Molecular Mechanisms and Crosstalk Signaling in Soybean’s Response to Water Deficit and Excess: Implications for Stress Resilience and Productivity
Abstract
1. Introduction
2. Results
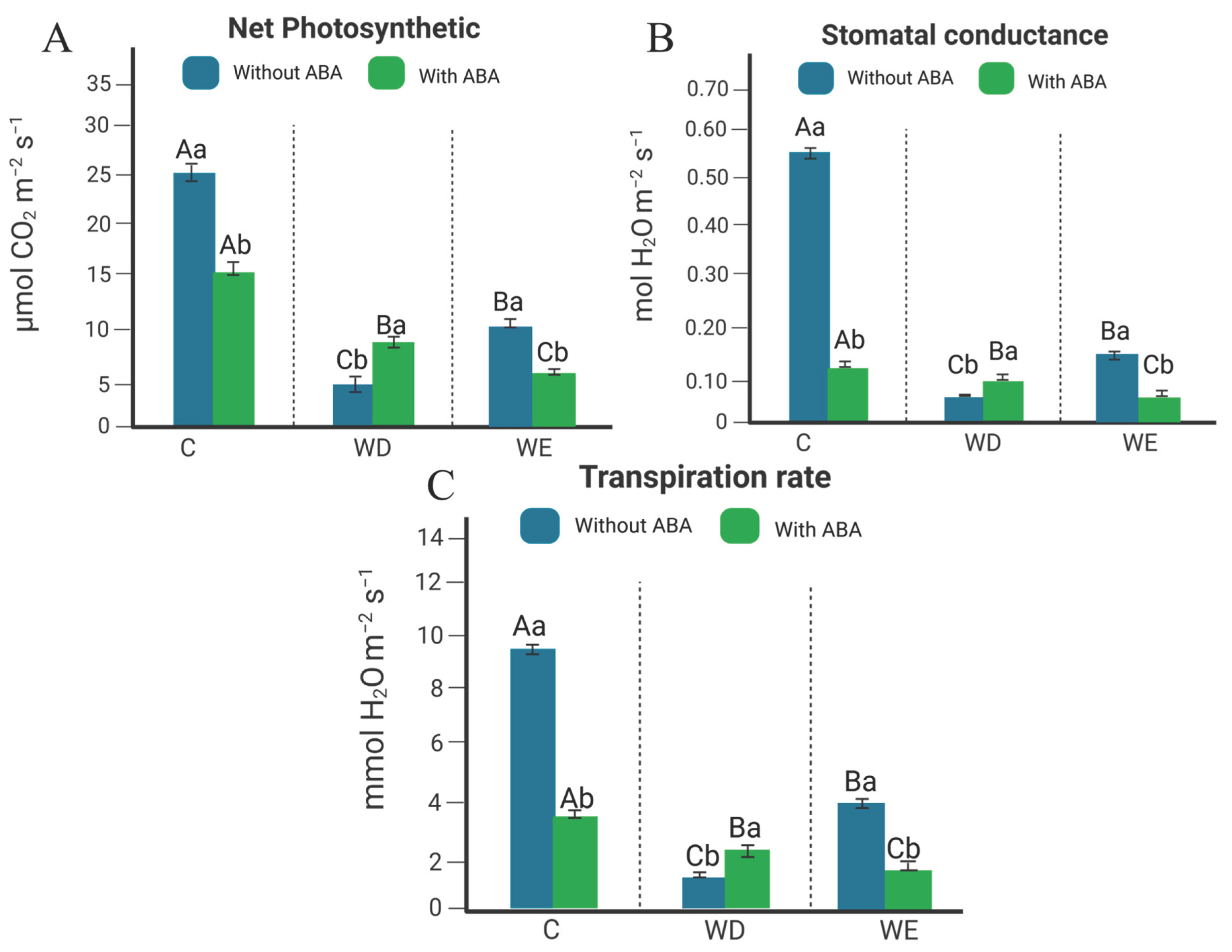
2.1. Gas Exchange Analysis in Leaves of Williams 82 Soybean Cultivar
2.2. Root Morphology
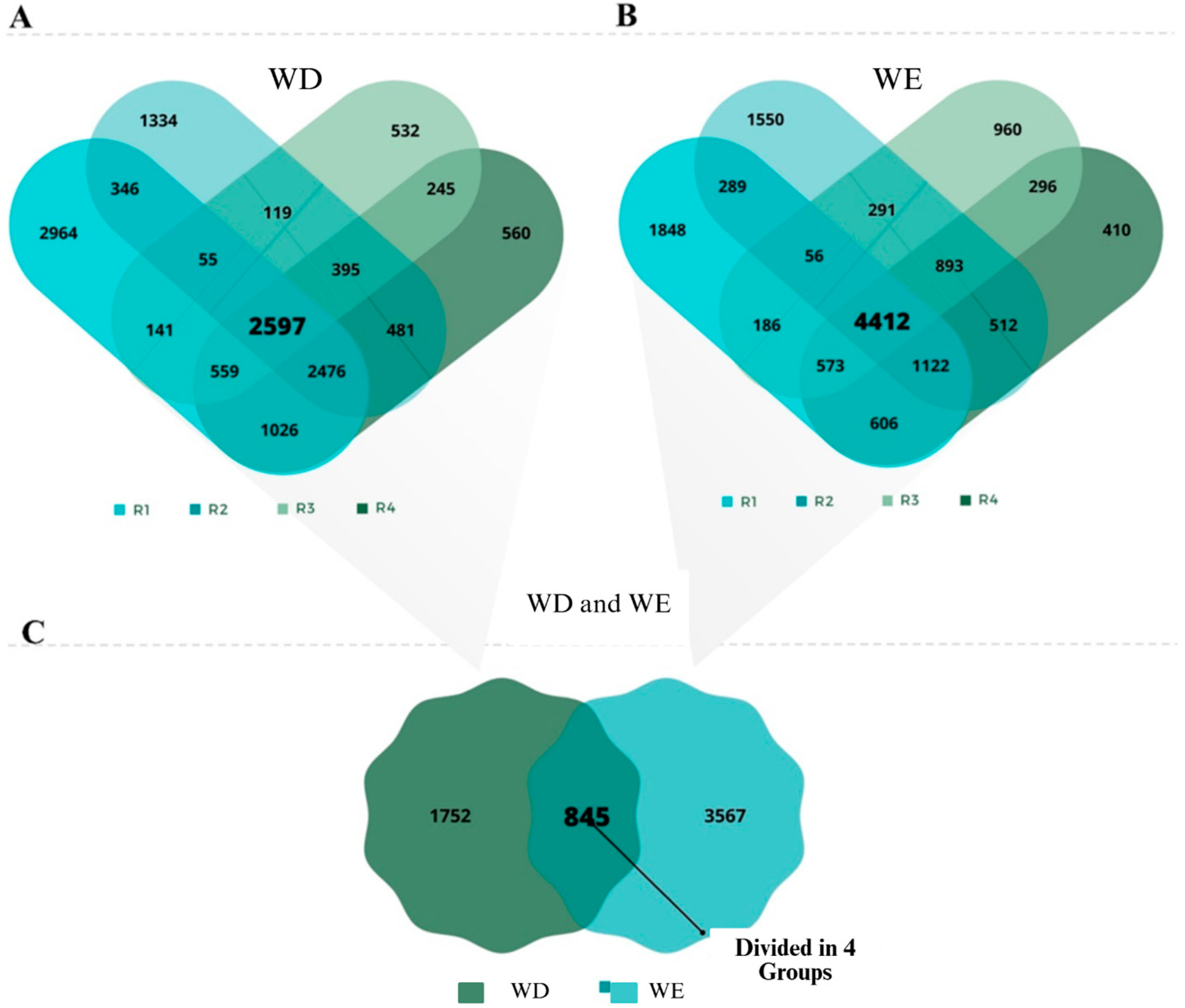
2.3. Soybean Differentially Expressed Genes Under WD and WE Conditions
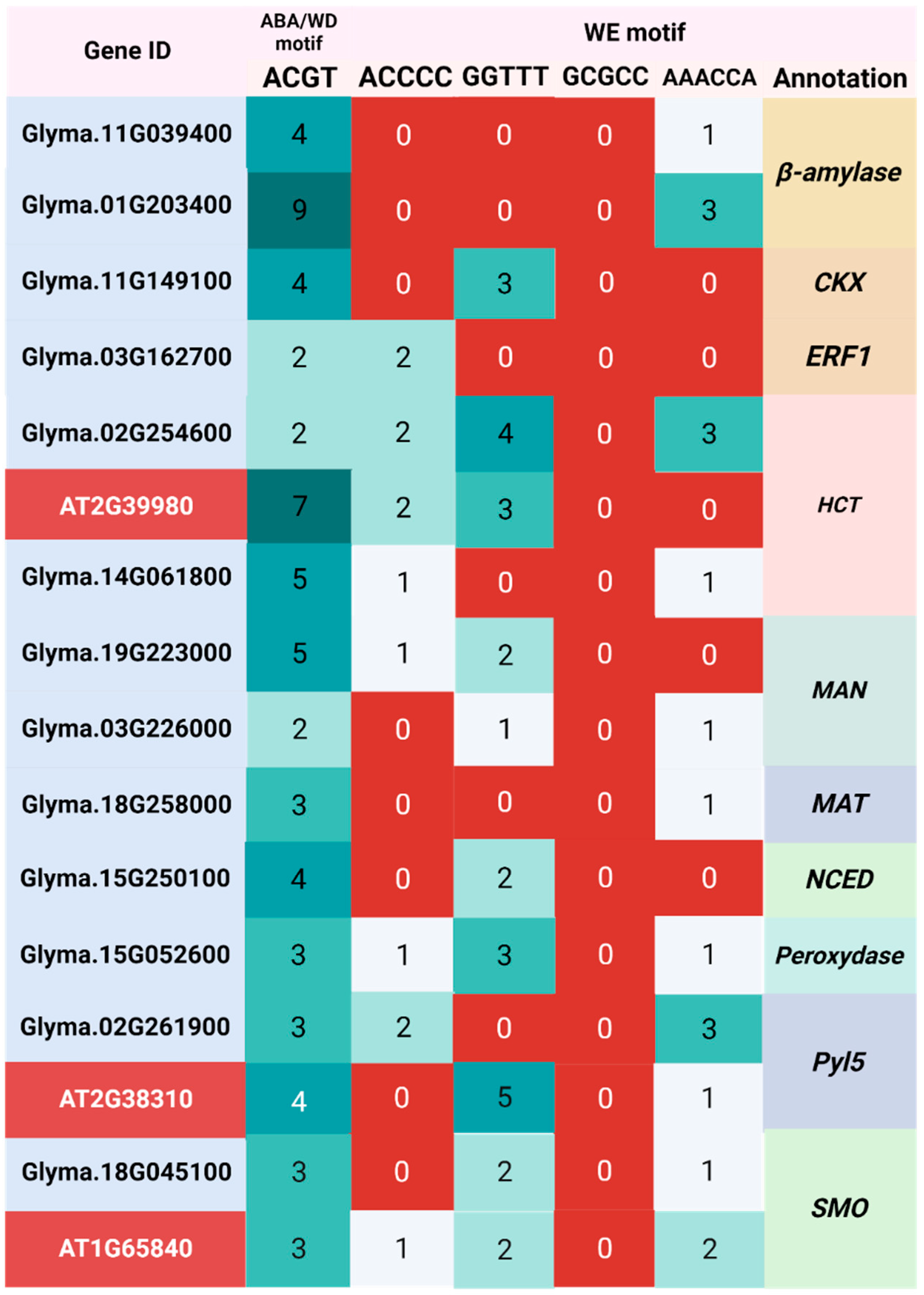
2.4. Identification of WD, and WE Responsive Motifs
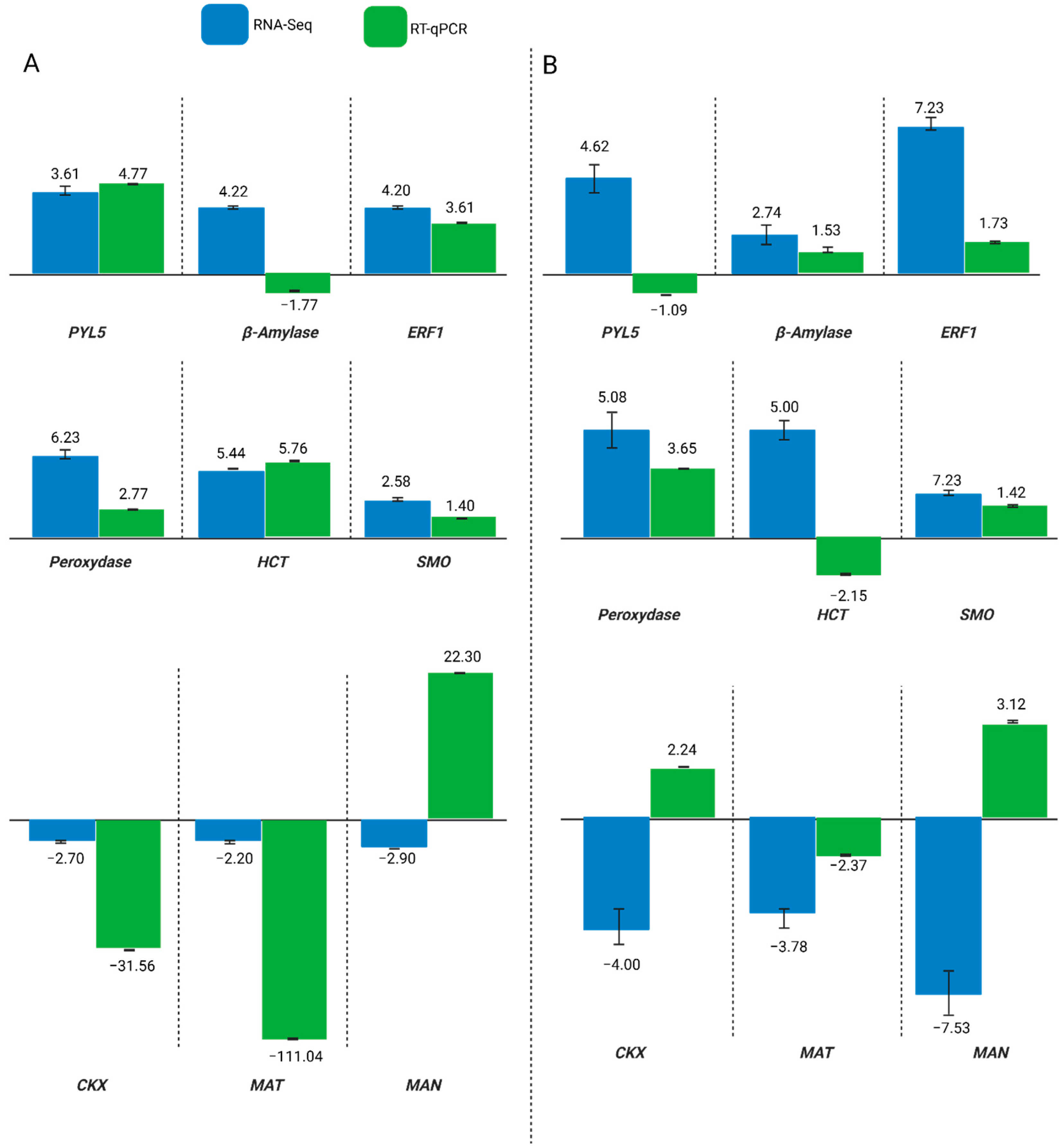
2.5. Comparative Analysis of Gene Expression in Response to Water Treatments: Consistent Regulation Patterns Between RNA-Seq and RT-qPCR
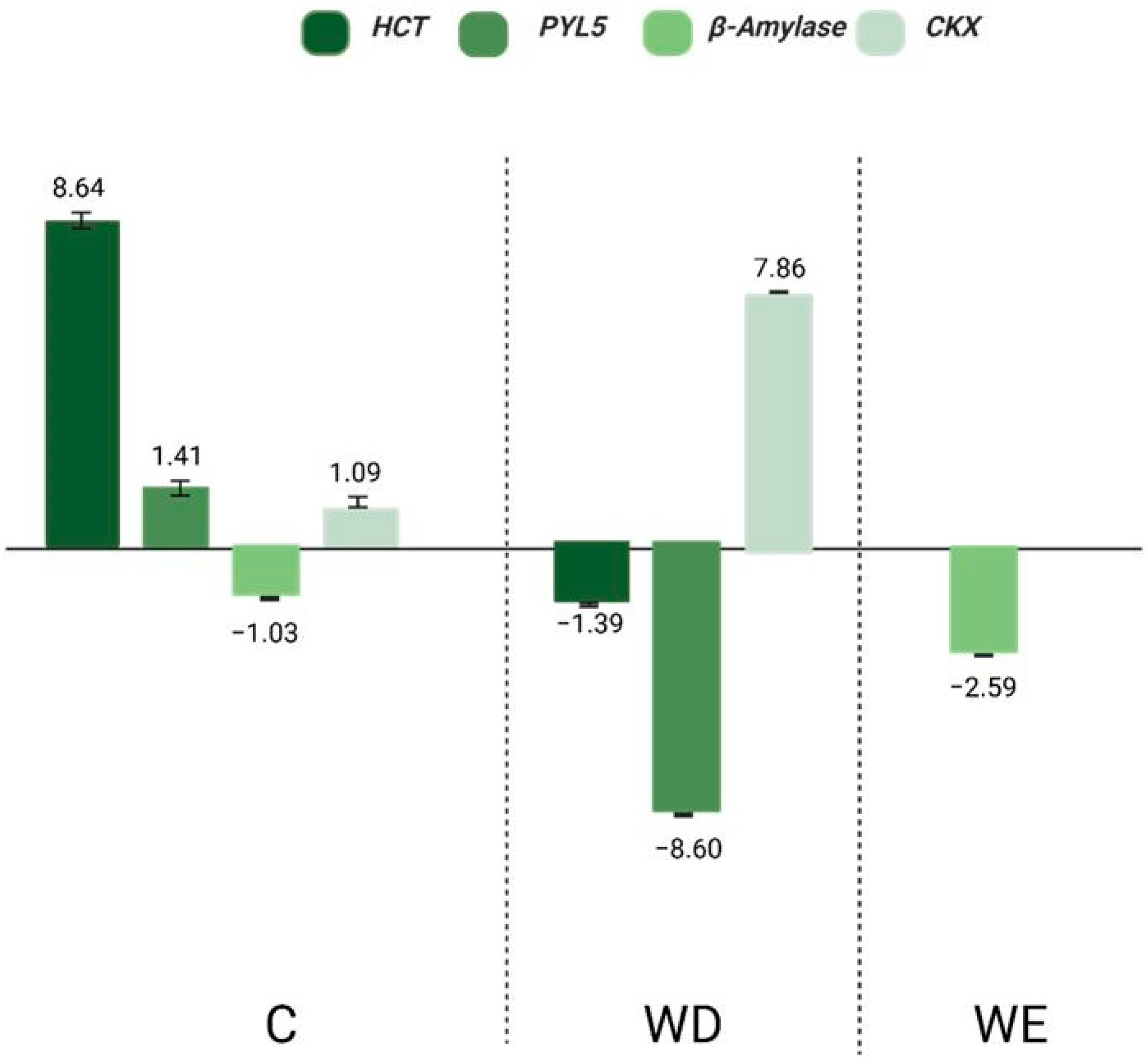
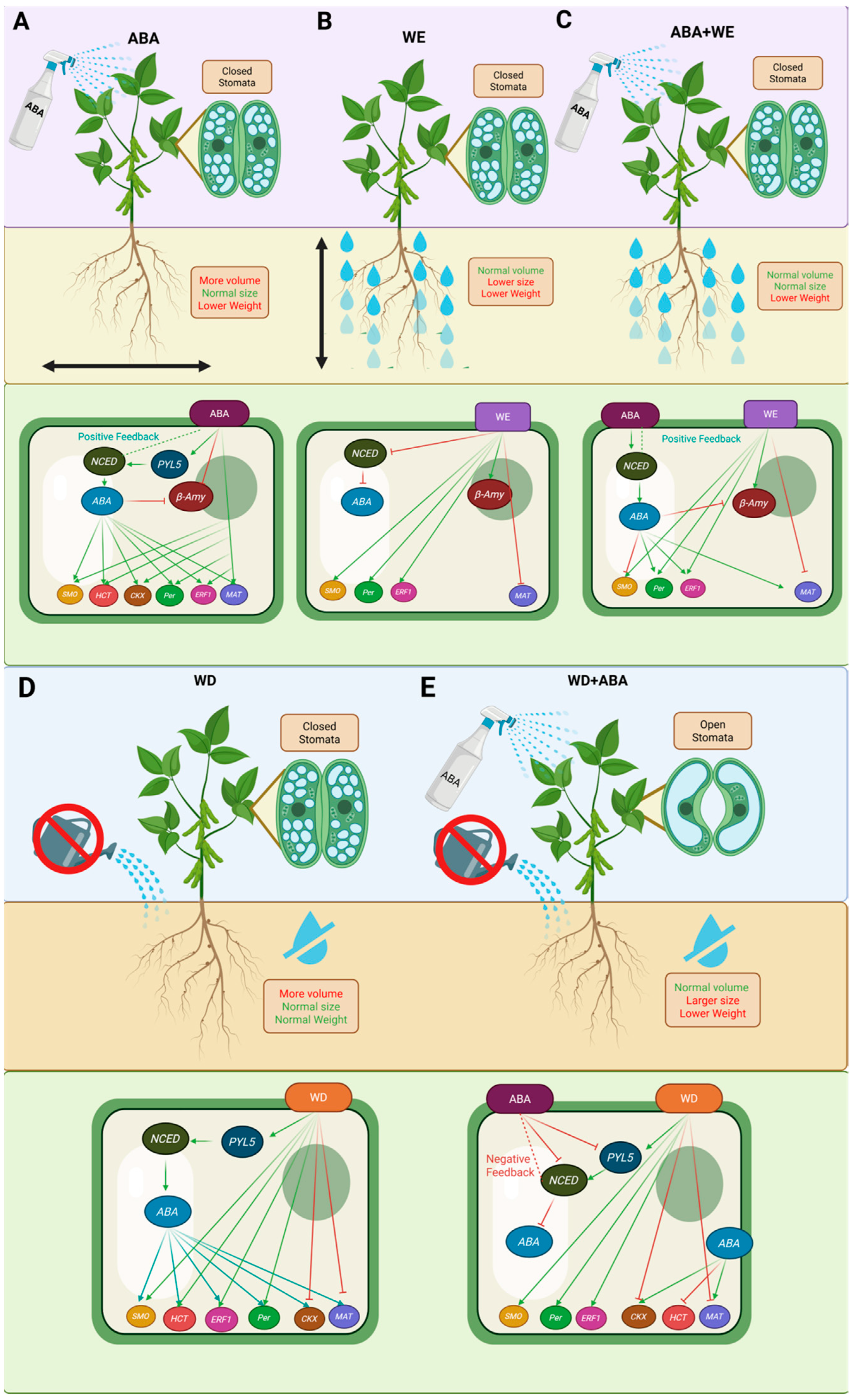
2.6. Effect of ABA on Gene Expression Under WD and WE Conditions
2.7. Gene Copies, Transcripts, and Orthologs in Soybean (Glycine max) and Arabidopsis thaliana: Insights into Conservation and Functional Implications
3. Discussion
3.1. Physiological and Transcriptomics Insights into WD and WE Stress
3.2. Discussion of Gene Regulation in Relation to ABA, WD, and WE
4. Materials and Methods
4.1. Greenhouse Experiment
4.2. Gas Exchange Analysis
4.3. Root Length and Dry Mass Measurements
4.4. Root Analysis by Image
4.5. Identification of Genes Responsive to Water Deficit and Excess in rna-seq Libraries
4.6. Identification of Conserved Motifs in Promoter Regions Responsive to ABA and Abiotic Factors
4.7. System Biology
4.8. Gene Expression Analysis by RT-qPCR
4.9. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| WD | Water Deficit |
| WE | Water Excess |
| ABA | abscisic acid |
References
- Valliyodan, B.; Ye, H.; Song, L.; Murphy, M.; Grover Shannon, J.; Nguyen, H.T. Genetic diversity and genomic strategies for improving drought and waterlogging tolerance in soybeans. J. Exp. Bot. 2017, 68, 1835–1849. [Google Scholar] [CrossRef]
- Ploschuk, R.A.; Miralles, D.J.; Striker, G.G. A quantitative review of soybean responses to waterlogging: Agronomical, morpho-physiological and anatomical traits of tolerance. Plant Soil 2022, 475, 237–252. [Google Scholar] [CrossRef]
- de Sousa, L.F.; de Menezes-Silva, P.E.; Lourenço, L.L.; Galmés, J.; Guimarães, A.C.; da Silva, A.F.; Lima, A.P.d.R.; Henning, L.M.M.; Costa, A.C.; Silva, F.G.; et al. Improving water use efficiency by changing hydraulic and stomatal characteristics in soybean exposed to drought: The involvement of nitric oxide. Physiol. Plant 2020, 168, 576–589. [Google Scholar] [CrossRef] [PubMed]
