Fabaceae Flavonoids Beyond the Commonplace: A Review of Chemical Diversity, Pharmacological Activities, Mass Spectrometric Profiling and In Silico Insights into Their Subclasses
Abstract
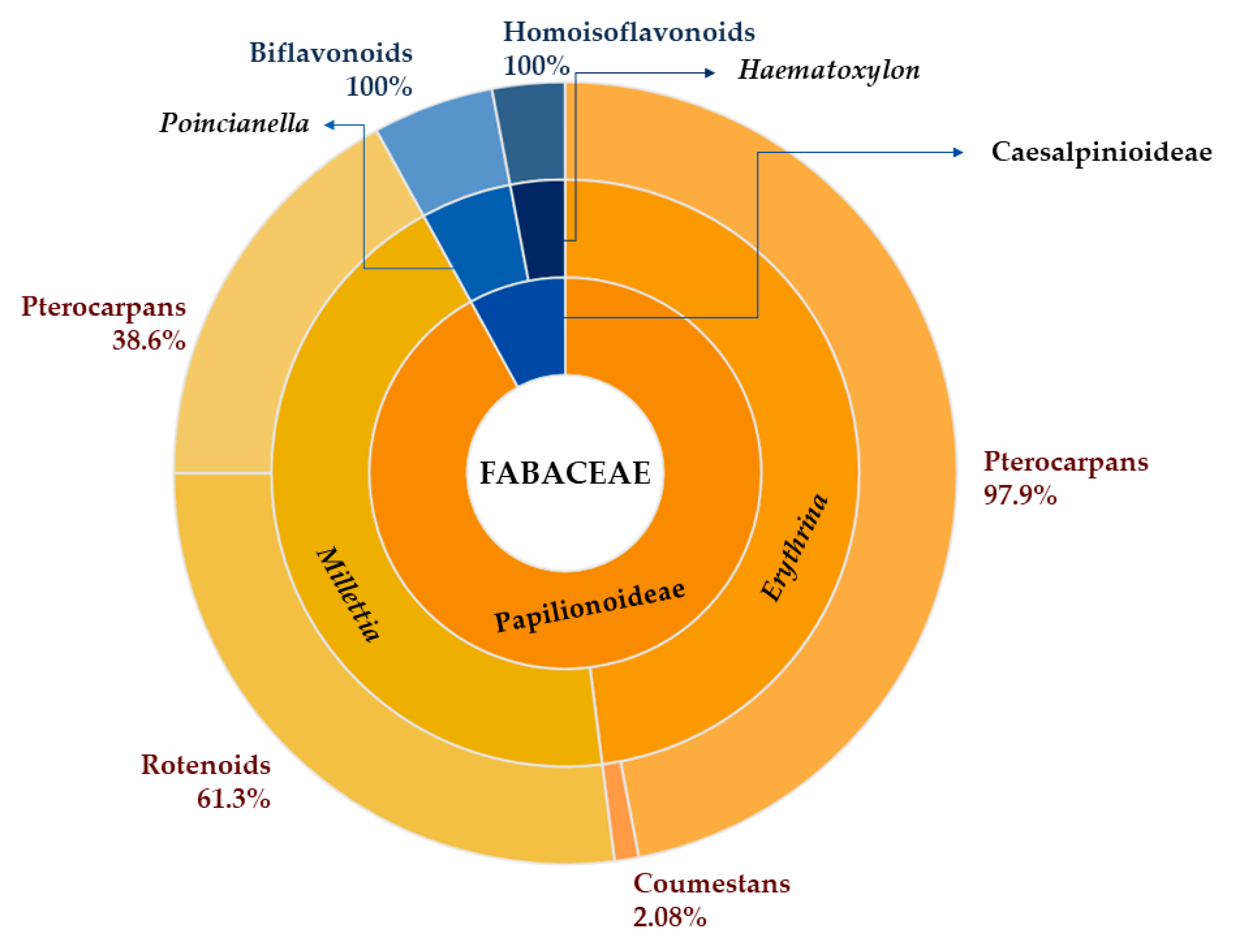
1. Introduction
2. Methodology
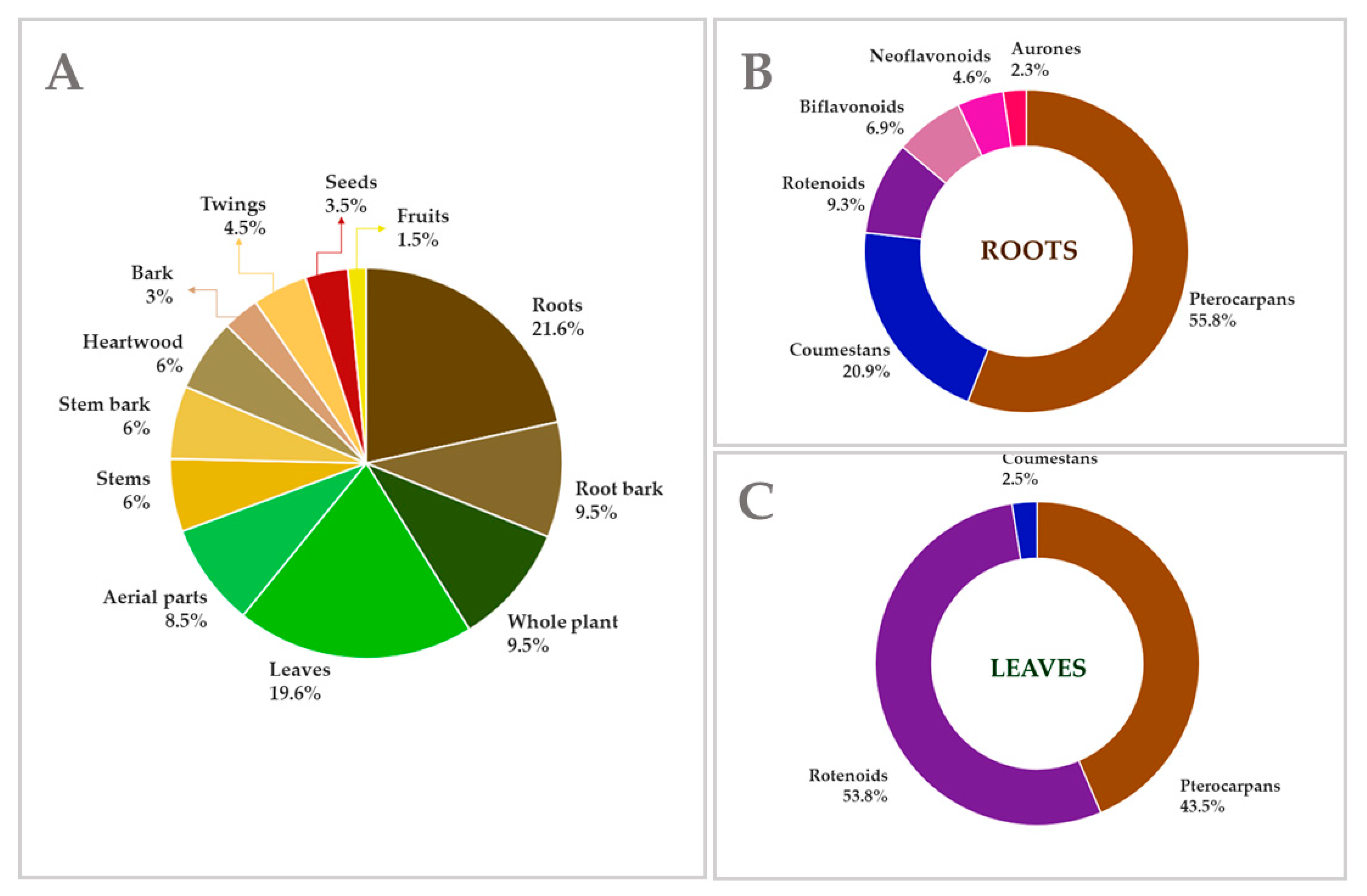
2.1. Data Collection
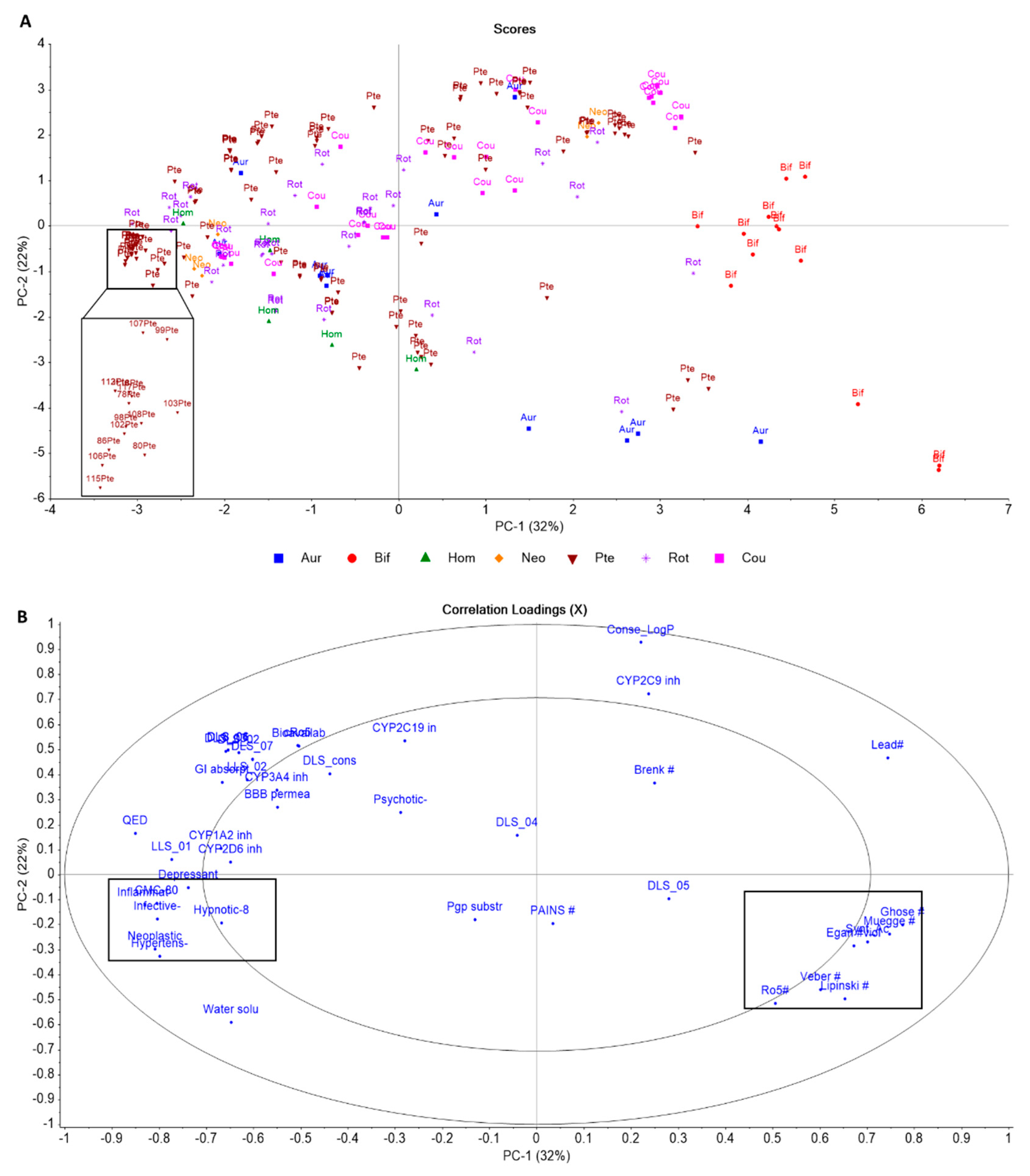
2.2. Fabaceae Flavonoids ADMET and Drug-likeness
3. Biosynthesis
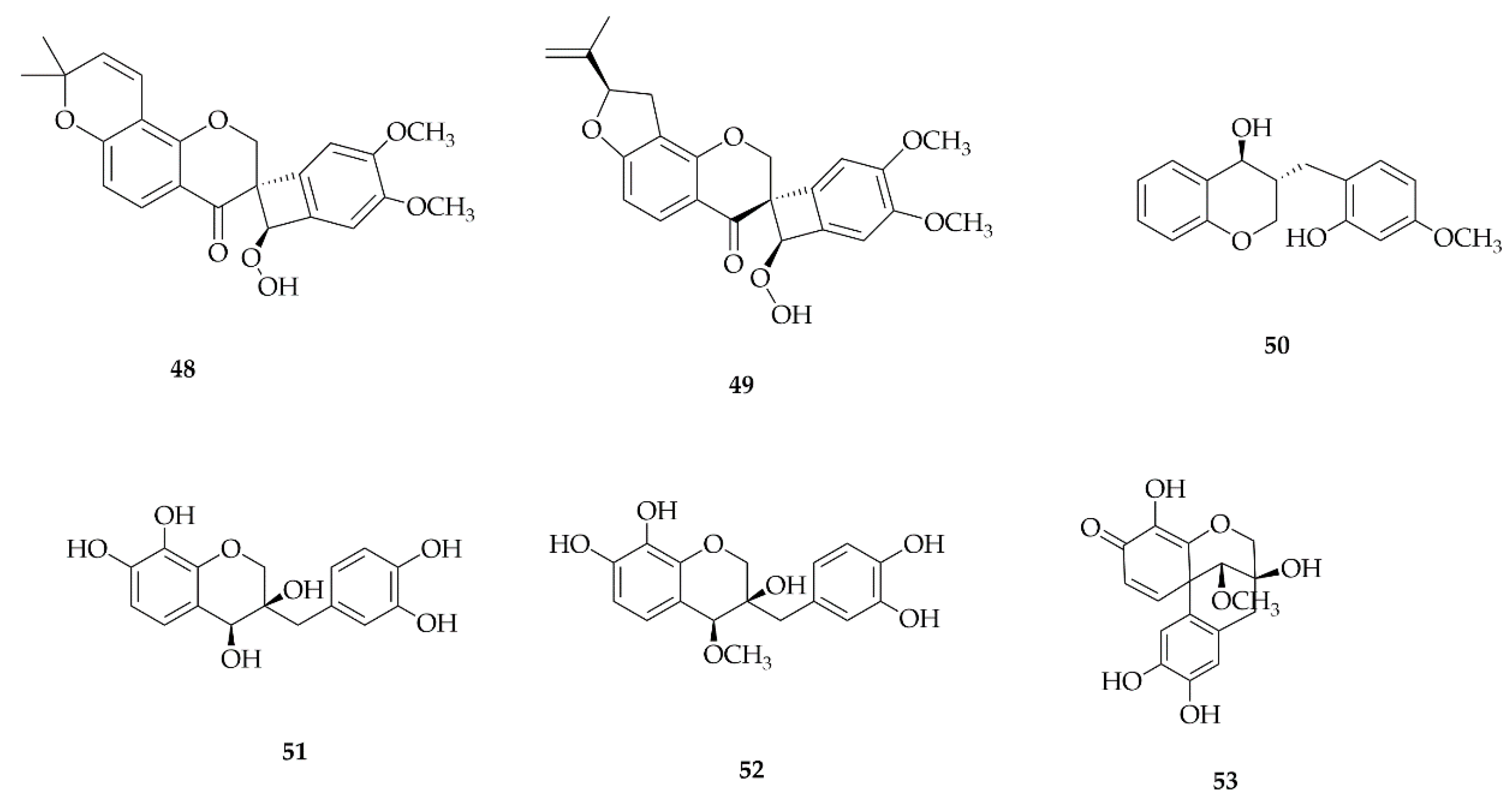
4. Extraction and Isolation
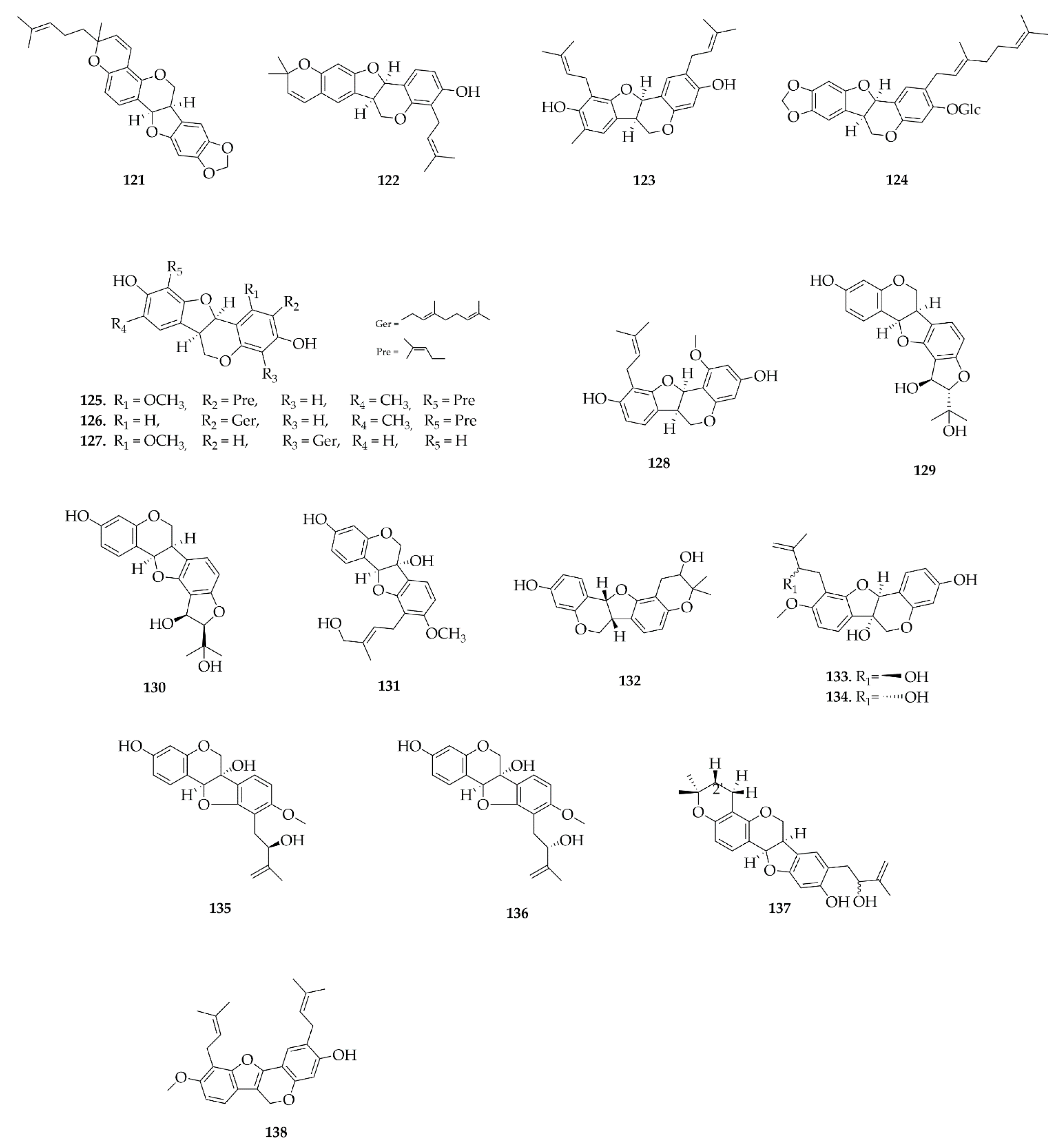
4.1. Aurones
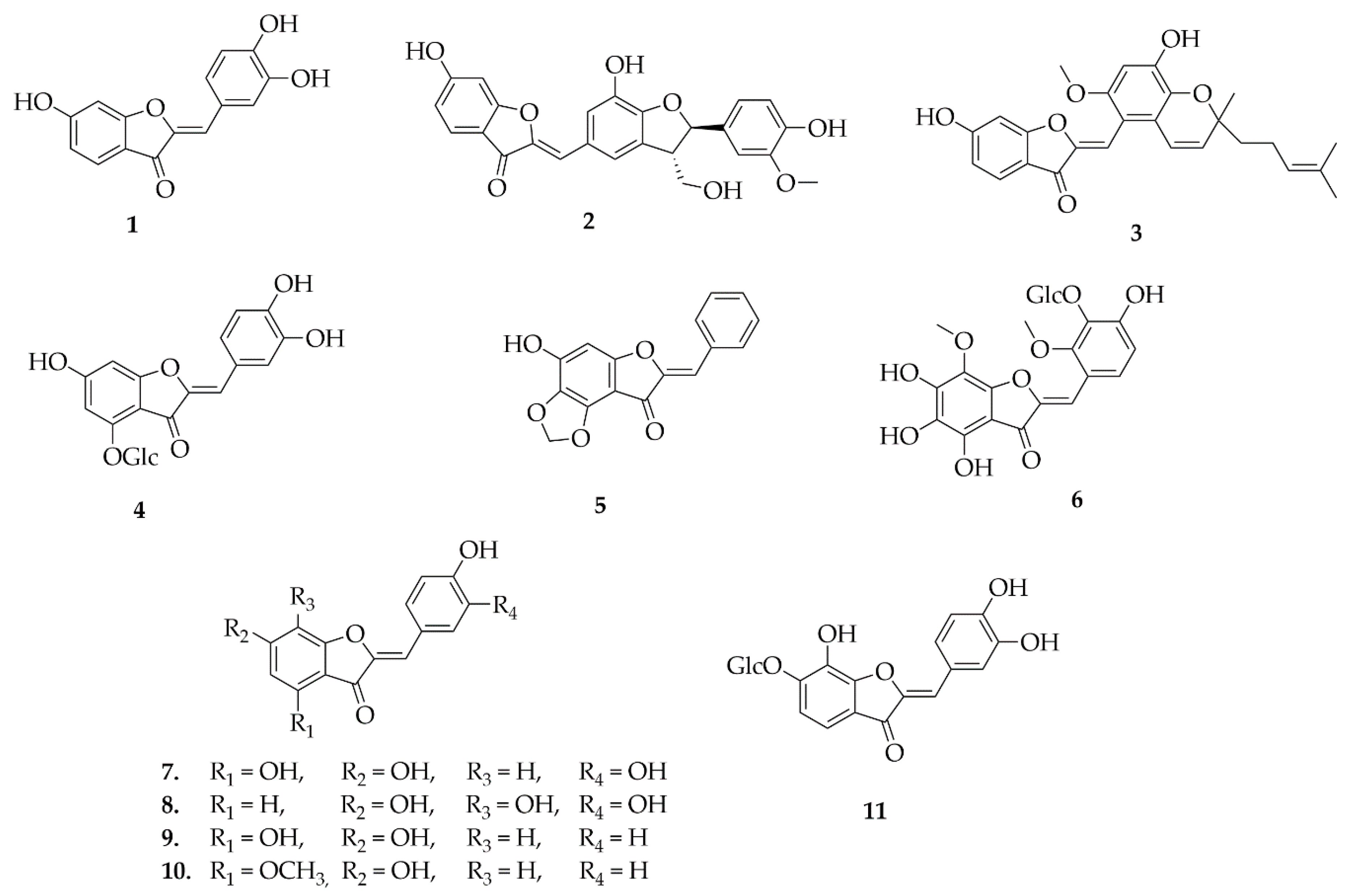
4.2. Biflavonoids
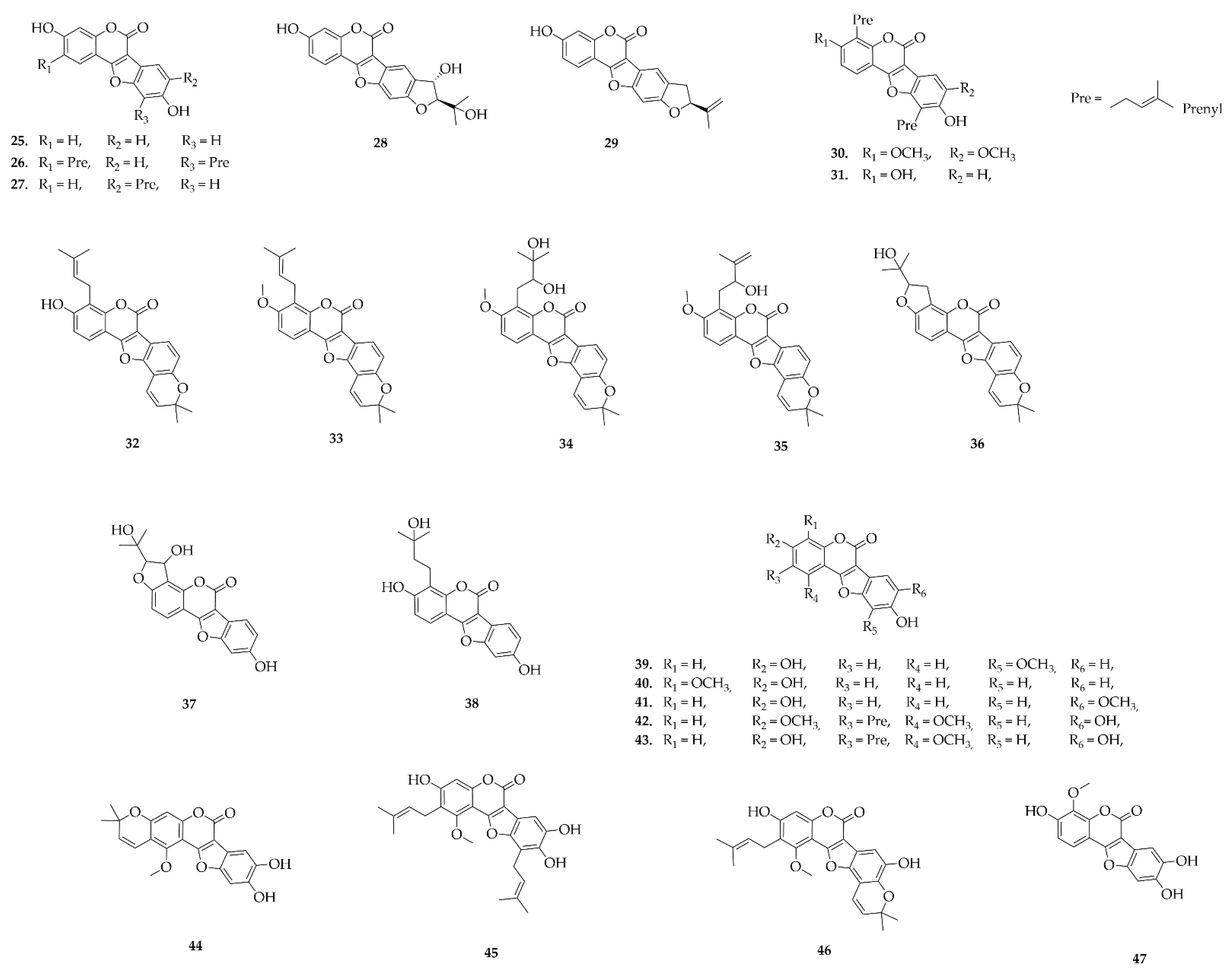
4.3. Coumestans
4.4. Homoisoflavonoids
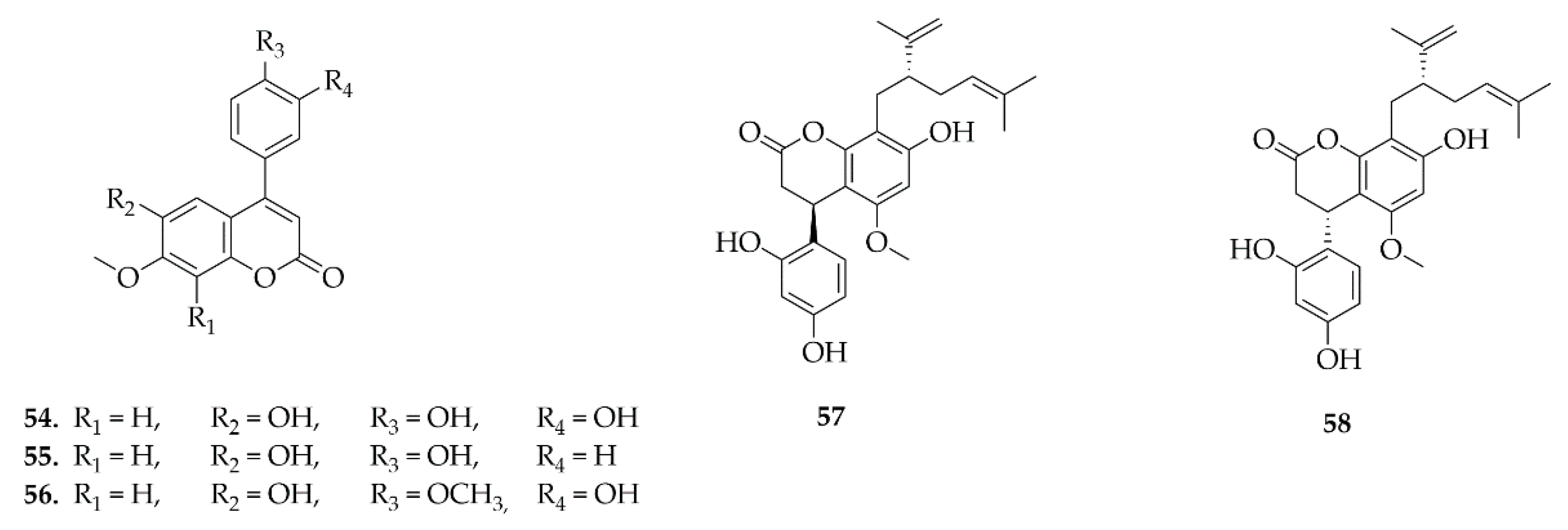
4.5. Neoflavonoids
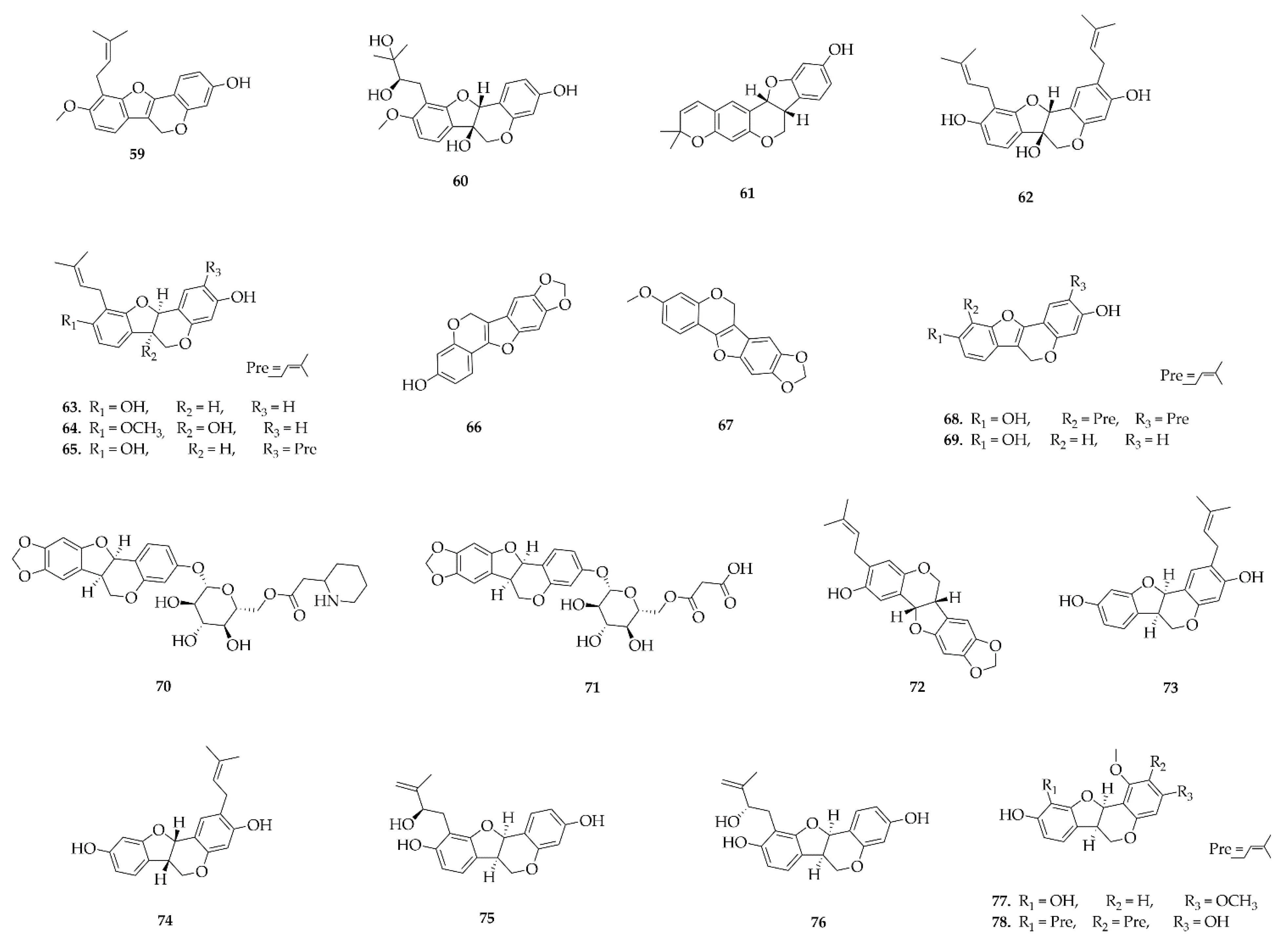
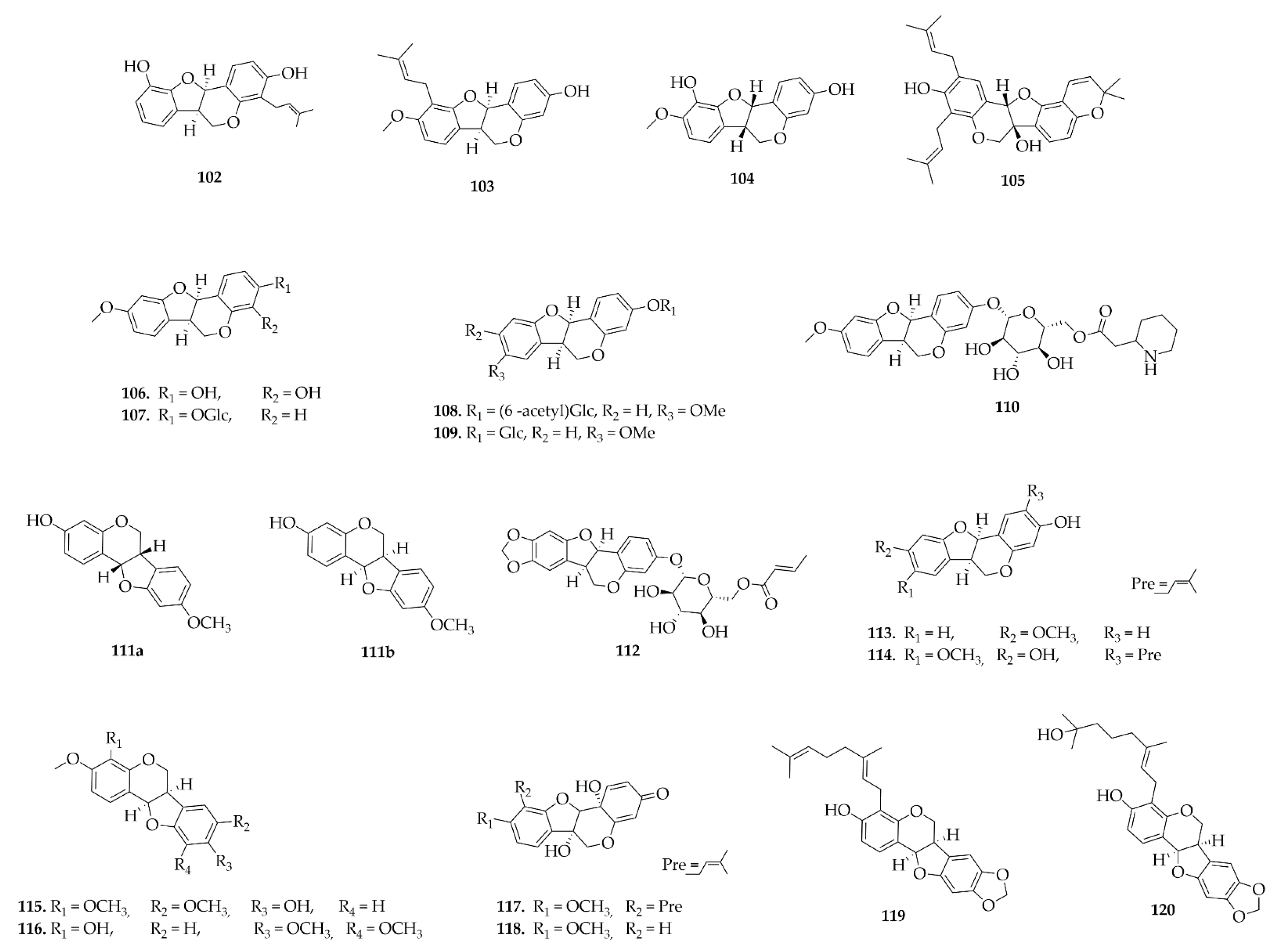
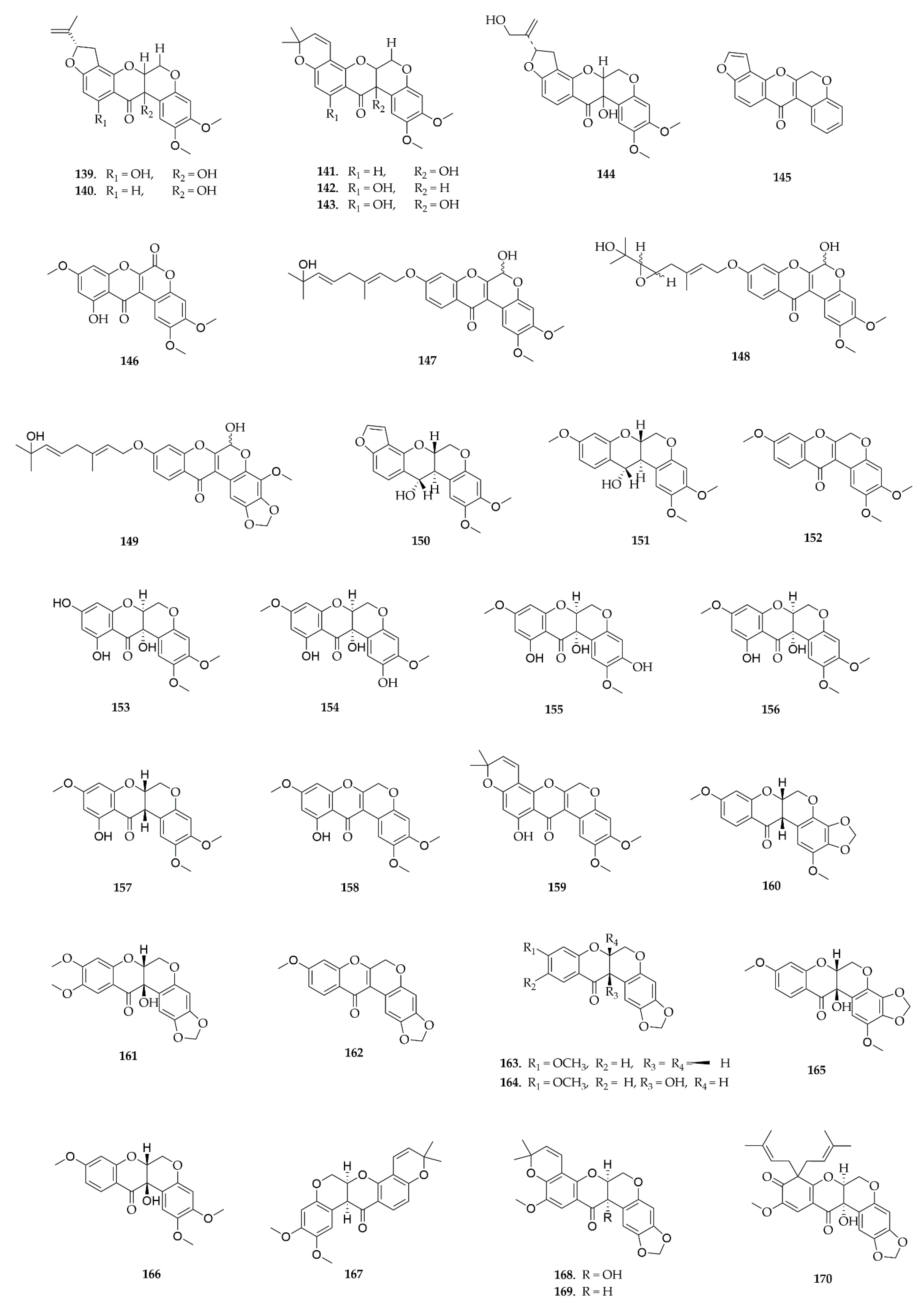
4.6. Pterocarpans
4.7. Rotenoids
5. Pharmacological Properties
5.1. Aurones
5.2. Biflavonoids
5.3. Coumestans
5.4. Homoisoflavonoids
5.5. Neoflavonoids
5.6. Pterocarpans
5.6.1. Antitumoral Activity
5.6.2. Antimicrobial Activity
5.6.3. Other Activities
5.7. Rotenoids
5.7.1. Cytotoxic Activity
5.7.2. Antimicrobial Activity
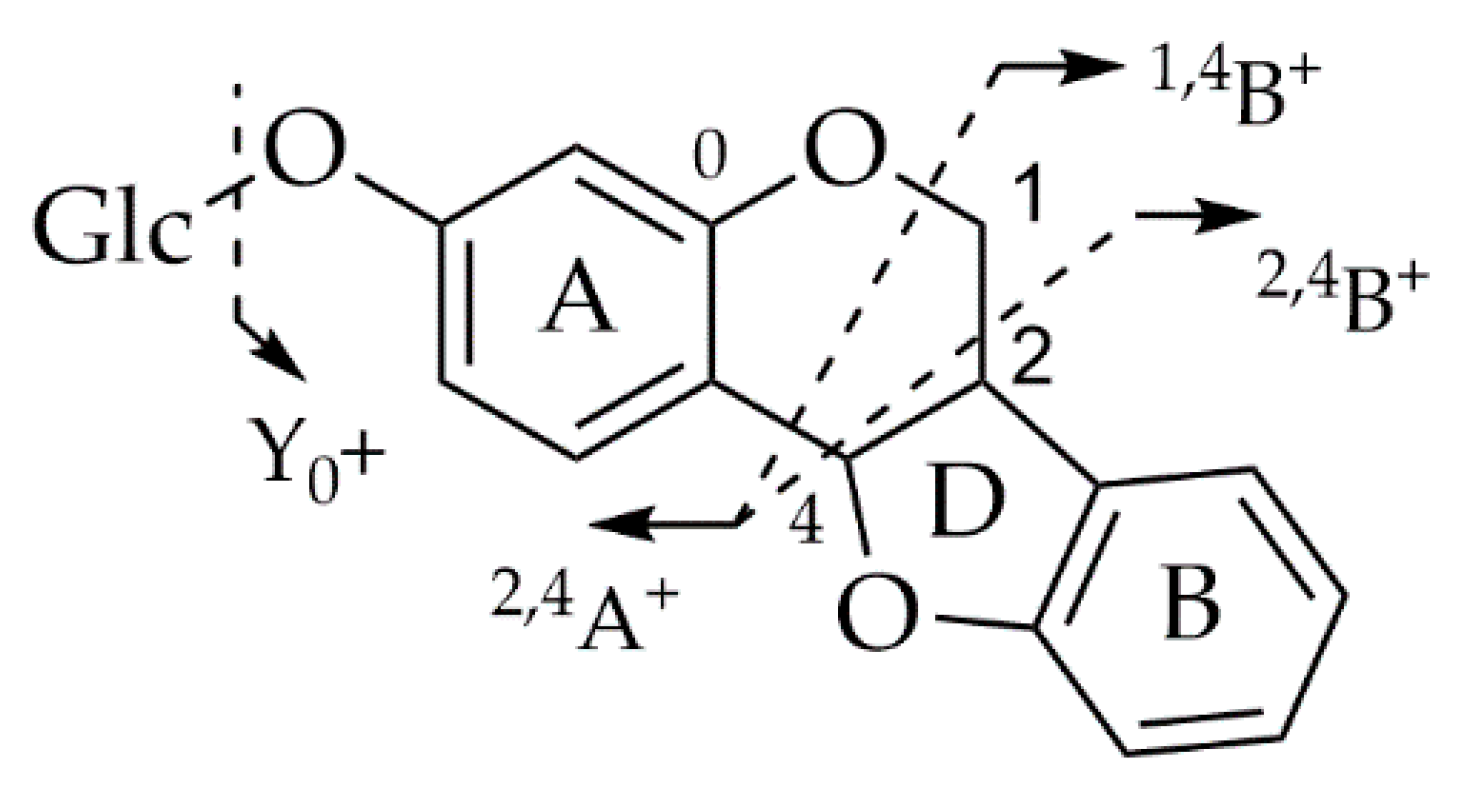
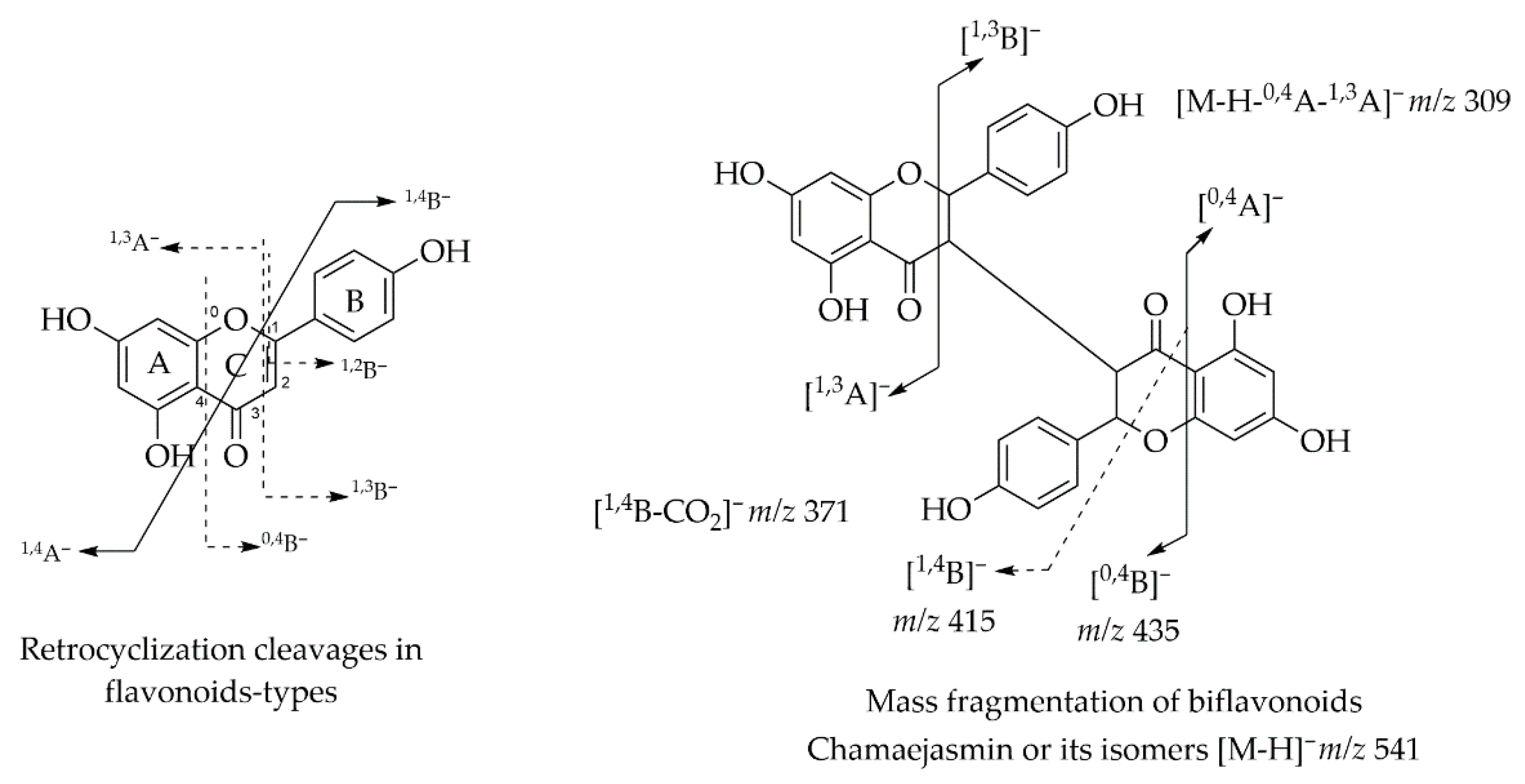
6. LC-MS/MS Analysis of Rare Flavonoid Types from Fabaceae
| Sample | Flavonoid-Type Rare Analyzed | Analytical Technique | Ionization Mode | Instrument Condition | Detector Conditions | Remarks | Ref. |
|---|---|---|---|---|---|---|---|
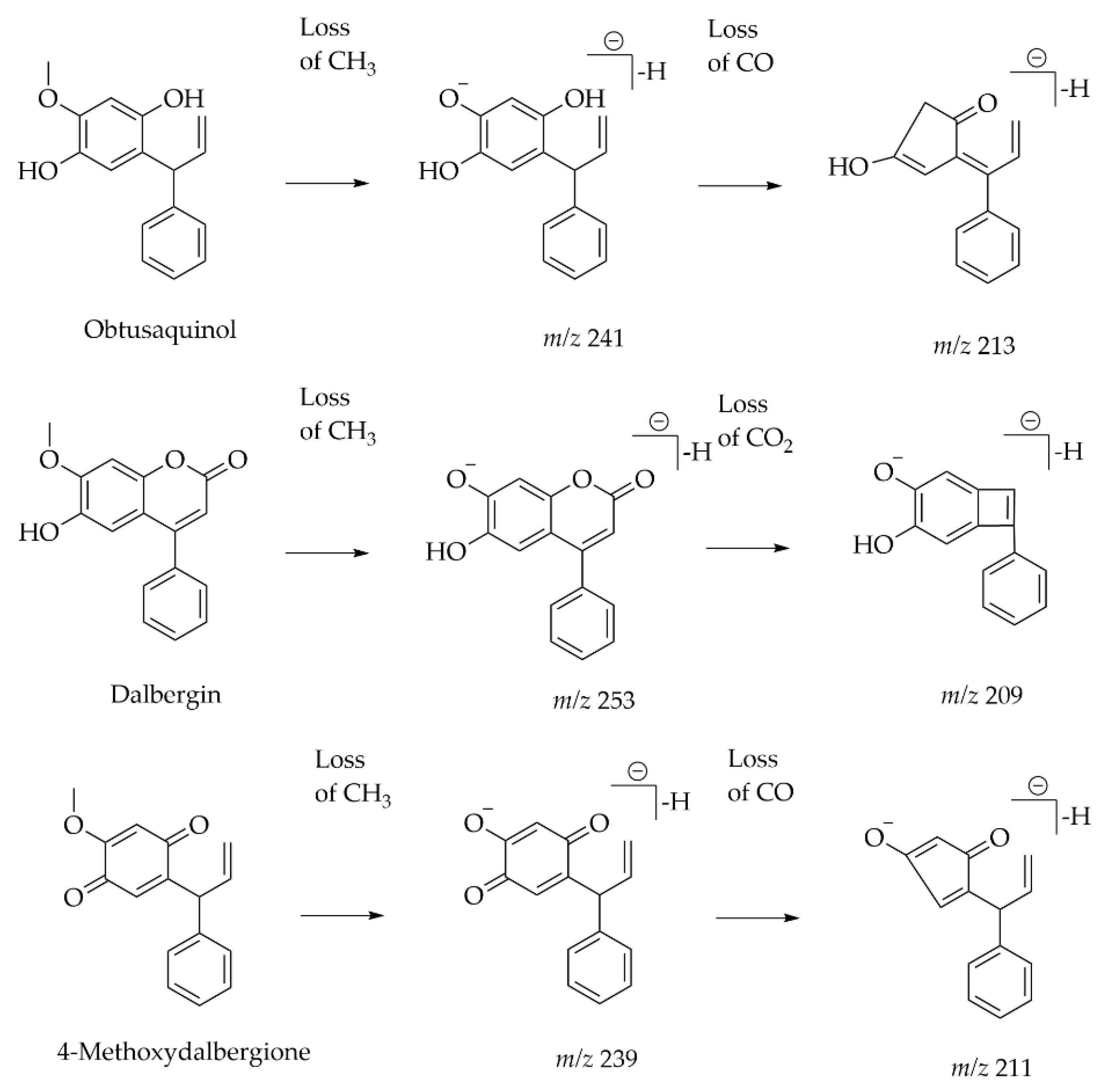
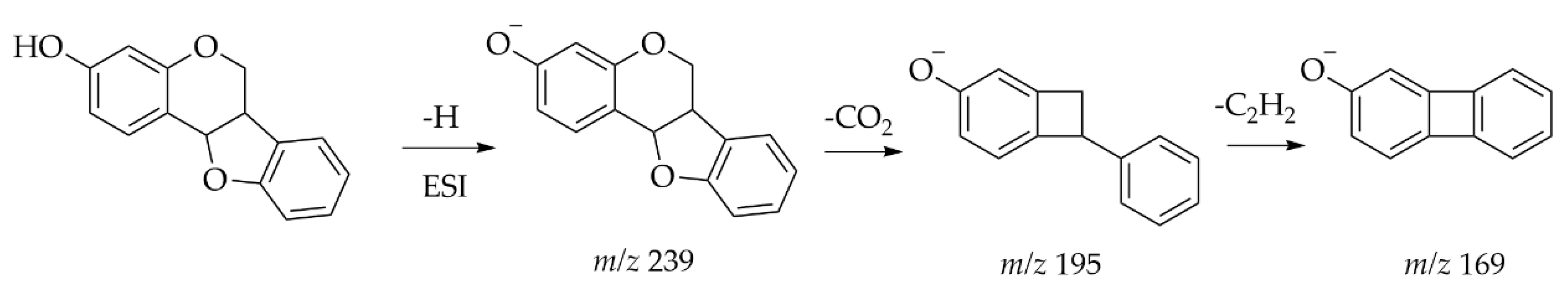
| Heartwood of Dalbergia odorifera | Neoflavonoids | UPLC-ESI-MS | [M − H] − | C18 (2.1 mm × 100 mm, 1.8 μm) Mobile phase: 0.05% acetic acid in water; 0.05% acetic acid in CAN | UV and Orbitrap MS | A total of 13 compounds were classified as the skeleton of neoflavones with target analysis. Neutral losses of CH3, CO, H2O and CO2 are easily found in the skeletons of neoflavonoids. | [49] |
| Heartwook of Pterocarpus santalinus | Coumestans | UPLC- HRESI-MS- | [M + H] + | C18 (2.1 mm × 150 mm, 1.7 μm) Mobile phase: water + 0.1% formic acid; ACN + 0.1% formic acid | UV and QTOF | The method allowed the guided isolation of the compound 3,9-O-dimethylcoumestrol. | [67] |
| Roots of Glycyrrhiza inflata, G. uralensis and G. glabra | Coumestans | HPLC-ESI-MS | [M + H] + | C18 (4.6 mm × 250 mm, 5 μm) Mobile phase: water acidified with 0.2% v/v formic acid; acetonitrile | PDA and TripleTOF | A total of 39 compounds were identified, including two coumestans, glycycoumarin and neoglycyrol. | [68] |
| Root of Ononis spinosa L. | Pterocarpans | LC-ESI-MS | [M + H] + | C18 (3.0 mm × 150 mm, 3.5 μm) Mobile phase: 0.3% (v/v) formic acid and methanol | PDA, QTOF and Ion Trap | The method made it possible to differentiate fragmentation patterns of isomeric forms of Maackiain and described the fragmentation pattern of Medicarpin for the first time. | [69] |
| Roots of Milletia speciosa | Pterocarpans | UPLC-ESI-MS | [M − H] − | C18 (2.1 mm × 100 mm, 1.7 μm) Mobile phase: water acidified with 0.1% v/v formic acid and ACN | PDA and QTOF | A total of 38 components, including Maackiain pterocarpan were unambiguously identified or tentatively assigned. | [70] |
| Stems of Acosmium diffusissimum | Pterocarpans | LC-ESI-MS | [M + H] + | C18 (4.6 mm × 250 mm 5 μm) Mobile phase: water acidified with 0.1% v/v formic acid and methanol | PDA and Ion Trap | The method enabled the isolation of new pterocarpans through the analysis of fragmentation patterns and the molecular network approach. | [66] |
7. ADMET Analysis
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, S.; Wang, X.; Cheng, Y.; Gao, H.; Chen, X. A review of classification, biosynthesis, biological activities and potential applications of flavonoids. Molecules 2023, 28, 4982. [Google Scholar] [CrossRef] [PubMed]
- Donadio, G.; Mensitieri, F.; Santoro, V.; Parisi, V.; Bellone, M.L.; De Tommasi, N.; Izzo, V.; Dal Piaz, F. Interactions with microbial proteins driving the antibacterial activity of flavonoids. Pharmaceutics 2021, 13, 660. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez De Luna, S.L.; Ramírez-Garza, R.E.; Serna Saldívar, S.O. Environmentally friendly methods for flavonoid extraction from plant material: Impact of their operating conditions on yield and antioxidant properties. Sci. World J. 2020, 1, 6792069. [Google Scholar] [CrossRef]
