Regulatory Effects of Different Doses of Penoxsulam on Endogenous Hormones and Antioxidant System in Foxtail Millet
Abstract
1. Introduction
2. Results
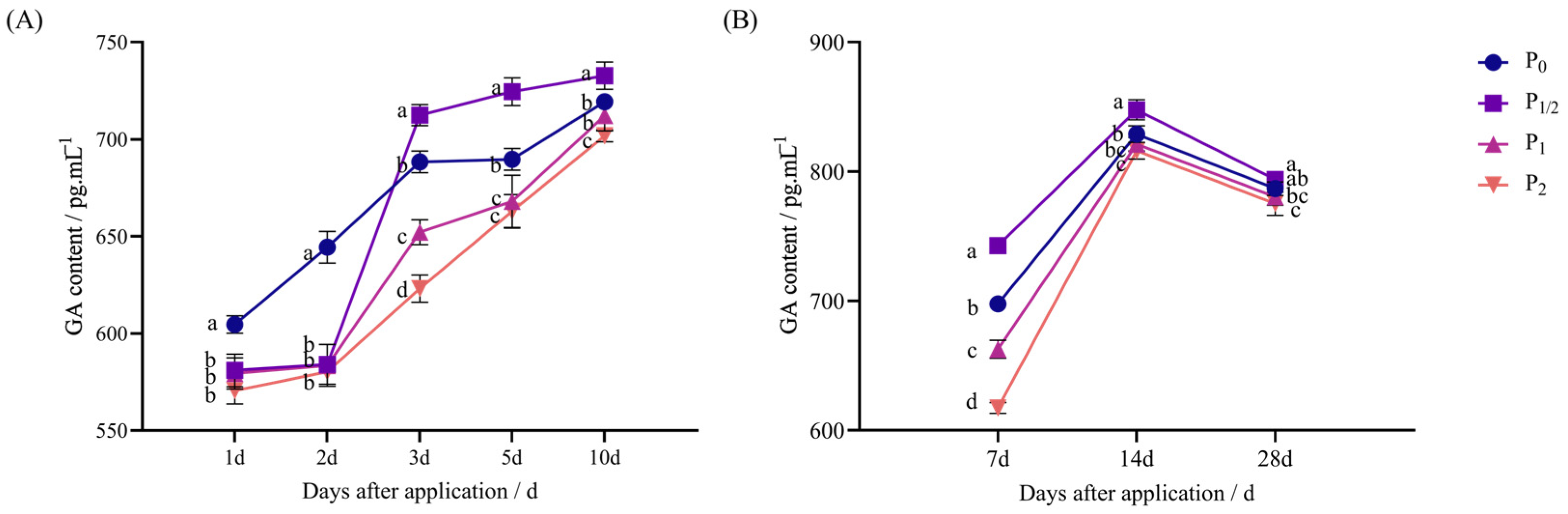
2.1. Effects of Penoxsulam Spraying on Endogenous Hormones of Foxtail Millet
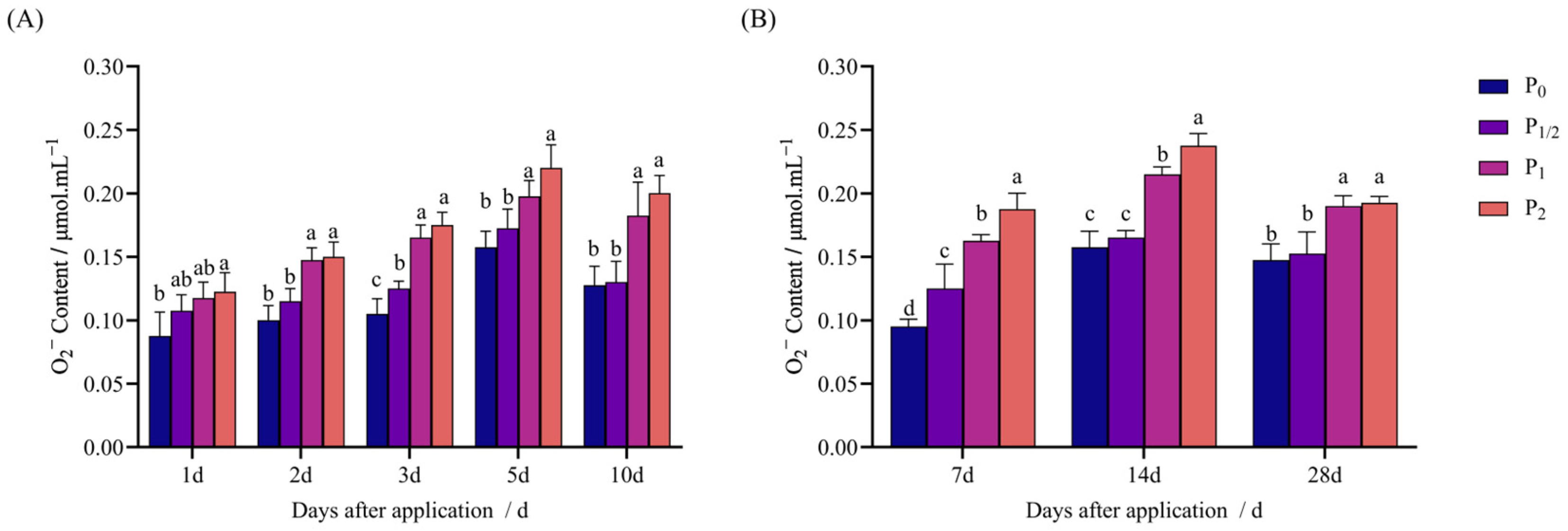
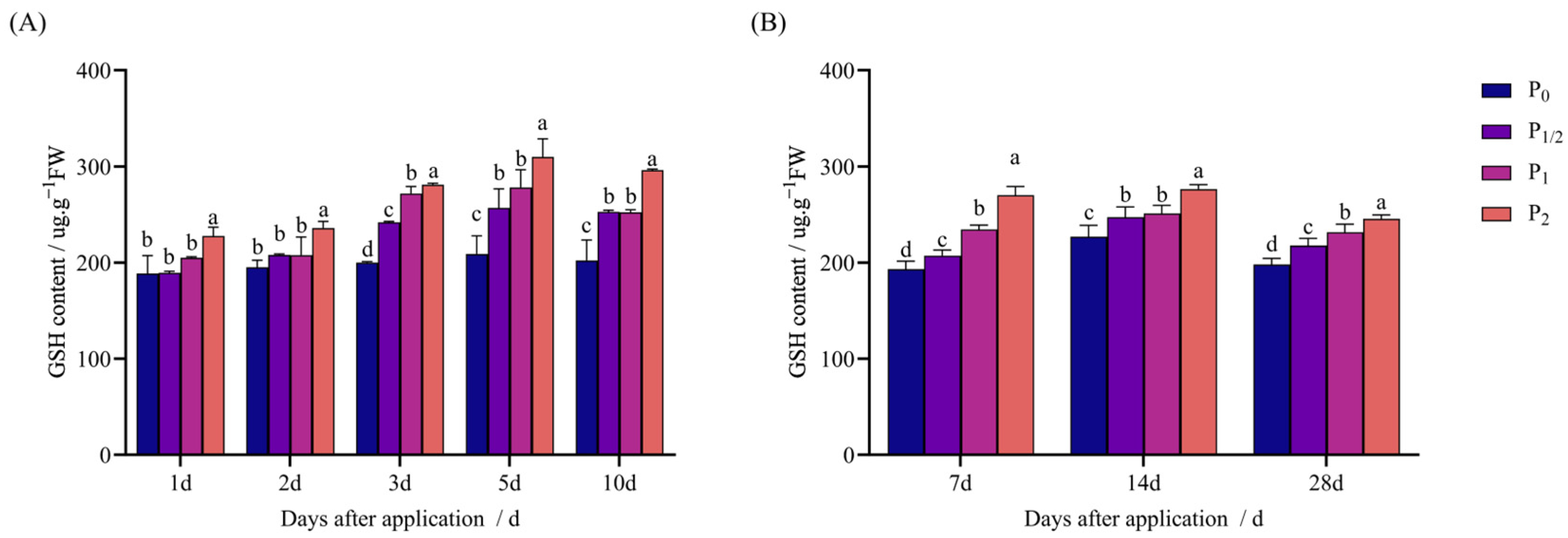
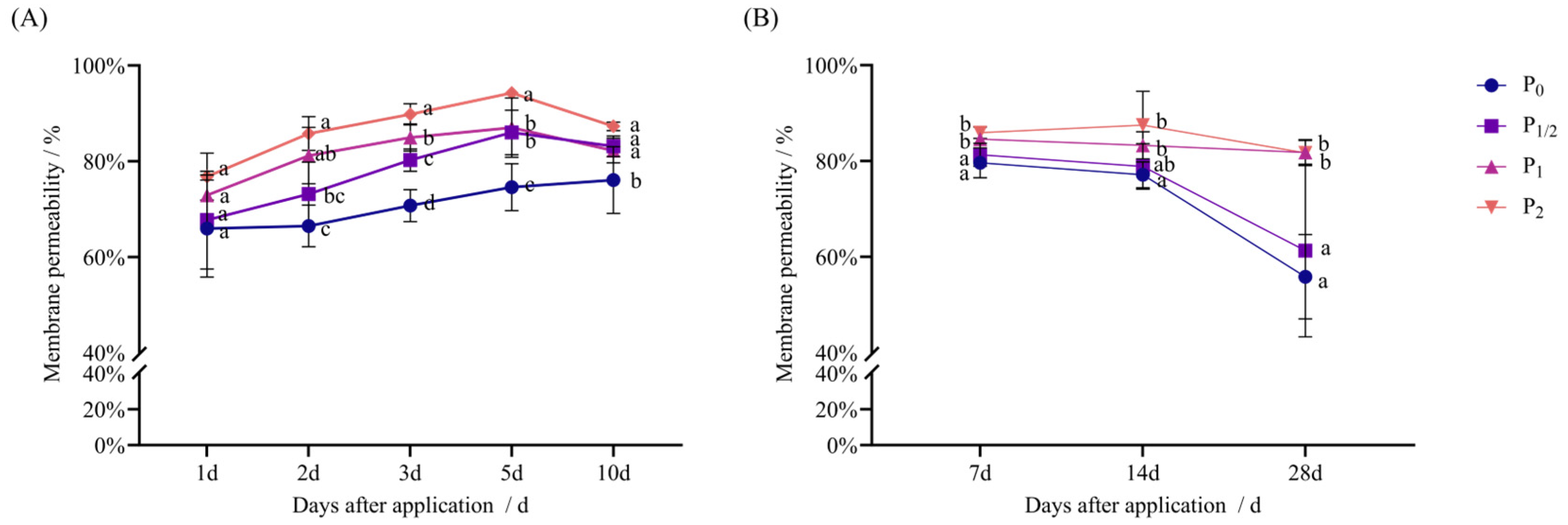
2.2. Effects of Penoxsulam Spraying on Antioxidant System of Foxtail Millet
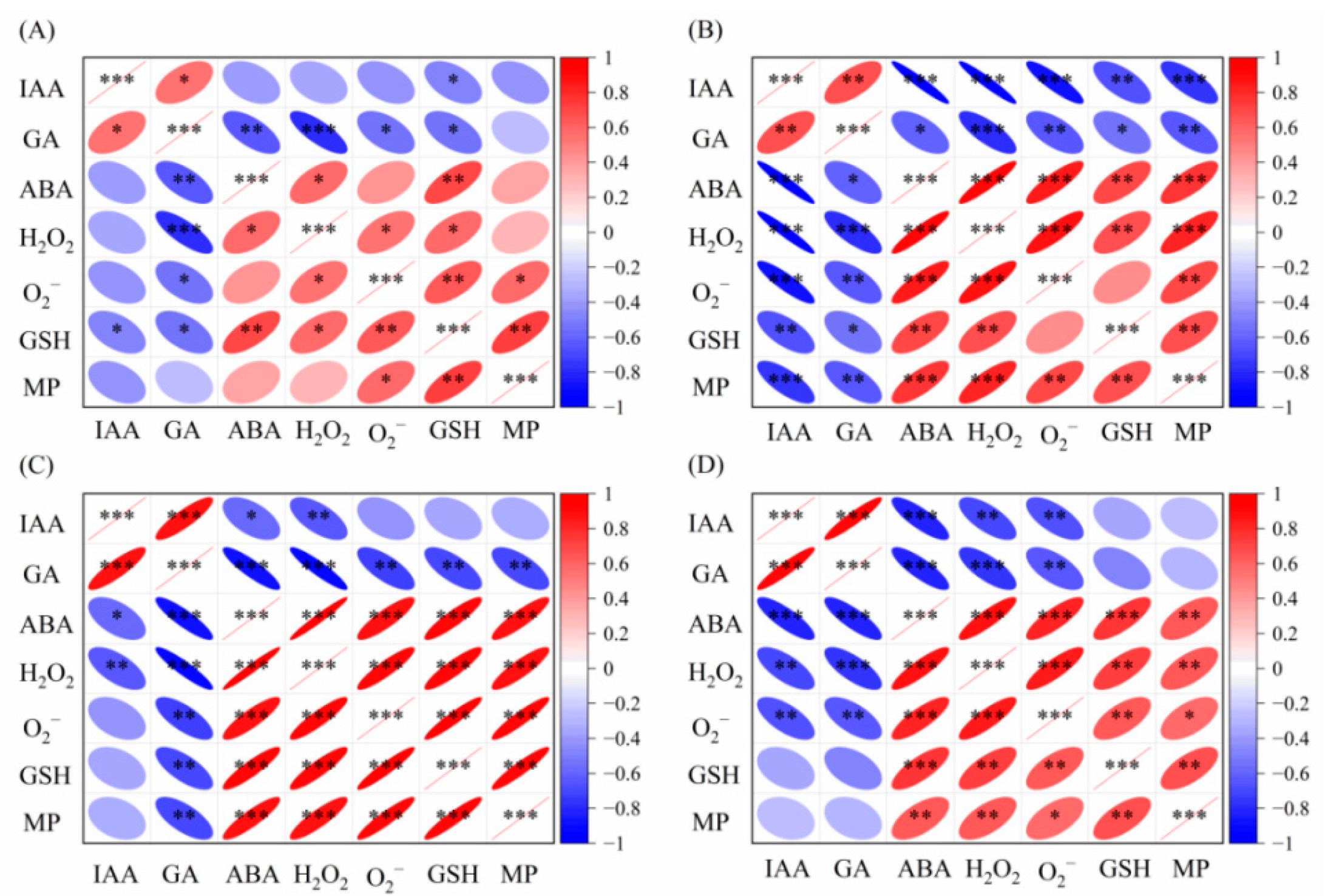
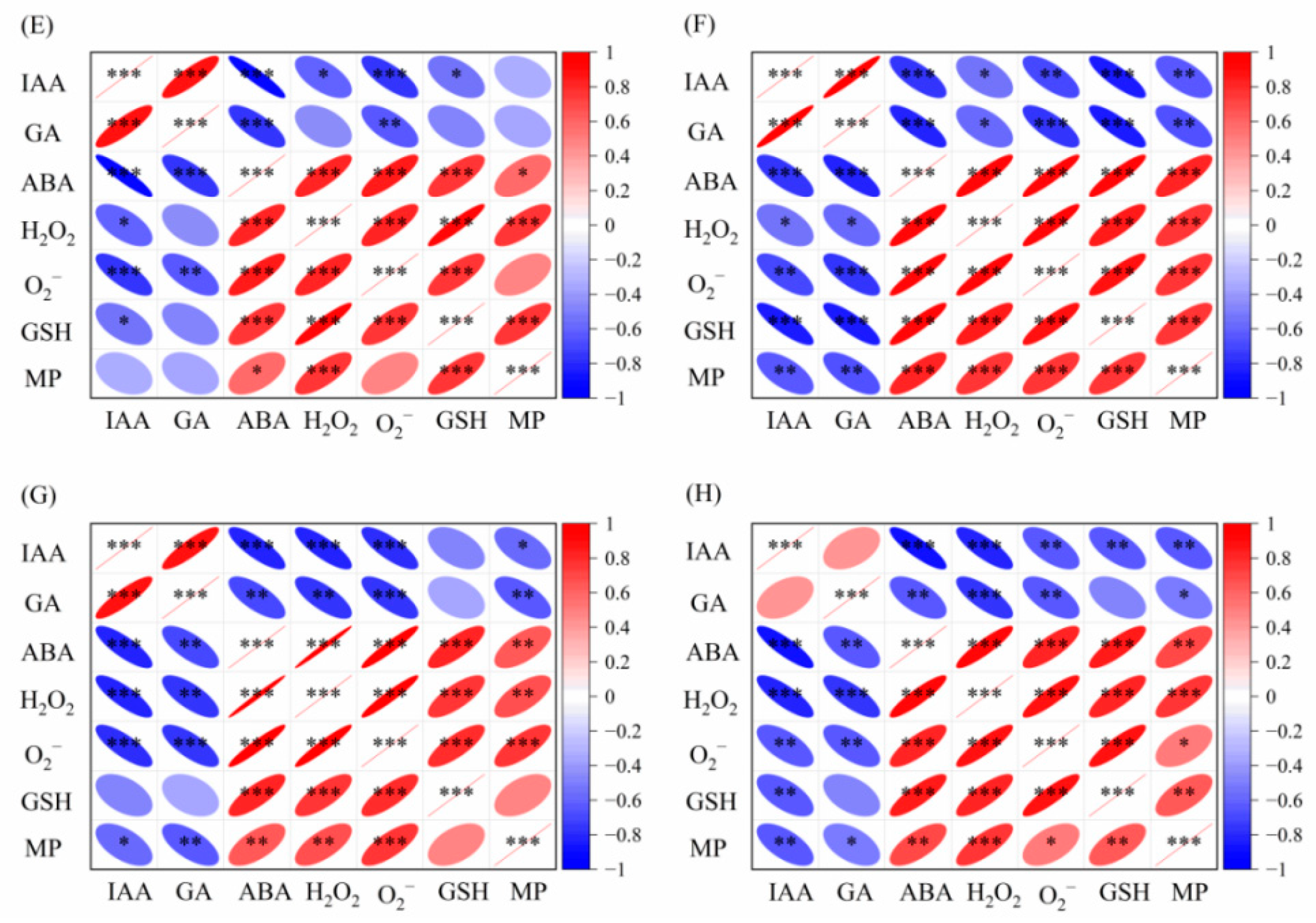
2.3. Relationship Between Endogenous Hormone and Antioxidant System of Foxtail Millet
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Experimental Design
4.2.1. Pot Experiment
4.2.2. Field Experiment
4.3. Determination Indexes and Methods
4.3.1. Determination of Endogenous Hormone Contents
4.3.2. Determination of H2O2 Content
4.3.3. Determination of O2− Content
4.3.4. Determination of GSH Content
4.3.5. Determination of MP
4.4. Data Processing
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sondhia, S.; Rajput, S.; Varma, R.J.; Kumar, A. Biodegradation of the herbicide penoxsulam (triazolopyrimidine sulphonamide) by fungal strains of Aspergillus in soil. Appl. Soil Ecol. 2016, 105, 196–206. [Google Scholar] [CrossRef]
- Kogan, M.; Araya, M.; Alister, C. Water and sediment dynamics of penoxsulam and molinate in paddy fields: Field and lysimeter studies. Pest Manag. Sci. 2012, 68, 399–403. [Google Scholar] [CrossRef]
