Melatonin Enhances Growth and Glucosinolate-Associated Nutritional Quality of Mustard Sprouts Under Moderate Salinity Stress
Abstract
1. Introduction
2. Results
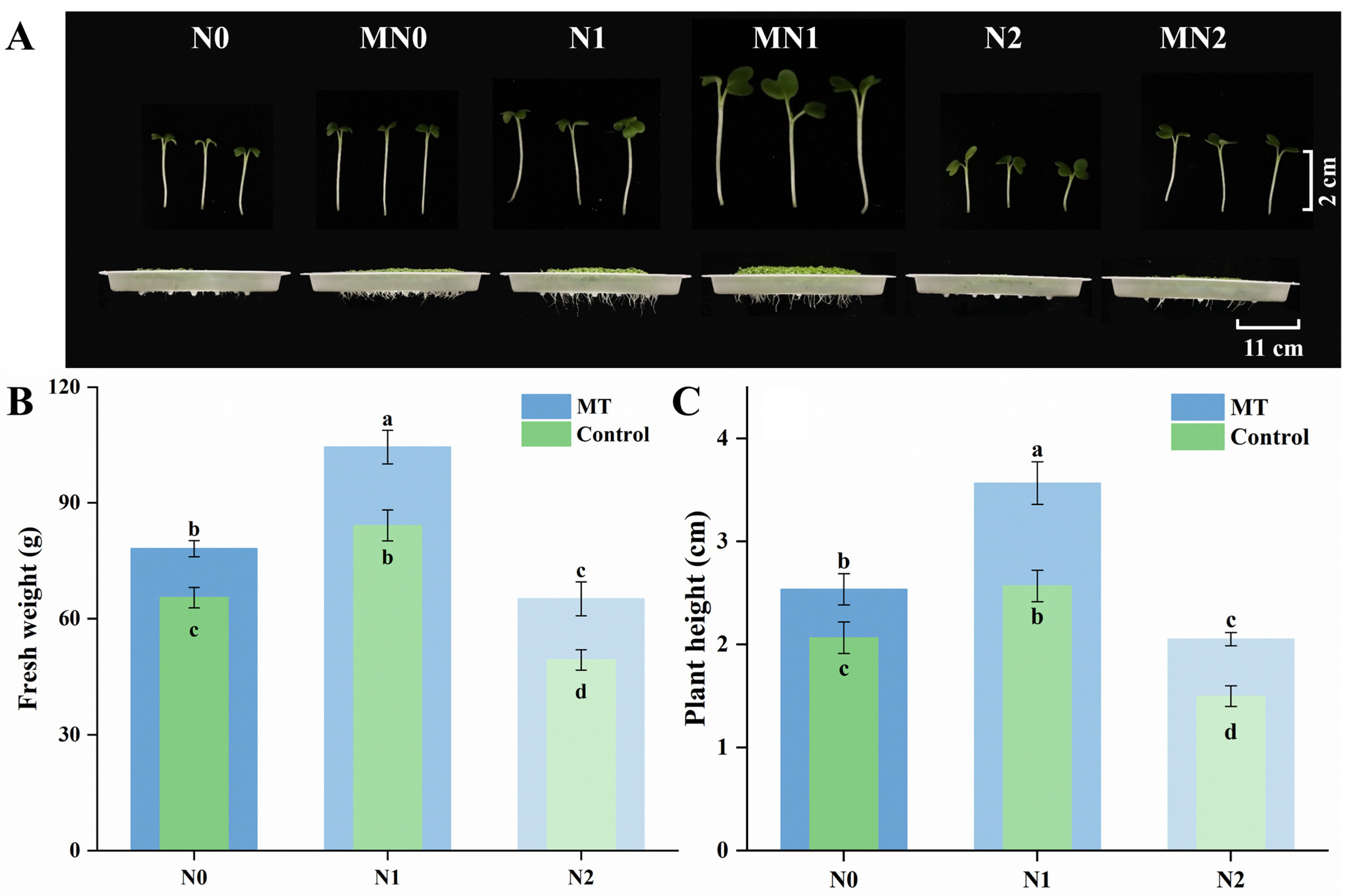
2.1. Appearance and Growth Performance
2.2. Soluble Sugar and Protein Content
2.3. Ascorbic Acid and Total Phenolic Content
2.4. Antioxidant Capacity
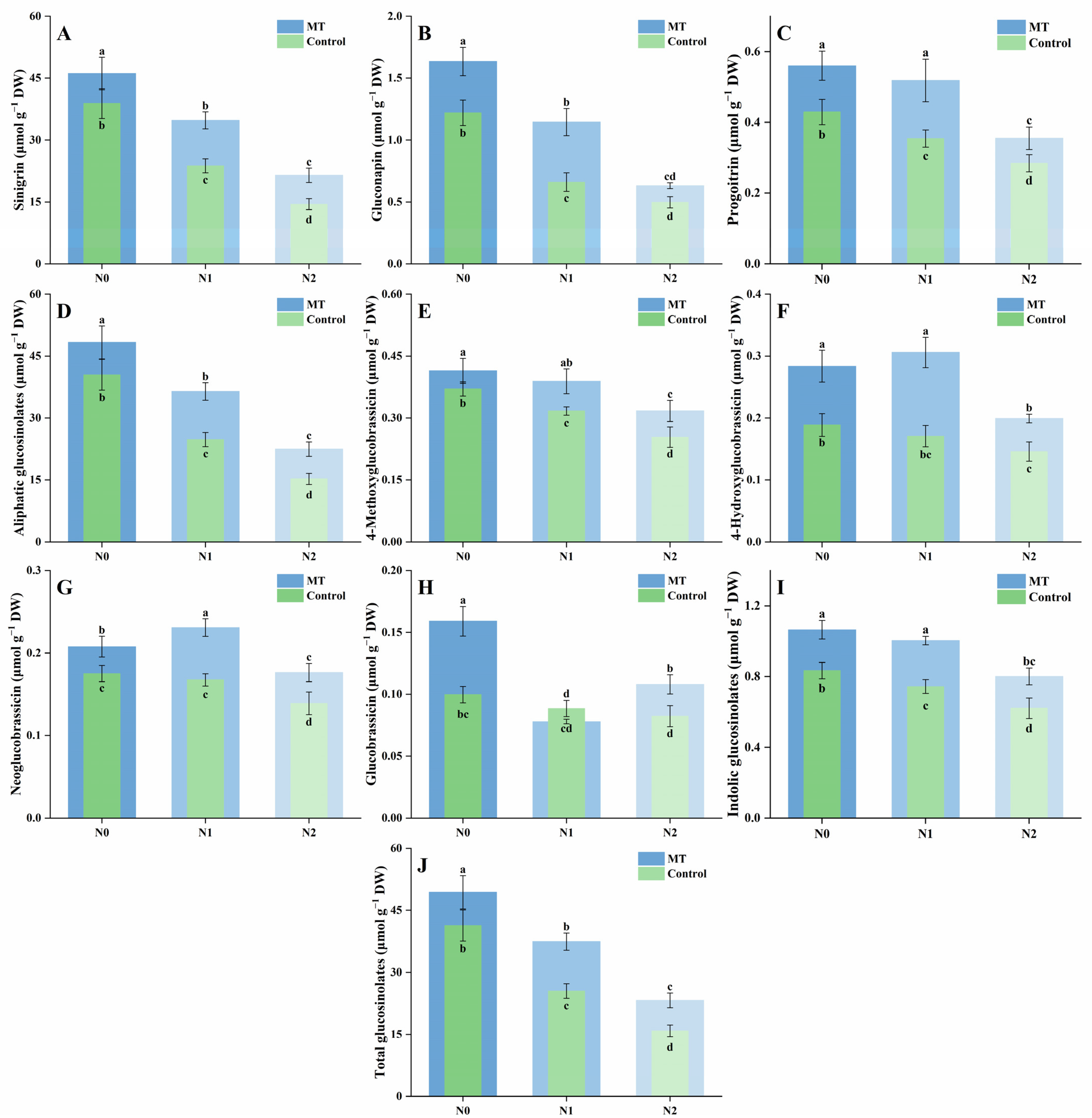
2.5. Glucosinolate Composition and Content
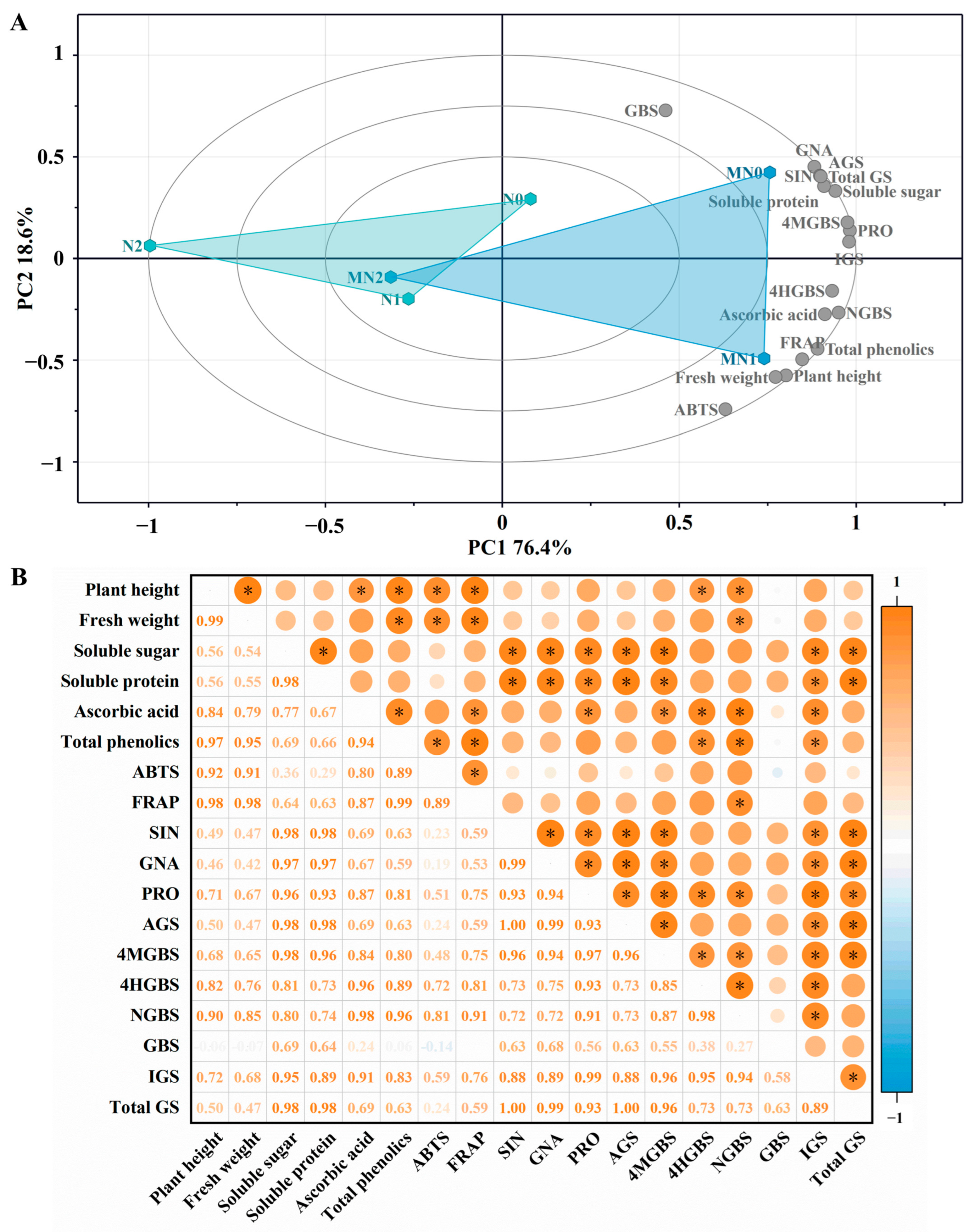
2.6. Principal Component Analysis
2.7. Correlation Analysis
2.8. Membership Function Analysis and Comprehensive Score
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Treatments
4.2. Growth Parameters
4.3. Soluble Sugar Content
4.4. Soluble Protein Content
4.5. Ascorbic Acid Content
4.6. Total Phenolics Content
4.7. 2,2-Azinobis (3-Ethyl-benzothiazoline-6-sulfonic Acid) (ABTS+) Assay
4.8. Ferric Reducing Antioxidant Power (FRAP)
4.9. Glucosinolate Content
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ortiz, I.; Zhu, X.; Shakoomahally, S.; Wu, W.; Kunle-Rabiu, O.; Turner, E.R.; Yang, T. Effects of harvest day after first true leaf emergence of broccoli and radish microgreen yield and quality. Technol. Hortic. 2024, 4, e003. [Google Scholar] [CrossRef]
- Bansal, S.; Lakra, N.; Mishra, S.; Ahlawat, Y.K. Unraveling the potential of glucosinolates for nutritional enhancement and stress tolerance in Brassica crops. Veg. Res. 2024, 4, e015. [Google Scholar] [CrossRef]
- Cheng, B.; Ran, R.; Qu, Y.; Verkerk, R.; Henry, R.; Dekker, M.; He, H. Advancements in balancing glucosinolate production in plants to deliver effective defense and promote human health. Agric. Commun. 2024, 2, 100040. [Google Scholar] [CrossRef]
- Wang, M.; Li, Y.; Yang, Y.; Tao, H.; Mustafa, G.; Meng, F.; Sun, B.; Wang, J.; Zhao, Y.; Zhang, F. Biofortification of health-promoting glucosinolates in cruciferous sprouts along the whole agro-food chain. Trends Food Sci. Technol. 2023, 140, 104164. [Google Scholar] [CrossRef]
