Integration of Transcriptome, miRNA-Omics, and Hormone Metabolism Analysis Reveals the Regulatory Network of Camellia drupifera Fruit Maturation
Abstract
1. Introduction
2. Results
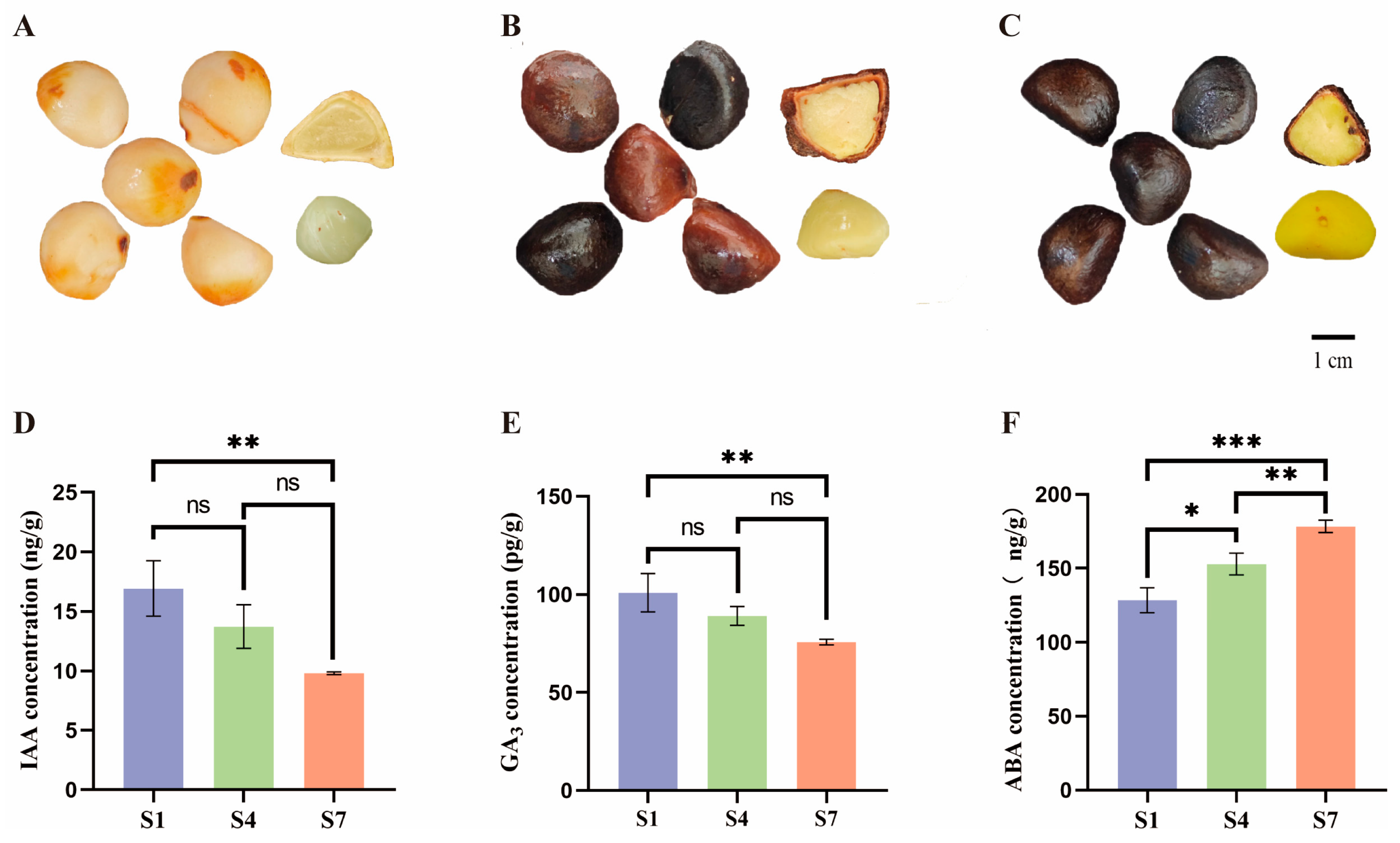
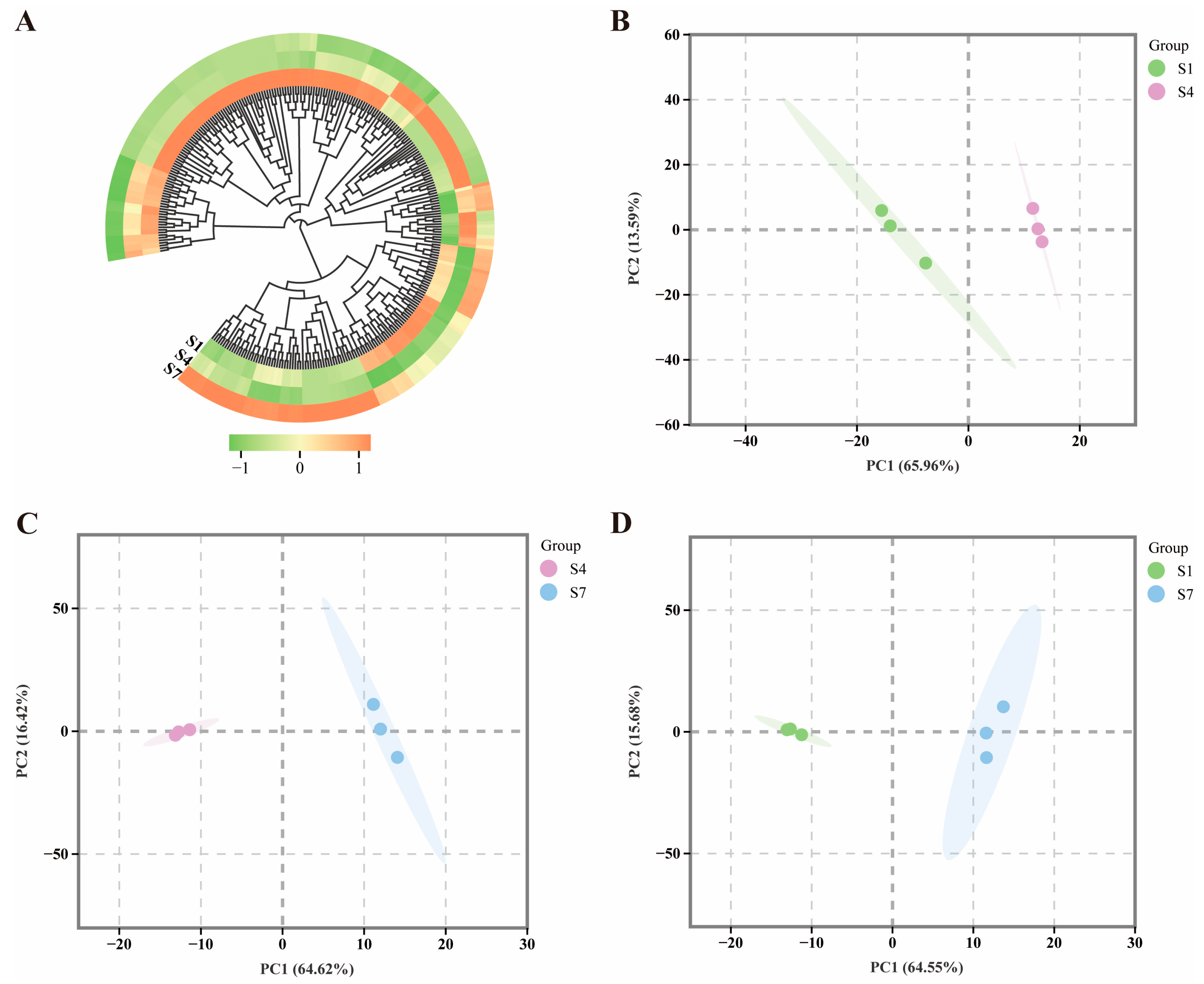
2.1. Analysis of Hormone Levels at Different Periods During the Ripening Process
2.2. Comparative Analysis of Genes Related to Plant Hormone Signal Transduction
2.3. Comparative Analysis of microRNAs Related to Plant Hormone Signal Transduction
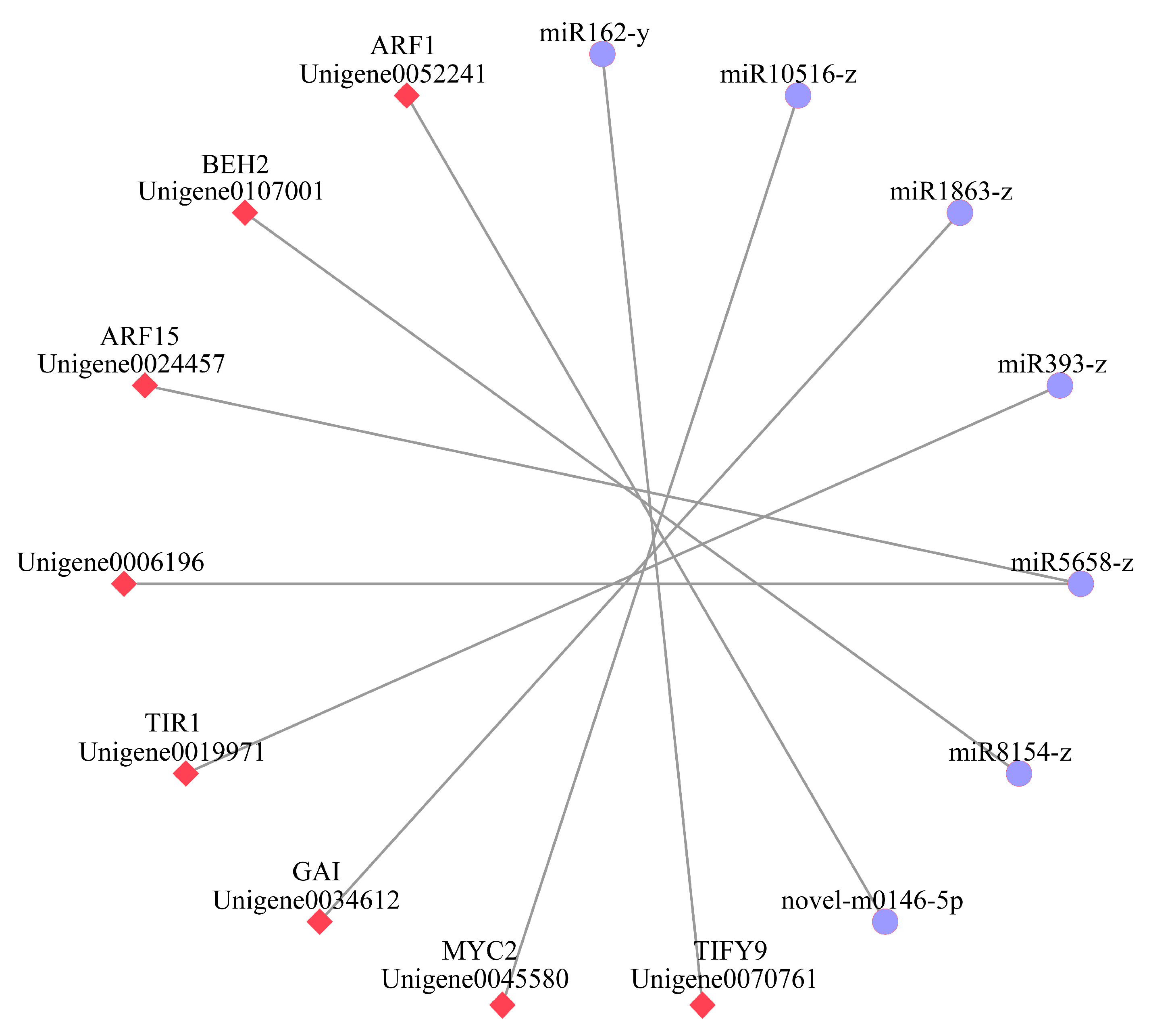
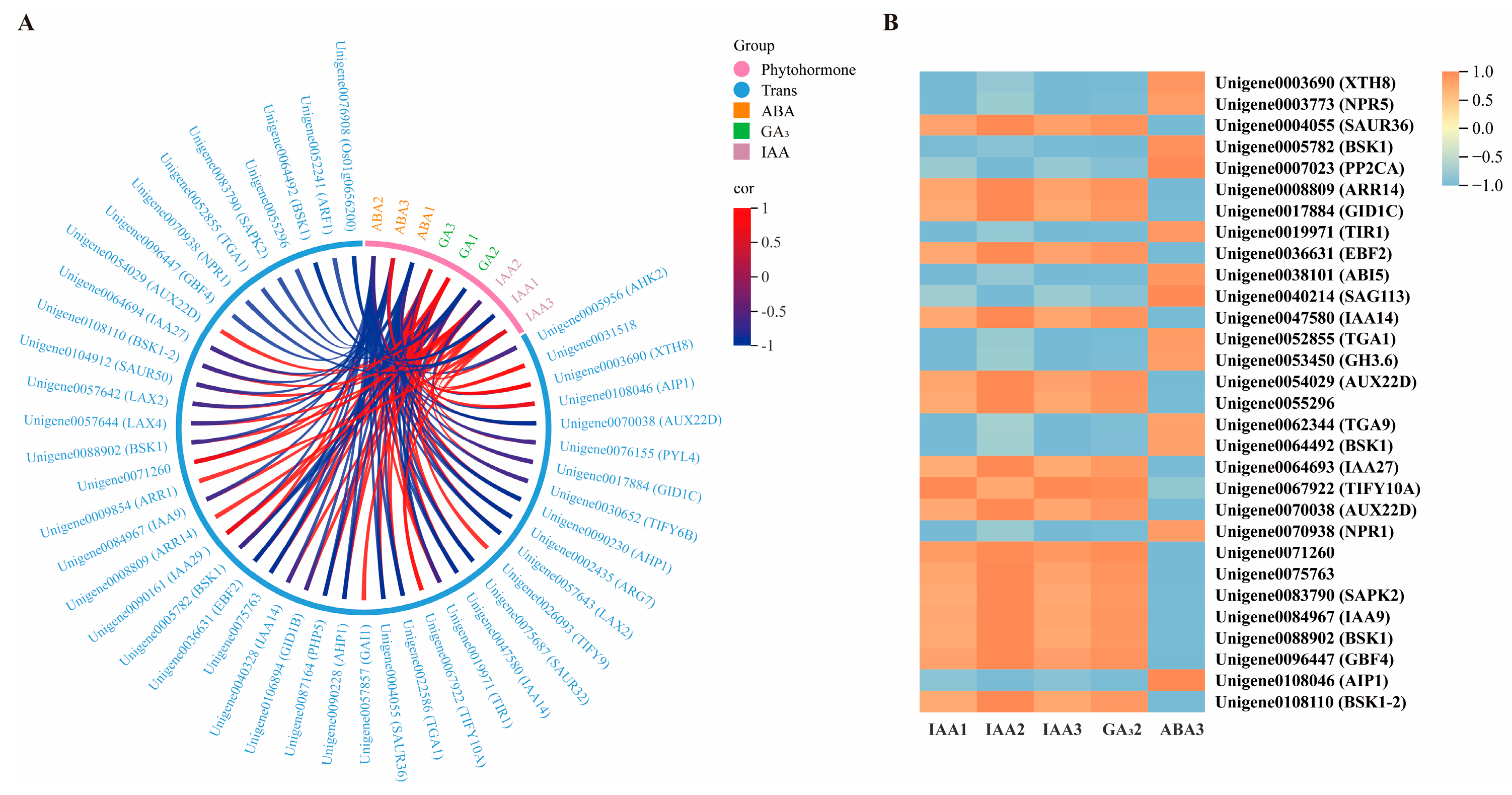
2.4. Correlation Analysis of Differential Genes and Plant Hormone Content
2.5. Analysis of Plant Hormone Signal Transduction Pathways in C. drupifera Fruit
3. Discussion
3.1. Changes in the Hormone Content
3.2. Changes in Oil Content and Hormone Content
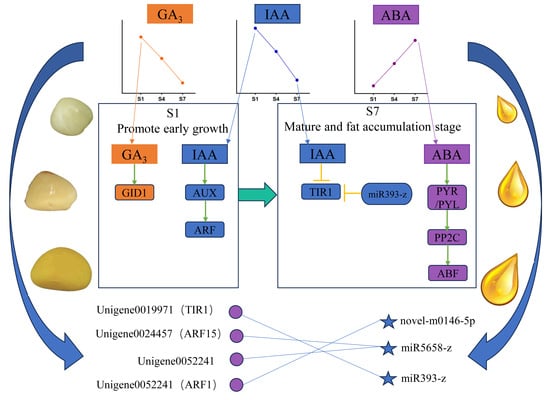
3.3. Combined Analysis of mRNA and microRNA in the IAA Pathway
3.4. Combined Analysis of mRNA and microRNA in Other Hormone Pathways
4. Materials and Methods
4.1. Plant Material
4.2. Determination of Endogenous Hormones in C. drupifera Fruits at Different Stages
4.3. RNA Extraction, Library Construction, and Sequencing
4.4. Construction and Sequencing of miRNA Libraries
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, F.; Feng, L.; Lin, P.; Jia, J.; Gao, L. Chromosome-Scale Genome Assembly of Oil-Tea Tree Camellia crapnelliana. Sci. Data 2024, 11, 599. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, Y.; Gong, W.; Zhao, G.; Xiao, S.; Lin, H.; Li, Y.; Liao, Z.; Zhang, S.; Hu, G.; et al. The Tetraploid Camellia oleifera Genome Provides Insights into Evolution, Agronomic Traits, and Genetic Architecture of Oil Camellia Plants. Cell Rep. 2024, 43, 114902. