematic Review and Meta-Analysis. Agronomy 2022, 12, 2928. [Google Scholar] [CrossRef]
- Marușca, T.; Păcurar, F.S.; Scrob, N.; Vaida, I.; Nicola, N.; Taulescu, E.; Dragoș, M.; Lukács, Z. Contributions to the Assessment of Grasslands Productivity of the Apuseni Natural Park (Rosci 0002). Rom. J. Grassl. Forage Crops 2021, 24, 23. [Google Scholar]
- Păcurar, F.; Marușca, T.; Scrob, N.; Vaida, I.; Nicola, N. The Ecological and Agronomic Study of Some Grasslands Phytocenoses from the Site Natura 2000 ROSCI0002 Apuseni. Rom. J. Grassl. Forage Crops 2023, 28, 31–54. [Google Scholar]
- Bohner, A. Soil chemical properties as indicators of plant species richness in grassland communities. Grassl. Sci. Eur. 2005, 10, 48–51. [Google Scholar]
- Păcurar, F. Specii Indicator Pentru Evaluarea şi Elaborarea Managementului Sistemelor de Pajişti cu Înaltă Valoare Naturală-HNV; Casa Cărţii de Ştiinţă: Cluj-Napoca, Romania, 2020. [Google Scholar]
- Toth, G.; Rotar, I.; Păcurar, F.; Vidican, R.; Vaida, I.; Pleșa, A. Characterization of Some Grassland from Transilvanian Plateau. RIULS Home 2017, 60, 189–192. Available online: https://repository.iuls.ro/xmlui/handle/20.500.12811/759 (accessed on 15 September 2025).
- Păcurar, F.; Rotar, I. Metode de Stud. şi Interpret. A Veg. Pajiştilor; Risoprint: Burlington, MA, USA, 2014. [Google Scholar]
- Păcurar, F.; Balázsi, Á.; Rotar, I.; Vaida, I.; Reif, A.; Vidican, R.; Rușdea, E.; Stoian, V.; Sângeorzan, D. Technologies Used for Maintaining Oligotrophic Grasslands and Their Biodiversity in a Mountain Landscape. Rom. Biotechnol. Lett. 2018, 23, 1128–1135. [Google Scholar] [CrossRef]
- Michler, B.; Rotar, I.; Pacurar, F.; Stoie, A. Arnica Montana, an Endangered Species and a Traditional Medicinal Plant: The Biodiversity and Productivity of Its Typical Grasslands Habitats. Grassl. Sci. Eur. 2005, 10, 336–339. [Google Scholar]
- Gianelle, D.; Guastella, F.; Vescovo, L. Cattle management for biodiversity conservation in an alpine pasture. Integr. Effic. Grassl. Farming Biodivers. 2005, 112. [Google Scholar]
- Bohner, A.; Karrer, J.; Walcher, R.; Brandl, D.; Michel, K.; Arnberger, A.; Frank, T.; Zaller, J.G. Ecological Responses of Semi-Natural Grasslands to Abandonment: Case Studies in Three Mountain Regions in the Eastern Alps. Folia Geobot. 2019, 54, 211–225. [Google Scholar] [CrossRef]
- Fartyal, A.; Chaturvedi, R.K.; Bargali, S.S.; Bargali, K. The Relationship between Phenological Characteristics and Life Forms within Temperate Semi-Natural Grassland Ecosystems in the Central Himalaya Region of India. Plants 2025, 14, 835. [Google Scholar] [CrossRef]
- Andreatta, D.; Bachofen, C.; Dalponte, M.; Klaus, V.H.; Buchmann, N. Extracting Flowering Phenology from Grassland Species Mixtures Using Time-Lapse Cameras. Remote Sens. Environ. 2023, 298, 113835. [Google Scholar] [CrossRef]
- McCune, B.; Grace, J.B. Analysis of Ecological Communities; Mjm Software Design: Gleneden Beach, OR, USA, 2002. [Google Scholar]
- McCune, B.; Mefford, M.J. PC-ORD: Multivariate Analysis of Ecological Data, Version 7; MjM Software Design: Gleneden Beach, OR, USA, 2016. [Google Scholar]