- Azani, N.; Babineau, M.; Bailey, C.D.; Banks, H.; Barbosa, A.R.; Pinto, R.B.; Boatwright, J.S.; Borges, L.M.; Brown, G.K.; Bruneau, A.; et al. A new subfamily classification of the Leguminosae based on a taxonomically comprehensive phylogeny. Taxon 2017, 66, 44–77. [Google Scholar] [CrossRef]
- Morante-Carriel, J.; Nájera, H.; Samper-Herrero, A.; Živković, S.; Martínez-Esteso, M.J.; Martínez-Márquez, A.; Sellés-Marchart, S.; Obrebska, A.; Bru-Martínez, R. Therapeutic Potential of Prenylated Flavonoids of the Fabaceae Family in Medicinal Chemistry: An Updated Review. Int. J. Mol. Sci. 2024, 25, 13036. [Google Scholar] [CrossRef]
- Mazziotti, I.; Petrarolo, G.; La Motta, C. Aurones: A golden resource for active compounds. Molecules 2021, 27, 2. [Google Scholar] [CrossRef]
- Tu, Y.; Yang, Y.; Li, Y.; He, C. Naturally occurring coumestans from plants, their biological activities and therapeutic effects on human diseases. Pharmacol. Res. 2021, 169, 105615. [Google Scholar] [CrossRef]
- Garazd, M.M.; Garazd, Y.L.; Khilya, V.P. Neoflavones. 1. Natural distribution and spectral and biological properties. Chem. Nat. Compd. 2003, 39, 54–121. [Google Scholar] [CrossRef]
- Santos, R.A.; David, J.M.; David, J.P. Detection and quantification of rotenoids from Clitoria fairchildiana and its lipids profile. Nat. Prod. Commun. 2016, 11, 631–632. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Important flavonoids and their role as a therapeutic agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef] [PubMed]
- Liga, S.; Paul, C.; Péter, F. Flavonoids: Overview of biosynthesis, biological activity, and current extraction techniques. Plants 2023, 12, 2732. [Google Scholar] [CrossRef]
- Torres-Rêgo, M.; Aquino-Vital, A.K.S.; Cavalcanti, F.F.; Rocha, E.E.A.; Daniele-Silva, A.; Furtado, A.A.; Silva, D.P.; Ururahy, M.A.G.; Silveira, E.R.; Fernandes-Pedrosa, M.F.; et al. Phytochemical analysis and preclinical toxicological, antioxidant, and anti-inflammatory evaluation of hydroethanol extract from the roots of Harpalyce brasiliana Benth (Leguminosae). J. Ethnopharmacol. 2022, 294, 115364. [Google Scholar] [CrossRef]
- Demarque, D.P.; Crotti, A.E.; Vessecchi, R.; Lopes, J.L.; Lopes, N.P. Fragmentation reactions using electrospray ionization mass spectrometry: An important tool for the structural elucidation and characterization of synthetic and natural products. Nat. Prod. Rep. 2016, 33, 432–455. [Google Scholar] [CrossRef]
- Souza, T.A.; Pereira, L.H.; Alves, A.F.; Dourado, D.; Lins, J.D.S.; Scotti, M.T.; Scotti, L.; Abreu, L.S.; Tavares, J.F.; Silva, M.S. Jatropha Diterpenes: An Updated Review Concerning Their Structural Diversity, Therapeutic Performance, and Future Pharmaceutical Applications. Pharmaceuticals 2024, 17, 1399. [Google Scholar] [CrossRef] [PubMed]
- Dewick, P.M. Medicinal Natural Products: A Biosynthetic Approach, 3rd ed.; Wiley: Chichester, UK, 2009. [Google Scholar] [CrossRef]
- Winkel-Shirley, B. Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol. 2001, 126, 485–493. [Google Scholar] [CrossRef]
- Vogt, T. Phenylpropanoid biosynthesis. Mol. Plant 2010, 3, 2–20. [Google Scholar] [CrossRef]
- Dixon, R.A.; Paiva, N.L. Stress-induced phenylpropanoid metabolism. Plant Cell 1995, 7, 1085–1097. [Google Scholar] [CrossRef]
- Ollis, W.D. The neoflavanoids, a new class of natural products. Experientia 1966, 22, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Seshadri, K.; Abad, A.N.; Nagasawa, K.K.; Yost, K.M.; Johnson, C.W.; Dror, M.J.; Tang, Y. Synthetic Biology in Natural Product Biosynthesis. Chem. Rev. 2025, 125, 3814–3931. [Google Scholar] [CrossRef]
- Wang, M.; Chen, L.; Zhang, Z.; Wang, Q. Recent advances in genome mining and synthetic biology for discovery and biosynthesis of natural products. Crit. Rev. Biotechnol. 2025, 45, 236–256. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.; Mathesius, U. The role of flavonoids in root–rhizosphere signalling: Opportunities and challenges for improving plant–microbe interactions. J. Exp. Bot. 2012, 63, 3429–3444. [Google Scholar] [CrossRef]
- Buer, C.S.; Imin, N.; Djordjevic, M.A. Flavonoids: New roles for old molecules. J. Integr. Plant Biol. 2010, 52, 98–111. [Google Scholar] [CrossRef]