- Di, H.; Ma, J.; Zhang, Y.; Wei, J.; Yang, J.; Ma, J.; Bian, J.; Xu, J.; Huang, Z.; Tang, Y. Correlations between flavor and glucosinolates and changes in quality-related physiochemical characteristics of Guizhou suancai during the fermentation process. Food Chem. 2023, 405, 134965. [Google Scholar] [CrossRef]
- Sun, Y.; Niu, G.; Masabni, J.G. Growth, gas exchange, and mineral nutrition of ‘Wonderful’ pomegranate irrigated with saline water. Technol. Hortic. 2024, 4, e002. [Google Scholar] [CrossRef]
- Plocek, G.; Kathi, S.; Simpson, C. Effects of eustress induced by low concentrations of salinity on broccoli (Brassica oleracea) and purslane (Portulaca oleracea) microgreens. Technol. Hortic. 2023, 3, 4. [Google Scholar] [CrossRef]
- Tian, L.; Li, X.; Yang, R.; Gu, Z. NaCl treatment improves reactive oxygen metabolism and antioxidant capacity in broccoli sprouts. Hortic. Environ. Biotechnol. 2016, 57, 640–648. [Google Scholar] [CrossRef]
- Trasmundi, F.; Galieni, A.; Eugelio, F.; Fanti, F.; Benincasa, P.; Carlo, M.D.; Sergi, M.; Stagnari, F. Salt elicitation to enhance phytochemicals in durum wheat seedlings. J. Sci. Food Agr. 2024, 104, 249–256. [Google Scholar] [CrossRef]
- Koodkaew, I. NaCl and glucose improve health-promoting properties in mung bean sprouts. Sci. Hortic. 2019, 247, 235–241. [Google Scholar] [CrossRef]
- Benincasa, P.; Bravi, E.; Marconi, O.; Lutts, S.; Tosti, G.; Falcinelli, B. Transgenerational effects of salt stress imposed to rapeseed (Brassica napus var. oleifera del.) plants involve greater phenolic content and antioxidant activity in the edible sprouts obtained from offspring seeds. Plants 2021, 10, 932. [Google Scholar]
- Wu, T.; Li, H.; Li, J.; Hao, J. Nutrient composition of germinated foxtail millet flour treated with mixed salt solution and slightly acidic electrolyzed water. Foods 2022, 12, 75. [Google Scholar] [CrossRef]