- Murussi, C.R.; Thorstenberg, M.L.; Leitemperger, J.; Costa, M.; Clasen, B.; Santi, A.; Menezes, C.; Engers, V.K.; Loro, V.L. Toxic effects of penoxsulam herbicide in two fish species reared in Southern Brazil. Bull. Environ. Contam. Toxicol. 2014, 92, 81–84. [Google Scholar] [CrossRef]
- Wei, L.; Shao, W.W.; Ding, G.H.; Fan, X.L.; Yu, M.L.; Lin, Z.H. Acute and joint toxicity of three agrochemicals to Chinese tiger frog (Hoplobatrachus chinensis) tadpoles. Dongwuxue Yanjiu 2014, 35, 272–279. [Google Scholar]
- Damalas, C.A.; Dhima, K.V.; Eleftherohorinos, I.G. Control of early watergrass (Echinochloa Oryzoides) and late watergrass (Echinochloa Phyllopogon) with cyhalofop, clefoxydim, and penoxsulam applied alone and in mixture with broadleaf herbicides. Weed Technol. 2006, 20, 992–998. [Google Scholar] [CrossRef]
- Shiraishi, I. Development of a New rice herbicide penoxsulam (DASH-001SC) and its charact eristics. J. Pestic. Sci. 2005, 30, 265–268. [Google Scholar] [CrossRef]
- Acar, A.; Singh, D. Monitoring genotoxic, biochemical and morphotoxic potential of penoxsulam and the protective role of European blueberry (Vaccinium myrtillus L.) extract. Sci. Rep. 2023, 13, 6787. [Google Scholar] [CrossRef]
- Song, X.; Wang, H.; Dong, Q.; Qiu, T.; Shi, C.; Li, X.; Dong, S.; Zhao, J.; Guo, P.; Yuan, X. Comprehensive evaluation and main identification indexes of herbicide resistance of high-quality foxtail millet (Setaria italica L.). Agronomy 2023, 13, 3033. [Google Scholar] [CrossRef]
- Peleg, Z.; Blumwald, E. Hormone balance and abiotic stress tolerance in crop plants. Curr. Opin. Plant Biol. 2011, 14, 290–295. [Google Scholar] [CrossRef]
