Storage-Induced Fruit Breakdown in Cryptocarya alba: Implications for the Conservation of a Keystone Mediterranean Recalcitrant Species
Abstract
1. Introduction
2. Results
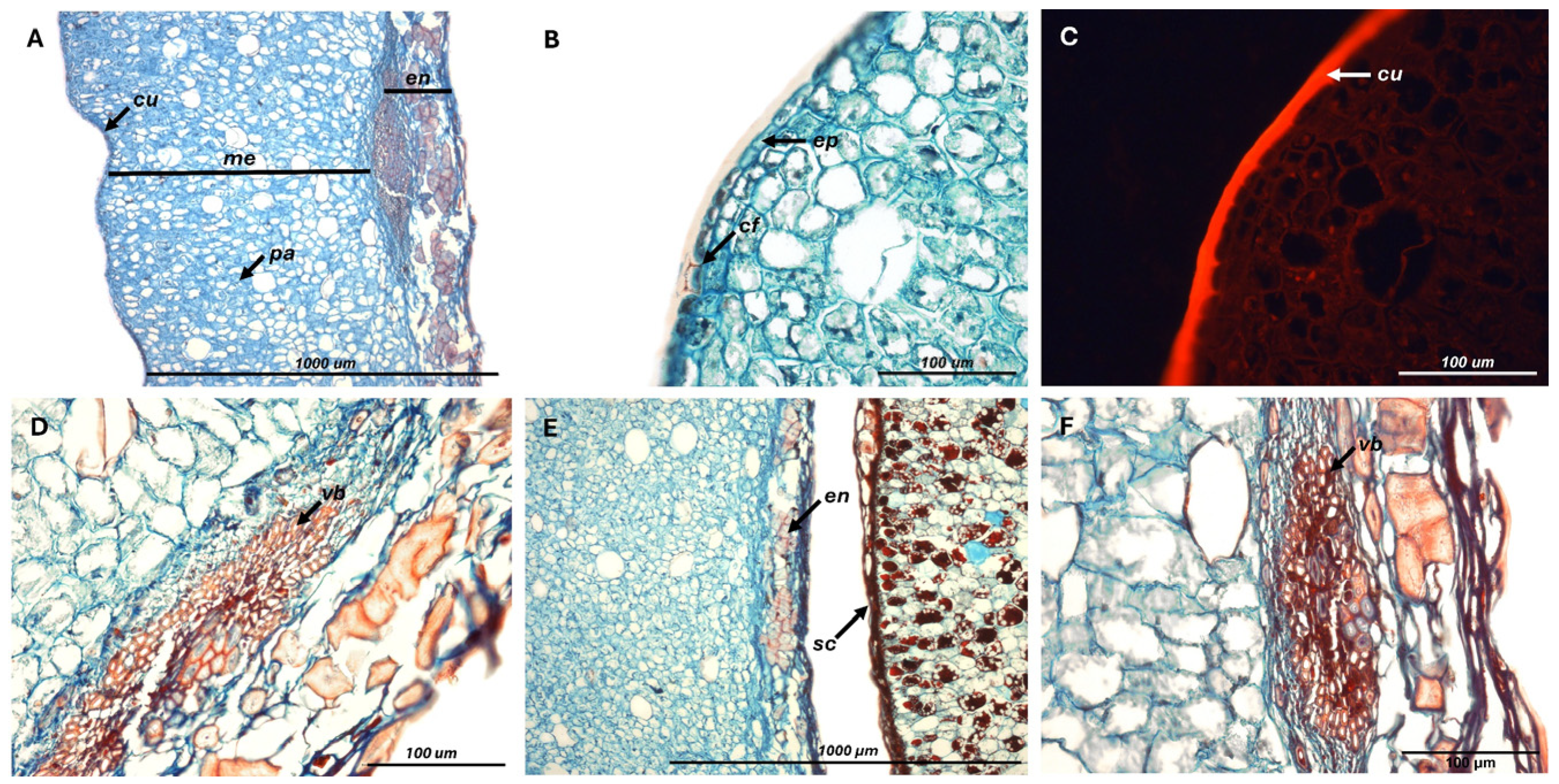
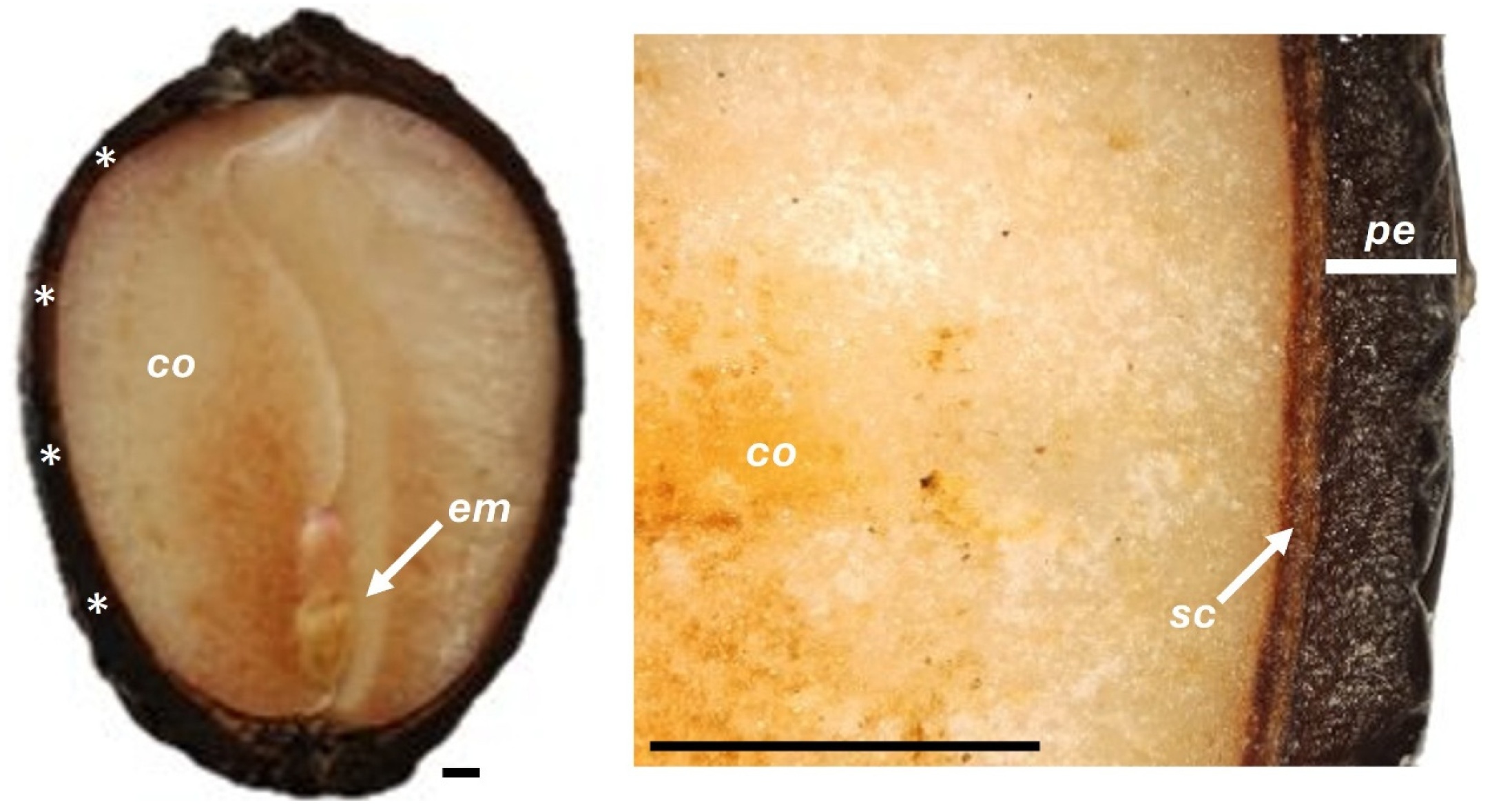
2.1. Fruit Characterization
2.2. Storage-Induced Breakdown
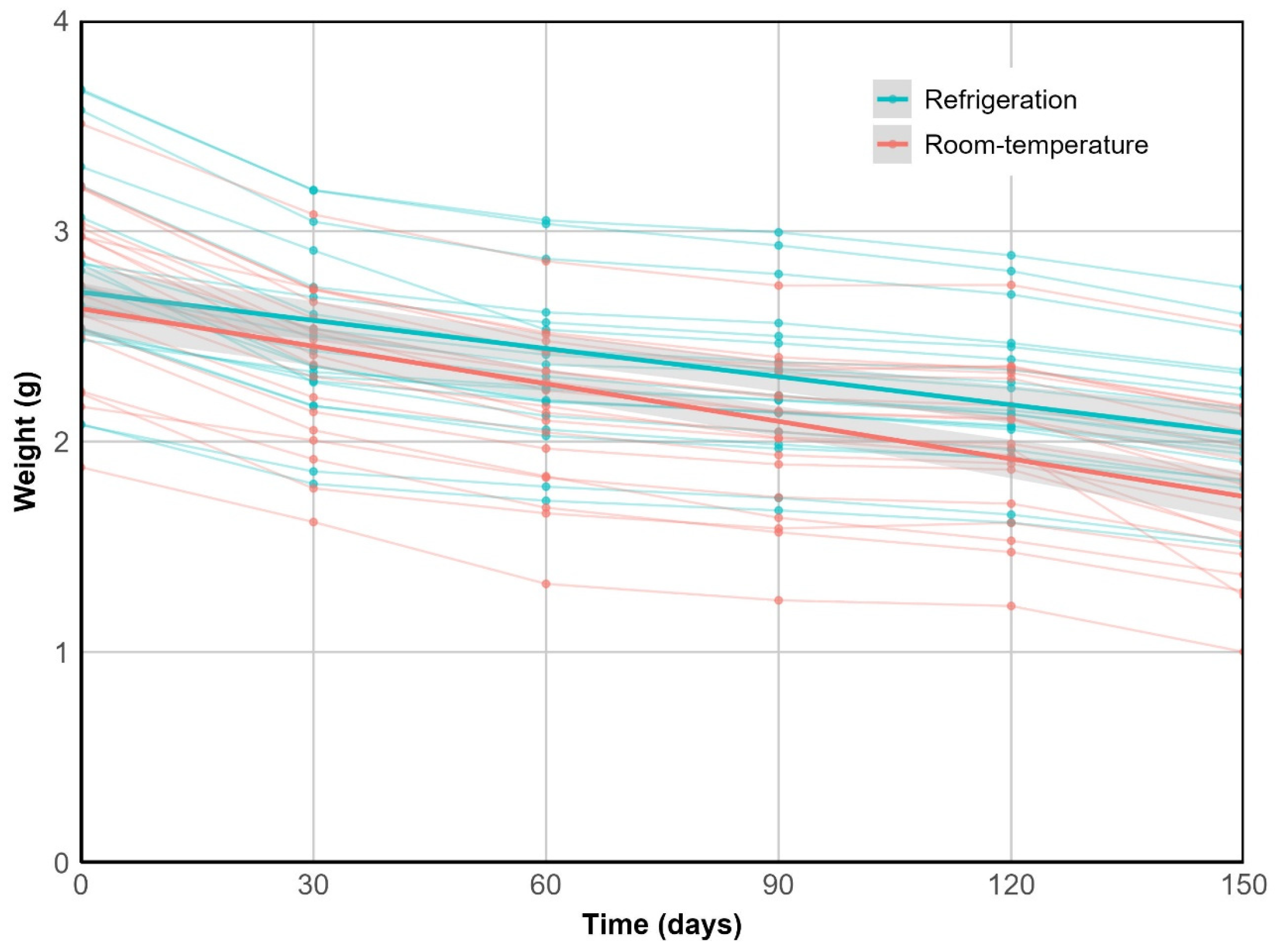
2.2.1. Weight Loss
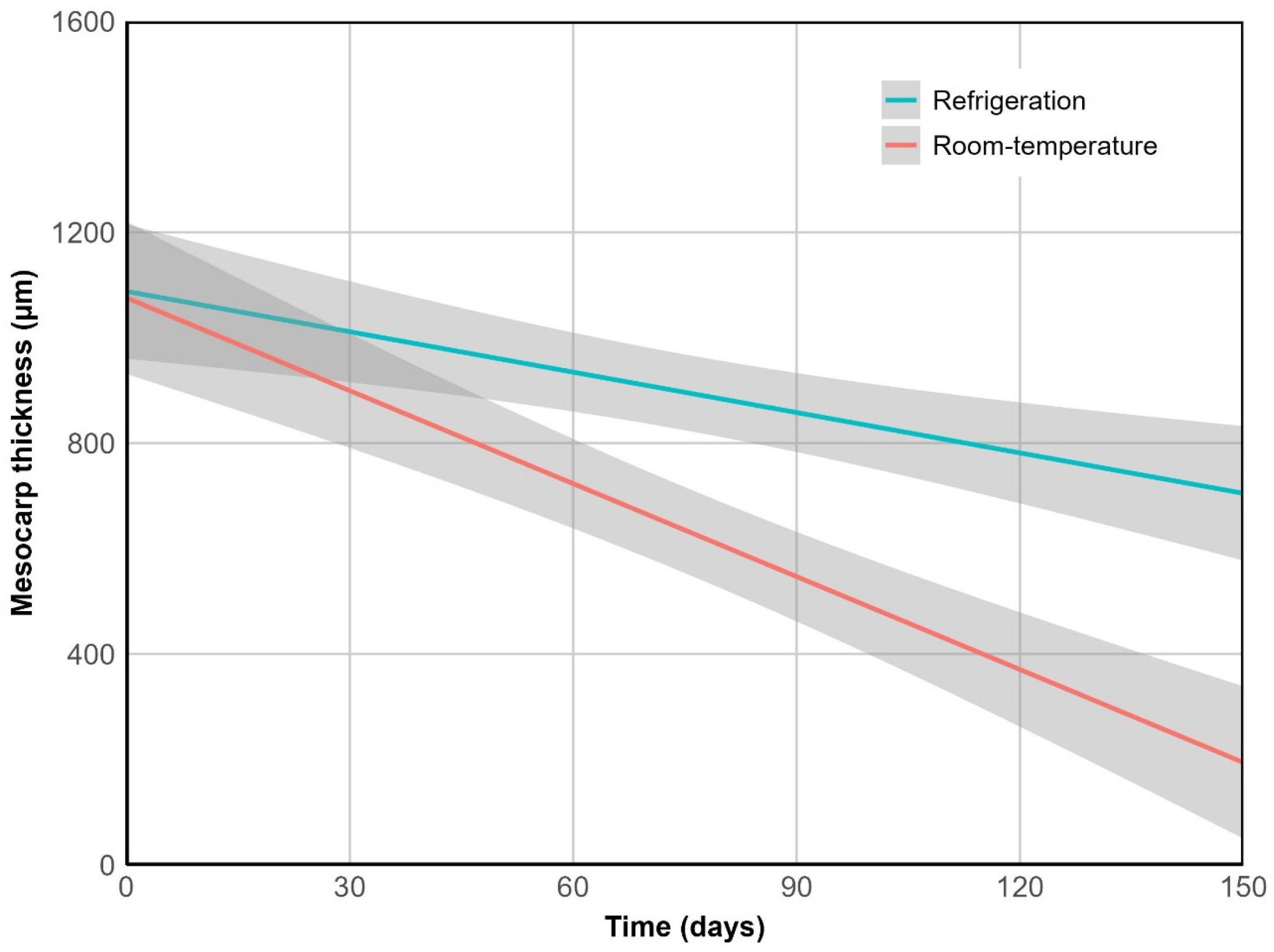
2.2.2. Desiccation
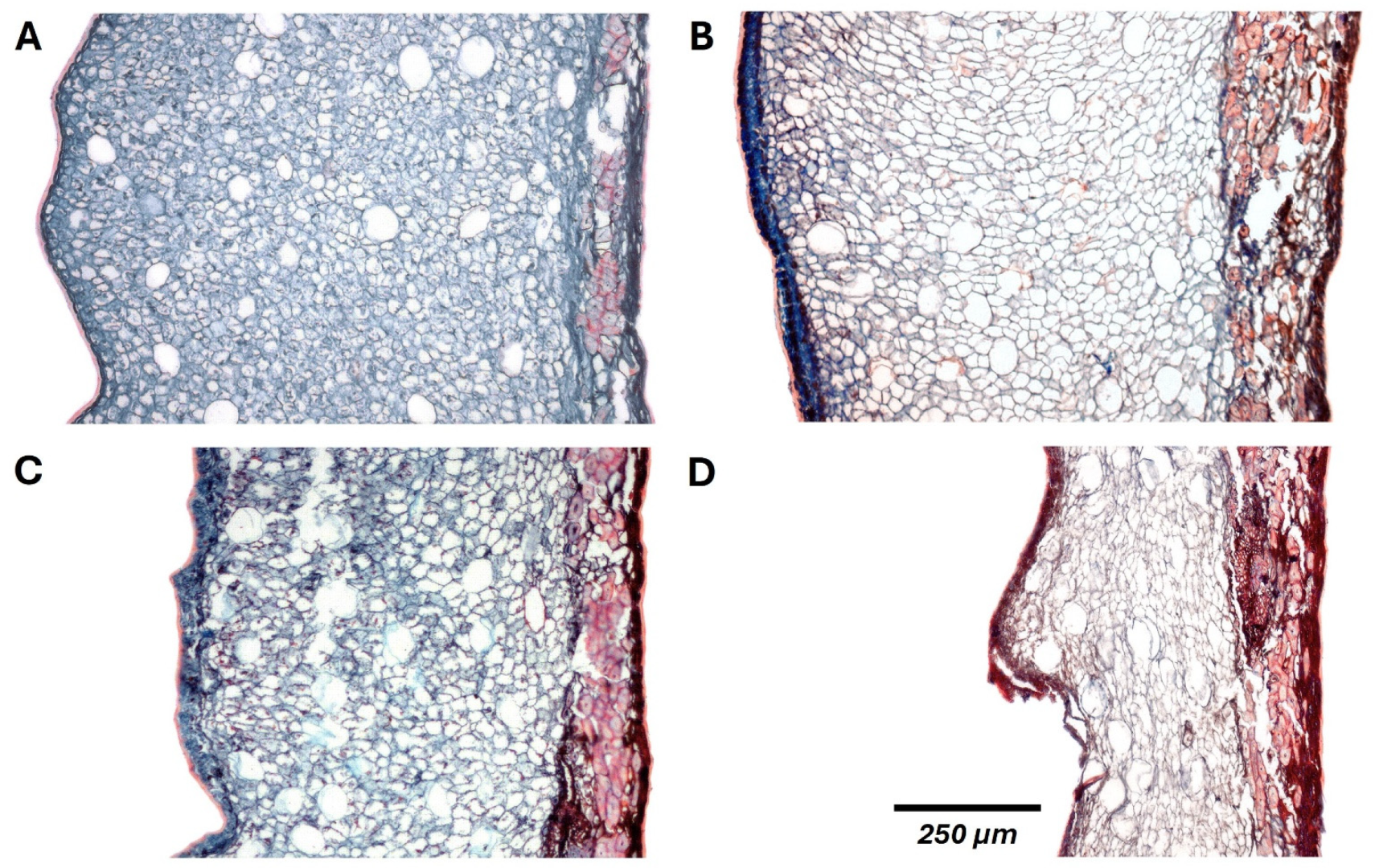
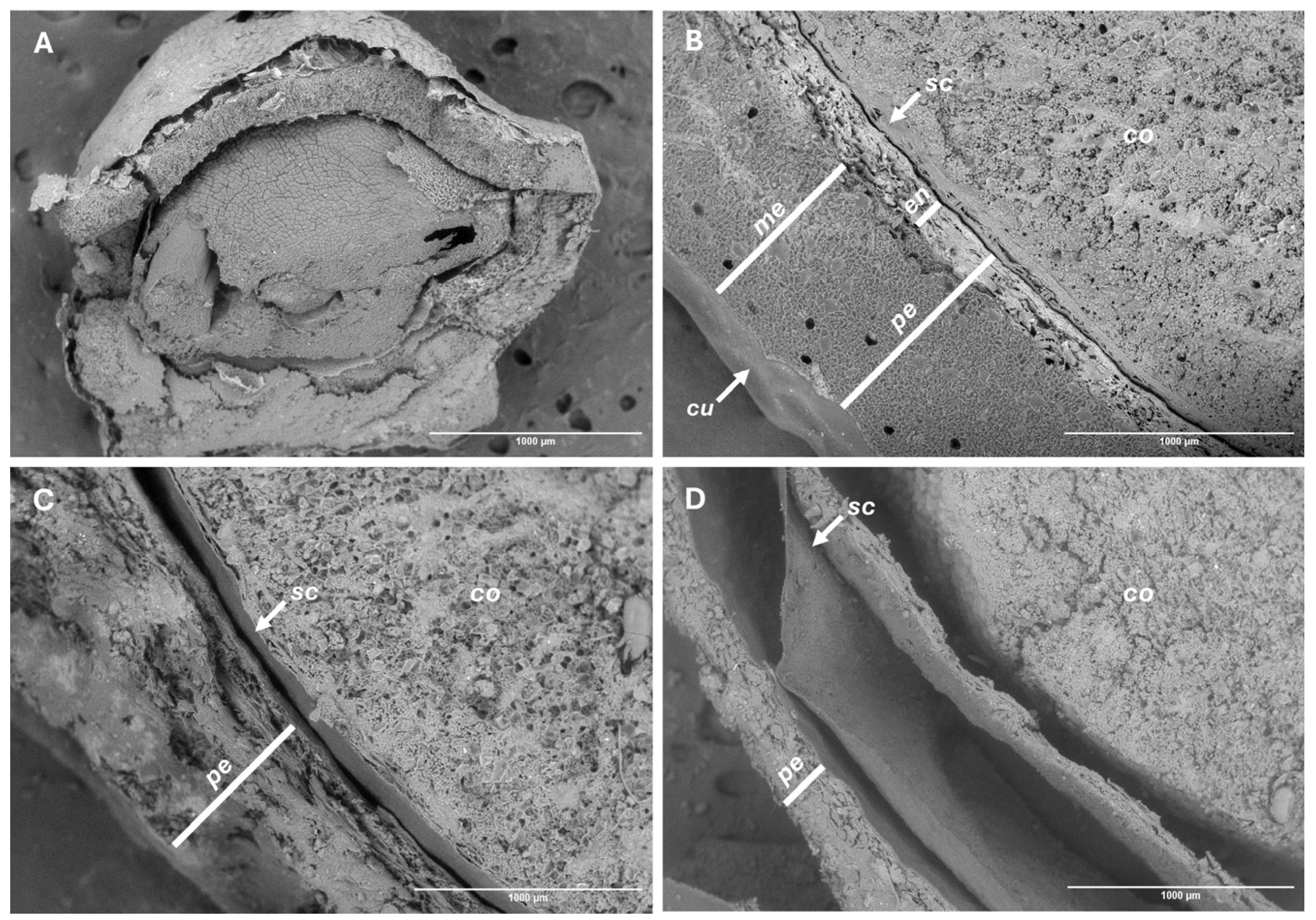
2.2.3. Tissue Degradation
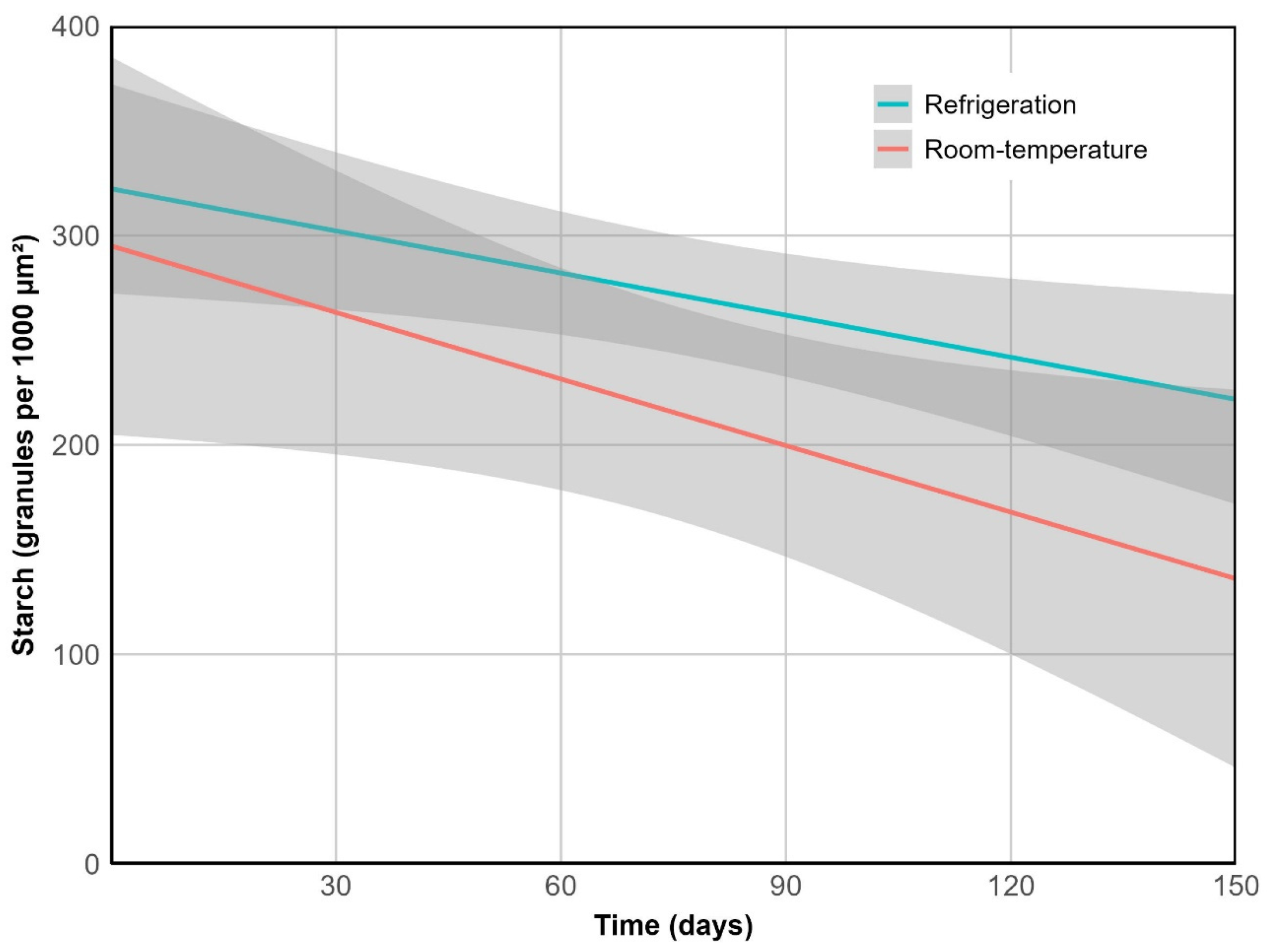
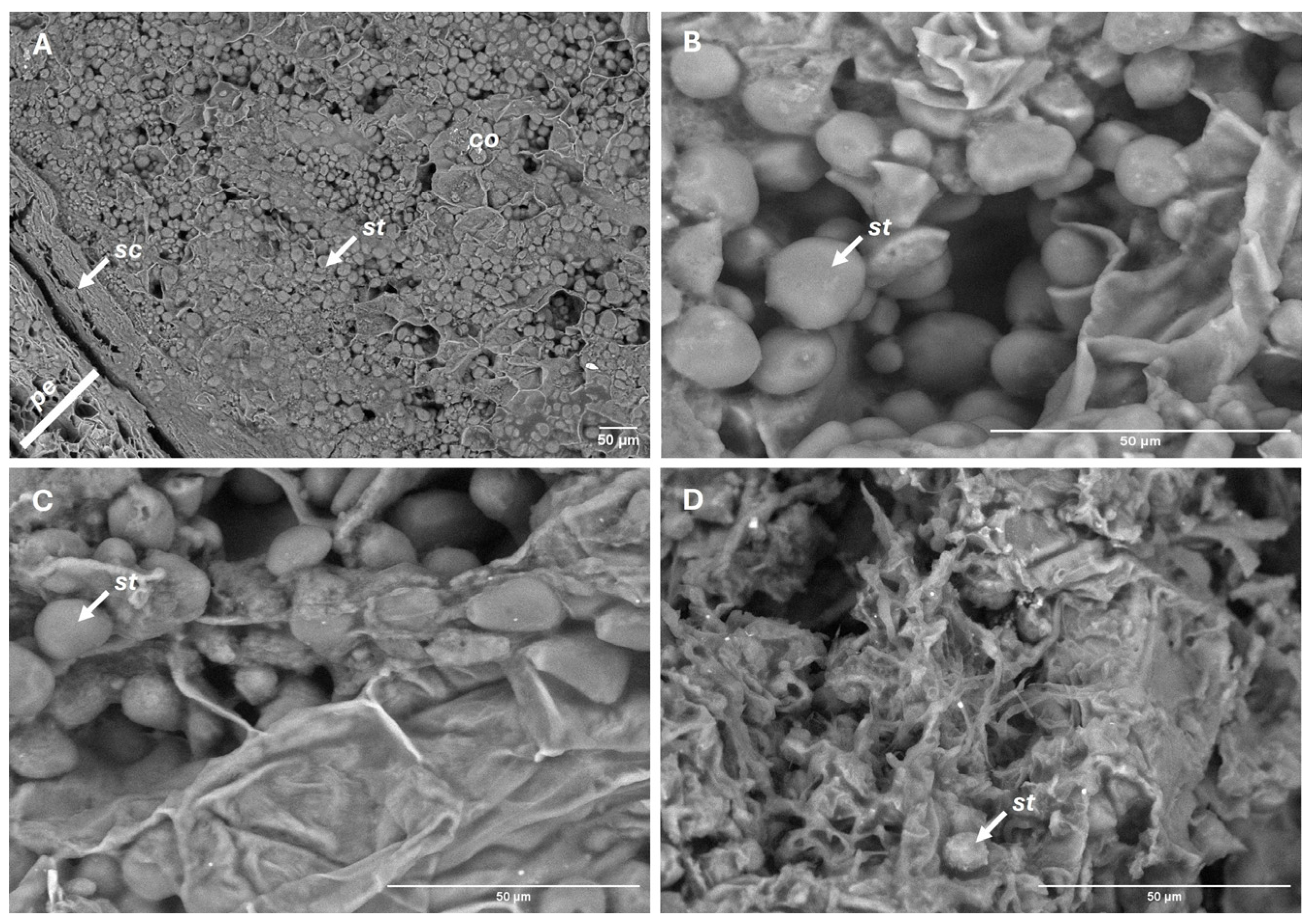
2.2.4. Reserve Depletion
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Physical Analysis
4.3. Histological Analysis
4.4. Ultrastructural Analysis
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AIC | Akaike information criterion |
| BSE | Backscattered electron |
| FAA | Formalin–acetic acid–alcohol |
| LRT | Likelihood ratio test |
| RH | Relative humidity |
| SEM | Scanning electron microscopy |
References
- Underwood, E.C.; Viers, J.H.; Klausmeyer, K.R.; Cox, R.L.; Shaw, M.R. Threats and biodiversity in the mediterranean biome. Divers. Distrib. 2009, 15, 188–197. [Google Scholar] [CrossRef]
- Miranda, A.; Altamirano, A.; Cayuela, L.; Lara, A.; González, M. Native forest loss in the Chilean biodiversity hotspot: Revealing the evidence. Reg. Environ. Change 2017, 17, 285–297. [Google Scholar] [CrossRef]
