How Warmer and Drier Conditions Drive Forest Dieback and Tree Death: A Review and Conceptual Model for Silver Fir
Abstract
1. Introduction: Silver Fir Decline in a Global Context
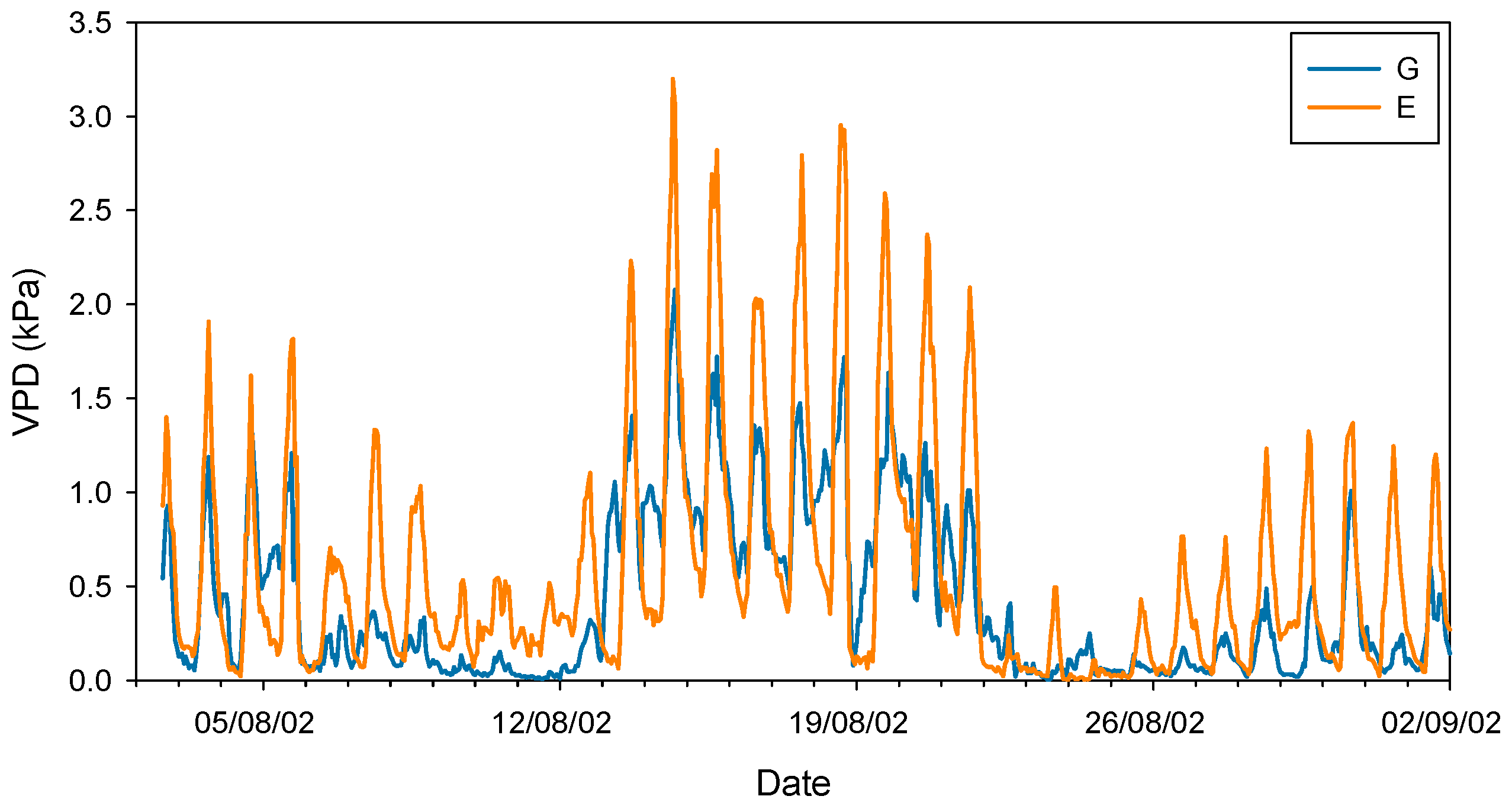
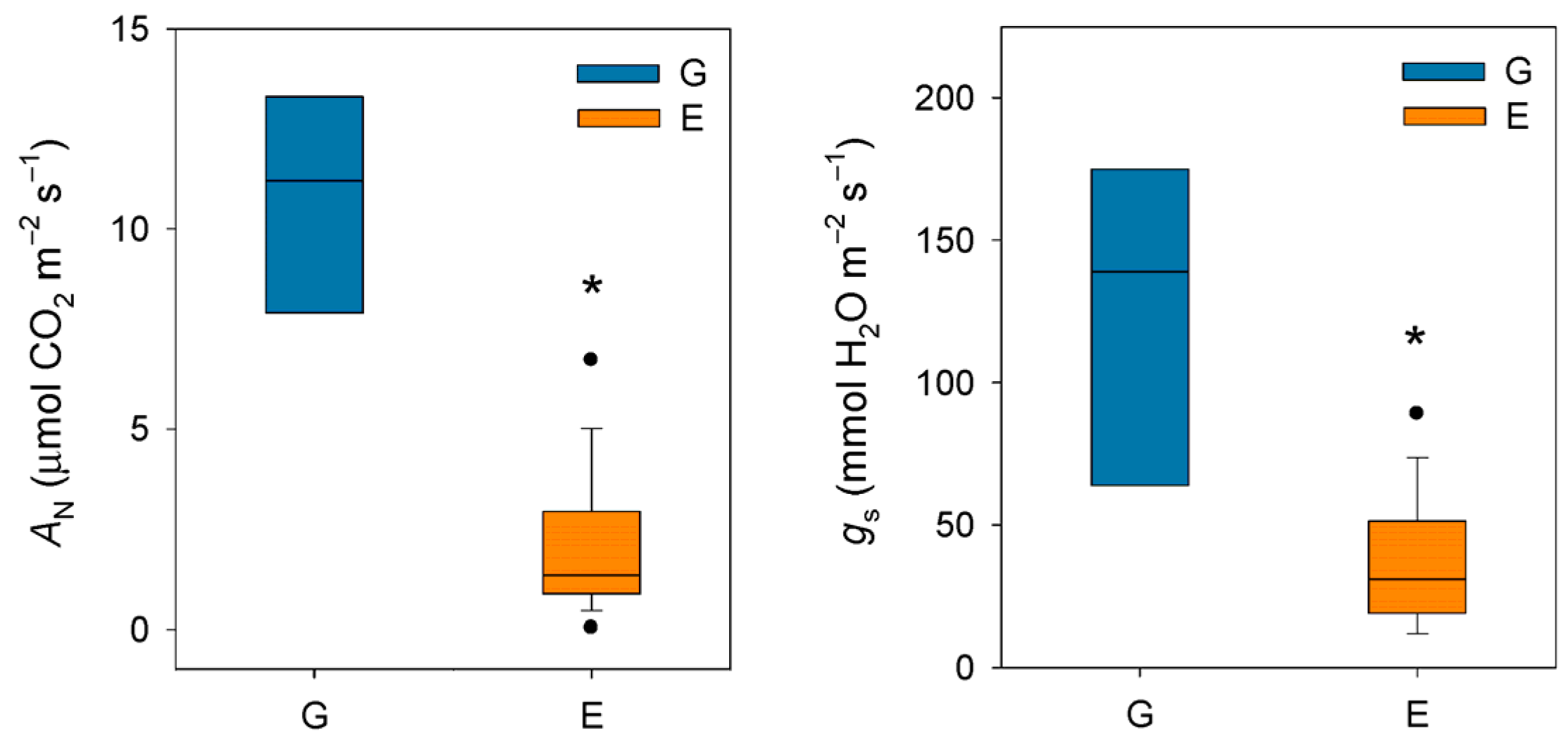
2. The Effect of Atmospheric Drought on Stomatal and Photosynthesis Rates in Conifers with a Focus on Silver Fir
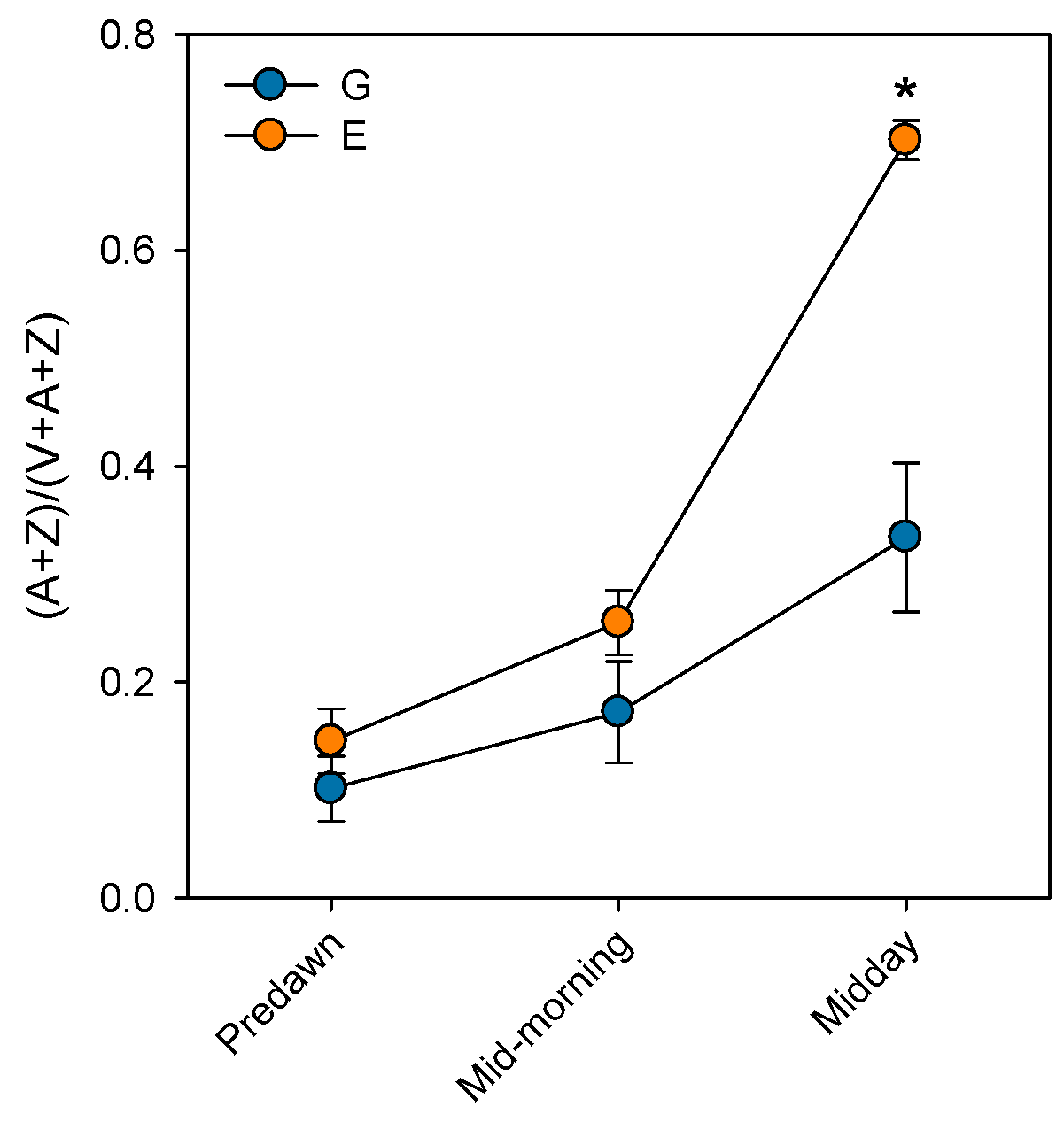
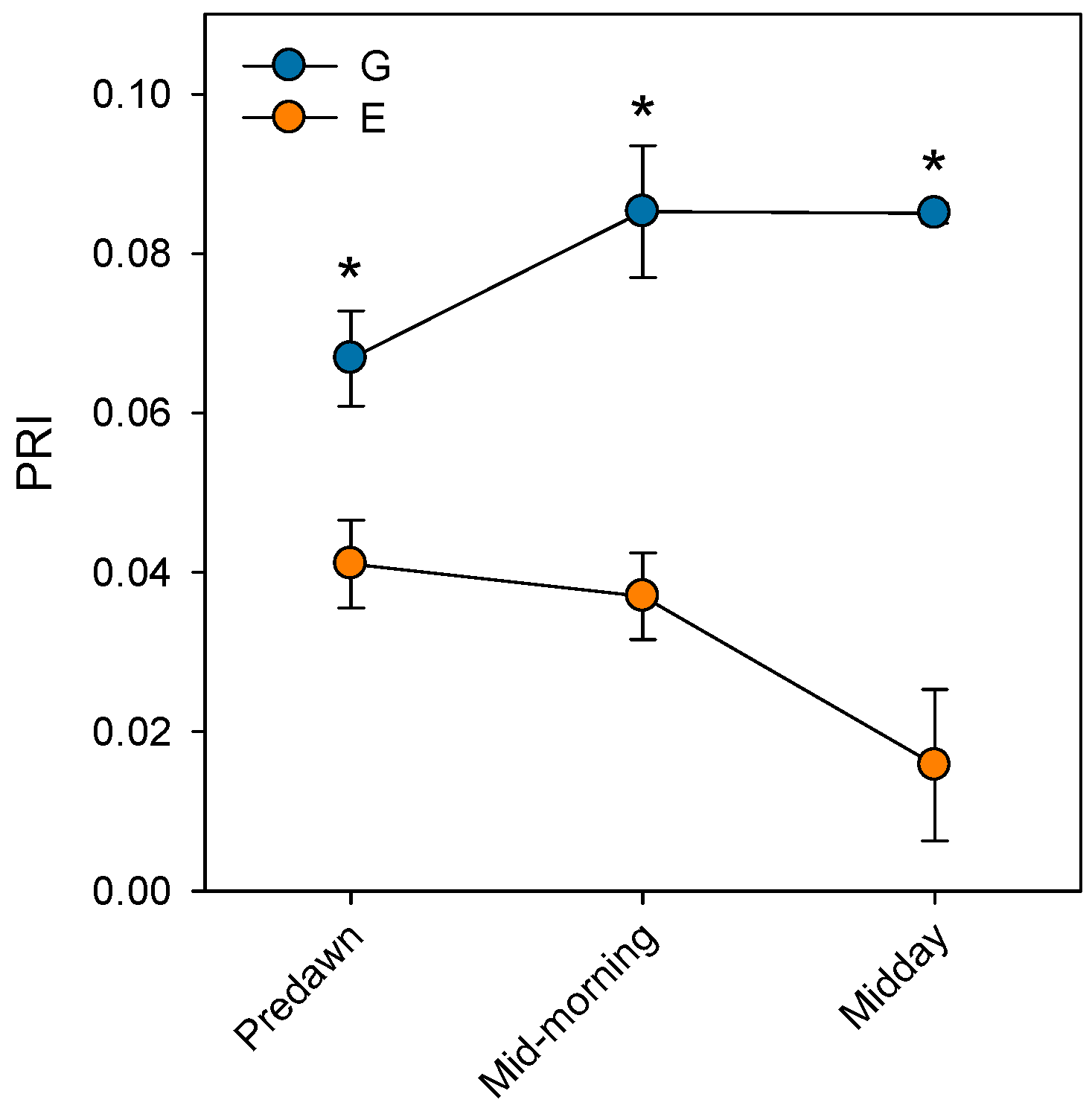
3. Impacts of Atmospheric Drought on Photosystem II (PSII) Performance in Silver Fir
4. Xylem Vulnerability to Embolism Under Soil Water Deficit: What Are the Critical Water Potential Values?
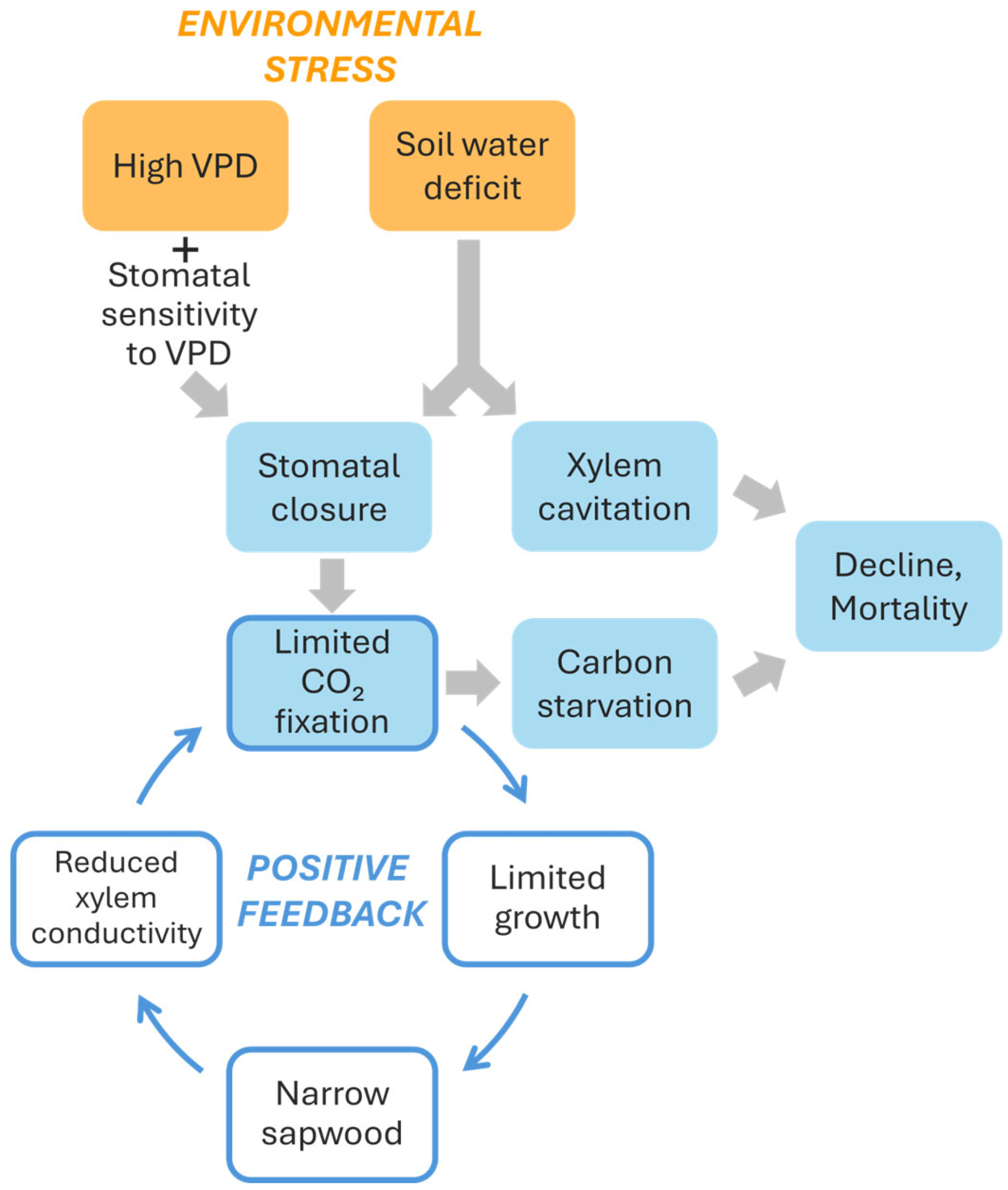
5. A Conceptual Model of Silver Fir Decline Based on High VPD and Soil Water Deficit
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Breshears, D.D.; Allen, C.D. The importance of rapid, disturbance-induced losses in carbon management and sequestration. Glob. Ecol. Biogeogr. 2002, 11, 1–5. [Google Scholar] [CrossRef]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G.; et al. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef]
- van Mantgem, P.J.; Stephenson, N.L. Apparent climatically induced increase of tree mortality rates in a temperate forest. Ecol. Lett. 2007, 10, 909–916. [Google Scholar] [CrossRef]
- Manion, P.D. Evolution of concepts in forest pathology. Phytopathology 2003, 93, 1052–1055. [Google Scholar] [CrossRef]
- Manion, P.D. Tree Disease Concepts; Prentice Hall: Englewood Cliffs, NJ, USA, 1981. [Google Scholar]
- Suarez, M.L.; Ghermandi, L.; Kitzberger, T. Factors predisposing episodic drought-induced tree mortality in Nothofagus-site, climatic sensitivity and growth trends. J. Ecol. 2004, 92, 954–966. [Google Scholar] [CrossRef]
- Bigler, C.; Braker, O.U.; Bugmann, H.; Dobbertin, M.; Rigling, A. Drought as an inciting mortality factor in Scots pine stands of the Valais, Switzerland. Ecosystems 2006, 9, 330–343. [Google Scholar] [CrossRef]
- Frelich, L.E. Forest Dynamics and Disturbance Regimes: Studies from Temperate Evergreen-Deciduous Forests; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- Skelly, J.M.; Innes, J.L. Waldsterben in the forests of Central Europe and Eastern North America: Fantasy or reality? Plant Dis. 1994, 78, 1021–1032. [Google Scholar] [CrossRef]
- Camarero, J.J.; Bigler, C.; Linares, J.C.; Gil-Pelegrín, E. Synergistic effects of past historical logging and drought on the decline of Pyrenean silver fir forests. For. Ecol. Manag. 2011, 262, 759–769. [Google Scholar] [CrossRef]
