Larvicidal Activities of Juniperus chinensis var. kaizuka Leaf Essential Oil and Its Constituents Against Dengue Vector Mosquitoes, Aedes aegypti and Ae. albopictus
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Constituents of Juniperus chinensis var. kaizuka Leaf Essential Oil
| RT (min) | Compound | Formula | KI a | rKI b | Relative Content (%) |
|---|---|---|---|---|---|
| 7.06 | α-Pinene | C10H16 | 935 | 939 | 0.98 ± 0.04 |
| 7.62 | Camphene | C10H16 | 952 | 954 | 0.93 ± 0.04 |
| 8.45 | Sabinene | C10H16 | 974 | 975 | 3.54 ± 0.13 |
| 9.11 | β-Myrcene | C10H16 | 990 | 990 | 8.11 ± 0.25 |
| 10.16 | α-Terpinene | C10H16 | 1017 | 1017 | 0.73 ± 0.03 |
| 10.77 | Limonene | C10H16 | 1033 | 1029 | 33.33 ± 1.14 |
| 11.90 | γ-Terpinene | C10H16 | 1059 | 1059 | 1.12 ± 0.04 |
| 13.08 | Terpinolene | C10H16 | 1085 | 1088 | 1.44 ± 0.05 |
| 13.74 | Linalool | C10H18O | 1098 | 1096 | 0.11 ± 0.02 |
| 17.34 | Terpinen-4-ol | C10H18O | 1179 | 1177 | 1.23 ± 0.02 |
| 22.14 | Bornyl acetate | C12H20O2 | 1284 | 1285 | 23.71 ± 0.73 |
| 26.28 | β-Elemene | C15H24 | 1388 | 1390 | 0.49 ± 0.01 |
| 30.31 | Cubebol | C15H26O | 1515 | 1515 | 1.41 ± 0.03 |
| 31.20 | β-Elemol | C15H26O | 1549 | 1549 | 14.99 ± 1.73 |
| 33.33 | γ-Eudesmol | C15H26O | 1630 | 1632 | 0.54 ± 0.38 |
| Monoterpenes | 50.19 ± 2.10 | ||||
| Oxygenated monoterpenes | 25.05 ± 0.91 | ||||
| Sesquiterpenes | 0.49 ± 0.02 | ||||
| Oxygenated sesquiterpenes | 16.94 ± 2.52 | ||||
| Total identified | 92.67 ± 0.41 | ||||
2.2. Brine Shrimp Lethality Activity of Juniperus chinensis var. kaizuka Leaf Essential Oil
2.3. Brine Shrimp Lethality Activity of J. chinensis var. kaizuka Leaf Essential Oil Against Dengue Vector Mosquitoes
2.4. Mosquito Larvicidal Activity of J. chinensis var. kaizuka Leaf Essential Oil and Its Constituents
3. Materials and Methods
3.1. Plant Material
3.2. Hydrodistillation of Essential Oil
3.3. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
3.4. Mosquito Larvicidal Assay
3.5. Brine Shrimp Lethality Assay
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Belov, T.; Terenzhev, D.; Bushmeleva, K.N.; Davydova, L.; Burkin, K.; Fitsev, I.; Gatiyatullina, A.; Egorova, A.; Nikitin, E. comparative analysis of chemical profile and biological activity of Juniperus communis L. Berry extracts. Plants 2023, 12, 3401. [Google Scholar] [CrossRef]
- Höferl, M.; Stoilova, I.; Schmidt, E.; Wanner, J.; Jirovetz, L.; Trifonova, D.; Krastev, L.; Krastanov, A. Chemical composition and antioxidant properties of Juniper berry (Juniperus communis L.) Essential Oil. Action of the essential oil on the antioxidant Protection of Saccharomyces cerevisiae model organism. Antioxidants 2014, 3, 81–98. [Google Scholar] [CrossRef]
- Hrytsyna, M.; Salamon, I.; Peleno, R.; Vargova, V. Identification and analysis of the content of biologically active substances of juniper cone berries and their antioxidant activity. Horticulturae 2024, 10, 1237. [Google Scholar] [CrossRef]