- Klaus, G. Auxin herbicides: Current status of mechanism and mode of action. Pest Manag. Sci. 2010, 66, 113–120. [Google Scholar]
- Sauer, M.; Robert, S.; Kleine-Vehn, J. Auxin: Simply complicated. J. Exp. Bot. 2013, 64, 2565–2577. [Google Scholar] [CrossRef]
- Shah, S.H.; Islam, S.; Mohammad, F.; Siddiqui, M.H. Gibberellic acid: A versatile regulator of plant growth, development and stress responses. J. Plant Growth Regul. 2023, 42, 7352–7373. [Google Scholar] [CrossRef]
- Cutler, S.R.; Rodriguez, P.L.; Finkelstein, R.R.; Abrams, S.R. Abscisic acid: Emergence of a core signaling network. Annu. Rev. Plant Biol. 2010, 61, 651–679. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Shi, L.; Chen, D.; Ren, A.; Gao, T.; Zhao, M. Functional analysis of the role of glutathione peroxidase (GPx) in the ROS signaling pathway, hyphal branching and the regulation of ganoderic acid biosynthesis in Ganoderma Lucidum. Fungal Genet. Biol. 2015, 82, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Ahanger, M.A.; Tomar, N.S.; Tittal, M.; Argal, S.; Agarwal, R.M. Plant growth under water/salt stress: ROS production; antioxidants and significance of added potassium under such conditions. Physiol. Mol. Biol. Plants 2017, 23, 731–744. [Google Scholar] [CrossRef]
- Cezary, W.; Melanie, C.; Jaakko, K. Reactive oxygen species in plant signaling. Annu. Rev. Plant Biol. 2018, 69, 209–236. [Google Scholar] [CrossRef]