- Benincasa, P.; D’Amato, R.; Falcinelli, B.; Troni, E.; Fontanella, M.C.; Frusciante, S.; Guiducci, M.; Beone, G.M.; Businelli, D.; Diretto, G. Grain endogenous selenium and moderate salt stress work as synergic elicitors in the enrichment of bioactive compounds in maize sprouts. Agronomy 2020, 10, 735. [Google Scholar] [CrossRef]
- Sakamoto, M.; Suzuki, T. Methyl jasmonate and salinity increase anthocyanin accumulation in radish sprouts. Horticulturae 2019, 5, 62. [Google Scholar] [CrossRef]
- Hao, X.; Sun, B.; Song, Y.; Zhang, J.; Wu, J.; Zhang, N.; Zhang, X.; Yao, W.; Xu, W. Melatonin-mediated physiological and molecular responses to abiotic stress in horticultural crops. Hortic. Plant J. 2024, 11, 1381–1396. [Google Scholar] [CrossRef]
- Zhou, A.; Zhang, Y.; Li, L.; Di, H.; Bian, J.; Ma, J.; Escalona, V.H.; Hong, H.; Li, H.; Tang, Y. Effects of combined treatment of light quality and melatonin on health-promoting compounds in Chinese kale sprouts. LWT 2024, 199, 116137. [Google Scholar] [CrossRef]
- Posmyk, M.M.; Bałabusta, M.; Wieczorek, M.; Sliwinska, E.; Janas, K. Melatonin applied to cucumber (Cucumis sativus L.) seeds improves germination during chilling stress. J. Pineal Res. 2009, 46, 214–223. [Google Scholar] [CrossRef]
- Zrig, A.; Saleh, A.M.; Sheteiwy, M.S.; Hamouda, F.; Selim, S.; Abdel-Mawgoud, M.; Almuhayawi, M.S.; Okla, M.K.; Abbas, Z.K.; Al-Qahtani, W.H. Melatonin priming as a promising approach to improve biomass accumulation and the nutritional values of Chenopodium quinoa sprouts: A genotype-based study. Sci. Hortic. 2022, 301, 111088. [Google Scholar] [CrossRef]
- Xue, J.; Quan, X.; Yang, J.; Fang, W.; Yin, Y. Study on the mechanism of flavonoid enrichment in black soybean sprouts by abscisic acid/melatonin under slight acid treatment. Foods 2024, 13, 3567. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Fanourakis, D.; Tsaniklidis, G.; Li, K.; Yang, Q.; Li, T. Contrary to red, blue monochromatic light improves the bioactive compound content in broccoli sprouts. Agronomy 2021, 11, 2139. [Google Scholar] [CrossRef]