- Tang, J.; Dunshea, F.R.; Suleria, H.A.R. LC-ESI-QTOF/MS Characterization of phenolic compounds from medicinal plants (Hops and Juniper Berries) and their antioxidant activity. Foods 2019, 9, 7. [Google Scholar] [CrossRef]
- Mërtiri, I.; Păcularu-Burada, B.; Stănciuc, N. Phytochemical characterization and antibacterial activity of Albanian Juniperus communis and Juniperus oxycedrus berries and needle leaves extracts. Antioxidants 2024, 13, 345. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, D.I.; Nedialkov, P.T.; Tashev, A.N.; Olech, M.; Nowak, R.; Ilieva, Y.E.; Kokanova-Nedialkova, Z.K.; Atanasova, T.N.; Angelov, G.; Najdenski, H.M. Junipers of Various Origins as Potential Sources of the Anticancer Drug Precursor Podophyllotoxin. Molecules 2021, 26, 5179. [Google Scholar] [CrossRef] [PubMed]
- Alhayyani, S.; Akhdhar, A.; Asseri, A.H.; Mohammed, A.M.A.; Hussien, M.A.; Roselin, L.S.; Hosawi, S.; AlAbbasi, F.; Alharbi, K.H.; Baty, R.S.; et al. Potential Anticancer Activity of Juniperus procera and Molecular Docking Models of Active Proteins in Cancer Cells. Molecules 2023, 28, 2041. [Google Scholar] [CrossRef] [PubMed]
- Meringolo, L.; Bonesi, M.; Sicari, V.; Rovito, S.; Passalacqua, N.G.; Loizzo, M.R.; Tundis, R. Essential Oils and Extracts of Juniperus macrocarpa Sm. and Juniperus oxycedrus L.: Comparative Phytochemical Composition and Anti-Proliferative and Antioxidant Activities. Plants 2022, 11, 1025. [Google Scholar] [CrossRef]
- Raasmaja, A.; Stenius, U.; Ghalali, A. The water extract of Juniperus communis l. induces cell death and sensitizes cancer cells to cytostatic Drugs through p53 and PI3K/Akt Pathways. Int. J. Mol. Sci. 2019, 20, 2054. [Google Scholar] [CrossRef]
- Raina, R.; Verma, P.K.; Peshin, R.; Kour, H. Potential of Juniperus communis L as a nutraceutical in human and veterinary medicine. Heliyon 2019, 5, e02376. [Google Scholar] [CrossRef]
- Hribar, L.J.; Boehmler, M.B.; Murray, H.L.; Pruszynski, C.A.; Leal, A.L. Mosquito Surveillance and Insecticide Resistance Monitoring Conducted by the Florida Keys Mosquito Control District, Monroe County, Florida, USA. Insects 2022, 13, 927. [Google Scholar] [CrossRef]
- Lees, R.S.; Fornadel, C.; Snetselaar, J.; Wagman, J.; Spiers, A. Insecticides for Mosquito Control: Improving and Validating Methods to Strengthen the Evidence Base. Insects 2023, 14, 116. [Google Scholar] [CrossRef]
- Borovsky, D. Biosynthesis and control of mosquito gut proteases. IUBMB Life 2003, 55, 435–441. [Google Scholar] [CrossRef]
- Chen, W.J. Dengue outbreaks and the geographic distribution of dengue vectors in Taiwan: A 20-year epidemiological analysis. Biomed. J. 2018, 41, 283–289. [Google Scholar] [CrossRef] [PubMed]
- See, K.C. Dengue Vaccination: A Practical Guide for Clinicians. Vaccines 2025, 13, 145. [Google Scholar] [CrossRef] [PubMed]
- Anumanthan, G.; Sahay, B.; Mergia, A. Current Dengue virus vaccine developments and future directions. Viruses 2025, 17, 212. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Dengue and Severe Dengue. Available online: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue (accessed on 28 June 2024).