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Chen, S.; Li, J.; Ouyang, X. Transcriptome Analysis Unravels Key Pathways and Hub Genes Related to Immature Fruit Abscission in Camellia oleifera. Front. Plant Sci. 2024, 15, 1418358. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yan, H.; Haq, M.Z.U.; Liu, Y.; Wu, Y.; Yu, J.; Xia, P. Physiological and Transcriptional Analysis Provides Insights into Tea Saponin Biosynthesis and Regulation in Response to SA in Camellia vietnamensis Huang. Horticulturae 2023, 10, 8. [Google Scholar] [CrossRef]
- Umemura, H.; Nakajima, M.; Ishii, H.; Kurokura, T.; Asami, T.; Shimada, Y.; Nakamura, A. Analysis of the Effect of Each Plant Hormone on the Maturation of Woodland Strawberry Fruit in Auxin-Induced Parthenocarpic Fruit. Biosci. Biotechnol. Biochem. 2023, 87, 592–604. [Google Scholar] [CrossRef] [PubMed]
- Teh, H.F.; Neoh, B.K.; Wong, Y.C.; Kwong, Q.B.; Ooi, T.E.K.; Ng, T.L.M.; Tiong, S.H.; Low, J.Y.S.; Danial, A.D.; Ersad, M.A.; et al. Hormones, Polyamines, and Cell Wall Metabolism during Oil Palm Fruit Mesocarp Development and Ripening. J. Agric. Food Chem. 2014, 62, 8143–8152. [Google Scholar] [CrossRef]
- Solano, C.; Artola, A.; Barrena, R.; Ballardo, C.; Sánchez, A. Effect of the Exogenous Application of Different Concentrations of Indole-3-Acetic Acid as a Growth Regulator on Onion (Allium cepa L.) Cultivation. Agronomy 2023, 13, 2204. [Google Scholar] [CrossRef]
- Lordan, J.; Vilardell, P.; Peris, M.; Torres, E.; Alegre, S.; Asín, L. Post Petal Fall Applications of Gibberellins Improve Fruit Set on Pear. Sci. Hortic. 2019, 252, 149–155. [Google Scholar] [CrossRef]
- Qiao, Q.; Shen, B.; Lin, K.; Zhu, D.; Hong, P.; Zhang, L.; Sun, J.; Sun, S.; Gao, Y.; Zhang, S.; et al. Detecting the Physiological and Molecular Mechanisms by Which Abscisic Acid (ABA) Regulates the Consistency of Sweet Cherry Fruit Maturity. Sci. Rep. 2025, 15, 6311. [Google Scholar] [CrossRef]