- Bolwell, G.P.; Bindschedler, L.V.; Blee, K.A.; Butt, V.S.; Davies, D.R.; Gardner, S.L.; Gerrish, C.; Minibayeva, F. The apoplasticoxidative burst in response to biotic stress in plants:a three-component system. J. Exp. Bot. 2002, 53, 1367–1376. [Google Scholar]
- Vranová, E.; Inzé, D.; Van Breusegem, F. Signal transductionduring oxidative stress. J. Exp. Bot. 2002, 53, 1227–1236. [Google Scholar] [CrossRef]
- Dumanović, J.; Nepovimova, E.; Natić, M.; Kuča, K.; Jaćević, V. The significance of reactive oxygen species and antioxidant defense system in plants: A concise overview. Front. Plant Sci. 2021, 11, 552969. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Grillet, L.; Ouerdane, L.; Flis, P.; Hoang, M.T.; Isaure, M.P.; Lobinski, R.; Curie, C.; Mari, S. Ascorbate efflux as a new strategy for iron reduction and transport in plants. J. Biol. Chem. 2014, 289, 2515–2525. [Google Scholar] [CrossRef] [PubMed]
- Amini, S.; Maali-Amiri, R.; Kazemi-Shahandashti, S.S.; López-Gómez, M.; Sadeghzadeh, B.; Sobhani-Najafabadi, A.; Kariman, K. Effect of cold stress on polyamine metabolism and antioxidant responses in chickpea. J. Plant Physiol. 2021, 258–259, 153387. [Google Scholar] [CrossRef]
- Peng, R.; Zhang, B. Foxtail millet: A new model for C4 plants. Trends Plant Sci. 2021, 26, 199–201. [Google Scholar] [CrossRef]
- Kalsing, A.; Rossi, C.V.S.; Lucio, F.R.; Zobiole, L.H.S.; Cunha, L.C.V.; Minozzi, G.B. Effect of formulations and spray nozzles on 2, 4-D spray drift under field conditions. Weed Technol. 2018, 32, 379–384. [Google Scholar] [CrossRef]