- Osanloo, M.; Sedaghat, M.M.; Sanei-Dehkordi, A.; Amani, A. Plant-Derived Essential Oils; Their Larvicidal Properties and Potential Application for Control of Mosquito-Borne Diseases. Galen Med J. 2019, 8, e1532. [Google Scholar] [CrossRef]
- Senthil-Nathan, S. A Review of resistance mechanisms of synthetic insecticides and botanicals, phytochemicals, and essential oils as alternative larvicidal agents against mosquitoes. Front. Physiol. 2020, 10, 1591. [Google Scholar] [CrossRef]
- Malijan, R.P.B.; Angeles, J.R.; Apilado, A.M.A.; Ammugauan, M.A.T.; Salazar, F.V. Insecticide Resistance in Aedes aegypti from the National Capital Region of the Philippines. Insects 2024, 15, 782. [Google Scholar] [CrossRef]
- Farag, M.R.; Alagawany, M.; Bilal, R.M.; Gewida, A.G.A.; Dhama, K.; Abdel-Latif, H.M.R.; Amer, M.S.; Rivero-Perez, N.; Zaragoza-Bastida, A.; Binnaser, Y.S.; et al. An overview on the potential hazards of pyrethroid insecticides in fish, with Special emphasis on cypermethrin toxicity. Animals 2021, 11, 1880. [Google Scholar] [CrossRef]
- Ahamad, A.; Kumar, J. Pyrethroid pesticides: An overview on classification, toxicological assessment and monitoring. J. Hazard. Mater. Adv. 2023, 10, 100284. [Google Scholar] [CrossRef]
- Frezza, C.; Venditti, A.; De Vita, D.; Toniolo, C.; Franceschin, M.; Ventrone, A.; Tomassini, L.; Foddai, S.; Guiso, M.; Nicoletti, M.; et al. Phytochemistry, Chemotaxonomy, and Biological Activities of the Araucariaceae Family-A Review. Plants 2020, 9, 888. [Google Scholar] [CrossRef]
- Carroll, J.F.; Tabanca, N.; Kramer, M.; Elejalde, N.M.; Wedge, D.E.; Bernier, U.R.; Coy, M.; Becnel, J.J.; Demirci, B.; Başer, K.H.; et al. Essential oils of Cupressus funebris, Juniperus communis, and J. chinensis (Cupressaceae) as repellents against ticks (Acari: Ixodidae) and mosquitoes (Diptera: Culicidae) and as toxicants against mosquitoes. J. Vector Ecol. 2011, 36, 258–268. [Google Scholar] [CrossRef]
- Luz, T.R.S.A.; de Mesquita, L.S.S.; Amaral, F.M.M.D.; Coutinho, D.F. Essential oils and their chemical constituents against Aedes aegypti L. (Diptera: Culicidae) larvae. Acta Trop. 2020, 212, 105705. [Google Scholar] [CrossRef] [PubMed]
- Araujo, M.O.; Perez-Castillo, Y.; Oliveira, L.H.G.; Nunes, F.C.; de Sousa, D.P. Larvicidal activity of cinnamic acid derivatives: Investigating alternative products for Aedes aegypti L. control. Molecules 2021, 26, 61. [Google Scholar] [CrossRef] [PubMed]