- Wu, W.; Bao, Z.; Xiong, C.; Shi, L.; Chen, W.; Yin, X.; Yang, Z. The Softening of Persimmon Fruit Was Inhibited by Gibberellin via DkDELLA1/2. J. Agric. Food Chem. 2024, 73, 1159–1166. [Google Scholar] [CrossRef]
- Wu, W.; Sun, N.; Xu, Y.; Chen, Y.; Liu, X.; Shi, L.; Chen, W.; Zhu, Q.; Gong, B.; Yin, X.; et al. Exogenous Gibberellin Delays Maturation in Persimmon Fruit through Transcriptional Activators and Repressors. Plant Physiol. 2023, 193, 840–854. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Guo, X.; Zhao, D.; Zhang, Q.; Jiang, Y.; Wang, Y.; Peng, X.; Wei, Y.; Zhai, Z.; Zhao, W.; et al. Cloning and Expression Profiling of the PacSnRK2 and PacPP2C Gene Families during Fruit Development, ABA Treatment, and Dehydration Stress in Sweet Cherry. Plant Physiol. Biochem. 2017, 119, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Ji, K.; Mo, W.; Wang, L.; Chen, L.; Gao, L.; Gong, W.; Yuan, D. Dynamics of Sugars, Endogenous Hormones, and Oil Content during the Development of Camellia Oleifera Fruit. Botany 2021, 99, 515–529. [Google Scholar] [CrossRef]
- Zhu, Y.; Huo, D.; Zhang, M.; Wang, G.; Xiao, F.; Xu, J.; Li, F.; Zeng, Q.; Wei, Y.; Xu, J. Integrated Transcriptome and Endogenous Hormone Analyses Reveal the Factors Affecting the Yield of Camellia oleifera. BMC Genom. 2024, 25, 887. [Google Scholar] [CrossRef]
- Rafiq, M.; Guo, M.; Shoaib, A.; Yang, J.; Fan, S.; Xiao, H.; Chen, K.; Xie, Z.; Cheng, C. Unraveling the Hormonal and Molecular Mechanisms Shaping Fruit Morphology in Plants. Plants 2025, 14, 974. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, É.S.d.; Dias, A.N.; Sousa, R.M.; Germano, T.A.; Sousa, R.O.d.; Miranda, R.d.S.; Costa, J.H.; Santos, C.P.D. Genome and Transcriptome Analyses of Genes Involved in Ascorbate Biosynthesis in Pepper Indicate Key Genes Related to Fruit Development, Stresses, and Phytohormone Exposures. Plants 2023, 12, 3367. [Google Scholar] [CrossRef]