- Crespo-Antia, J.P.; González de Andrés, E.; Gazol, A.; Camarero, J.J.; Linares, J.C. Tree-Level Climate Sensitivity Reveals Size Effects and Impending Growth Decline in Silver Fir Affected by Dieback. Forests 2024, 15, 999. [Google Scholar] [CrossRef]
- Vejpustková, M.; Čihák, T.; Fišer, P. The Increasing Drought Sensitivity of Silver Fir (Abies alba Mill.) Is Evident in the Last Two Decades. J. For. Sci. 2023, 69, 67–79. [Google Scholar] [CrossRef]
- Dong, P.-B.; Wang, L.-Y.; Wang, L.-J.; Jia, Y.; Li, Z.-H.; Bai, G.; Zhao, R.-M.; Liang, W.; Wang, H.-Y.; Guo, F.-X.; et al. Distributional Response of the Rare and Endangered Tree Species Abies chensiensis to Climate Change in East Asia. Biology 2022, 11, 1659. [Google Scholar] [CrossRef]
- Nelson, K.N.; O’Dean, E.; Knapp, E.E.; Parker, A.J.; Bisbing, S.M. Persistent Yet Vulnerable: Resurvey of an Abies Ecotone Reveals Few Differences but Vulnerability to Climate Change. Ecology 2021, 102, e03525. [Google Scholar] [CrossRef]
- Bledý, M.; Vacek, S.; Brabec, P.; Vacek, Z.; Cukor, J.; Černý, J.; Ševčík, R.; Brynychová, K. Silver Fir (Abies alba Mill.): Review of Ecological Insights, Forest Management Strategies, and Climate Change’s Impact on European Forests. Forests 2024, 15, 998. [Google Scholar] [CrossRef]
- Walder, D.; Krebs, P.; Bugmann, H.; Manetti, M.C.; Pollastrini, M.; Anzillotti, S.; Conedera, M. Silver fir (Abies alba Mill.) is able to thrive and prosper under meso-Mediterranean conditions. For. Ecol. Manag. 2021, 498, 119537. [Google Scholar] [CrossRef]
- Henne, P.D.; Elkin, C.; Colombaroli, D.; Samartin, S.; Bugmann, H.; Heiri, O.; Tinner, W. Impacts of changing climate and land use on vegetation dynamics in a Mediterranean ecosystem: Insights from paleoecology and dynamic modeling. Landsc. Ecol. 2013, 28, 819–833. [Google Scholar] [CrossRef]
- Henne, P.D.; Elkin, C.; Franke, J.; Colombaroli, D.; Calò, C.; La Mantia, T.; Pasta, S.; Conedera, M.; Dermody, O.; Tinner, W. Reviving extinct Mediterranean forest communities may improve ecosystem potential in a warmer future. Front. Ecol. Environ. 2015, 13, 356–362. [Google Scholar] [CrossRef]
- Tinner, W.; Colombaroli, D.; Heiri, O.; Henne, P.D.; Steinacher, M.; Untenecker, J.; Vescovi, E.; Allen, J.R.M.; Carraro, G.; Conedera, M.; et al. The past ecology of Abies alba provides new perspectives on future responses of silver fir forests to global warming. Ecol. Monogr. 2013, 83, 419–439. [Google Scholar] [CrossRef]
- Motta, R.; Garbarino, M.; Berretti, R.; Bono, A.; Curovic, M.; Dukić, V.; Nola, P. Monastic Silviculture Legacies and Current Old-Growthness of Silver Fir (Abies alba) Forests in the Northern Apennines (Italy). Front. For. Glob. Change 2023, 6, 1252462. [Google Scholar] [CrossRef]