- Karunamoorthi, K.; Girmay, A.; Fekadu, S. Larvicidal efficacy of Ethiopian ethnomedicinal plant Juniperus procera essential oil against Afrotropical malaria vector Anopheles arabiensis (Diptera: Culicidae). Asian Pac. J. Trop. Biomed. 2014, 4 (Suppl. 1), S99–S106. [Google Scholar] [CrossRef]
- Ho, Y.T.; Liu, I.H.; Chang, S.T.; Wang, S.Y.; Chang, H.T. In vitro and in vivo antimelanogenesis effects of leaf essential oil from Agathis dammara. Pharmaceutics 2023, 15, 2269. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; p. 804. ISBN 978-1932633214. [Google Scholar]
- Niksic, H.; Becic, F.; Koric, E.; Gusic, I.; Omeragic, E.; Muratovic, S.; Miladinovic, B.; Duric, K. Cytotoxicity screening of Thymus vulgaris L. essential oil in brine shrimp nauplii and cancer cell lines. Sci. Rep. 2021, 11, 13178. [Google Scholar] [CrossRef]
- Meyer, B.N.; Ferrigni, N.R.; Putnam, J.E.; Jacobsen, L.B.; Nichols, D.E.; McLaughlin, J.L. Brine shrimp: A convenient general bioassay for active plant constituents. Planta Med. 1982, 45, 31–34. [Google Scholar] [CrossRef]
- Sousa, O.V.; Del-Vechio-Vieira, G.; Alves, M.S.; Araújo, A.A.; Pinto, M.A.; Amaral, M.P.; Rodarte, M.P.; Kaplan, M.A. Chemical composition and biological activities of the essential oils from Duguetia lanceolata St. Hil. barks. Molecules 2012, 17, 11056–11066. [Google Scholar] [CrossRef]
- Bıtgen, N.; Onder, G.O.; Baran, M.; Yay, A. Cytotoxicity screening of Thymus vulgaris L. in breast cancer: In vitro study. Toxicol. Res. 2023, 12, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Waghulde, S.; Kale, M.K.; Patil, V.R. Brine shrimp lethality assay of the aqueous and ethanolic extracts of the selected species of medicinal plants. Proceedings 2019, 41, 47. [Google Scholar] [CrossRef]
- Blažíčková, M.; Blaško, J.; Kubinec, R.; Kozics, K. Newly synthesized thymol derivative and its effect on colorectal cancer cells. Molecules 2022, 27, 22. [Google Scholar] [CrossRef] [PubMed]
- Moola, S.; Orchard, A.; van Vuuren, S. The Antimicrobial and toxicity influence of six carrier oils on essential oil compounds. Molecules 2023, 28, 30. [Google Scholar] [CrossRef]
- Reddy, P.G.; Domb, A.J. Bioactive phenolate salts: Thymol salts. Chem. Med. Chem. 2023, 18, e202300045. [Google Scholar] [CrossRef]