- Falcioni, R.; de Oliveira, C.A.; Vedana, N.G.; Mendonça, W.A.; Gonçalves, J.V.F.; Haubert, D.d.F.d.S.; de Matos, D.H.S.; Reis, A.S.; Antunes, W.C.; Crusiol, L.G.T.; et al. Progressive Water Deficit Impairs Soybean Growth, Alters Metabolic Profiles, and Decreases Photosynthetic Efficiency. Plants 2025, 14, 2615. [Google Scholar] [CrossRef]
- Wang, C.; Sun, A.; Zhu, L.J.; Liu, M.; Zhang, Q.; Wang, L.; Gao, X. Drought and rewatering effects on soybean photosynthesis, physiology and yield. PeerJ 2025, 13, e19658. [Google Scholar] [CrossRef]
- Adegoye, G.A.; Olorunwa, O.J.; Alsajri, F.A.; Walne, C.H.; Wijewandana, C.; Kethireddy, S.R.; Reddy, K.N.; Reddy, K.R. Waterlogging Effects on Soybean Physiology and Hyperspectral Reflectance during the Reproductive Stage. Agriculture 2023, 13, 844. [Google Scholar] [CrossRef]
- Goergen, P.C.H.; Lopes, S.J.; Zanon, A.J.; Lago, I.; Pohlmann, V.; Dalcin, M.S.; Bittencourt, P.N.; Saccol, V.G. Tolerância de cultivares de soja ao estresse por alagamento em estádios de crescimento vegetativo. Pesqui. Agropecuária Bras. 2023, 58, e03058. [Google Scholar] [CrossRef]
- Wu, C.; Florez-Palacios, L.; Acuna, A.; Harrison, D.; Rogers, D.; Carlin, J.; Mozzoni, L.; Nguyen, H.T.; Shannon, G.; Vieira, C.C. Impact of flooding at the early reproductive growth stage on soybean yield and seed composition. Crop Sci. 2025, 65, e21397. [Google Scholar] [CrossRef]
- Munemasa, S.; Hauser, F.; Park, J.; Waadt, R.; Brandt, B.; Schroeder, J.I. Mechanisms of abscisic acid-mediated control of stomatal aperture. Curr. Opin. Plant Biol. 2015, 28, 154. [Google Scholar] [CrossRef]
- Muhammad Aslam, M.; Waseem, M.; Jakada, B.H.; Okal, E.J.; Lei, Z.; Saqib, H.S.A.; Yuan, W.; Xu, W.; Zhang, Q. Mechanisms of Abscisic Acid-Mediated Drought Stress Responses in Plants. Int. J. Mol. Sci. 2022, 23, 1084. [Google Scholar] [CrossRef]
- Molinari, M.D.C.; Fuganti-Pagliarini, R.; Barbosa, D.d.A.; Barbosa, E.G.G.; Kafer, J.M.; Marin, D.R.; Marin, S.R.R.; Mertz-Henning, L.M.; Nepomuceno, A.L. Comparative ABA-Responsive Transcriptome in Soybean Cultivars Submitted to Different Levels of Drought. Plant Mol. Biol. Report. 2022, 41, 260–276. [Google Scholar] [CrossRef]
- Voesenek, L.A.C.J.; Bailey-Serres, J. Flood adaptive traits and processes: An overview. New Phytol. 2015, 206, 57–73. [Google Scholar] [CrossRef]