- Lin, S.; Wu, B.; Xiong, Y.; Huang, L.; Lin, D.; Lin, J.; Lin, S.; Wu, J. Integrated Endogenous Hormones and Transcriptome Analysis Contribute to Fruit Development Related Gene Mining in Eriobotrya japonica. Sci. Rep. 2025, 15, 14794. [Google Scholar] [CrossRef]
- Jiang, T.; Luo, C.; Mo, X.; Zhang, X.; Li, X.; Li, J.; He, X. Transcriptome Analysis to Identify Candidate Genes That Response to GA3 and CPPU Treatments for Mango Fruit Development. Plant Growth Regul. 2024, 104, 885–897. [Google Scholar] [CrossRef]
- Moya-León, M.A.; Stappung, Y.; Mattus-Araya, E.; Herrera, R. Insights into the Genes Involved in ABA Biosynthesis and Perception during Development and Ripening of the Chilean Strawberry Fruit. Int. J. Mol. Sci. 2023, 24, 8531. [Google Scholar] [CrossRef]
- Wu, B.; Wang, L.; Pan, G.; Li, T.; Li, X.; Hao, J. Genome-Wide Characterization and Expression Analysis of the Auxin Response Factor (ARF) Gene Family during Melon (Cucumis melo L.) Fruit Development. Protoplasma 2020, 257, 979–992. [Google Scholar] [CrossRef]
- Liu, N. Effects of IAA and ABA on the Immature Peach Fruit Development Process. Hortic. Plant J. 2019, 5, 145–154. [Google Scholar] [CrossRef]
- Li, W.-F.; Zhou, Q.; Ma, Z.-H.; Zuo, C.-W.; Chu, M.-Y.; Mao, J.; Chen, B.-H. Regulatory Mechanism of GA3 Application on Grape (Vitis vinifera L.) Berry Size. Plant Physiol. Biochem. 2024, 210, 108543. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.G.; Hong, Y.; Lee, E.J. Analysis of Eight Phytohormone Concentrations, Expression Levels of ABA Biosynthesis Genes, and Ripening-Related Transcription Factors during Fruit Development in Strawberry. J. Plant Physiol. 2019, 239, 52–60. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Zhang, Y.; Zhang, A.; You, C.-X. Regulation of Fleshy Fruit Ripening: From Transcription Factors to Epigenetic Modifications. Hortic. Res. 2022, 9, uhac013. [Google Scholar] [CrossRef]