- Lillo-Robles, F.; Tapia-Gatica, J.; Díaz-Siefer, P.; Moya, H.; Youlton, C.; Celis-Diez, J.L.; Santa-Cruz, J.; Ginocchio, R.; Sauvé, S.; Brykov, V.A.; et al. Which soil Cu pool governs phytotoxicity in field-collected soils contaminated by copper smelting activities in central Chile? Chemosphere 2020, 242, 125176. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Arroyo, M.; Marquet, P.; Marticorena, C.; Cavieres, L.; Squeo, F.; Simonetti, J.; Rozzi, R.; Massardo, F. El hotspot chileno, prioridad mundial para la conservación. In Diversidad de Chile: Patrimonios y Desafíos; Comisión Nacional del Medio Ambiente, Ed.; Ministerio del Medio Ambiente de Chile: Santiago, Chile, 2006; pp. 94–97. [Google Scholar]
- Figueroa, J.A.; Jaksic, F.M. Latencia y banco de semillas en plantas de la región mediterránea de Chile central. Rev. Chil. Hist. Nat. 2004, 77, 201–215. [Google Scholar] [CrossRef]
- Kremer, K.N.; Promis, Á.A.; Mancilla, G.; Magni, C.R. Leaf litter and irrigation can increase seed germination and early seedling survival of the recalcitrant-seeded tree Beilschmiedia miersii. Austral Ecol. 2019, 44, 86–94. [Google Scholar] [CrossRef]
- Magni, C.R.; Saavedra, N.; Espinoza, S.E.; Yáñez, M.A.; Quiroz, I.; Faúndez, Á.; Grez, I.; Martinez-Herrera, E. The recruitment of the recalcitrant-seeded Cryptocarya alba (Mol.) Looser, established via direct seeding is mainly affected by the seed source and forest cover. Plants 2022, 11, 2918. [Google Scholar] [CrossRef]
- Berjak, P.; Pammenter, N.W. Implications of the lack of desiccation tolerance in recalcitrant seeds. Front. Plant Sci. 2013, 4, 478. [Google Scholar] [CrossRef]
- Walters, C.; Berjak, P.; Pammenter, N.; Kennedy, K.; Raven, P. Preservation of recalcitrant seeds. Science 2013, 339, 915–916. [Google Scholar] [CrossRef] [PubMed]