- Oliva, J.; Colinas, C. Decline of silver fir (Abies alba Mill.) stands in the Spanish Pyrenees: Role of management, historic dynamics and pathogens. For. Ecol. Manag. 2007, 252, 84–97. [Google Scholar] [CrossRef]
- Puddu, A.; Luisi, N.; Capretti, P.; Santini, A. Environmental factors related to damage by Heterobasidion abietinum in Abies alba forests in Southern Italy. For. Ecol. Manag. 2003, 180, 37–44. [Google Scholar] [CrossRef]
- Tripathy, K.P.; Mukherjee, S.; Mishra, A.K.; Mann, M.E.; Williams, A.P. Climate change will accelerate the high-end risk of compound drought and heatwave events. Proc. Natl. Acad. Sci. USA 2023, 120, e2219825120. [Google Scholar] [CrossRef]
- Hammond, W.M.; Williams, A.P.; Abatzoglou, J.T.; Adams, H.D.; Klein, T.; López, R.; Sáenz-Romero, C.; Hartmann, H.; Breshears, D.D.; Allen, C.D. Global field observations of tree die-off reveal hotter-drought fingerprint for Earth’s forests. Nat. Commun. 2022, 13, 1761. [Google Scholar] [CrossRef] [PubMed]
- Seddon, N. Harnessing the potential of nature-based solutions for mitigating and adapting to climate change. Science 2022, 376, 1410–1416. [Google Scholar] [CrossRef]
- Liu, Q.; Peng, C.; Schneider, R.; Cyr, D.; McDowell, N.G.; Kneeshaw, D. Drought-induced increase in tree mortality and corresponding decrease in the carbon sink capacity of Canada’s boreal forests from 1970 to 2020. Glob. Chang Biol. 2023, 29, 2274–2285. [Google Scholar] [CrossRef]
- Guo, W.; Huang, S.; Liu, L.; Leng, G.; Huang, Q.; Chen, D.; Li, J.; Li, P.; Wang, Y.; Zhu, X.; et al. Global Critical Drought Thresholds of Terrestrial Carbon Sink–Source Transition. Glob. Change Biol. 2025, 31, e70129. [Google Scholar] [CrossRef] [PubMed]
- Gazol, A.; Camarero, J.J. Compound climate events increase tree drought mortality across European forests. Sci. Total Environ. 2022, 816, 151604. [Google Scholar] [CrossRef] [PubMed]
- Grossiord, C.; Buckley, T.N.; Cernusak, L.A.; Novick, K.A.; Poulter, B.; Siegwolf, R.T.W.; Sperry, J.S.; McDowell, N.G. Plant responses to rising vapor pressure deficit. New Phytol. 2020, 226, 1550–1566. [Google Scholar] [CrossRef]
- Griebel, A.; Bennett, L.T.; Metzen, D.; Pendall, E.; Lane, P.N.J.; Arndt, S.K. Trading water for carbon: Maintaining photosynthesis at the cost of increased water loss during high temperatures in a temperate forest. J. Geophys. Res. Biogeosci. 2020, 125, e2019JG005239. [Google Scholar] [CrossRef]