- Domínguez-Yescas, R.; Vázquez-García, J.A.; Muñiz-Castro, M.Á.; Hernández-Vera, G.; Salcedo-Pérez, E.; Rodríguez-Pérez, C.; Gallardo-Yobal, S.I. Small-Scale Environmental Drivers of Plant Community Structure and Diversity in Neotropical Montane Cloud Forests Harboring Threatened Magnolia Dealbata in Southern Mexico. Diversity 2020, 12, 444. [Google Scholar] [CrossRef]
- Marcin, M.; Raschmanová, N.; Miklisová, D.; Šupinskỳ, J.; Kaňuk, J.; Kováč, L. Karst Dolines Support Highly Diversified Soil Collembola Communities—Possible Refugia in a Warming Climate? Diversity 2022, 14, 1037. [Google Scholar] [CrossRef]
- Morabito, A.; Musarella, C.M.; Spampinato, G. Diversity and Ecological Assessment of Grasslands Habitat Types: A Case Study in the Calabria Region (Southern Italy). Land 2024, 13, 719. [Google Scholar] [CrossRef]
- Terzi, M.; Jasprica, N.; Pesaresi, S. Syntaxonomic Diversity of Rocky Dry Grasslands of the Chrysopogono Grylli-Koelerion Splendentis Along the East Adriatic. Diversity 2024, 16, 718. [Google Scholar] [CrossRef]
- Hao, M.; Corral-Rivas, J.J.; González-Elizondo, M.S.; Ganeshaiah, K.N.; Nava-Miranda, M.G.; Zhang, C.; Zhao, X.; Von Gadow, K. Assessing Biological Dissimilarities between Five Forest Communities. For. Ecosyst. 2019, 6, 30. [Google Scholar] [CrossRef]
- Xu, W.; Dai, W.; Ding, Y.; Song, S.; Liu, Q.; Yang, W. Drivers of Spontaneous Plant Communities in Urban Parks: A Case from Nanjing, China. Sustainability 2024, 16, 3841. [Google Scholar] [CrossRef]
- Mei, L.; Liu, Y.; Wang, Z.; Xiong, Z.; Wang, Y.; Jin, T.; Yang, X. Diversity and Community Structure of Rhizosphere Arbuscular Mycorrhizal Fungi in Songnen Grassland Saline–Alkali-Tolerant Plants: Roles of Environmental Salinity and Plant Species Identity. Agronomy 2025, 15, 2070. [Google Scholar] [CrossRef]
- Manu, M.; Băncilă, R.I.; Onete, M. Soil Fauna-Indicators of Ungrazed Versus Grazed Grassland Ecosystems in Romania. Diversity 2025, 17, 323. [Google Scholar] [CrossRef]
- Li, G.; Liu, J.; Zhang, W.; Hu, J.; Shi, P.; Wei, G. Fertilization Alters Indicator Species Serving as Bioindicators for Evaluating Agricultural Practices Related to Maize Grain Yield. Microorganisms 2025, 13, 1384. [Google Scholar] [CrossRef]
- Fan, S.; Tang, Y.; Yang, H.; Hu, Y.; Zeng, Y.; Wang, Y.; Zhao, Y.; Chen, X.; Wu, Y.; Wang, G. Effects of Fertilization and Planting Modes on Soil Organic Carbon and Microbial Community Formation of Tree Seedlings. Plants 2024, 13, 2665. [Google Scholar] [CrossRef]
- Caldararu, S.; Rolo, V.; Stocker, B.D.; Gimeno, T.E.; Nair, R. Ideas and Perspectives: Beyond Model Evaluation–Combining Experiments and Models to Advance Terrestrial Ecosystem Science. Biogeosci. Discuss. 2023, 2023, 3637–3649. [Google Scholar] [CrossRef]
- Peck, J.E. Multivariate Analysis for Community Ecologists: Step-by-Step Using PC-ORD; Mjm Software Design: Gleneden Beach, OR, USA, 2010. [Google Scholar]
- Hautier, Y.; Zhang, P.; Loreau, M.; Wilcox, K.R.; Seabloom, E.W.; Borer, E.T.; Byrnes, J.E.; Koerner, S.E.; Komatsu, K.J.; Lefcheck, J.S. General Destabilizing Effects of Eutrophication on Grassland Productivity at Multiple Spatial Scales. Nat. Commun. 2020, 11, 5375. [Google Scholar] [CrossRef]