- Safaei Far, A.; Mousavi-Fard, S.; Rezaei Nejad, A.; Shahbazi, F.; Ahmadi-Majd, M.; Fanourakis, D. Nano silver and melatonin effectively delay the senescence of cut carnation flowers under simulated vibrational stress. J. Hortic. Sci. Biotechnol. 2024, 99, 597–608. [Google Scholar] [CrossRef]
- Hao, X.; Ren, J.; Xu, M.; Sun, B.; Li, R.; Yang, S.; Xu, W. Melatonin in plant pathogen defense: A review of its role in horticultural crops. Hortic. Res. 2025, 12, uhaf150. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Li, W.; Wang, J.; Li, Q.; Shen, Y.; Cheng, Y.; Li, T.; Wang, T.; Wang, Y.; Song, L. NaCl stress on physio-biochemical, phenolics synthesis and antioxidant system of pea (Pisum sativum L.) sprouts. LWT 2024, 210, 12. [Google Scholar] [CrossRef]
- Yin, Y.; Hu, J.; Yang, Z.; Fang, W.; Yang, J. Effects of methyl jasmonate and NaCl treatments on the resveratrol accumulation and defensive responses in germinated peanut (Arachis hypogaea L.). Plant Physiol. Biochem. 2023, 194, 664–673. [Google Scholar] [CrossRef]
- Younis, M.E.; Rizwan, M.; Tourky, S.M.N. Assessment of early physiological and biochemical responses in chia (Salvia hispanica L.) sprouts under salt stress. Acta Physiol. Plant 2021, 43, 121. [Google Scholar] [CrossRef]
- An, R.; Cheng, Y.; Tang, N. Elucidating the impact of calcium supplementation on biomass accumulation, polyphenol composition, and oxidative stress response in common vetch (Vicia sativa L.) sprouts. Food Biosci. 2025, 66, 106315. [Google Scholar] [CrossRef]
- Park, K.; Kim, Y.; Lee, H.; Jang, B.-K.; Kim, D.; Cho, J.-S. Seed priming with melatonin enhances chilling stress tolerance in tomato seedlings. Hortic. Plant J. 2025; in press. [Google Scholar] [CrossRef]
- Zhang, Q.; Qin, B.; Wang, G.D.; Zhang, W.J.; Li, M.; Yin, Z.G.; Yuan, X.; Sun, H.Y.; Du, J.D.; Du, Y.L.; et al. Exogenous melatonin enhances cell wall response to salt stress in common bean (Phaseolus vulgaris) and the development of the associated predictive molecular markers. Front. Plant Sci. 2022, 13, 1012186. [Google Scholar] [CrossRef]