- Sowndhararajan, K.; Seo, M.; Kim, S. Comparative analysis of the composition of essential oils from the needles, twigs and berries of Juniperus chinensis L. in Korea. J. Appl. Pharm. Sci. 2016, 6, 122–126. [Google Scholar] [CrossRef]
- Jayaraman, M.; Senthilkumar, A.; Venkatesalu, V. Evaluation of some aromatic plant extracts for mosquito larvicidal potential against Culex quinquefasciatus, Aedes aegypti, and Anopheles stephensi. Parasitol. Res. 2015, 114, 1511–1518. [Google Scholar] [CrossRef]
- Imran, M.; Jan, H.; Faisal, S.; Ali Shah, S.; Shah, S.; Naeem Khan, M.; Taj Akbar, M.; Rizwan, M.; Jan, F.; Syed, S. In vitro examination of anti-parasitic, anti-Alzheimer, insecticidal and cytotoxic potential of Ajuga bracteosa Wallich leaves extracts. Saudi J. Biol. Sci. 2021, 28, 3031–3036. [Google Scholar] [CrossRef]
- Nondo, R.S.; Mbwambo, Z.H.; Kidukuli, A.W.; Innocent, E.M.; Mihale, M.J.; Erasto, P.; Moshi, M.J. Larvicidal, antimicrobial and brine shrimp activities of extracts from Cissampelos mucronata and Tephrosia villosa from coast region, Tanzania. BMC Complement. Altern. Med. 2011, 11, 33. [Google Scholar] [CrossRef]
- Moshi, M.J.; Innocent, E.; Magadula, J.J.; Otieno, D.F.; Weisheit, A.; Mbabazi, P.K.; Nondo, R.S. Brine shrimp toxicity of some plants used as traditional medicines in Kagera Region, north western Tanzania. Tanzan. J. Health Res. 2010, 12, 63–67. [Google Scholar] [CrossRef]
- Sayono, S.; Anwar, R.; Sumanto, D. Larvicidal activity of ethyl acetate extract of Derris elliptica Root against the Third-Instar Larvae of cypermethrin-resistant Aedes aegypti Offspring. J. Arthropod-Borne Dis. 2020, 14, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, M.; Rajeswary, M.; Benelli, G. δ-Cadinene, Calarene and δ-4-Carene from Kadsura heteroclita Essential Oil as Novel Larvicides Against Malaria, Dengue and Filariasis Mosquitoes. Comb. Chem. High Throughput Screen. 2016, 19, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lin, X.; Qian, Y.; Qin, M.; Zhang, S.; Wang, L.; Luo, Y. Preparation and characterization of efficient and safe Rotenone solid nanodispersion by self-emulsifying technique. Nanomaterials 2025, 15, 1056. [Google Scholar] [CrossRef]