- Nakayama, T.J.; Rodrigues, F.A.; Neumaier, N.; Marcolino-Gomes, J.; Molinari, H.B.C.; Santiago, T.R.; Formighieri, E.F.; Basso, M.F.; Farias, J.R.B.; Emygdio, B.M.; et al. Insights into soybean transcriptome reconfiguration under hypoxic stress: Functional, regulatory, structural, and compositional characterization. PLoS ONE 2017, 12, e0187920. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Nishimura, M.; Hajika, M.; Komatsu, S. Quantitative proteomics reveals the flooding-tolerance mechanism in mutant and abscisic acid-treated soybean. J. Proteome Res. 2016, 15, 2008–2025. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yan, C.; Cao, Y.; Wang, C.; Sun, X.; Zhang, L.; Wang, W.; Song, S. Comparative physiological and transcriptomic analysis of two contrasting soybean genotypes reveals complex mechanisms involved in drought avoidance. Crop Sci. 2024, 64, 788–802. [Google Scholar] [CrossRef]
- da Silva, A.A.; Silva, C.O.; do Rosario Rosa, V.; Santos, M.F.S.; Kuki, K.N.; Dal-Bianco, M.; Bueno, R.D.; de Oliveira, J.A.; Brito, D.S.; Costa, A.C.; et al. Metabolic adjustment and regulation of gene expression are essential for increased resistance to severe water deficit and resilience post-stress in soybean. PeerJ 2022, 10, e13118. [Google Scholar] [CrossRef]
- Hwang, S.Y.; Vantoai, T.T. Abscisic Acid Induces Anaerobiosis Tolerance in Corn. Plant Physiol. 1991, 97, 593. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Du, J.; Chen, L.; Zeng, Y.; Tan, X.; Shi, Q.; Pan, X.; Wu, Z.; Zeng, Y. Transcriptomic, proteomic, and physiological comparative analyses of flooding mitigation of the damage induced by low-temperature stress in direct seeded early indica rice at the seedling stage. BMC Genom. 2021, 22, 176. [Google Scholar] [CrossRef]
- Harris, J.M. Abscisic Acid: Hidden Architect of Root System Structure. Plants 2015, 4, 548. [Google Scholar] [CrossRef]
- Ding, Z.; De Smet, I. Localised ABA signalling mediates root growth plasticity. Trends Plant Sci. 2013, 18, 533. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.R.; Wang, Y.B.; He, S.B.; Hao, F.S. Mechanisms for Abscisic Acid Inhibition of Primary Root Growth. Plant Signal. Behav. 2018, 13, e1500069. [Google Scholar] [CrossRef] [PubMed]
- Jhan, L.-H.; Yang, C.-Y.; Huang, C.-M.; Lai, M.-C.; Huang, Y.-H.; Baiya, S.; Kao, C.-F. Integrative pathway and network analysis provide insights on flooding-tolerance genes in soybean. Sci. Rep. 2023, 13, 1980. [Google Scholar] [CrossRef]
- Tamang, B.G.; Li, S.; Rajasundaram, D.; Lamichhane, S.; Fukao, T. Overlapping and stress-specific transcriptomic and hormonal responses to flooding and drought in soybean. Plant J. 2021, 107, 100–117. [Google Scholar] [CrossRef]