- Yin, Y.; Tian, X.; He, X.; Yang, J.; Yang, Z.; Fang, W. Exogenous melatonin stimulated isoflavone biosynthesis in NaCl-stressed germinating soybean (Glycine max L.). Plant Physiol. Biochem. 2022, 185, 123–131. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, X.; Lu, X.; Zhao, B.; Liu, J. Integrative analysis of transcriptome and metabolome reveal mechanism of tolerance to salt stress in oat (Avena sativa L.). Plant Physiol. Biochem. 2021, 160, 315–328. [Google Scholar] [CrossRef]
- Keunen, E.; Peshev, D.; Vangronsveld, J.; Van Den Ende, W.; Cuypers, A. Plant sugars are crucial players in the oxidative challenge during abiotic stress: Extending the traditional concept. Plant Cell Environ. 2013, 36, 1242–1255. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Qian, J.; Zhang, H.; Lu, H.; Li, O.; Li, R.; Yu, Y.; Wen, J.; Zhao, L.; Yi, B.; et al. Combined transcriptomics and metabolomics analysis reveals the molecular mechanism of salt tolerance of Huayouza 62, an elite cultivar in rapeseed (Brassica napus L.). Int. J. Mol. Sci. 2022, 23, 1279. [Google Scholar] [CrossRef] [PubMed]
- Farhat, M.B.; Amor, G.B.; Beji-Serairi, R.; Selmi, S.; Khammassi, S.; Saidani-Tounsi, M.; Abdelly, C. Enhancement of nutritional quality and antioxidant properties of Lepidium sativum L. sprouts by salt treatment and domestic cooking. Int. J. Gastron. Food Sci. 2023, 32, 100736. [Google Scholar] [CrossRef]
- Li, Z.-G.; Xu, Y.; Bai, L.-K.; Zhang, S.-Y.; Wang, Y. Melatonin enhances thermotolerance of maize seedlings (Zea mays L.) by modulating antioxidant defense, methylglyoxal detoxification, and osmoregulation systems. Protoplasma 2019, 256, 471–490. [Google Scholar] [CrossRef]