- Faúndez, Á.; Magni, C.R.; Martínez-Herrera, E.; Espinoza, S.; Vaswani, S.; Yañez, M.A.; Gréz, I.; Seguel, O.; Abarca-Rojas, B.; Quiroz, I. Effect of the soil matric potential on the germination capacity of Prosopis chilensis, Quillaja saponaria and Cryptocarya alba from contrasting geographical origins. Plants 2022, 11, 2963. [Google Scholar] [CrossRef] [PubMed]
- Wyse, S.V.; Dickie, J.B.; Willis, K.J. Seed banking not an option for many threatened plants. Nat. Plants 2018, 4, 848–850. [Google Scholar] [CrossRef]
- Pritchard, H.W.; Sershen; Tsan, F.Y.; Wen, B.; Jaganathan, G.K.; Calvi, G.; Pence, V.C.; Mattana, E.; Ferraz, I.D.K.; Seal, C.E. Regeneration in recalcitrant-seeded species and risks from climate change. In Plant Regeneration from Seeds; Baskin, C.C., Baskin, J.M., Eds.; Elsevier: New York, NY, USA, 2022; pp. 259–273. [Google Scholar] [CrossRef]
- Delpiano, C.A.; Vargas, S.; Ovalle, J.F.; Cáceres, C.; Zorondo-Rodríguez, F.; Miranda, A.; Pohl, N.; Rojas, C.; Squeo, F.A. Unveiling emerging interdisciplinary research challenges in the highly threatened sclerophyllous forests of central Chile. Rev. Chil. Hist. Nat. 2024, 97, 7. [Google Scholar] [CrossRef]
- Pedrini, S.; Dixon, K.W. International principles and standards for native seeds in ecological restoration. Restor. Ecol. 2020, 28, 286–303. [Google Scholar] [CrossRef]
- Fernández, A.; León-Lobos, P.; Contreras, S.; Ovalle, J.F.; Sershen; Van Der Walt, K.; Ballesteros, D. The potential impacts of climate change on ex situ conservation options for recalcitrant-seeded species. Front. For. Glob. Change 2023, 6, 1110431. [Google Scholar] [CrossRef]
- Xia, K.; Daws, M.I.; Stuppy, W.; Zhou, Z.-K.; Pritchard, H.W. Rates of water loss and uptake in recalcitrant fruits of Quercus species are determined by pericarp anatomy. PLoS ONE 2012, 7, e47368. [Google Scholar] [CrossRef]