- Parvini, F.; Sicardo, M.D.; Hosseini-Mazinani, M.; Martínez-Rivas, J.M.; Hernández, M.L. Transcriptional Analysis of Stearoyl-Acyl Carrier Protein Desaturase Genes from Olive (Olea europaea) in Relation to the Oleic Acid Content of the Virgin Olive Oil. J. Agric. Food Chem. 2016, 64, 7770–7781. [Google Scholar] [CrossRef] [PubMed]
- Garighan, J.; Dvorak, E.; Estevan, J.; Loridon, K.; Huettel, B.; Sarah, G.; Farrera, I.; Leclercq, J.; Grynberg, P.; Togawa, R.C.; et al. The Identification of Small RNAs Differentially Expressed in Apple Buds Reveals a Potential Role of the Mir159-MYB Regulatory Module during Dormancy. Plants 2021, 10, 2665. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, H.; Li, J.; Li, G.; Zahid, M.S.; Li, D.; Ma, C.; Xu, W.; Song, S.; Li, X.; et al. Transcriptome Analysis Revealed the Expression Levels of Genes Related to Abscisic Acid and Auxin Biosynthesis in Grapevine (Vitis vinifera L.) under Root Restriction. Front. Plant Sci. 2022, 13, 959693. [Google Scholar] [CrossRef] [PubMed]
- López-Galiano, M.J.; García-Robles, I.; González-Hernández, A.I.; Camañes, G.; Vicedo, B.; Real, M.D.; Rausell, C. Expression of miR159 Is Altered in Tomato Plants Undergoing Drought Stress. Plants 2019, 8, 201. [Google Scholar] [CrossRef]
- Wu, Q.; Chen, Y.; Bi, W.; Tong, B.; Wang, A.; Zhan, J.; He, L.; Xiao, D. Comprehensive Analysis of Small RNA, Transcriptome, and Degradome Sequencing: Mapping the miRNA-Gene Regulatory Network for the Development of Sweet Potato Tuber Roots. Plant Physiol. Biochem. 2025, 220, 109510. [Google Scholar] [CrossRef]
- Liu, X.-X.; Luo, X.-F.; Luo, K.-X.; Liu, Y.-L.; Pan, T.; Li, Z.-Z.; Duns, G.J.; He, F.-L.; Qin, Z.-D. Small RNA Sequencing Reveals Dynamic microRNA Expression of Important Nutrient Metabolism during Development of Camellia oleifera Fruit. Int. J. Biol. Sci. 2019, 15, 416–429. [Google Scholar] [CrossRef]