- Cheng, M.C.; Liao, P.M.; Kuo, W.W.; Lin, T.P. The Arabidopsis ETHYLENE RESPONSE FACTOR1 Regulates Abiotic Stress-Responsive Gene Expression by Binding to Different cis-Acting Elements in Response to Different Stress Signals. Plant Physiol. 2013, 162, 1566. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Chen, M.; Li, L.; Xu, Z.; Chen, X.; Guo, J.; Ma, Y. Overexpression of the soybean GmERF3 gene, an AP2/ERF type transcription factor for increased tolerances to salt, drought, and diseases in transgenic tobacco. J. Exp. Bot. 2009, 60, 3781–3796. [Google Scholar] [CrossRef]
- Fang, X.; Ma, J.; Guo, F.; Qi, D.; Zhao, M.; Zhang, C.; Wang, L.; Song, B.; Liu, S.; He, S.; et al. The AP2/ERF GmERF113 Positively Regulates the Drought Response by Activating GmPR10-1 in Soybean. Int. J. Mol. Sci. 2022, 23, 8159. [Google Scholar] [CrossRef]
- Sofo, A.; Scopa, A.; Nuzzaci, M.; Vitti, A. Ascorbate Peroxydase and Catalase Activities and Their Genetic Regulation in Plants Subjected to Drought and Salinity Stresses. Int. J. Mol. Sci. 2015, 16, 13561. [Google Scholar] [CrossRef]
- Zhai, X.; Yan, X.; Zenda, T.; Wang, N.; Dong, A.; Yang, Q.; Zhong, Y.; Xing, Y.; Duan, H. Overexpression of the Peroxydase gene ZmPRX1 increases maize seedling drought tolerance by promoting root development and lignification. Crop. J. 2024, 12, 753–765. [Google Scholar] [CrossRef]
- Jardim-Messeder, D.; Caverzan, A.; Balbinott, N.; Menguer, P.K.; Paiva, A.L.S.; Lemos, M.; Cunha, J.R.; Gaeta, M.L.; Costa, M.; Zamocky, M.; et al. Stromal Ascorbate Peroxydase (OsAPX7) Modulates Drought Stress Tolerance in Rice (Oryza sativa). Antioxidants 2023, 12, 387. [Google Scholar] [CrossRef]
- Moschou, P.N.; Paschalidis, K.A.; Delis, I.D.; Andriopoulou, A.H.; Lagiotis, G.D.; Yakoumakis, D.I.; Roubelakis-Angelakis, K.A. Spermidine Exodus and Oxidation in the Apoplast Induced by Abiotic Stress Is Responsible for H2O2 Signatures That Direct Tolerance Responses in Tobacco. Plant Cell 2008, 20, 1708–1724. [Google Scholar] [CrossRef]
- Sagor, G.H.M.; Inoue, M.; Kusano, T.; Berberich, T. Expression profile of seven polyamine oxidase genes in rice (Oryza sativa) in response to abiotic stresses, phytohormones and polyamines. Physiol. Mol. Biol. Plants 2021, 27, 1353–1359. [Google Scholar] [CrossRef]
- Yu, Z.; Jia, D.; Liu, T. Polyamine Oxidases Play Various Roles in Plant Development and Abiotic Stress Tolerance. Plants 2019, 8, 184. [Google Scholar] [CrossRef]
- Pottosin, I.; Velarde-Buendía, A.M.; Bose, J.; Zepeda-Jazo, I.; Shabala, S.; Dobrovinskaya, O. Cross-talk between reactive oxygen species and polyamines in regulation of ion transport across the plasma membrane: Implications for plant adaptive responses. J. Exp. Bot. 2014, 65, 1271–1283. [Google Scholar] [CrossRef]