- Wyse, S.V.; Dickie, J.B. Taxonomic affinity, habitat and seed mass strongly predict seed desiccation response: A boosted regression trees analysis based on 17,539 species. Ann. Bot. 2018, 121, 71–83. [Google Scholar] [CrossRef]
- Salvador, H.F.; Mazzottini-dos-Santos, H.C.; Dias, D.S.; Azevedo, A.M.; Lopes, P.S.N.; Nunes, Y.R.F.; Ribeiro, L.M. The dynamics of Mauritia flexuosa (Arecaceae) recalcitrant seed banks reveal control of their persistence in marsh environments. For. Ecol. Manag. 2022, 511, 120155. [Google Scholar] [CrossRef]
- Ley-López, J.M.; Wawrzyniak, M.K.; Chacón-Madrigal, E.; Chmielarz, P. Seed traits and tropical arboreal species conservation: A case study of a highly diverse tropical humid forest region in Southern Costa Rica. Biodivers. Conserv. 2023, 32, 1573–1590. [Google Scholar] [CrossRef]
- Lah, N.H.C.; El Enshasy, H.A.; Mediani, A.; Azizan, K.A.; Aizat, W.M.; Tan, J.K.; Afzan, A.; Noor, N.M.; Rohani, E.R. An insight into the behaviour of recalcitrant seeds by understanding their molecular changes upon desiccation and low temperature. Agronomy 2023, 13, 2099. [Google Scholar] [CrossRef]
- Rossetti, C.; Rosa, C.P.D.; Pagel, G.D.O.; Aumonde, T.Z.; Tunes, L.V.M. Challenges for storing recalcitrant seeds. Colloq. Agrar. 2023, 19, 352–362. [Google Scholar] [CrossRef]
- Luebert, F.; Pliscoff, P. Sinopsis Bioclimática y Vegetacional de Chile, 2nd ed.; Editorial Universitaria: Santiago, Chile, 2018; p. 384. [Google Scholar]
- Gipoulou-Zúñiga, T.; Rojas-Badilla, M.; LeQuesne, C.; Rozas, V. Endemic threatened tree species in the Mediterranean forests of central Chile are highly sensitive to ENSO-driven water availability and drought. For. Ecosyst. 2025, 13, 100324. [Google Scholar] [CrossRef]