- Zhang, Y.; Su, Z.; Luo, L.; Wang, P.; Zhu, X.; Liu, J.; Wang, C. Exogenous Auxin Regulates the Growth and Development of Peach Fruit at the Expansion Stage by Mediating Multiple-Hormone Signaling. BMC Plant Biol. 2023, 23, 499. [Google Scholar] [CrossRef]
- Zang, Y.-X.; Chun, I.-J.; Zhang, L.-L.; Hong, S.-B.; Zheng, W.-W.; Xu, K. Effect of Gibberellic Acid Application on Plant Growth Attributes, Return Bloom, and Fruit Quality of Rabbiteye Blueberry. Sci. Hortic. 2016, 200, 13–18. [Google Scholar] [CrossRef]
- Xue, Z.; Yang, R.; Wang, Y.; Ma, Y.; Lin, Y.; Li, Z.; Song, Y.; Feng, X.; Li, L. Candidate Gene Mining of GA-Mediated Regulation of Pear Fruit Shape. Hortic. Environ. Biotechnol. 2024, 65, 403–412. [Google Scholar] [CrossRef]
- Nguyen, T.Q.; Kisiala, A.B.; Hai, N.N.; Narine, S.; Emery, R.J.N. Phytohormone Dynamics Impact Fatty Acid and Oil Accumulation during Soybean Seed Maturation. Seed Sci. Res. 2021, 31, 278–291. [Google Scholar] [CrossRef]
- Yeap, W.-C.; Lee, F.-C.; Shabari Shan, D.K.; Musa, H.; Appleton, D.R.; Kulaveerasingam, H. WRI1-1, ABI5, NF-YA3 and NF-YC2 Increase Oil Biosynthesis in Coordination with Hormonal Signaling during Fruit Development in Oil Palm. Plant J. 2017, 91, 97–113. [Google Scholar] [CrossRef]
- Sun, X.; Haq, M.Z.U.; Liu, Y.; Yang, D.; Yang, H.; Wu, Y. Gene Expression Dynamics of Sugar Metabolism and Accumulation during Fruit Ripening in Camellia drupifera. Plants 2025, 14, 817. [Google Scholar] [CrossRef] [PubMed]
- Fenn, M.A.; Giovannoni, J.J. Phytohormones in Fruit Development and Maturation. Plant J. 2021, 105, 446–458. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, K.; Luo, L.; Lv, Y.; Li, Y.; Zhu, S.; Luo, B.; Wan, Y.; Zhang, X.; Liu, F. Identification of Peanut Aux/IAA Genes and Functional Prediction during Seed Development and Maturation. Plants 2022, 11, 472. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Lyu, S.; Chen, Y.; Xu, S.; Ye, J.; Chen, G.; Wu, S.; Li, X.; Chen, J.; Pan, D. MicroRNAs and Transcripts Associated with an Early Ripen-Ing Mutant of Pomelo (Citrus grandis Osbeck). Int. J. Mol. Sci. 2021, 22, 9348. [Google Scholar] [CrossRef]
- Hu, J.; Israeli, A.; Ori, N.; Sun, T.-P. The Interaction between DELLA and ARF/IAA Mediates Crosstalk between Gibberellin and Auxin Signaling to Control Fruit Initiation in Tomato. Plant Cell 2018, 30, 1710–1728. [Google Scholar] [CrossRef]
- Yue, K.; Li, L.; Xie, J.; Effah, Z.; Anwar, S.; Wang, L.; Meng, H.; Li, L. Integrating microRNAs and mRNAs Reveals the Hormones Synthesis and Signal Transduction of Maize under Different N Rates. J. Integr. Agric. 2023, 22, 2673–2686. [Google Scholar] [CrossRef]
- Gabriele, S.; Rizza, A.; Martone, J.; Circelli, P.; Costantino, P.; Vittorioso, P. The Dof Protein DAG1 Mediates PIL5 Activity on Seed Germination by Negatively Regulating GA Biosynthetic Gene AtGA3ox1. Plant J. 2009, 61, 312–323. [Google Scholar] [CrossRef]
- Seo, M.; Koshiba, T. Complex Regulation of ABA Biosynthesis in Plants. Trends Plant Sci. 2002, 7, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.; Yamaguchi, S.; Hu, J.; Yusuke, J.; Jung, B.; Paik, I.; Lee, H.-S.; Sun, T.; Kamiya, Y.; Choi, G. PIL5, a Phytochrome-Interacting bHLH Protein, Regulates Gibberellin Responsiveness by Binding Directly to the GAI and RGA Promoters in Arabidopsis Seeds. Plant Cell 2007, 19, 1192–1208. [Google Scholar] [CrossRef]
- Bi, C.; Ma, Y.; Wang, X.-F.; Zhang, D.-P. Overexpression of the Transcription Factor NF-YC9 Confers Abscisic Acid Hypersensitivity in Arabidopsis. Plant Mol. Biol. 2017, 95, 425–439. [Google Scholar] [CrossRef]