- Sánchez-Salguero, R.; Camarero, J.J.; Gutiérrez, E.; González Rouco, F.; Gazol, A.; Sangüesa-Barreda, G.; Andreu-Hayles, L.; Linares, J.C.; Seftigen, K. Assessing forest vulnerability to climate warming using a process-based model of tree growth: Bad prospects for rear-edges. Glob. Change Biol. 2017, 23, 2705–2719. [Google Scholar] [CrossRef]
- Sheriff, D.W. Stomatal aperture and the sensing of the environment by guard cells. Plant Cell Environ. 1979, 2, 15–22. [Google Scholar] [CrossRef]
- Raschke, K. Stomatal responses to pressure changes and interruptions in the water supply of detached leaves of Zea mays L. Plant Physiol. 1970, 45, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Lange, O.L.; Lösch, R.; Schulze, E.D.; Kappen, L. Responses of stomata to changes in humidity. Planta 1971, 100, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Bunce, J.A. Does transpiration control stomatal responses to water vapour pressure deficit? Plant Cell Environ. 1997, 20, 131–135. [Google Scholar] [CrossRef]
- Bunce, J.A. How do leaf hydraulics limit stomatal conductance at high water vapour pressure deficits? Plant Cell Environ. 2006, 29, 1644–1650. [Google Scholar] [CrossRef]
- Petek-Petrik, A.; Húdoková, H.; Fleischer, P.; Jamnická, G.; Kurjak, D.; Sliacka Konôpková, A.; Petrík, P. The combined effect of branch position, temperature, and VPD on gas exchange and water-use efficiency of Norway spruce. Biol. Plant. 2023, 67, 136–141. [Google Scholar] [CrossRef]
- Slot, M.; Rifai, S.W.; Eze, C.E.; Winter, K. The stomatal response to vapor pressure deficit drives the apparent temperature response of photosynthesis in tropical forests. New Phytol. 2024, 244, 1238–1249. [Google Scholar] [CrossRef]
- Sandford, A.P.; Jarvis, P.G. Stomatal responses to humidity in selected conifers. Tree Physiol. 1986, 2, 89–103. [Google Scholar] [CrossRef]
- Grieu, P.; Guehl, J.M.; Aussenac, G. The effects of soil and atmospheric drought on photosynthesis and stomatal control of gas exchange in three coniferous species. Physiol. Plant. 1988, 73, 97–104. [Google Scholar] [CrossRef]
- Peguero-Pina, J.J.; Flexas, J.; Galmés, J.; Niinemets, Ü.; Sancho-Knapik, D.; Barredo, G.; Villarroya, D.; Gil-Pelegrín, E. Leaf anatomical properties in relation to differences in mesophyll conductance to CO2 and photosynthesis in two related Mediterranean Abies species. Plant Cell Environ. 2012, 35, 2121–2129. [Google Scholar] [CrossRef]
- Peguero-Pina, J.J.; Sancho-Knapik, D.; Flexas, J.; Galmés, J.; Niinemets, Ü.; Gil-Pelegrín, E. Light acclimation of photosynthesis in two closely related firs (Abies pinsapo Boiss. and Abies alba Mill.): The role of leaf anatomy and mesophyll conductance to CO2. Tree Physiol. 2016, 36, 300–310. [Google Scholar] [CrossRef]