- Dodd, I.C. Hormonal interactions and stomatal responses. J. Plant Growth Regul. 2003, 22, 32–46. [Google Scholar] [CrossRef]
- Yuan, S.Z.; Yuan, S.; Zhou, W.Y. Effects of ethametsulfuron on protective enzymes and endogenous hormones of rice seedling. Agric. Sci. Technol. 2012, 13, 173–176. [Google Scholar]
- Doganlar, Z.B. Physiological and genetic responses to pesticide mixture treatment of Veronica beccabunga. Water Air Soil Pollut. 2012, 223, 6201–6212. [Google Scholar] [CrossRef]
- Mansour, F.A.; Badaway, A.M.; Nemat, A.M. Comparative effects of alachlor-metolachlor-atrazine-fluometuron and trifluralin on the endogenous phytohormones in maize and soybean. Agrochimica 1994, 38, 118–131. [Google Scholar]
- Locher, R.; Pilet, P.E. Trifluralin uptake and its effect on ABA content in growing maize and pea roots. J. Plant Physiol. 1995, 146, 569–571. [Google Scholar] [CrossRef]
- Li, X.Y.; Tong, W.; Huang, H.L. Atrazine accumulation and toxic responses in maize (Zea mays). J. Environ. Sci. 2012, 24, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- GogoiI, K.; Gogoi, H.; Borgohain, M.; Saikia, R.; Chikkaputtaiah, C.; Hiremath, S.; Basu, U. The molecular dynamics between reactive oxygen species (ROS), reactive nitrogen species (RNS) and phytohormones in plant’s response to biotic stress. Plant Cell Rep. 2024, 43, 263. [Google Scholar] [CrossRef]
- Cordon, G.; Valino, I.L.; Prieto, A.; Costa, C.; Marchi, M.C.; Diz, V. Effects of the nanoherbicide made up of atrazine-chitosan on the primary events of photosynthesis. J. Photochem. Photobiol. 2022, 12, 100144. [Google Scholar] [CrossRef]