- Yan, H.; Li, F.; Liu, G. Diminishing Influence of Negative Relationship between Species Richness and Evenness on the Modeling of Grassland α-Diversity Metrics. Front. Ecol. Evol. 2023, 11, 1108739. [Google Scholar] [CrossRef]
- Guo, P.; Lu, Q.; Li, S. Productivity and Species Diversity of Plant Communities Are Higher inside than Outside the West Ordos National Nature Reserve, Northern China. Plants 2024, 13, 660. [Google Scholar] [CrossRef]
- Chen, W.; Ye, M.; Pan, X.; Li, M.; Zeng, G.; Zhang, X.; He, Q.; Gu, X.; Qian, J.; Lv, Y. Relationships and Changes in Grassland Community Diversity and Biomass in the Pastoral Areas of the Two Rivers under Grazing Disturbance. Agronomy 2024, 14, 1336. [Google Scholar] [CrossRef]
- Sang, Y.; Gu, H.; Meng, Q.; Men, X.; Sheng, J.; Li, N.; Wang, Z. An Evaluation of the Performance of Remote Sensing Indices as an Indication of Spatial Variability and Vegetation Diversity in Alpine Grassland. Remote Sens. 2024, 16, 4726. [Google Scholar] [CrossRef]
- Magurran, A.E. Measuring Biological DPeetersiversity; Blackwell Publishing: Malden, MA, USA, 2004. [Google Scholar]
- Cislaghi, A.; Giupponi, L.; Tamburini, A.; Giorgi, A.; Bischetti, G.B. The effects of mountain grazing abandonment on plant community, forage value and soil properties: Observations and field measurements in an alpine area. Catena 2019, 181, 104086. [Google Scholar] [CrossRef]
- Weigelt, A.; Weisser, W.W.; Buchmann, N.; Scherer-Lorenzen, M. Biodiversity for multifunctional grasslands: Equal productivity in high-diversity low-input and low-diversity high-input systems. Biogeosciences 2009, 6, 1695–1706. [Google Scholar] [CrossRef]
- Samuil, C.; Nazare, A.I.; Sîrbu, C.; Grigoraş, B.; Vîntu, V. The Impact of Fertilizer Gradient on High Nature Value Mountain Grassland. Plants 2025, 14, 3397. [Google Scholar] [CrossRef]
- Poux, X.; Aubert, P.M. Permanent grassland and ruminants are a key component of the agroecological transition in Europe-findings from the’Ten Years for Agroecology’scenario. Grassl. Sci. Eur. 2022, 27, 16–28. [Google Scholar]
- Rancane, S.; Karklins, A.; Lazdina, D. Grass dry matter yield and plant nutrient removal following fertilization with wood ash and digestate. Grassl. Heart Circ. Sustain. Food Syst. 2022, 27, 259. [Google Scholar]
- Lomba, A.; McCracken, D.; Herzon, I. High nature value farming systems in Europe. Ecol. Soc. 2023, 28, 20. [Google Scholar] [CrossRef]
- Keenleyside, C.; Beaufoy, G.; Tucker, G.; Jones, G. High Nature Value farming throughout EU-27 and its financial support under the CAP. Inst. Eur. Environ. Policy 2014, 10, 91086. [Google Scholar]
- Bartolini, F.; Brunori, G. Understanding linkages between common agricultural policy and High Nature Value (HNV) farmland provision: An empirical analysis in Tuscany Region. Agric. Food Econ. 2014, 2, 13. [Google Scholar] [CrossRef]
- Peeters, A. Past and Future of European Grasslands. 124. Available online: https://www.researchgate.net/publication/234001775_Past_and_future_of_European_grasslands_The_challenge_of_the_CAP_towards_2020 (accessed on 1 August 2025).