- Guehl, J.M.; Aussenac, G.; Bouachrine, J.; Zimmermann, J.R.; Pennes, J.M.; Ferhi, A.; Grieu, P. Sensitivity of leaf gas exchange to atmospheric drought, soil drought, and water-use efficiency in some Mediterranean Abies species. Can. J. For. Res. 1991, 21, 1507–1515. [Google Scholar] [CrossRef]
- Guehl, J.M.; Aussenac, G. Photosynthesis decrease and stomatal control of gas exchange in Abies alba Mill. in response to vapor pressure difference. Plant Physiol. 1987, 83, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Guicherd, P. Water relations of European silver fir (Abies alba Mill.) in two natural stands in the French Alps subject to contrasting climatic conditions. Ann. For. Sci. 1994, 51, 599–611. [Google Scholar] [CrossRef]
- Sancho-Knapik, D.; Mendoza-Herrer, O.; Alonso-Forn, D.; Saz, M.A.; Martín-Sánchez, R.; dos Santos Silva, J.V.; Ogee, J.; Peguero-Pina, J.J.; Gil-Pelegrín, E.; Ferrio, J.P. Vapor pressure deficit constrains transpiration and photosynthesis in holm oak: A comparison of three methods during summer drought. Agric. For. Meteorol. 2022, 327, 109218. [Google Scholar] [CrossRef]
- Peguero-Pina, J.J.; Sisó, S.; Sancho-Knapik, D.; Díaz-Espejo, A.; Flexas, J.; Galmés, J.; Gil-Pelegrín, E. Leaf morphological and physiological adaptations of a deciduous oak (Quercus faginea Lam.) to the Mediterranean climate: A comparison with a closely related temperate species (Quercus robur L.). Tree Physiol. 2016, 36, 287–299. [Google Scholar] [CrossRef]
- Peguero-Pina, J.J.; Sancho-Knapik, D.; Cochard, H.; Barredo, G.; Villarroya, D.; Gil-Pelegrín, E. Hydraulic traits are associated with the distribution range of two closely related Mediterranean firs, Abies alba Mill. and Abies pinsapo Boiss. Tree Physiol. 2011, 31, 1067–1075. [Google Scholar] [CrossRef]
- Peguero-Pina, J.J.; Camarero, J.J.; Abadía, A.; Martín, E.; González-Cascón, E.; Morales, F.; Gil-Pelegrín, E. Physiological performance of silver-fir (Abies alba Mill.) populations under contrasting climates near the south-western distribution limit of the species. Flora 2007, 202, 226–236. [Google Scholar] [CrossRef]
- Konôpková, A.; Pšidová, E.; Kurjak, D.; Stojnić, S.; Petrík, P.; Fleischer, P.; Kučerová, J.; Ježík, M.; Petek, A.; Gömöry, D.; et al. Photosynthetic performance of silver fir (Abies alba) of different origins under suboptimal growing conditions. Funct. Plant Biol. 2020, 47, 1007–1018. [Google Scholar] [CrossRef]