- Armero, J.; Requejo, R.; Jorrín, J.; López-Valbuena, R.; Tena, M. Release of phytoalexins and related isoflavonoids from intact chickpea seedlings elicited with reduced glutathione at root level. Plant Physiol. Biochem. 2021, 39, 785–795. [Google Scholar] [CrossRef]
- Selvam, C.; Jordan, B.C.; Prakash, S.; Mutisya, D.; Thilagavathi, R. Pterocarpan scaffold: A natural lead molecule with diverse pharmacological properties. Eur. J. Med. Chem. 2017, 128, 219–236. [Google Scholar] [CrossRef]
- Narbona, E.; del Valle, J.C.; Arista, M.; Buide, M.L.; Ortiz, P.L. Major flower pigments originate different colour signals to pollinators. Front. Ecol. Evol. 2021, 9, 743850. [Google Scholar] [CrossRef]
- Ma, Q.; Wei, R.; Sang, Z. Neuroprotective aurones from Sophora japonica. Chem. Nat. Compd. 2019, 55, 265–268. [Google Scholar] [CrossRef]
- Li, J.Q.; Xiao, C.J.; Li, Y.M.; Tian, X.Y.; Dong, X.; Jiang, B. Astrernestin, a novel aurone-phenylpropanoid adduct from the roots of Astragalus ernestii. Nat. Prod. Res. 2020, 34, 2894–2899. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.J.; Zhang, Y.; Qiu, L.; Dong, X.; Jiang, B. Schistosomicidal and antioxidant flavonoids from Astragalus englerianus. Planta Medica 2014, 80, 1727–1731. [Google Scholar] [CrossRef]
- Ferreira, R.O.; Carvalho, M.G.D.; Silva, T.M.S.S. Ocorrência de biflavonoides em Clusiaceae: Aspectos químicos e farmacológicos. Química Nova 2012, 35, 2271–2277. [Google Scholar] [CrossRef]
- Oliveira, J.C.S.; David, J.P.; David, J.M. Biflavonoids from the bark roots of Poincianella pyramidalis (Fabaceae). Phytochem. Lett. 2016, 16, 18–22. [Google Scholar] [CrossRef]
- Chalo, D.M.; Kakudidi, E.; Origa-Oryem, H.; Namukobe, J.; Franke, K.; Yenesew, A.; Wessjohann, L.A. Chemical constituents of the roots of Ormocarpum sennoides subsp. zanzibaricum. Biochem. Syst. Ecol. 2020, 93, 104142. [Google Scholar] [CrossRef]
- Kamto, E.L.D.; Carvalho, T.S.; Mbing, J.N.; Matene, M.C.; Pegnyemb, D.E.; Leitão, G.G. Alternating isocratic and step gradient elution high-speed counter-current chromatography for the isolation of minor phenolics from Ormocarpum kirkii bark. J. Chromatogr. A 2017, 1480, 50–61. [Google Scholar] [CrossRef]
- Gontijo, V.S.; Santos, M.H.; Viegas Jr, C. Biological and chemical aspects of natural biflavonoids from plants: A brief review. Mini Rev. Med. Chem. 2017, 17, 834–862. [Google Scholar] [CrossRef] [PubMed]
- Nyamboki, D.K.; Wanga, L.A. Review of the phytochemical and pharmacological studies of the genus Ormocarpum. Pharmacogn. Rev. 2022, 16, 95–99. [Google Scholar] [CrossRef]
- Govindachari, T.R.; Nagarajan, K.; Pai, B.R. 126. Chemical examination of Wedelia calendulacea. Part I. Structure of wedelolactone. J. Chem. Soc. 1956, 629–632. [Google Scholar] [CrossRef]
- Naik, M.M.; Patre, R.E.; Tilve, S.G. Coumestans: Synthesis, isolation, and bioactivity. Asian J. Org. Chem. 2025, 14, e202400564. [Google Scholar] [CrossRef]
- Davies, K.M.; Marshall, G.B.; Bradley, J.M.; Schwinn, K.E.; Bloor, S.J.; Winefield, C.S.; Martin, C.R. Characterisation of aurone biosynthesis in Antirrhinum majus. Physiol. Plant. 2006, 128, 593–603. [Google Scholar] [CrossRef]
- Akinmoladun, A.C.; Olaleye, M.T.; Komolafe, K.; Adetuyi, A.O.; Akindahunsi, A.A. Effect of homopterocarpin, an isoflavonoid from Pterocarpus erinaceus, on indices of liver injury and oxidative stress in acetaminophen-provoked hepatotoxicity. J. Basic Clin. Physiol. Pharmacol. 2015, 26, 555–562. [Google Scholar] [CrossRef]
- Morimoto, M.; Fukumoto, H.; Hiratani, M.; Chavasiri, W.; Komai, K. Insect Antifeedants, Pterocarpans and Pterocarpol, in Heartwood of Pterocarpus macrocarpus Kruz. Biosci. Biotechnol. Biochem. 2006, 70, 1864–1868. [Google Scholar] [CrossRef]
- Mottaghipisheh, J.; Stuppner, H. A comprehensive review on chemotaxonomic and phytochemical aspects of homoisoflavonoids, as rare flavonoid derivatives. Int. J. Mol. Sci. 2021, 22, 2735. [Google Scholar] [CrossRef]
- Escobar-Ramos, A.; Lobato-García, C.E.; Zamilpa, A.; Gómez-Rivera, A.; Tortoriello, J.; González-Cortazar, M. Homoisoflavonoids and Chalcones Isolated from Haematoxylum campechianum L., with Spasmolytic Activity. Molecules 2017, 22, 1405. [Google Scholar] [CrossRef]
- Kite, G.C.; Green, P.W.; Veitch, N.C.; Groves, M.C.; Gasson, P.E.; Simmonds, M.S. Dalnigrin, a neoflavonoid marker for the identification of Brazilian rosewood (Dalbergia nigra) in CITES enforcement. Phytochemistry 2010, 71, 1122–1131. [Google Scholar] [CrossRef]