- Persson, T.; Szulc, W.; Rutkowska, B.; Höglind, M.; Hanslin, H.M.; Sæbø, A. Impacts of organic soil amendments on forage grass production under different soil conditions. Agric. Food Sci. 2020, 29, 482–493. [Google Scholar] [CrossRef]
- Rotar, I.; Păcurar, F.; Roxana, V.; Sima, N. The influence of organic and organo-mineral fertilization on biodiversity of the Festuca rubra meadows in Apuseni Mountains. Lucr. Ştiinţifice 2005, 55, 2012. [Google Scholar]
- Rotar, I.; Păcurar, F.; Vidican, R. The influence of organic fertilisers on the biodiversity of a Festuca rubra meadow. Grassland Productivity. Grassl. Sci. Eur. 2006, 11, 363–366. [Google Scholar]
- Vidican, R.; Stoian, V.; Rotar, I.; Păcurar, F. Use of Non-metric Ordination and Group Analysis for the Assessment of Influence Level of Climatic and Technological Factors over Mycorrhizal Parameters. Rom. J. Grasslallds Forage Crops 2013, 8, 67. [Google Scholar]
- Huygens, D.; Vidican, R.; Păcurar, F.; Carlier, L.; Mălinaș, A. The influence of different type of management upon the floristic structure of grasslands systems, with special attention on Arnica montana L. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Agric. 2013, 70, 2013. [Google Scholar] [CrossRef]
- Marușca, T. Contributions to the evaluation of the productivity of Natura 2000 grassland habitats in the Apuseni Mountains. Ann. Acad. Rom. Sci. 2025, 14, 41–51. [Google Scholar] [CrossRef]
- Tzonev, R.; Gussev, C.; Popgeorgiev, G. Scrub, grassland and rocky habitats in Ponor Special Protection Area (Natura 2000), western Bulgaria: Mapping and assessment of conservation status. Acta Zool. Bulg. 2014, 5, 21–32. [Google Scholar]
- Proorocu, M.; Miclăuş, M.; Pauliuc, S.; Bodan, S.; Popa, A. The Conservation Measures of NATURA 2000 “Someşul Rece” Site Management Plan. Bull. UASVM Ser. Agric. 2016, 73, 296–299. [Google Scholar] [CrossRef]
- Brinkmann, K.; Reif, A. Vegetation, landuse and landscape in the Apuseni Mountains, Romania. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Agric. 2007, 62, 1–13. [Google Scholar]


| Axis | Degree of Participation (r) | Cumulative |
|---|---|---|
| 1 | 0.896 | 0.896 |
| 2 | 0.066 | 0.962 |
| Treatments | T | A | p Value |
|---|---|---|---|
| V1_2015 vs. V1_2016 | −0.063 | 0.001 | 0.429 |
| V1_2015 vs. V1_2017 | −0.342 | 0.010 | 0.330 |
| V2_2015 vs. V2_2016 | −0.555 | 0.014 | 0.262 |
| V2_2015 vs. V2_2017 | −0.592 | 0.016 | 0.263 |
| V3_2015 vs. V3_2016 | −0.204 | 0.009 | 0.326 |
| V3_2015 vs. V3_2017 | 0.950 | −0.038 | 0.829 |
| V4_2015 vs. V4_2016 | 0.825 | −0.058 | 0.808 |
| V4_2015 vs. V4_2017 | −1.216 | 0.063 | 0.115 |