- Inoue, T.; Sunaga, M.; Ito, M.; Yuchen, Q.; Matsushima, Y.; Sakoda, K.; Wataru, Y. Minimizing VPD fluctuations maintains higher stomatal conductance and photosynthesis, resulting in improvement of plant growth in lettuce. Front. Plant Sci. 2021, 12, 646144. [Google Scholar] [CrossRef] [PubMed]
- Demmig-Adams, B.; Adams, W.W. Photoprotection in an ecological context: The remarkable complexity of thermal energy dissipation. New Phytol. 2006, 172, 11–21. [Google Scholar] [CrossRef]
- Hernández, I.; Munné-Bosch, S. Linking phosphorus availability with photooxidative stress in plants. J. Exp. Bot. 2015, 66, 2889–2900. [Google Scholar] [CrossRef] [PubMed]
- Kitao, M.; Harayama, H.; Han, Q.; Agathokleous, E.; Uemura, A.; Furuya, N.; Ishibashi, S. Springtime photoinhibition constrains regeneration of forest floor seedlings of Abies sachalinensis after a removal of canopy trees during winter. Sci. Rep. 2018, 8, 6310. [Google Scholar] [CrossRef]
- Je, S.M.; Kim, S.H.; Woo, S.Y. Responses of the photosynthetic apparatus of Abies koreana to drought under different light conditions. Ecol. Res. 2018, 33, 413–423. [Google Scholar] [CrossRef]
- Jin, C.; Zha, T.; Bourque, C.P.-A.; Liu, P.; Jia, X.; Tian, Y.; Li, X.; Liu, X.; Guo, X.; Xu, M.; et al. Key stress indicators from chlorophyll fluorescence in five desert plant species. Ecol. Indic. 2022, 145, 109679. [Google Scholar] [CrossRef]
- Marozas, V.; Augustaitis, A.; Pivoras, A.; Baumgarten, M.; Mozgeris, G.; Sasnauskienė, J.; Dautarte, A.; Abraitiené, A.; Bicenkiené, S.; Mordas, G.; et al. Comparative analyses of gas exchange characteristics and chlorophyll fluorescence of three dominant tree species during the vegetation season in hemi-boreal zone, Lithuania. J. Agric. Meteorol. 2019, 75, 3–12. [Google Scholar] [CrossRef]
- Urli, M.; Porté, A.J.; Cochard, H.; Guengant, Y.; Burlett, R.; Delzon, S. Xylem embolism threshold for catastrophic hydraulic failure in angiosperm trees. Tree Physiol. 2013, 33, 672–683. [Google Scholar] [CrossRef]
- Maherali, H.; Pockman, W.T.; Jackson, R.B. Adaptive variation in the vulnerability of woody plants to xylem cavitation. Ecology 2004, 85, 2184–2199. [Google Scholar] [CrossRef]
- Tang, Z.; Zhai, B.; Wang, G.; Gessler, A.; Sun, S.; Hu, Z. Elevational variations in stem hydraulic efficiency and safety of Abies fabri. Funct. Ecol. 2023, 37, 2570–2582. [Google Scholar] [CrossRef]
- Cochard, H. Vulnerability of several conifers to air embolism. Tree Physiol. 1992, 11, 73–83. [Google Scholar] [CrossRef]