- Chen, L.; Lu, B.; Liu, L.; Duan, W.; Jiang, D.; Li, J.; Zhang, K.; Sun, H.; Zhang, Y.; Li, C. Melatonin promotes seed germination under salt stress by regulating ABA and GA3 in cotton (Gossypium hirsutum L.). Plant Physiol. Biochem. 2021, 162, 506–516. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, X.-k.; Cheng, Y. Exogenous application of a low concentration of melatonin enhances salt tolerance in rapeseed (Brassica napus L.) seedlings. J. Integr. Agric. 2018, 17, 328–335. [Google Scholar] [CrossRef]
- Lan, G.; Xuan, C.; Guo, Y.; Huang, X.; Feng, M.; Yuan, L.; Li, H.; Ma, J.; Zhang, Y.; Wang, Z. The transcription factor ClWRKY61 interacts with ClLEA55 to enhance salt tolerance in watermelon. Hortic. Res. 2025, 12, uhae320. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, W.; Zhang, C.; Huang, H.; Yang, S.; Wang, Y.; Huang, Z.; Tang, Y.; Li, X.; Lian, H. Variation in the main health-promoting compounds and antioxidant capacity of three leafy vegetables in Southwest China. Molecules 2023, 28, 4780. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, A.; Xu, B.; Wang, H.; Yu, J.; Liu, J.; Jian, L.; Quan, C.; Du, J. Exogenous melatonin enhances salt tolerance by regulating the phenylpropanoid biosynthesis pathway in common bean at sprout stage. Plant Stress 2024, 14, 100589. [Google Scholar] [CrossRef]
- Wu, S.-Q.; Wang, Y.-X.; Beta, T.; Wang, S.-Y.; Mendez-Zamora, G.; Laborda, P.; Herrera-Balandrano, D.D. Effect of exogenous melatonin on the isoflavone content and antioxidant properties of soybean sprouts. LWT 2023, 175, 114498. [Google Scholar] [CrossRef]
- Yin, Y.; Xu, J.; He, X.; Yang, Z.; Fang, W.; Tao, J. Role of exogenous melatonin involved in phenolic acid metabolism of germinated hulless barley under NaCl stress. Plant Physiol. Biochem. 2022, 170, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Wang, X.; Guo, R.; Wang, Q. Effect of salt stress on phenolic compounds, glucosinolates, myrosinase and antioxidant activity in radish sprouts. Food Chem. 2010, 121, 1014–1019. [Google Scholar] [CrossRef]