- Gutiérrez Caro, B. Criterios prácticos para la recolección y almacenamiento de semillas forestales en programas de conservación, restauración y mejoramiento genético. Cienc. Invest. For. 2025, 31, 113–123. [Google Scholar] [CrossRef]
- Vita, A. Reforestación por Siembra Directa con Quillay (Quillaja saponaria Mol.) y Peumo (Cryptocarya alba (Mol.) Looser). Bachelor’s Thesis, Universidad de Chile, Santiago, Chile, 1966. [Google Scholar]
- Ramírez, B. Factores que Afectan la Germinación y la Producción de Plantas de Cryptocarya alba (Mol.) Looser. Bachelor’s Thesis, Universidad de Chile, Santiago, Chile, 1997. [Google Scholar]
- Doll, U. Domesticación de Especies Nativas Ornamentales de Potencial uso Industrial (FIA-PI-C-1996-1-F-007); Fundación para la Innovación Agraria: Talca, Chile, 2000; p. 207. [Google Scholar]
- Rodríguez, M.; Aguilera, M. Antecedentes silviculturales del peumo. In Recopilación de Experiencias Silvícolas en el “Bosque nativo Maulino”; Aguilera, M., Benavides, G., Eds.; Corporación Nacional Forestal: Santiago, Chile, 2005; pp. 121–127. [Google Scholar]
- Vogel, H.; Razmilic, I.; San Martín, J.; Doll, U.; González, B. Plantas Medicinales Chilenas: Experiencias de Domesticación y Cultivo de Boldo, Matico, Bailahuén, Canelo, Peumo y Maqui, 2nd ed.; Universidad de Talca: Talca, Chile, 2008; p. 193. [Google Scholar]
- Rodríguez-Cerda, L.; Guedes, L.M.; Torres, S.; Gavilán, E.; Aguilera, N. Phenolic antioxidant protection in the initial growth of Cryptocarya alba: Two different responses against two invasive Fabaceae. Plants 2023, 12, 3584. [Google Scholar] [CrossRef]