- Chang, H.T.; Chang, M.L.; Chen, Y.T.; Chang, S.T.; Hsu, F.L.; Wu, C.C.; Ho, C.K. Evaluation of motor coordination and anti-depressant activities of Cinnamomum osmophloeum ct. linalool leaf oil in rodent model. Molecules 2021, 26, 3037. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.R.; Chang, M.L.; Chang, S.T.; Ho, Y.T.; Chang, H.T. Cytotoxicity and apoptosis induction of 6,7-dehydroroyleanone from Taiwania cryptomerioides bark essential oil in hepatocellular carcinoma cells. Pharmaceutics 2022, 14, 351. [Google Scholar] [CrossRef]
- Huang, C.Y.; Chang, Y.Y.; Chang, S.T.; Chang, H.T. Xanthine oxidase inhibitory activity and chemical composition of Pistacia chinensis leaf essential oil. Pharmaceutics 2022, 14, 1982. [Google Scholar] [CrossRef]
- Chang, Y.-Y.; Huang, Y.-M.; Chang, H.-T. Using Headspace Gas Chromatography–Mass Spectrometry to Investigate the Volatile Terpenoids Released from the Liquidambar formosana Leaf and Its Essential Oil. Forests 2024, 15, 1495. [Google Scholar] [CrossRef]
- Huang, C.Y.; Yeh, T.F.; Hsu, F.L.; Lin, C.Y.; Chang, S.T.; Chang, H.-T. Xanthine oxidase inhibitory activity and thermostability of cinnamaldehyde-chemotype leaf oil of Cinnamomum osmophloeum microencapsulated with β-cyclodextrin. Molecules 2018, 23, 1107. [Google Scholar] [CrossRef]
- Wu, C.C.; Huang, S.L.; Ko, C.H.; Chang, H.T. Antifungal sesquiterpenoids from Michelia formosana leaf essential oil against wood-rotting fungi. Molecules 2022, 27, 2136. [Google Scholar] [CrossRef]
- Chaita, E.; Lambrinidis, G.; Cheimonidi, C.; Agalou, A.; Beis, D.; Trougakos, I.; Mikros, E.; Skaltsounis, A.L.; Aligiannis, N. Anti-melanogenic properties of greek plants. a novel depigmenting agent from Morus alba wood. Molecules 2017, 22, 514. [Google Scholar] [CrossRef]
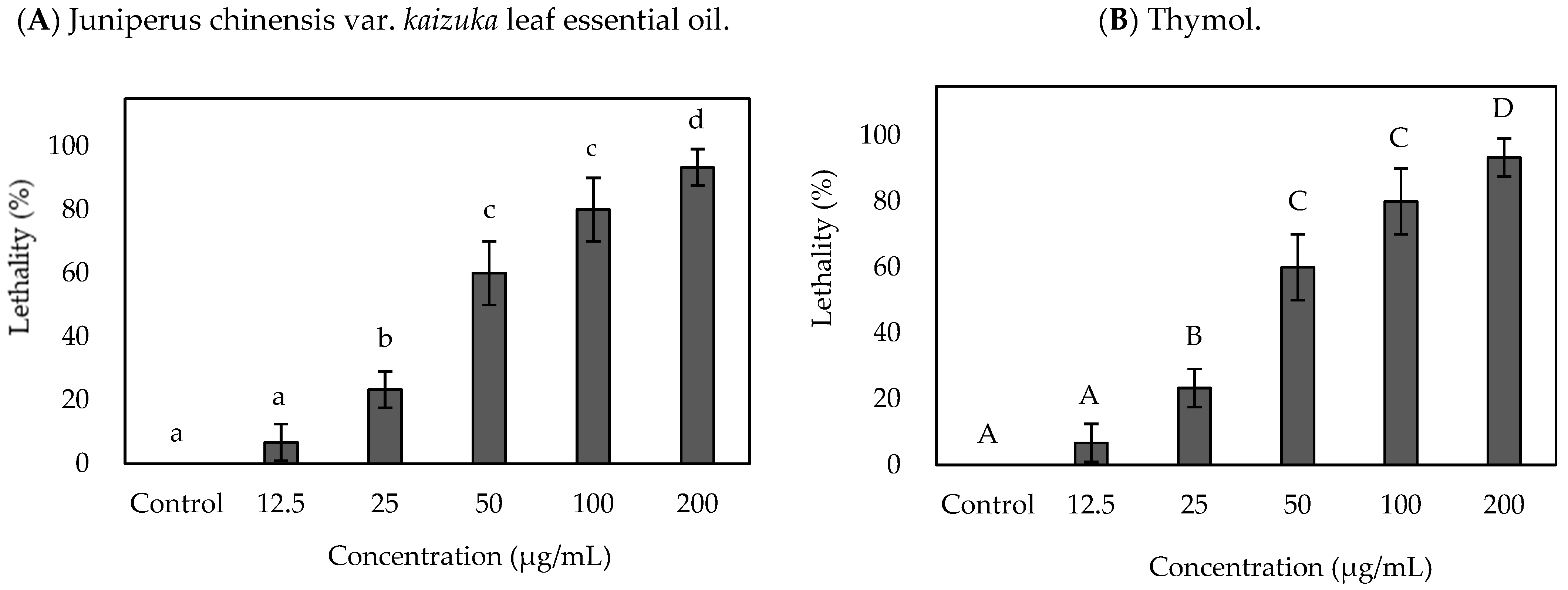
| Specimen | LC50 (μg/mL) | LC90 (μg/mL) |
|---|---|---|
| Leaf essential oil | 43.06 ± 1.97 b | 88.20 ± 2.92 B |
| Thymol * | 8.43 ± 1.46 a | 15.99 ± 1.75 A |
| Specimen | 24 h | 48 h | ||
|---|---|---|---|---|
| LC50 | LC90 | LC50 | LC90 | |
| Leaf essential oil | 213.52 ± 11.23 c | 272.70 ± 22.18 d | 155.04 ± 8.44 b | 207.07 ± 6.98 c |
| Rotenone * | 4.89 ± 0.66 a | - | 1.61 ± 0.15 a | 5.32 ± 0.61 a |
| Specimen | LC50 | LC90 | ||
|---|---|---|---|---|
| 24 h | 48 h | 24 h | 48 h | |
| Leaf oil | 27.51 ± 1.25 bc | 25.46 ± 0.86 bc | 44.44 ± 4.95 B | 44.40 ± 1.98 B |
| Limonene | 35.72 ± 3.91 cd | 28.73 ± 8.95 c | 76.01 ± 2.38 C | 74.07 ± 2.10 C |