- Guijarro-Real, C.; Hernández-Cánovas, L.; Abellán-Victorio, Á.; Ben-Romdhane, O.; Moreno, D.A. The Combination of monochromatic LEDs and elicitation with stressors enhances the accumulation of glucosinolates in mustard sprouts with species-dependency. Plants 2022, 11, 2961. [Google Scholar] [CrossRef] [PubMed]
- Petretto, G.L.; Urgeghe, P.P.; Massa, D.; Melito, S. Effect of salinity (NaCl) on plant growth, nutrient content, and glucosinolate hydrolysis products trends in rocket genotypes. Plant Physiol. Biochem. 2019, 141, 30–39. [Google Scholar] [CrossRef]
- Esfandiari, A.; Saei, A.; McKenzie, M.J.; Matich, A.J.; Babalar, M.; Hunter, D.A. Preferentially enhancing anti-cancer isothiocyanates over glucosinolates in broccoli sprouts: How NaCl and salicylic acid affect their formation. Plant Physiol. Biochem. 2017, 115, 343–353. [Google Scholar] [CrossRef]
- Tian, X.; Liu, C.; Yang, Z.; Zhu, J.; Fang, W.; Yin, Y. Crosstalk between ethylene and melatonin activates isoflavone biosynthesis and antioxidant systems to produce high-quality soybean sprouts. Plant Sci. 2024, 347, 112197. [Google Scholar] [CrossRef]
- Miao, H.; Zeng, W.; Zhao, M.; Wang, J.; Wang, Q. Effect of melatonin treatment on visual quality and health-promoting properties of broccoli florets under room temperature. Food Chem. 2020, 319, 126498. [Google Scholar] [CrossRef]
- Sun, B.; Tian, Y.-X.; Jiang, M.; Yuan, Q.; Chen, Q.; Zhang, Y.; Luo, Y.; Zhang, F.; Tang, H.-R. Variation in the main health-promoting compounds and antioxidant activity of whole and individual edible parts of baby mustard (Brassica juncea var. gemmifera). RSC Adv. 2018, 8, 33845–33854. [Google Scholar] [CrossRef]
- Yan, C.; Song, S.; Wang, W.; Wang, C.; Li, H.; Wang, F.; Li, S.; Sun, X. Screening diverse soybean genotypes for drought tolerance by membership function value based on multiple traits and drought-tolerant coefficient of yield. BMC Plant Biol. 2020, 20, 321. [Google Scholar] [CrossRef] [PubMed]