- Molinari, M.D.C.; Fuganti-Pagliarini, R.; Marin, S.R.R.; Ferreira, L.C.; Barbosa, D.d.A.; Marcolino-Gomes, J.; de Oliveira, M.C.N.; Mertz-Henning, L.M.; Kanamori, N.; Takasaki, H.; et al. Overexpression of AtNCED3 gene improved drought tolerance in soybean in greenhouse and field conditions. Genet. Mol. Biol. 2020, 43, e20190292. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, F.K.; Da-Silva, C.J.; Garcia, N.; Agualongo, D.A.P.; de Oliveira, A.C.B.; Kanamori, N.; Takasaki, H.; Urano, K.; Shinozaki, K.; Nakashima, K.; et al. The overexpression of NCED results in waterlogging sensitivity in soybean. Plant Stress 2022, 3, 100047. [Google Scholar] [CrossRef]
- Iuchi, S.; Kobayashi, M.; Taji, T.; Naramoto, M.; Seki, M.; Kato, T.; Tabata, S.; Kakubari, Y.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Regulation of drought tolerance by gene manipulation of 9-cis-epoxycarotenoid dioxygenase, a key enzyme in abscisic acid biosynthesis in Arabidopsis. Plant J. 2001, 27, 325–333. [Google Scholar] [CrossRef]
- Wan, X.R.; Li, L. Regulation of ABA level and water-stress tolerance of Arabidopsis by ectopic expression of a peanut 9-cis-epoxycarotenoid dioxygenase gene. Biochem. Biophys. Res. Commun. 2006, 347, 1030–1038. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Oh, M.W.; Komatsu, S. Characterization of S-adenosylmethionine synthetases in soybean under flooding and drought stresses. Biol. Plant 2016, 60, 269–278. [Google Scholar] [CrossRef]
- Arraes, F.B.M.; Beneventi, M.A.; Lisei de Sa, M.E.; Paixao, J.F.R.; Albuquerque, E.V.S.; Marin, S.R.R.; Purgatto, E.; Nepomuceno, A.L.; Grossi-de-Sa, M.F. Implications of ethylene biosynthesis and signaling in soybean drought stress tolerance. BMC Plant Biol. 2015, 15, 213. [Google Scholar] [CrossRef]
- Santiago, J.; Rodrigues, A.; Saez, A.; Rubio, S.; Antoni, R.; Dupeux, F.; Park, S.; Márquez, J.A.; Cutler, S.R.; Rodriguez, P.L. Modulation of drought resistance by the abscisic acid receptor PYL5 through inhibition of clade A PP2Cs. Plant J. 2009, 60, 575–588. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, K.; Hwang, H.; Bhatnagar, N.; Kim, D.-Y.; Yoon, I.S.; Byun, M.-O.; Kim, S.T.; Jung, K.-H.; Kim, B.-G. Overexpression of PYL5 in rice enhances drought tolerance, inhibits growth, and modulates gene expression. J. Exp. Bot. 2014, 65, 453. [Google Scholar] [CrossRef] [PubMed]
- Quan, W.; Hu, Y.; Mu, Z.; Shi, H.; Chan, Z. Overexpression of AtPYL5 under the control of guard cell specific promoter improves drought stress tolerance in Arabidopsis. Plant Physiol. Biochem. 2018, 129, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Guzman, M.; Pizzio, G.A.; Antoni, R.; Vera-Sirera, F.; Merilo, E.; Bassel, G.W.; Fernández, M.A.; Holdsworth, M.J.; Perez-Amador, M.A.; Kollist, H.; et al. Arabidopsis PYR/PYL/RCAR receptors play a major role in quantitative regulation of stomatal aperture and transcriptional response to abscisic acid. Plant Cell 2012, 24, 2483–2496. [Google Scholar] [CrossRef]