- Liu, Y.; Shu, J.C.; Wang, M.F.; Xu, Z.J.; Yang, L.; Meng, X.W.; Duan, W.B.; Zhang, N.; Shao, F.; Liu, R.H.; et al. Melanoxylonin A-G, neoflavonoids from the heartwood of Dalbergia melanoxylon and their cardioprotective effects. Phytochemistry 2021, 189, 112845. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, K.Z.; Yang, Y.N.; Feng, Z.M.; Yuan, X.; Jiang, J.S.; Zhang, P.C. Six undescribed lavandulylated flavonoids with PTP1B inhibition from the roots of Sophora flavescens. Phytochemistry 2023, 216, 113889. [Google Scholar] [CrossRef] [PubMed]
- Blatt, C.T.; Chávez, D.; Chai, H.; Graham, J.G.; Cabieses, F.; Farnsworth, N.R.; Cordell, G.A.; Pezzuto, J.M.; Kinghorn, A.D. Cytotoxic flavonoids from the stem bark of Lonchocarpus aff. fluvialis. Phytother. Res. 2002, 16, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, M.; Nakanishi, K.; Darko, L.L.; Vick, J.A. Structures of cabenegrins AI and A-II, potent anti-snake venoms. Tetrahedron Lett. 1982, 23, 3855–3858. [Google Scholar] [CrossRef]
- Pontes, M.C.N.D.; Torres-Rêgo, M.; Aquino, A.K.S.; Aquino, N.C.; Teles, Y.C.F.; Ximenes, R.M.; Monteiro, H.S.A.; Silveira, E.R.; Araújo, R.M. Harpalyce brasiliana Benth: A prolific source of bioactive flavonoids with antiophidic potential. Phytochem. Lett. 2021, 41, 158–167. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Y.; Zhang, L.; Tian, Z.; Zhang, H.; Jiang, H. Rapid identification of chemical components and screening of acetylcholinesterase inhibitors from Dalbergia odorifera based on mass defect and diagnostic ion filtering strategy, affinity ultrafiltration, and liquid chromatography--tandem mass spectrometry. J. Sep. Sci. 2024, 47, 2400288. [Google Scholar] [CrossRef] [PubMed]
- Lo, W.L.; Wu, C.C.; Chang, F.R.; Wang, W.Y.; Khalil, A.T.; Lee, K.H.; Wu, Y.C. Antiplatelet and Anti-HIV Constituents from Euchresta Formosana. Nat. Prod. Res. 2003, 17, 91–97. [Google Scholar] [CrossRef]
- Sarkar, J.; Singh, R.; Chandel, S. Understanding LC/MS--Based Metabolomics: A Detailed Reference for Natural Product Analysis. Proteom.–Clin. Appl. 2025, 19, e202400048. [Google Scholar] [CrossRef]
- Saito, T.; Kurosu, Y.; Sato, H.; Katoh, E.; Hosoya, T. Analysis of the components of Cassia nomame and their antioxidant activity. Nat. Prod. Res. 2024, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Paramashivam, S.K.; Dhiraviam, K.N. Deciphering the Anti-metastatic Efficacy of Semi-purified Indigocarpan by Modulating Matrix Metalloproteinases and Promoting Cell Death. Rev. Bras. De Farmacogn. 2024, 34, 979–990. [Google Scholar] [CrossRef]
- Benhabrou, H.; Bitam, F.; Cristino, L.; Nicois, A.; Carbone, M.; Ammar, D.; Gavagnin, M.; Ciavatta, M.L. Prenyl Pterocarpans from Algerian Bituminaria bituminosa and Their Effects on Neuroblastoma. Molecules 2024, 29, 3678. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Šamec, D.; Karalija, E.; Dahija, S.; Hassan, S.T. Biflavonoids: Important contributions to the health benefits of Ginkgo (Ginkgo biloba L.). Plants 2022, 11, 1381. [Google Scholar] [CrossRef]
- Nascimento, B.O.; David, J.M. Chemical composition, biological activities and traditional uses of plants from the segregated genus Caesalpinia sensu lato. Phytochem. Rev. 2024, 23, 1–93. [Google Scholar] [CrossRef]
- Rahman, M.; Riaz, M.; Desai, U.R. Synthesis of biologically relevant biflavanoids–a review. Chem. Biodivers. 2007, 4, 2495–2527. [Google Scholar] [CrossRef]
- Šamec, D.; Jurčević Šangut, I.; Karalija, E.; Šarkanj, B.; Zelić, B.; Šalić, A. 3′-8 ″-Biflavones: A review of their structural diversity, natural occurrence, role in plants, extraction and identification. Molecules 2024, 29, 4634. [Google Scholar] [CrossRef]
- Pereira, R.; Ribeiro, D.; Silva, V.L.; Fernandes, E. Synthetic flavonoid dimers: Synthesis strategies and biological activities. Eur. J. Med. Chem. 2025, 291, 117669. [Google Scholar] [CrossRef]
- Yan, W.; Yang, J.; Tang, H.; Xue, L.; Chen, K.; Wang, L.; Zhao, M.; Tang, M.; Peng, A.; Long, C.; et al. Flavonoids from the stems of Millettia pachyloba Drake mediate cytotoxic activity through apoptosis and autophagy in cancer cells. J. Adv. Res. 2019, 20, 117–127. [Google Scholar] [CrossRef]