- Wortemann, R.; Herbette, S.; Barigah, T.S.; Fumanal, B.; Alia, R.; Ducousso, A.; Gomory, D.; Roeckel-Drevet, P.; Cochard, H. Genotypic variability and phenotypic plasticity of cavitation resistance in Fagus sylvatica L. across Europe. Tree Physiol. 2011, 31, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Nourtier, M.; Chanzy, A.; Cailleret, M.; Yinge, X.; Huc, R.; Davi, H. Transpiration of silver fir (Abies alba Mill.) during and after drought in relation to soil properties in a Mediterranean mountain area. Ann. For. Sci. 2014, 71, 683–695. [Google Scholar] [CrossRef]
- McDowell, N.G. Mechanisms linking drought, hydraulics, carbon metabolism, and vegetation mortality. Plant Physiol. 2011, 155, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
- McDowell, N.G.; Beerling, D.J.; Breshears, D.D.; Fisher, R.A.; Raffa, K.F.; Stitt, M. The interdependence of mechanisms underlying climate-driven vegetation mortality. Trends Ecol. Evol. 2011, 26, 523–532. [Google Scholar] [CrossRef]
- Sevanto, S.; McDowell, N.G.; Dickman, L.T.; Pangle, R.; Pockman, W.T. How do trees die? A test of the hydraulic failure and carbon starvation hypotheses. Plant Cell Environ. 2014, 37, 153–161. [Google Scholar] [CrossRef]
- Hajek, P.; Link, R.M.; Nock, C.A.; Bauhus, J.; Gebauer, T.; Gessler, A.; Kovach, K.; Messier, C.; Paquette, A.; Saurer, M.; et al. Mutually inclusive mechanisms of drought-induced tree mortality. Glob. Change Biol. 2022, 28, 3365–3378. [Google Scholar] [CrossRef]
- Sala, A.; Piper, F.; Hoch, G. Physiological mechanisms of drought-induced tree mortality are far from being resolved. New Phytol. 2010, 186, 274–281. [Google Scholar] [CrossRef]
- Rowland, L.; da Costa, A.C.; Galbraith, D.R.; Oliveira, R.S.; Binks, O.J.; Oliveira, A.A.; Pullen, A.M.; Doughty, C.E.; Metcalfe, D.B.; Vasconcelos, S.S.; et al. Death from drought in tropical forests is triggered by hydraulics not carbon starvation. Nature 2015, 528, 119–122. [Google Scholar] [CrossRef]
- Adams, H.D.; Zeppel, M.J.B.; Anderegg, W.R.L.; Hartmann, H.; Landhäusser, S.M.; Tissue, D.T.; Huxman, T.E.; Hudson, P.J.; Franz, T.E.; Allen, C.D.; et al. A multi-species synthesis of physiological mechanisms in drought-induced tree mortality. Nat. Ecol. Evol. 2017, 1, 1285–1291. [Google Scholar] [CrossRef]
- Galiano, L.; Martínez-Vilalta, J.; Lloret, F. Carbon reserves and canopy defoliation determine the recovery of Scots pine 4 yr after a drought episode. New Phytol. 2011, 190, 750–759. [Google Scholar] [CrossRef]
- Camarero, J.J.; Gazol, A.; Sangüesa-Barreda, G.; Oliva, J.; Vicente-Serrano, S.M. To die or not to die: Early warnings of tree dieback in response to a severe drought. J. Ecol. 2015, 103, 44–57. [Google Scholar] [CrossRef]