- Hoffmann, L.; Besseau, S.; Geoffroy, P.; Ritzenthaler, C.; Meyer, D.; Lapierre, C.; Pollet, B.; Legrand, M. Silencing of Hydroxycinnamoyl-Coenzyme A Shikimate/Quinate Hydroxycinnamoyltransferase Affects Phenylpropanoid Biosynthesis. Plant Cell 2004, 16, 1446–1465. [Google Scholar] [CrossRef]
- Chen, Y.; Yi, N.; Yao, S.B.; Zhuang, J.; Fu, Z.; Ma, J.; Yin, S.; Jiang, X.; Liu, Y.; Gao, L.; et al. CsHCT-Mediated Lignin Synthesis Pathway Involved in the Response of Tea Plants to Biotic and Abiotic Stresses. J. Agric. Food Chem. 2021, 69, 10069–10081. [Google Scholar] [CrossRef]
- Coutinho, F.S.; Mesquita, R.O.; Rodrigues, J.M.; Zanotti, A.; Faustino, V.A.; Barros, E.; Vital, C.E.; Oliveira, M.G.d.A.; Meira, R.M.S.A.; Williams, T.C.R.; et al. Alterations in the root phenylpropanoid pathway and root–shoot vessel system as main determinants of the drought tolerance of a soybean genotype. Physiol. Mol. Biol. Plants 2023, 29, 559. [Google Scholar] [CrossRef]
- Köllmer, I.; Novák, O.; Strnad, M.; Schmülling, T.; Werner, T. Overexpression of the cytosolic cytokinin oxidase/dehydrogenase (CKX7) from Arabidopsis causes specific changes in root growth and xylem differentiation. Plant J. 2014, 78, 359–371. [Google Scholar] [CrossRef]
- Werner, T.; Motyka, V.; Laucou, V.; Smets, R.; Van Onckelen, H.; Schmülling, T. Cytokinin-Deficient Transgenic Arabidopsis Plants Show Multiple Developmental Alterations Indicating Opposite Functions of Cytokinins in the Regulation of Shoot and Root Meristem Activity. Plant Cell 2003, 15, 2532. [Google Scholar] [CrossRef]
- Rashid, A.; Achary, V.M.M.; Abdin, M.Z.; Karippadakam, S.; Parmar, H.; Panditi, V.; Prakash, G.; Bhatnagar-Mathur, P.; Reddy, M.K. Cytokinin oxidase2-deficient mutants improve panicle and grain architecture through cytokinin accumulation and enhance drought tolerance in indica rice. Plant Cell Rep. 2024, 43, 207. [Google Scholar] [CrossRef]
- Masoabi, M.; Snyman, S.; Pols, S.; Hills, P.N.; van der Vyver, C. Response of sugarcane plants with modified cytokinin homeostasis under water deficit conditions. Plant Stress 2023, 10, 100240. [Google Scholar] [CrossRef]
- Le, D.T.; Nishiyama, R.; Watanabe, Y.; Vankova, R.; Tanaka, M.; Seki, M.; Ham, L.H.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Tran, L.-S.P. Identification and Expression Analysis of Cytokinin Metabolic Genes in Soybean under Normal and Drought Conditions in Relation to Cytokinin Levels. PLoS ONE 2012, 7, e42411. [Google Scholar] [CrossRef]