- Lin, S.; Ganesan, A.R.; Vargas--De--La--Cruz, C.; Ortiz--Viedma, J.; Xiao, J. Advances in resources, biosynthesis pathway, bioavailability, bioactivity, and pharmacology of ochnaflavone. Future Postharvest Food 2024, 1, 47–60. [Google Scholar] [CrossRef]
- Su, Q.; Krai, P.; Goetz, M.; Cassera, M.; Kingston, D. Antiplasmodial Isoflavanes and Pterocarpans from Apoplanesia paniculata. Planta Medica 2015, 81, 1128–1132. [Google Scholar] [CrossRef]
- Woo, H.S.; Lee, K.H.; Park, K.H.; Kim, D.W. Flavonoids Derived from the Roots of Lespedeza bicolor Inhibit the Activity of SARS-CoV Papain-like Protease. Plants 2024, 13, 3319. [Google Scholar] [CrossRef]
- Srinivas, K.V.N.S.; Rao, Y.K.; Mahender, I.; Das, B.; Krishna, K.R.; Kishore, K.H.; Murty, U.S.N. Flavanoids from Caesalpinia pulcherrima. Phytochemistry 2003, 63, 789–793. [Google Scholar] [CrossRef]
- Liu, R.; Ye, M.; Guo, H.; Bi, K.; Guo, D. Liquid chromatography/electrospray ionization mass spectrometry for the characterization of twenty-three flavonoids in the extract of Dalbergia odorifera. Rapid Commun. Mass. Spectrom. 2005, 19, 1557–1565. [Google Scholar] [CrossRef] [PubMed]
- Sousa, G.R.; Teles, N.R.L.; Silva, C.V.A.; Aragão, M.C.; Cardoso, D.B.O.S.; Gadelha, F.A.A.F.; Piuvezam, M.R.; Tavares, J.F.; Silva, M.S.; Barbosa-Filho, J.M. Target Isolation of Prenylated Isoflavonoids and Pterocarpans from Acosmium diffusissimum Using LC−MS/MS-Based Molecular Networking. ACS Omega 2025, 10, 13645–13654. [Google Scholar] [CrossRef]
- Wasilewicz, A.; Zwirchmayr, J.; Kirchweger, B.; Bojkova, D.; Cinatl, J., Jr.; Rabenau, H.F.; Rollinger, J.M.; Beniddir, M.A.; Grienke, U. Discovery of anti-SARS-CoV-2 secondary metabolites from the heartwood of Pterocarpus santalinus using multi-informative molecular networking. Front. Mol. Biosci. 2023, 10, 1202394. [Google Scholar] [CrossRef]
- Fang, S.; Qu, Q.; Zheng, Y.; Zhong, H.; Shan, C.; Wang, F.; Li, C.; Peng, G. Structural characterization and identification of flavonoid aglycones in three Glycyrrhiza species by liquid chromatography with photodiode array detection and quadrupole time-of-flight mass spectrometry. J. Sep. Sci. 2016, 39, 2068–2078. [Google Scholar] [CrossRef]
- Gampe, N.; Darcsi, A.; Lohner, S.; Béni, S.; Kursinszki, L. Characterization and identification of isoflavonoid glycosides in theroot of Spiny restharrow (Ononis spinosa L.) by HPLC-QTOF-MS, HPLC–MS/MS and NMR. J. Pharm. Biomed. Anal. 2016, 123, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Liang, X. Characterization and Identification of Isoflavonoids in the Roots of Millettia speciosa Champ. by UPLC-Q-TOF-MS/MS. Curr. Pharm. Anal. 2019, 15, 580–591. [Google Scholar] [CrossRef]
- Goel, A.; Kumar, A.; Raghuvanshi, A. Synthesis, Stereochemistry, Structural Classification, and Chemical Reactivity of Natural Pterocarpans. Chem. Rev. 2013, 113, 1614–1640. [Google Scholar] [CrossRef]
- Yao, H.; Chen, B.; Zhang, Y.; Ou, H.; Li, Y.; Li, S.; Shi, P.; Lin, X. Analysis of the Total Biflavonoids Extract from Selaginella doederleinii by HPLC-QTOF-MS and Its In Vitro and In Vivo Anticancer Effects. Molecules 2017, 22, 325. [Google Scholar] [CrossRef]
- Ko, Y.; Feng, H.; Lee, R.; Lee, M. The determination of flavonoids in Wikstroemia indica C. A. Mey. by liquid chromatography with photo-diode array detection and negative electrospray ionization tandem mass spectrometry. Rapid Commun. Mass. Spectrom. 2013, 27, 59–67. [Google Scholar] [CrossRef]
- Durán-Iturbide, N.A.; Díaz-Eufracio, B.I.; Medina-Franco, J.L. In silico ADME/Tox profiling of natural products: A focus on BIOFACQUIM. ACS Omega 2020, 5, 16076–16084. [Google Scholar] [CrossRef]
- Yi, J.C.; Yang, Z.Y.; Zhao, W.T.; Yang, Z.J.; Zhang, X.C.; Wu, C.K.; Lu, A.P.; Cao, D.S. ChemMORT: An automatic ADMET optimization platform using deep learning and multi-objective particle swarm optimization. Brief. Bioinform. 2024, 25, bbae008. [Google Scholar] [CrossRef]
- Kenakin, T. Know your molecule: Pharmacological characterization of drug candidates to enhance efficacy and reduce late-stage attrition. Nat. Rev. Drug Discov. 2024, 23, 626–644. [Google Scholar] [CrossRef] [PubMed]
- Kar, S.; Leszczynski, J. Open access in silico tools to predict the ADMET profiling of drug candidates. Expert Opin. Drug Discov. 2020, 15, 1473–1487. [Google Scholar] [CrossRef] [PubMed]