- Michelet, L.; Zaffagnini, M.; Marchand, C.; Collin, V.; Decottignies, P.; Tsan, P.; Lancelin, J.; Trost, P.; Miginiac-Maslow, M.; Noctor, G.; et al. Glutathionylation of chloroplast thioredoxin f is a redox signaling mechanism in plants. Proc. Natl. Acad. Sci. USA 2005, 102, 16478–16483. [Google Scholar] [CrossRef]
- Žaltauskaité, J.; Brazaityté, V. Assessment of the effects of sulfonylureas herbicide amidosulfuron application on target and non-target organisms. Fresenius Environ. Bull. 2013, 22, 1977–1982. [Google Scholar]
- Pan, D.; Li, Q.X.; Lin, Z.; Chen, Z.; Tang, W.; Pan, C.; Tan, H.; Zeng, D. Interactions between salicylic acid and antioxidant enzymes tilting the balance of H2O2 from photorespiration in nontarget-crops under halosulf uron-methyl stress. Pestic. Biochem. Physiol. 2017, 143, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Zhang, L.; Ning, N.; Wen, Y.; Dong, S.; Yin, M.; Guo, M.; Wang, B.; Feng, L.; Guo, P. Photosynthetic physiological response of Radix Isatidis (Isatis indigotica Fort.) Seedlings to Nicosulfuron. PLoS ONE 2014, 9, e105310. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.L.; Cui, J.; Tao, L.; Yang, H. Fluroxypyr triggers oxidative damage by producing superoxide and hydrogen peroxide in rice (Oryza sativa). Ecotoxicology 2010, 19, 124–132. [Google Scholar] [CrossRef]