| Experimental Factors | Axis 1 | Axis 2 | ||
|---|---|---|---|---|
| r | Significance | r | Significance | |
| V1 Control | 0.660 | −0.601 | ||
| V2 50N25P25K | 0.344 | * | 0.771 | *** |
| V3 100N50P50K | −0.372 | * | 0.140 | ns |
| V4 150N75P75K | −0.608 | *** | −0.289 | ns |
| Treatments | T | A | p |
|---|---|---|---|
| V1 vs. V2 | −15.333 | 0.398 | <0.001 |
| V1 vs. V3 | −15.714 | 0.645 | <0.001 |
| V1 vs. V4 | −15.836 | 0.730 | <0.001 |
| V2 vs. V3 | −15.588 | 0.603 | <0.001 |
| V2 vs. V4 | −15.808 | 0.718 | <0.001 |
| V3 vs. V4 | −14.393 | 0.343 | <0.001 |
| Species | Axis 1 | Axis 2 | ||||||
|---|---|---|---|---|---|---|---|---|
| r | r-sq | tau | Signif. | r | r-sq | tau | Signif. | |
| Agrostis capillaris L. | 0.983 | 0.966 | 0.901 | *** | 0.160 | 0.025 | 0.076 | ns |
| Anthoxanthum odoratum L. s. str. | −0.792 | 0.627 | −0.694 | *** | 0.049 | 0.002 | 0.010 | ns |
| Briza media L. | −0.462 | 0.213 | −0.545 | ** | 0.429 | 0.184 | 0.527 | * |
| Cynosurus cristatus L. | −0.760 | 0.577 | −0.574 | *** | 0.005 | 0.000 | 0.082 | ns |
| Festuca pratensis Huds. s. l. | 0.230 | 0.053 | 0.134 | ns | 0.049 | 0.002 | 0.052 | ns |
| Festuca rubra L. | −0.886 | 0.786 | −0.764 | *** | 0.397 | 0.157 | 0.145 | * |
| Trisetum flavescens (L.) P. Beauv. | −0.150 | 0.023 | −0.124 | ns | −0.953 | 0.908 | −0.811 | *** |
| Carex pallescens L. | −0.937 | 0.877 | −0.711 | *** | −0.077 | 0.006 | 0.041 | ns |
| Luzula multiflora (Ehrh.) Lej. | −0.957 | 0.915 | −0.715 | *** | −0.151 | 0.023 | −0.017 | ns |
| Lotus corniculatus L. | −0.613 | 0.375 | −0.662 | *** | 0.015 | 0.000 | −0.010 | ns |
| Trifolium pratense L. | −0.535 | 0.287 | −0.445 | ** | 0199 | 0.040 | 0.265 | ns |
| Trifolium repens L. | −0.748 | 0.560 | −0.582 | *** | 0.039 | 0.001 | 0.154 | ns |
| Vicia cracca L. s. str. | −0.036 | 0.001 | −0.059 | ns | −0.052 | 0.003 | −0.032 | ns |
| Achillea millefolium L. | 0.200 | 0.040 | 0.053 | ns | 0.071 | 0.005 | 0.109 | ns |
| Alchemilla vulgaris L. | −0.303 | 0.092 | −0.209 | ns | −0.558 | 0.311 | −0.419 | ns |
| Campanula patula L. | −0.088 | 0.008 | −0.246 | ns | −0.043 | 0.002 | −0.014 | ns |
| Carlina acaulis L. | −0.537 | 0.288 | −0.476 | ** | 0.472 | 0.223 | 0.427 | ** |
| Centaurea pseudophrygia C. A. Mey. | −0.659 | 0.434 | −0.529 | *** | −0.189 | 0.036 | −0.087 | ns |
| Cerastium holosteoides Fr. | −0.439 | 0.193 | −0.379 | * | 0.411 | 0.169 | 0.360 | * |
| Colchium autumnale L. | −0.335 | 0.112 | −0.349 | * | 0.130 | 0.017 | 0.089 | ns |
| Crepis biennis L. | −0.401 | 0.160 | −0.446 | * | −0.078 | 0.006 | 0.138 | ns |
| Gentianella lutescens (Velen.) Holub | −0.513 | 0.264 | −0.441 | ** | 0.492 | 0.242 | 0.448 | ** |