- Miura, K.; Lee, J.; Jin, J.B.; Yoo, C.Y.; Miura, T.; Hasegawa, P.M. Sumoylation of ABI5 by the Arabidopsis SUMO E3 Ligase SIZ1 Negatively Regulates Abscisic Acid Signaling. Proc. Natl. Acad. Sci. USA 2009, 106, 5418–5423. [Google Scholar] [CrossRef]
- Li, X.; Zhang, D.; Pan, X.; Kakar, K.U.; Nawaz, Z. Regulation of Carotenoid Metabolism and ABA Biosynthesis during Blueberry Fruit Ripening. Plant Physiol. Biochem. 2024, 206, 108232. [Google Scholar] [CrossRef]
- Sun, X.; Ke, L.; Wu, Y.; Haq, M.Z.U.; Yang, D.; Liu, Y.; Yang, H. Identification and Expression Analysis of miRNA-mRNA Regulatory Modules Related to Lipid Accumulation in Camellia drupifera. Int. J. Biol. Macromol. 2025, 318, 145147. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Sun, X.; Yao, Y.; Liu, Y.; Yang, D.; Yang, H.; Yu, J.; Zheng, D.; Wu, Y. Integration of Transcriptome, miRNA-Omics, and Hormone Metabolism Analysis Reveals the Regulatory Network of Camellia drupifera Fruit Maturation. Plants 2025, 14, 3282. https://doi.org/10.3390/plants14213282
Zhao J, Sun X, Yao Y, Liu Y, Yang D, Yang H, Yu J, Zheng D, Wu Y. Integration of Transcriptome, miRNA-Omics, and Hormone Metabolism Analysis Reveals the Regulatory Network of Camellia drupifera Fruit Maturation. Plants. 2025; 14(21):3282. https://doi.org/10.3390/plants14213282
Chicago/Turabian StyleZhao, Jin, Xue Sun, Yanqiang Yao, Ya Liu, Dongmei Yang, Huageng Yang, Jing Yu, Daojun Zheng, and Yougen Wu. 2025. "Integration of Transcriptome, miRNA-Omics, and Hormone Metabolism Analysis Reveals the Regulatory Network of Camellia drupifera Fruit Maturation" Plants 14, no. 21: 3282. https://doi.org/10.3390/plants14213282
APA StyleZhao, J., Sun, X., Yao, Y., Liu, Y., Yang, D., Yang, H., Yu, J., Zheng, D., & Wu, Y. (2025). Integration of Transcriptome, miRNA-Omics, and Hormone Metabolism Analysis Reveals the Regulatory Network of Camellia drupifera Fruit Maturation. Plants, 14(21), 3282. https://doi.org/10.3390/plants14213282