| Sabinene | 55.62 ± 10.65 e | 49.94 ± 17.36 de | 137.99 ± 26.21 D | 131.61 ± 30.50 D |
| β-Myrcene | 36.10 ± 1.41 d | 36.40 ± 1.53 d | 70.77 ± 2.51 C | 70.41 ± 2.34 C |
| Bornyl acetate | 111.63 ± 20.85 f | 102.43 ± 8.66 f | 183.45 ± 6.06 A | 181.00 ± 4.98 A |
| Rotenone * | 21.11 ± 5.77 bc | 10.78 ± 2.71 ab | - ** | - |
| Specimen | LC50 | LC90 | ||
|---|---|---|---|---|
| 24 h | 48 h | 24 h | 48 h | |
| Leaf oil | 46.74 ± 10.62 b | 46.64 ± 10.79 b | 65.94 ± 8.85 B | 66.36 ± 8.01 B |
| Sabinene | 59.57 ± 3.97 c | 58.63 ± 3.53 c | 113.05 ± 9.12 D | 114.67 ± 12.08 D |
| β-Myrcene | 57.13 ± 5.97 bc | 53.52 ± 7.46 bc | 105.43 ± 14.33 CE | 94.25 ± 14.51 C |
| Limonene | 24.12 ± 0.65 a | 23.86 ± 1.15 a | 42.30 ± 1.67 A | 41.80 ± 2.55 A |
| Bornyl acetate | 150.02 ± 12.77 d | 146.57 ± 11.20 e | 194.09 ± 2.94 E | 193.39 ± 3.45 E |
| Rotenone * | - ** | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, J.-Y.; Tsai, K.-H.; Huang, Y.-M.; Chang, Y.-Y.; Huang, C.-S.; Ho, Y.-T.; Wang, S.-Y.; Chang, M.-L.; Chang, H.-T. Larvicidal Activities of Juniperus chinensis var. kaizuka Leaf Essential Oil and Its Constituents Against Dengue Vector Mosquitoes, Aedes aegypti and Ae. albopictus. Plants 2025, 14, 3321. https://doi.org/10.3390/plants14213321
Chang J-Y, Tsai K-H, Huang Y-M, Chang Y-Y, Huang C-S, Ho Y-T, Wang S-Y, Chang M-L, Chang H-T. Larvicidal Activities of Juniperus chinensis var. kaizuka Leaf Essential Oil and Its Constituents Against Dengue Vector Mosquitoes, Aedes aegypti and Ae. albopictus. Plants. 2025; 14(21):3321. https://doi.org/10.3390/plants14213321
Chicago/Turabian StyleChang, Ji-Yun, Kun-Hsien Tsai, Yu-Mei Huang, Yu-Yi Chang, Chong-Syuan Huang, Yu-Tung Ho, Sheng-Yang Wang, Mei-Ling Chang, and Hui-Ting Chang. 2025. "Larvicidal Activities of Juniperus chinensis var. kaizuka Leaf Essential Oil and Its Constituents Against Dengue Vector Mosquitoes, Aedes aegypti and Ae. albopictus" Plants 14, no. 21: 3321. https://doi.org/10.3390/plants14213321
APA StyleChang, J.-Y., Tsai, K.-H., Huang, Y.-M., Chang, Y.-Y., Huang, C.-S., Ho, Y.-T., Wang, S.-Y., Chang, M.-L., & Chang, H.-T. (2025). Larvicidal Activities of Juniperus chinensis var. kaizuka Leaf Essential Oil and Its Constituents Against Dengue Vector Mosquitoes, Aedes aegypti and Ae. albopictus. Plants, 14(21), 3321. https://doi.org/10.3390/plants14213321