| Gymnadenia conopsea (L.) R. Br. s. l. | −0.650 | 0.423 | −0.571 | *** | 0.606 | 0.367 | 0.559 | *** |
| Hieracium aurantiacum L. | −0.467 | 0.218 | −0.345 | ** | −0.189 | 0.036 | −0.104 | ns |
| Hypericum maculatum Crantz s. str. | −0.472 | 0.223 | −0.329 | ** | −0.486 | 0.237 | −0.236 | ** |
| Leontodon autumnalis L. | −0.712 | 0.507 | −0.579 | *** | 0.090 | 0.008 | 0.151 | ns |
| Leucanthemum vulgare Lam. s. str. | −0.698 | 0.487 | −0.575 | *** | 0.564 | 0.318 | 0.335 | ** |
| Pimpinella major (L.) Huds. | −0.416 | 0.173 | −0.383 | * | −0.025 | 0.001 | −0.042 | ns |
| Plantago lanceolata L. | −0.640 | 0.409 | −0.621 | *** | 0.532 | 0.283 | 0.340 | ** |
| Plantago media L. | −0.665 | 0.442 | −0.631 | *** | 0.547 | 0.299 | 0.340 | ** |
| Polygala vulgaris L. s. l. | −0.528 | 0.278 | −0.588 | ** | 0.381 | 0.145 | 0.440 | * |
| Potentilla erecta (L.) Raeusch. | −0.622 | 0.387 | −0.703 | *** | 0.314 | 0.098 | 0.024 | ns |
| Ranunculus acris L. | −0.604 | 0.365 | −0.557 | *** | 0.020 | 0.000 | 0.057 | ns |
| Ranunculus bulbosus L. | −0.449 | 0.201 | −0.494 | * | −0.295 | 0.087 | −0.197 | ns |
| Rhinanthus minor L. | −0.404 | 0.163 | −0.474 | * | −0.026 | 0.001 | −0.146 | ns |
| Rumex acetosa L. | 0.461 | 0.212 | 0.366 | ** | −0.504 | 0.254 | −0.497 | ** |
| Scabiosa columbaria L. | −0.501 | 0.251 | −0.611 | ** | 0.398 | 0.158 | 0.414 | * |
| Stellaria graminea L. | 0.183 | 0.034 | 0.155 | ns | −0.032 | 0.001 | −0.022 | ns |
| Taraxacum officinale Weber s. l. | 0.598 | 0.358 | 0.524 | ** | −0.132 | 0.017 | −0.185 | ns |
| Thymus pulegioides L. s. l. | −0.518 | 0.268 | −0.578 | ** | 0.471 | 0.221 | 0.564 | ** |
| Tragopogon pratensis L. s. l. | −0.754 | 0.568 | −0.556 | *** | −0.008 | 0.000 | 0.077 | ns |
| Veronica chamaedrys L. s. str. | 0.294 | 0.087 | 0.192 | ns | −0.063 | 0.004 | −0.124 | ns |
| Viola declinata L. | −0.874 | 0.764 | −0.645 | *** | −0.160 | 0.026 | −0.040 | ns |
| Variant | Species no. (S) | Shannon (H′) | Evenness (E) | Simpson (D) |
|---|---|---|---|---|
| V1 (Control) | 42.33 ± 0.49 a | 3.00 ± 0.08 a | 0.802 ± 0.025 a | 0.919 ± 0.016 a |
| V2 (Low-input) | 34.00 ± 0.00 b | 2.70 ± 0.05 b | 0.765 ± 0.015 a | 0.896 ± 0.008 a |
| V3 (Medium-input) | 22.00 ± 0.00 c | 1.81 ± 0.14 c | 0.586 ± 0.046 b | 0.680 ± 0.048 b |
| V4 (High-input) | 18.00 ± 0.00 d | 1.31 ± 0.20 d | 0.453 ± 0.070 c | 0.496 ± 0.078 c |
| F test | 24,928.00 | 416.53 | 132.76 | 445.97 |
| p.val | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 |
| Year | Months | Average | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | VI | VII | VIII | IX | X | XI | XII | ||
| 2015 | −0.8 | 2 | 4.7 | 3.8 | 10.8 | 13.5 | 16.5 | 16.5 | 12.7 | 7.7 | 3.4 | −0.2 | 7.7 |