- Valdenegro, M.; Bernales, M.; Knox, M.; Vinet, R.; Caballero, E.; Ayala-Raso, A.; Kučerová, D.; Kumar, R.; Viktorová, J.; Ruml, T.; et al. Characterization of fruit development, antioxidant capacity, and potential vasoprotective action of peumo (Cryptocarya alba), a native fruit of Chile. Antioxidants 2021, 10, 1997. [Google Scholar] [CrossRef]
- Bustamante, R.O.; Walkowiak, A.; Henríquez, C.A.; Serey, I. Bird frugivory and the fate of seeds of Cryptocarya alba (Lauraceae) in the Chilean matorral. Rev. Chil. Hist. Nat. 1996, 69, 357–363. [Google Scholar]
- Chacón, P. Efecto del Tamaño de la Semilla y del Pericarpio Sobre el Reclutamiento y Biomasa de Plántulas en Cryptocarya alba (Mol.) Looser (Lauraceae) en un año Lluvioso y en un año seco Simulados Experimentalmente. Master’s Thesis, Universidad de Chile, Santiago, Chile, 1998. [Google Scholar]
- Chacón, P.; Bustamante, R.O. The effects of seed size and pericarp on seedling recruitment and biomass in Cryptocarya alba (Lauraceae) under two contrasting moisture regimes. Plant Ecol. 2001, 152, 137–144. [Google Scholar] [CrossRef]
- Figueroa, C.; Ríos, D.; Fuentes, L.; Valdenegro, M.; Martínez, J.P.; Franco, W.; Vinet, R.; Sáez, F.; Ayala, A.; Paredes, K.; et al. Caracterización del Potencial Saludable y Agroalimentario de Frutos de Especies Arbóreas Nativas de la zona Centro sur del país (Proyecto CONAF 064/2011); CONAF: Santiago, Chile, 2012; p. 60. [Google Scholar]
- Benedetti, S. Monografía de Peumo Cryptocarya alba (Mol.) Looser; Instituto Forestal: Santiago, Chile, 2012; p. 77. [Google Scholar]
- Chung, P.; Gutiérrez, B.; Benedetti, S. Variabilidad morfológica de frutos de peumo (Cryptocarya alba (Mol.) Looser) de distintas localidades de la región del Bio Bio. Cienc. Invest. For. 2017, 23, 7–18. [Google Scholar] [CrossRef]