- Zanella, M.; Borghi, G.L.; Pirone, C.; Thalmann, M.; Pazmino, D.; Costa, A.; Santelia, D.; Trost, P.; Sparla, F. β-amylase 1 (BAM1) degrades transitory starch to sustain proline biosynthesis during drought stress. J. Exp. Bot. 2016, 67, 1819–1826. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Yang, X.; Wang, X.; Li, Q.; Guo, J.; Ma, T.; Zhao, C.; Tang, Y.; Qiao, L.; Wang, J.; et al. The sweetpotato β-amylase gene IbBAM1.1 enhances drought and salt stress resistance by regulating ROS homeostasis and osmotic balance. Plant Physiol. Biochem. 2021, 168, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Butsayawarapat, P.; Juntawong, P.; Khamsuk, O.; Somta, P. Comparative Transcriptome Analysis of Waterlogging-Sensitive and Tolerant Zombi Pea (Vigna vexillata) Reveals Energy Conservation and Root Plasticity Controlling Waterlogging Tolerance. Plants 2019, 8, 264. [Google Scholar] [CrossRef]
- Fehr, W.; Caviness, C. Stages of Soybean Development. 1977. Available online: https://dr.lib.iastate.edu/handle/20.500.12876/90239 (accessed on 7 September 2025).
- Chen, M.; Wang, Q.-Y.; Cheng, X.-G.; Xu, Z.-S.; Li, L.-C.; Ye, X.-G.; Xia, L.-Q.; Ma, Y.-Z. GmDREB2, a soybean DRE-binding transcription factor, conferred drought and high-salt tolerance in transgenic plants. Biochem. Biophys. Res. Commun. 2007, 353, 299–305. [Google Scholar] [CrossRef]
- Long, S.P.; Bernacchi, C.J. Gas exchange measurements, what can they tell us about the underlying limitations to photosynthesis? Procedures and sources of error. J. Exp. Bot. 2003, 54, 2393–2401. [Google Scholar] [CrossRef] [PubMed]
- Flexas, J.; Bota, J.; Loreto, F.; Cornic, G.; Sharkey, T.D. Diffusive and metabolic limitations to photosynthesis under drought and salinity in C3 plants. Plant Biol. 2004, 6, 269–279. [Google Scholar] [CrossRef]
- González-Guzmán, M.; Gómez-Cadenas, A.; Arbona, V. Abscisic Acid as an Emerging Modulator of the Responses of Plants to Low Oxygen Conditions. Front. Plant Sci. 2021, 12, 661789. [Google Scholar] [CrossRef]
- Hunt, R. Basic Growth Analysis; Springer Nature: Dordrecht, The Netherlands, 1990. [Google Scholar]
- Poorter, H.; Garnier, E. Ecological significance of inherent variation in relative growth rate and its components. In Functional Plant Ecology, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007; pp. 67–100. Available online: https://www.taylorfrancis.com/chapters/edit/10.1201/9781420007626-3/ecological-significance-inherent-variation-relative-growth-rate-components-hendrik-poorter-eric-garnier (accessed on 7 September 2025).