| 2016 | −5.4 | 0.1 | 1 | 8 | 8.7 | 14.8 | 16 | 15.3 | 10.9 | 4.6 | 0.4 | −5.4 | 5.7 |
| 2017 | −1.8 | 6 | 8.3 | 6.4 | 10 | 14.6 | 15.5 | 15.3 | 10.7 | 7.9 | 0.6 | −3.2 | 7.5 |
| Average values for the period 2001–2017 | |||||||||||||
| 2001–2017 | −4.5 | −2.7 | 0.2 | 5.3 | 10.5 | 14.5 | 15.9 | 15.4 | 11.3 | 6.0 | 1.4 | −3.1 | 5.8 |
| Year | Months | Total | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | VI | VII | VIII | IX | X | XI | XII | ||
| 2015 | 32.6 | 14.2 | 23.6 | 48.6 | 69.4 | 78.2 | 33.8 | 95 | 124 | 38.8 | 98.4 | 49.8 | 706.4 |
| 2016 | 120 | 111.4 | 79.4 | 108.4 | 67 | 165.4 | 58.8 | 49.4 | 56 | 86.6 | 127 | 0.2 | 1030 |
| 2017 | 112 | 0 | 86 | 11.4 | 116.6 | 95 | 37 | 35 | 88 | 100 | 98 | 36.2 | 815.2 |
| Average values for the period 2001–2017 | |||||||||||||
| 2001–2017 | 67.2 | 55.9 | 81.8 | 77.2 | 102.4 | 100.3 | 137.3 | 98.4 | 92.6 | 86.6 | 86.5 | 55.8 | 1042.1 |
| Class | Coverage Interval (%) | Class Central Value (%) | Sub-Note | Sub-Interval (%) | Central-Adjusted Value of Sub-Interval (%) |
|---|---|---|---|---|---|
| 5 | 75–100 | 87.5 | 5c | 92–100 | 96 |
| 5b | 83–92 | 87.5 | |||
| 5a | 75–83 | 79 | |||
| 4 | 50–75 | 62.5 | 4c | 67–75 | 71 |
| 4b | 58–67 | 62.5 | |||
| 4a | 50–58 | 54 | |||
| 3 | 25–50 | 37.5 | 3c | 42–50 | 46 |
| 3b | 33–42 | 37.5 | |||
| 3a | 25–33 | 29 | |||
| 2 | 10–25 | 17.5 | 2c | 20–25 | 22.25 |
| 2b | 15–20 | 17.5 | |||
| 2a | 10–15 | 12.5 | |||
| 1 | 1–10 | 5 | 1c | 6–10 | 8 |
| 1b | 4–6 | 5 | |||
| 1a | 1–4 | 2.5 | |||
| + | 0.1–1 | 0.5 | - | - | 0.5 |
| r | 0.01–0.1 | 0.05 | - | - | 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghețe, I.; Rotar, I.; Pleșa, A.; Ghețe, A.; Șerban, C.; Stoian, V. Effect of Mineral Fertilization on Vegetation of HNV Pastures in the Apuseni Mountains (Romania). Plants 2025, 14, 3564. https://doi.org/10.3390/plants14233564
Ghețe I, Rotar I, Pleșa A, Ghețe A, Șerban C, Stoian V. Effect of Mineral Fertilization on Vegetation of HNV Pastures in the Apuseni Mountains (Romania). Plants. 2025; 14(23):3564. https://doi.org/10.3390/plants14233564
Chicago/Turabian StyleGhețe, Ioana, Ioan Rotar, Anca Pleșa, Alexandru Ghețe, Claudiu Șerban, and Vlad Stoian. 2025. "Effect of Mineral Fertilization on Vegetation of HNV Pastures in the Apuseni Mountains (Romania)" Plants 14, no. 23: 3564. https://doi.org/10.3390/plants14233564
APA StyleGhețe, I., Rotar, I., Pleșa, A., Ghețe, A., Șerban, C., & Stoian, V. (2025). Effect of Mineral Fertilization on Vegetation of HNV Pastures in the Apuseni Mountains (Romania). Plants, 14(23), 3564. https://doi.org/10.3390/plants14233564