- Xiong, G.; Wu, Z.; Yi, J.; Fu, L.; Yang, Z.; Hsieh, C.; Yin, M.; Zeng, X.; Wu, C.; Lu, A.; et al. ADMETlab 2.0: An integrated online platform for accurate and comprehensive predictions of ADMET properties. Nucleic Acids Res. 2021, 49, W5–W14. [Google Scholar] [CrossRef] [PubMed]
- Moroy, G.; Martiny, V.Y.; Vayer, P.; Villoutreix, B.O.; Miteva, M.A. Toward in silico structure-based ADMET prediction in drug discovery. Drug Discov. Today 2012, 17, 44–55. [Google Scholar] [CrossRef]
- Ursu, O.; Rayan, A.; Goldblum, A.; Oprea, T.I. Understanding drug--likeness. WIREs Comput. Mol. Sci. 2011, 1, 760–781. [Google Scholar] [CrossRef]
- Pollastri, M.P. Overview on the Rule of Five. Curr. Protoc. Pharmacol. 2010, 49, 9–12. [Google Scholar] [CrossRef]
- Motiwale, M.; Verma, H.; Silakari, O.; Sapra, B. The utilization of descriptors in convoluted Lipinski’s rule of five. Comput. Drug Deliv. Mol. Simul. Pharm. Formul. 2024, 3, 39. [Google Scholar] [CrossRef]
- Ritchie, T.J.; Macdonald, S.J. How drug-like are ‘ugly’drugs: Do drug-likeness metrics predict ADME behaviour in humans? Drug Discov. Today 2014, 19, 489–495. [Google Scholar] [CrossRef]
- Bickerton, G.R.; Paolini, G.V.; Besnard, J.; Muresan, S.; Hopkins, A.L. Quantifying the chemical beauty of drugs. Nat. Chem. 2012, 4, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Wang, K.; Jia, X.; Tan, L.; Han, B.; Zhang, Q.; Li, Y.; He, C. Isolation and identification of Antiarthritic constituents from Glycine tabacina and network pharmacology-based prediction of their protective mechanisms against rheumatoid arthritis. J. Agric. Food Chem. 2020, 68, 10664–10677. [Google Scholar] [CrossRef] [PubMed]
- Chokchaisiri, S.; Suksamrarn, A. Chemical constituents of the roots of Erythrina subumbrans. Chem. Nat. Compd. 2014, 49, 1127–1128. [Google Scholar] [CrossRef]
- Chai, M.Y. A new bioactive coumestan from the seeds of Psoralea corylifolia. J. Asian Nat. Prod. Res. 2020, 22, 295–301. [Google Scholar] [CrossRef]
- Wen, R.; Lv, H.N.; Jiang, Y.; Tu, P.F. Anti-inflammatory pterocarpanoids from the roots of Pongamia pinnata. J. Asian Nat. Prod. Res. 2019, 21, 859–866. [Google Scholar] [CrossRef]
- Li, Y.; Chen, H.; Liu, J.; Luo, X.L.; Xiao, C.J.; Jiang, B. A new prenylated coumestan from the roots of Campylotropis hirtella. J. Asian Nat. Prod. Res. 2021, 23, 789–795. [Google Scholar] [CrossRef]
- Xie, Y.G.; Li, T.; Wang, G.; Ren, J.; Wang, X.; Pan, Y.; Li, H.; Yan, S.; Jin, H.; Zhang, W. Chemical constituents from Campylotropis hirtella. Planta Med. 2016, 82, 734–741. [Google Scholar] [CrossRef]
- Thuy, N.T.T.; Lee, J.E.; Yoo, H.M.; Cho, N. Antiproliferative pterocarpans and coumestans from Lespedeza bicolor. J. Nat. Prod. 2019, 82, 3025–3032. [Google Scholar] [CrossRef]
- Abdou, J.P.; Momeni, J.; Adhikari, A.; Tsabang, N.; Tchinda, A.T.; Choudhary, M.I.; Nkengfack, A.E. New coumestan and coumaronochromone derivatives from Dalbergia boehmii Taub. (Fabaceae). Phytochem. Lett. 2017, 21, 109–113. [Google Scholar] [CrossRef]
- Karthikeyan, M.; Baskar, B.; Kandasamy, V.; Balasundaram, U. Glutathione elicits enhanced biosynthesis of bonducellin, a homoisoflavonoid, in Caesalpinia bonducella leaf callus. Plant Cell Tissue Organ Cult. (PCTOC) 2023, 155, 57–65. [Google Scholar] [CrossRef]
- Felipe González, A.; Gutiérrez Gaitén, Y.I.; Scull Lizama, R.; Foubert, K.; Pieters, L.; Hernández, R.D. A New Homoisoflavonoid from Caesalpinia bahamensis. Rev. Bras. Farmacogn. 2020, 30, 733–736. [Google Scholar] [CrossRef]
- Mujahidin, D.; Noviany, I.; Hermawati, E.; Danova, A.; Heliawati, L.; Syah, Y.M. Isolation and inhibition study of eryvarin D and E from the root barks of Erythrina variegata against receptor tyrosine kinases. Rasayan J. Chem. 2024, 17, 832–837. [Google Scholar] [CrossRef]
- Nassief, S.M.; Amer, M.E.; Shawky, E.; Sishtla, K.; Mas-Claret, E.; Muniyandi, A.; Corson, T.W.; Mulholland, D.A.; El-Masry, S. Antiangiogenic Pterocarpan and Flavonoid Constituents of Erythrina lysistemon. J. Nat. Prod. 2023, 86, 759–766. [Google Scholar] [CrossRef]
- Azmi, A.S.; Mediani, A.; Abidin, W.A.M.W.Z.; Othman, W.N.N.W.; Cordell, G.A.; Salim, F. Phytochemical variation between hydrochloric and tartaric acid-derived alkaloidal extracts of Erythrina fusca Lour. leaves: A proton NMR-based. S. Afr. J. Bot. 2024, 168, 430–451. [Google Scholar] [CrossRef]