- Bonjovani, M.R.; Barbedo, C.J. Respiration and deterioration of Inga vera ssp. affinis embryos stored at different temperatures. J. Seed Sci. 2019, 41, 44–53. [Google Scholar] [CrossRef]
- Tonetti, O.A.O.; Pereira, W.V.S.; José, A.C.; Faria, J.M.R. Physiological and cellular changes of stored Cryptocarya aschersoniana Mez. seeds. Floresta Ambient. 2021, 28, e20200067. [Google Scholar] [CrossRef]
- Wawrzyniak, M.K.; Kalemba, E.M.; Wyka, T.P.; Chmielarz, P. Changes in reserve materials deposited in cotyledons of pedunculate oak (Quercus robur L.) seeds during 18 months of storage. Forests 2022, 13, 2142. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- International Seed Testing Association (ISTA). International Rules for Seed Testing; ISTA: Bassersdorf, Switzerland, 2016; p. 320. [Google Scholar]
- Johansen, D.A. Plant Microtechnique; McGraw-Hill: New York, NY, USA, 1940; p. 523. [Google Scholar]
- Singh, D.; Mathur, S.B. Histopathology of Seed-Borne Infections; CRC Press: Boca Raton, FL, USA, 2004; p. 296. [Google Scholar]
- Santa Cruz, J.; Calbucheo, D.; Valdebenito, S.; Cáceres, C.; Castillo, P.; Aguilar, M.; Hernández, I.; Allendes, H.; Vidal, K.; Peñaloza, P. Crushed, Squeezed, or Pressed? How Extraction Methods Influence Sap Analysis. Preprint. Available online: https://www.researchsquare.com/article/rs-6969389/v2 (accessed on 13 September 2025).
- Valdebenito, S.; Rubio, A.; Moller, A.; Santa Cruz, J.; Castillo, P.; Providell, M.L.; Cáceres, C.; Calbucheo, D.; Hernández, I.; Peñaloza, P. Histological and immunolabeling techniques in Arabidopsis thaliana: A practical guide and standardization roadmap. Agronomy 2025, 15, 2357. [Google Scholar] [CrossRef]