- Mondal, S.; Chakraborty, D. Root growth and physiological responses in wheat to topsoil and subsoil compaction with or without artificial vertical macropores. Heliyon 2023, 9, e18834. [Google Scholar] [CrossRef]
- Conesa, A.; Madrigal, P.; Tarazona, S.; Gomez-Cabrero, D.; Cervera, A.; McPherson, A.; Szcześniak, M.W.; Gaffney, D.J.; Elo, L.L.; Zhang, X.; et al. A survey of best practices for RNA-seq data analysis. Genome Biol. 2016, 17, 13. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.K.; Jain, M. NGS QC Toolkit: A Toolkit for Quality Control of Next Generation Sequencing Data. PLoS ONE 2012, 7, e30619. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Smith, A.D.; de Sena Brandine, G. Falco: High-speed FastQC emulation for quality control of sequencing data. F1000Res 2021, 8, 1874. [Google Scholar]
- Wen, G. A simple process of RNA-sequence analyses by Hisat2, Htseq and DESeq2. In Proceedings of the 2017 International Conference on Biomedical Engineering and Bioinformatics, Bangkok, Thailand, 14–16 September 2017; pp. 11–15. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R.; 1000 Genome Project Data Processing Subgroup. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- Feng, J.; Meyer, C.A.; Wang, Q.; Liu, J.S.; Liu, X.S.; Zhang, Y. GFOLD: A generalized fold change for ranking differentially expressed genes from RNA-seq data. Bioinformatics 2012, 28, 2782–2788. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Contreras-Moreira, B.; Castro-Mondragon, J.A.; Rioualen, C.; Cantalapiedra, C.P.; van Helden, J. RSAT::Plants: Motif Discovery Within Clusters of Upstream Sequences in Plant Genomes. Methods Mol. Biol. 2016, 1482, 279–295. [Google Scholar]
- Maruyama, K.; Todaka, D.; Mizoi, J.; Yoshida, T.; Kidokoro, S.; Matsukura, S.; Takasaki, H.; Sakurai, T.; Yamamoto, Y.Y.; Yoshiwara, K.; et al. Identification of cis-acting promoter elements in cold-and dehydration-induced transcriptional pathways in arabidopsis, rice, and soybean. DNA Res. 2012, 19, 37–49. [Google Scholar] [CrossRef]
- Gasch, P.; Fundinger, M.; Müller, J.T.; Lee, T.; Bailey-Serres, J.; Mustropha, A. Redundant ERF-VII Transcription Factors Bind to an Evolutionarily Conserved cis-Motif to Regulate Hypoxia-Responsive Gene Expression in Arabidopsis. Plant Cell 2016, 28, 160–180. [Google Scholar] [CrossRef]
- Wan, Q.; Chen, S.; Shan, Z.; Yang, Z.; Chen, L.; Zhang, C.; Yuan, S.; Hao, Q.; Zhang, X.; Qiu, D.; et al. Stability evaluation of reference genes for gene expression analysis by RT-qPCR in soybean under different conditions. PLoS ONE 2017, 12, e0189405. [Google Scholar] [CrossRef] [PubMed]


| Gene ID | SNC | Copy Gene | %S | STN | Annotation | Enzyme Code | Orthologs | ATC | ATN | %S |
|---|---|---|---|---|---|---|---|---|---|---|
| Glyma.15G250100 | na | na | 93 | 1 | NCED3 | EC 1.13.11.51 | na | na | na | na |
| Glyma.03G162700 | na | na | na | 1 | ERF1 | na | na | na | na | na |
| Glyma.02G254600 | 2 | Glyma.14G061800 | 92 | 1/1 | HCT | EC 2.3.1.133 | AT2G39980 | na | 1 | 70 |
| Glyma.02G261900 | na | na | na | 1 | PYL5 | Na | AT2G38310 | na | 1 | 69 |
| Glyma.11G039400 | 2 | Glyma.01G203400 | 94 | 1/1 | β-amylase | EC 3.2.1.2 | na | na | na | na |
| Glyma.18G045100 | na | na | na | 2 | SMO | EC 1.5.3.17 | AT1G65840 | na | 1 | 70 |
| Glyma.19G223000 | 2 | Glyma.03G226000 | 95 | 1/2 | MAN | EC 3.2.1.78 | na | na | na | na |
| Glyma.11G149100 | na | na | na | 1 | CKX | EC 1.5.99.12 | na | na | na | na |
| Glyma.18G258000 | na | na | na | 1 | MAT | EC 2.3.1.115 | na | na | na | na |
| Glyma.15G052600 | na | na | na | 1 | Peroxydase | EC 1.11.1.7 | na | na | na | na |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andreata, E.C.; Molinari, M.D.C.; Kafer, J.M.; Marin, S.R.R.; Marin, D.R.; Fuganti-Pagliarini, R.; Vanzela, A.L.L.; Rech, E.L.; Nepomuceno, A.L.; Mertz-Henning, L.M. Molecular Mechanisms and Crosstalk Signaling in Soybean’s Response to Water Deficit and Excess: Implications for Stress Resilience and Productivity. Plants 2025, 14, 3245. https://doi.org/10.3390/plants14213245
Andreata EC, Molinari MDC, Kafer JM, Marin SRR, Marin DR, Fuganti-Pagliarini R, Vanzela ALL, Rech EL, Nepomuceno AL, Mertz-Henning LM. Molecular Mechanisms and Crosstalk Signaling in Soybean’s Response to Water Deficit and Excess: Implications for Stress Resilience and Productivity. Plants. 2025; 14(21):3245. https://doi.org/10.3390/plants14213245
Chicago/Turabian StyleAndreata, Elizandra Carneiro, Mayla Daiane Correa Molinari, João Matheus Kafer, Silvana Regina Rockenbach Marin, Daniel Rockenbach Marin, Renata Fuganti-Pagliarini, André Luis Laforga Vanzela, Elibio Leopoldo Rech, Alexandre Lima Nepomuceno, and Liliane Marcia Mertz-Henning. 2025. "Molecular Mechanisms and Crosstalk Signaling in Soybean’s Response to Water Deficit and Excess: Implications for Stress Resilience and Productivity" Plants 14, no. 21: 3245. https://doi.org/10.3390/plants14213245
APA StyleAndreata, E. C., Molinari, M. D. C., Kafer, J. M., Marin, S. R. R., Marin, D. R., Fuganti-Pagliarini, R., Vanzela, A. L. L., Rech, E. L., Nepomuceno, A. L., & Mertz-Henning, L. M. (2025). Molecular Mechanisms and Crosstalk Signaling in Soybean’s Response to Water Deficit and Excess: Implications for Stress Resilience and Productivity. Plants, 14(21), 3245. https://doi.org/10.3390/plants14213245