- Zhang, X.H.; Liu, Y.H.; Liu, Q.; Zong, B.; Yuan, X.P.; Sun, H.E.; Wang, J.; Zang, L.; Ma, Z.Z.; Liu, H.M.; et al. Nitric oxide is involved in abscisic acid-induced photosynthesis and antioxidant system of tall fescue seedlings response to low-light stress. Environ. Exp. Bot. 2018, 155, 226–238. [Google Scholar] [CrossRef]
- Zhao, J.; Li, G.; Yi, G.X.; Wang, B.M.; Deng, A.X.; Nan, T.G.; Li, Z.H.; Li, Q.X. Comparison between conventional in direct competitive enzyme-linked immunosorbent assay (icELISA) and simplified icELISA for small molecules. Analytica. Chimica. Acta. 2006, 571, 79–85. [Google Scholar] [CrossRef]
- Qi Du, Q.; Zhao, X.H.; Xia, L.; Jiang, C.J.; Wang, X.G.; Han, Y.; Wang, J.; Yu, H.Q. Effects of potassium deficiency on photosynthesis, chloroplast ultrastructure, ROS, and antioxidant activities in maize (Zea mays L.). J. Integr. Agric. 2019, 18, 395–406. [Google Scholar] [CrossRef]
- Rauckman, E.J.; Rosen, G.M.; Kitchell, B.B. Superoxide radical as an intermediate in the oxidation of hydroxylamines by mixed function amine oxidase. Mol. Pharmacol. 1979, 15, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Sedlak, J.; Lindsay, R.H. Estimation of total, protein-bound, and nonprotein sulf-hydryl groups in tissue with Ellman’s reagent. Anal. Biochem. 1968, 25, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.X.; Lv, B.S.; Wang, M.M.; Ma, H.Y.; Yang, H.Y.; Liu, X.L.; Jiang, C.J.; Liang, Z.W. Priming effect of abscisic acid on alkaline stress tolerance in rice (Oryza sativa L.) seedlings. Plant Physiol. Biochem. 2015, 90, 50–57. [Google Scholar] [CrossRef]



| Variety | Treatment | |||
|---|---|---|---|---|
| P0 | P1/2 | P1 | P2 | |