- Gurmessa, G.T.; Kusari, S.; Laatsch, H.; Bojase, G.; Tatolo, G.; Masesane, I.B.; Spiteller, M.; Majinda, R.R.T. Chemical constituents of root and stem bark of Erythrina brucei. Phytochem. Lett. 2018, 25, 37–42. [Google Scholar] [CrossRef]
- Li, F.; Bi, D.; Luo, R.; Liang, X.; Zhuang, H.; Qin, H.; Wang, L. Isoprenoid pterocarpans, isoflavonoids and flavonoids from Erythrina stricta. Phytochem. Lett. 2021, 44, 160–163. [Google Scholar] [CrossRef]
- Luo, R.L.; Li, F.Q.; Zhuang, H.D.; Jiang, T.; Wang, L.Q. A new C22 polyacetylene and seven isoprenylated pterocarpans from Erythrina subumbrans. J. Asian Nat. Prod. Res. 2023, 26, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Raksat, A.; Maneerat, W.; Rujanapun, N.; Andersen, R.J.; Pyne, S.G.; Laphookhieo, S. Antibacterial and inhibitory activities against nitric oxide production of coumaronochromones and prenylated isoflavones from Millettia extensa. J. Nat. Prod. 2019, 82, 2343–2348. [Google Scholar] [CrossRef]
- Gampe, N.; Darcsi, A.; Kursinszki, L.; Béni, S. Separation and characterization of homopipecolic acid isoflavonoid ester derivatives isolated from Ononis spinosa L. root. J. Chromatogr. B 2018, 1091, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Ghribi, L.; Waffo-Téguo, P.; Cluzet, S.; Marchal, A.; Marques, J.; Mérillon, J.M.; Ben Jannet, H. Isolation and structure elucidation of bioactive compounds from the roots of the Tunisian Ononis angustissima L. Bioorganic Med. Chem. Lett. 2015, 25, 3825–3830. [Google Scholar] [CrossRef] [PubMed]
- Adem, F.A.; Kuete, V.; Mbaveng, A.T.; Heydenreich, M.; Koch, A.; Ndakala, A.; Irungu, B.; Yenessew, A.; Efferth, T. Cytotoxic flavonoids from two Lonchocarpus species. Nat. Prod. Res. 2018, 33, 2609–2617. [Google Scholar] [CrossRef]
- Jiang, G.; Yang, S.; Sun, Q.; Zhou, R.; Zhan, R.; Aisa, H.A.; Chen, Y. Compounds from the Leaves and Stems of Erythrina crista-galli. Chem. Nat. Compd. 2024, 60, 310–313. [Google Scholar] [CrossRef]
- Kaennakam, S.; Siripong, P.; Tip-pyang, S. Velucarpins A–C, three new pterocarpans and their cytotoxicity from the roots of Dalbergia velutina. Fitoterapia 2015, 105, 165–168. [Google Scholar] [CrossRef]
- Yin, C.; Zhou, J.; Wu, Y.; Cao, Y.; Wu, T.; Zhang, S.; Li, H.; Cheng, Z. Pterocarpans and triterpenoids from Gueldenstaedtia verna. Fitoterapia 2015, 106, 46–54. [Google Scholar] [CrossRef]
- Bandeira-Reidel, R.V.; Giovanelli, S.; Pipitone, A.; Minissale, P.; Pistelli, L. Phytochemical study of Bituminaria basaltica aerial parts, an Italian endemism. Nat. Prod. Res. 2017, 31, 2375–2380. [Google Scholar] [CrossRef]
- Pailee, P.; Mahidol, C.; Ruchirawat, S.; Prachyawarakorn, V. Diverse flavonoids from the roots of Millettia brandisiana. Phytochemistry 2019, 162, 157–164. [Google Scholar] [CrossRef]
- Lee, J.W.; Lee, J.H.; Lee, C.; Jin, Q.; Lee, D.; Kim, Y.; Hong, J.T.; Lee, M.K.; Hwang, B.Y. Inhibitory constituents of Sophora tonkinensis on nitric oxide production in RAW 264.7 macrophages. Bioorganic Med. Chem. Lett. 2015, 25, 960–962. [Google Scholar] [CrossRef]
- Santana, D.B.; Costa, R.C.; Araújo, R.M.; Paula, J.E.; Silveira, E.R.; Braz-Filho, R.; Espindola, L.S. Activity of Fabaceae species extracts against fungi and Leishmania: Vatacarpan as a novel potent anti-Candida agent. Rev. Bras. De Farmacogn. 2015, 25, 401–406. [Google Scholar] [CrossRef]
- Du, X.Y.; Li, G.X.; Chen, X.Q.; Li, R.T.; Zhang, Z.J. Pterocarpans and 2-arylbenzofurans from Sophora flavescens aiton and their chemotaxonomic significance. Biochem. Syst. Ecol. 2022, 100, 104357. [Google Scholar] [CrossRef]
- Mezrag, A.; Malafronte, N.; Bouheroum, M.; Travaglino, C.; Russo, D.; Milella, L.; Severino, L.; De Tommasi, N.; Braca, A.; Dal Piaz, F. Phytochemical and antioxidant activity studies on Ononis angustissima L. aerial parts: Isolation of two new flavonoids. Nat. Prod. Res. 2016, 31, 507–514. [Google Scholar] [CrossRef]
- Kiganda, I.; Bogaerts, J.; Wieske, L.H.; Deyou, T.; Atilaw, Y.; Uwamariya, C.; Miah, M.; Said, J.; Ndakala, A.; Akala, H.M.; et al. Antiviral Rotenoids and Isoflavones Isolated from Millettia oblata ssp. teitensis. J. Nat. Prod. 2024, 87, 1003–1012. [Google Scholar] [CrossRef]
- Meesakul, P.; Suthiphasilp, V.; Teerapongpisan, P.; Rujanapun, N.; Chaiyosang, B.; Tontapha, S.; Phukhatmuen, P.; Maneerat, T.; Charoensup, R.; Duangyod, T.; et al. Rotenoids and isoflavones from the leaf and pod extracts of Millettia brandisiana Kurz. Phytochemistry 2022, 204, 113440. [Google Scholar] [CrossRef]