- Hartmann, H.; Ziegler, W.; Trumbore, S. Lethal drought leads to reduction in nonstructural carbohydrates in Norway spruce tree roots but not in the canopy. Funct. Ecol. 2013, 27, 413–427. [Google Scholar] [CrossRef]
- Mátyás, C.; Beran, F.; Dostál, J.; Čáp, J.; Fulín, M.; Vejpustková, M.; Božič, G.; Balázs, P.; Frýdl, J. Surprising Drought Tolerance of Fir (Abies) Species between Past Climatic Adaptation and Future Projections Reveals New Chances for Adaptive Forest Management. Forests 2021, 12, 821. [Google Scholar] [CrossRef]
- Cailleret, M.; Jansen, S.; Robert, E.M.R.; Desoto, L.; Aakala, T.; Antos, J.A.; Beikircher, B.; Bigler, C.; Bugmann, H.; Caccianiga, M.; et al. A synthesis of radial growth patterns preceding tree mortality. Glob. Change Biol. 2017, 23, 1675–1690. [Google Scholar] [CrossRef]
- Gazol, A.; Camarero, J.J.; Gutiérrez, E.; Popa, I.; Andreu-Hayles, L.; Motta, R.; Nola, P.; Ribas, M.; Sangüesa-Barreda, G.; Urbinati, C.; et al. Distinct effects of climate warming on populations of silver fir (Abies alba) across Europe. J. Biogeogr. 2015, 42, 1150–1162. [Google Scholar] [CrossRef]
- Brodribb, T.J.; Holbrook, N.M.; Zwieniecki, M.A.; Palma, B. Leaf hydraulic capacity in ferns, conifers and angiosperms: Impacts on photosynthetic maxima. New Phytol. 2005, 165, 839–846. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gil-Pelegrín, E.; Peguero-Pina, J.J.; Sancho-Knapik, D.; Arrechea, E.; Camarero, J.J. How Warmer and Drier Conditions Drive Forest Dieback and Tree Death: A Review and Conceptual Model for Silver Fir. Plants 2025, 14, 3308. https://doi.org/10.3390/plants14213308
Gil-Pelegrín E, Peguero-Pina JJ, Sancho-Knapik D, Arrechea E, Camarero JJ. How Warmer and Drier Conditions Drive Forest Dieback and Tree Death: A Review and Conceptual Model for Silver Fir. Plants. 2025; 14(21):3308. https://doi.org/10.3390/plants14213308
Chicago/Turabian StyleGil-Pelegrín, Eustaquio, José Javier Peguero-Pina, Domingo Sancho-Knapik, Enrique Arrechea, and J. Julio Camarero. 2025. "How Warmer and Drier Conditions Drive Forest Dieback and Tree Death: A Review and Conceptual Model for Silver Fir" Plants 14, no. 21: 3308. https://doi.org/10.3390/plants14213308
APA StyleGil-Pelegrín, E., Peguero-Pina, J. J., Sancho-Knapik, D., Arrechea, E., & Camarero, J. J. (2025). How Warmer and Drier Conditions Drive Forest Dieback and Tree Death: A Review and Conceptual Model for Silver Fir. Plants, 14(21), 3308. https://doi.org/10.3390/plants14213308