| Treatments | Plant Height | Fresh Weight | Soluble Sugar | Soluble Protein | Ascorbic Acid | Total Phenolics |
|---|---|---|---|---|---|---|
| N0 | 0.27 | 0.29 | 0.64 | 0.68 | 0.43 | 0.42 |
| N1 | 0.52 | 0.63 | 0.32 | 0.42 | 0.21 | 0.44 |
| N2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| MN0 | 0.50 | 0.52 | 1.00 | 1.00 | 0.68 | 0.58 |
| MN1 | 1.00 | 1.00 | 0.66 | 0.63 | 1.00 | 1.00 |
| MN2 | 0.27 | 0.29 | 0.35 | 0.21 | 0.48 | 0.38 |
| Treatments | ABTS | FRAP | AGS | IGS | Comprehensive evaluation value | Rank |
| N0 | 0.15 | 0.45 | 0.76 | 0.48 | 0.50 | 3 |
| N1 | 0.51 | 0.56 | 0.29 | 0.28 | 0.34 | 4 |
| N2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 6 |
| MN0 | 0.39 | 0.55 | 1.00 | 1.00 | 0.82 | 1 |
| MN1 | 1.00 | 1.00 | 0.64 | 0.86 | 0.80 | 2 |
| MN2 | 0.53 | 0.35 | 0.22 | 0.41 | 0.32 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Yu, X.; Di, H.; Zhou, A.; Guan, Z.; Shi, P.; Wang, S.; Sun, B. Melatonin Enhances Growth and Glucosinolate-Associated Nutritional Quality of Mustard Sprouts Under Moderate Salinity Stress. Plants 2025, 14, 3553. https://doi.org/10.3390/plants14233553
Zhao X, Yu X, Di H, Zhou A, Guan Z, Shi P, Wang S, Sun B. Melatonin Enhances Growth and Glucosinolate-Associated Nutritional Quality of Mustard Sprouts Under Moderate Salinity Stress. Plants. 2025; 14(23):3553. https://doi.org/10.3390/plants14233553
Chicago/Turabian StyleZhao, Xiaoling, Xuena Yu, Hongmei Di, Aolian Zhou, Zhongrong Guan, Pingping Shi, Sen Wang, and Bo Sun. 2025. "Melatonin Enhances Growth and Glucosinolate-Associated Nutritional Quality of Mustard Sprouts Under Moderate Salinity Stress" Plants 14, no. 23: 3553. https://doi.org/10.3390/plants14233553
APA StyleZhao, X., Yu, X., Di, H., Zhou, A., Guan, Z., Shi, P., Wang, S., & Sun, B. (2025). Melatonin Enhances Growth and Glucosinolate-Associated Nutritional Quality of Mustard Sprouts Under Moderate Salinity Stress. Plants, 14(23), 3553. https://doi.org/10.3390/plants14233553