| Jingu 21 | 0 | 15 | 30 | 60 |
| Year | Total P (g/kg) | Total K (g/kg) | Total N (g/kg) | Available K (mg/kg) | Available N (mg/kg) | Available P (mg/kg) | Organic Matter (g/kg) | pH |
|---|---|---|---|---|---|---|---|---|
| 2024 | 1.058 | 22.6 | 1.053 | 307 | 70.4 | 12.4 | 24.4 | 8.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, C.; Chen, T.; Diao, C.; Dou, B.; Shang, S.; Li, S.; Wen, Y.; Song, X.; Zhao, J.; Cao, H.; et al. Regulatory Effects of Different Doses of Penoxsulam on Endogenous Hormones and Antioxidant System in Foxtail Millet. Plants 2025, 14, 3254. https://doi.org/10.3390/plants14213254
Hu C, Chen T, Diao C, Dou B, Shang S, Li S, Wen Y, Song X, Zhao J, Cao H, et al. Regulatory Effects of Different Doses of Penoxsulam on Endogenous Hormones and Antioxidant System in Foxtail Millet. Plants. 2025; 14(21):3254. https://doi.org/10.3390/plants14213254
Chicago/Turabian StyleHu, Chunyan, Tingting Chen, Chunxia Diao, Binglan Dou, Suqi Shang, Shuo Li, Yinyuan Wen, Xi’e Song, Juan Zhao, Hui Cao, and et al. 2025. "Regulatory Effects of Different Doses of Penoxsulam on Endogenous Hormones and Antioxidant System in Foxtail Millet" Plants 14, no. 21: 3254. https://doi.org/10.3390/plants14213254
APA StyleHu, C., Chen, T., Diao, C., Dou, B., Shang, S., Li, S., Wen, Y., Song, X., Zhao, J., Cao, H., & Dong, S. (2025). Regulatory Effects of Different Doses of Penoxsulam on Endogenous Hormones and Antioxidant System in Foxtail Millet. Plants, 14(21), 3254. https://doi.org/10.3390/plants14213254