- Kiernan, J.A. Histological and Histochemical Methods: Theory and Practice, 5th ed.; Scion: Banbury, UK, 2015; p. 571. [Google Scholar]
- Criswell, S.; Gaylord, B.; Pitzer, C.R. Histological methods for plant tissues. J. Histotechnol. 2025, 48, 58–67. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darricarrere, V.; Santa Cruz, J.; Calbucheo, D.; Valdebenito, S.; Providell, M.; Cisternas, M.; Muena, V.; Peñaloza, P. Storage-Induced Fruit Breakdown in Cryptocarya alba: Implications for the Conservation of a Keystone Mediterranean Recalcitrant Species. Plants 2025, 14, 3307. https://doi.org/10.3390/plants14213307
Darricarrere V, Santa Cruz J, Calbucheo D, Valdebenito S, Providell M, Cisternas M, Muena V, Peñaloza P. Storage-Induced Fruit Breakdown in Cryptocarya alba: Implications for the Conservation of a Keystone Mediterranean Recalcitrant Species. Plants. 2025; 14(21):3307. https://doi.org/10.3390/plants14213307
Chicago/Turabian StyleDarricarrere, Viviana, Javier Santa Cruz, Diego Calbucheo, Samuel Valdebenito, Mayra Providell, Mauricio Cisternas, Victoria Muena, and Patricia Peñaloza. 2025. "Storage-Induced Fruit Breakdown in Cryptocarya alba: Implications for the Conservation of a Keystone Mediterranean Recalcitrant Species" Plants 14, no. 21: 3307. https://doi.org/10.3390/plants14213307
APA StyleDarricarrere, V., Santa Cruz, J., Calbucheo, D., Valdebenito, S., Providell, M., Cisternas, M., Muena, V., & Peñaloza, P. (2025). Storage-Induced Fruit Breakdown in Cryptocarya alba: Implications for the Conservation of a Keystone Mediterranean Recalcitrant Species. Plants, 14(21), 3307. https://doi.org/10.3390/plants14213307