- Perumalsamy, H.; Jang, M.J.; Kim, J.R.; Kadarkarai, M.; Ahn, Y.J. Larvicidal activity and possible mode of action of four flavonoids and two fatty acids identified in Millettia pinnata seed toward three mosquito species. Parasit. Vectors 2015, 8, 237. [Google Scholar] [CrossRef]
- Mekuete, L.B.K.; Tsopgni, W.D.T.; Nkojap, A.K.; Kojom, J.J.W.; Stark, T.D.; Fouokeng, Y.; Dongmo, A.B.; Azeufack, L.T.; Azebaze, A.G.B. Rotenoids and isoflavones from Xeroderris stuhlmannii (taub.) mendonça & EP Souza and their biological activities. Molecules 2023, 28, 2846. [Google Scholar] [CrossRef] [PubMed]
- Sananboonudom, S.; Kaewnoi, A.; Pompimon, W.; Narakaew, S.; Jiajaroen, S.; Chainok, K.; Nuntasaen, N.; Suksen, K.; Chairoungdua, A.; Limthongkul, J.; et al. Study on the absolute configuration and biological activity of rotenoids from the leaves and twigs of Millettia pyrrhocarpa Mattapha, Forest & Hawkins, sp. Nov. BMC Complement. Med. Ther. 2023, 23, 147. [Google Scholar] [CrossRef]
- Ren, Y.; Benatrehina, P.A.; Acuña, U.M.; Yuan, C.; Chai, H.B.; Ninh, T.N.; Blanco, E.J.C.; Soejarto, D.D.; Kinghorn, A.D. Isolation of bioactive rotenoids and isoflavonoids from the fruits of Millettia caerulea. Planta Med. 2016, 82, 1096–1104. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Gao, Y.C.; Chen, Y.P.; Wang, L.Q. Chemical constituents of Erythrina speciosa. Nat. Prod. Res. 2025, 26, 394–398. [Google Scholar] [CrossRef]
- Innok, P.; Rukachaisirikul, T.; Phongpaichit, S.; Suksamrarn, A. Fuscacarpans A–C, new pterocarpans from the stems of Erythrina fusca. Fitoterapia 2010, 81, 518–523. [Google Scholar] [CrossRef]



| Compound | Cell Line | IC50 | Ref. |
|---|---|---|---|
| Indigocarpan (82) | MDA-MB-231, PC3, A549 | 92 μg/mL, 93 μg/mL, 135 μg/mL | [53] |
| Erybraedin C (87) | SH-SY5Y | 0.1976 µg/mL | [54] |
| Dehydromaackiain (66) | HeLa, HepG2, MCF-7, HCT-116, MDA-MB-231 | 22.50 ± 1.09 µM, 13.39 ± 1.41 µM, 21.21 ± 0.93 µM, 21.90 ± 1.73 µM, 25.45 ± 2.09 µM | [60] |
| Flemichapparin B (67) | 30.19 ± 0.54 µM, 25.38 ± 1.92 µM, 21.10 ± 1.65 µM, 27.03 ± 1.64 µM, 22.76 ± 3.54 µM | ||
| 3,9-dihydroxypterocarp-6a-en (69) | 36.15 ± 7.34 µM, 34.25 ± 1.87 µM, 30.34 ± 1.32 µM, 39.66 ± 2.06 µM, 36.78 ± 5.61 µM | ||
| Velucarpin A (119) | KB, HeLa | 15.77 µM, 18.96 µM | [61] |
| Velucarpin B (120) | 19.96 µM, 25.24 µM | ||
| Velucarpin C (121) | 8.22 µM, 8.09 µM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brilhante, A.R.R.d.A.S.; de Sousa, G.R.; de Aquino-Vital, A.K.S.; de Lima, N.T.R.; Souza, R.B.d.L.; de Souza, T.A.; Scotti, M.T.; Barbosa Filho, J.M.; Tavares, J.F.; da Silva, M.S. Fabaceae Flavonoids Beyond the Commonplace: A Review of Chemical Diversity, Pharmacological Activities, Mass Spectrometric Profiling and In Silico Insights into Their Subclasses. Plants 2025, 14, 3549. https://doi.org/10.3390/plants14233549
Brilhante ARRdAS, de Sousa GR, de Aquino-Vital AKS, de Lima NTR, Souza RBdL, de Souza TA, Scotti MT, Barbosa Filho JM, Tavares JF, da Silva MS. Fabaceae Flavonoids Beyond the Commonplace: A Review of Chemical Diversity, Pharmacological Activities, Mass Spectrometric Profiling and In Silico Insights into Their Subclasses. Plants. 2025; 14(23):3549. https://doi.org/10.3390/plants14233549
Chicago/Turabian StyleBrilhante, Ana Rita Rodrigues de Almeida Silva, Gabriela Ribeiro de Sousa, Ana Karoline Silva de Aquino-Vital, Natanael Teles Ramos de Lima, Ranna Beatris de Lima Souza, Thalisson Amorim de Souza, Marcus Tullius Scotti, José Maria Barbosa Filho, Josean Fechine Tavares, and Marcelo Sobral da Silva. 2025. "Fabaceae Flavonoids Beyond the Commonplace: A Review of Chemical Diversity, Pharmacological Activities, Mass Spectrometric Profiling and In Silico Insights into Their Subclasses" Plants 14, no. 23: 3549. https://doi.org/10.3390/plants14233549
APA StyleBrilhante, A. R. R. d. A. S., de Sousa, G. R., de Aquino-Vital, A. K. S., de Lima, N. T. R., Souza, R. B. d. L., de Souza, T. A., Scotti, M. T., Barbosa Filho, J. M., Tavares, J. F., & da Silva, M. S. (2025). Fabaceae Flavonoids Beyond the Commonplace: A Review of Chemical Diversity, Pharmacological Activities, Mass Spectrometric Profiling and In Silico Insights into Their Subclasses. Plants, 14(23), 3549. https://doi.org/10.3390/plants14233549







