Microbiome Analysis Reveals Biocontrol of Aspergillus and Mycotoxin Mitigation in Maize by the Growth-Promoting Fungal Endophyte Colletotrichum tofieldiae Ct0861
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Inocula
2.2. Plant Material and Ct0861 Applications
2.3. Site Description, Irrigation and Fertilization
2.4. Plant Growth and Yield Measurements
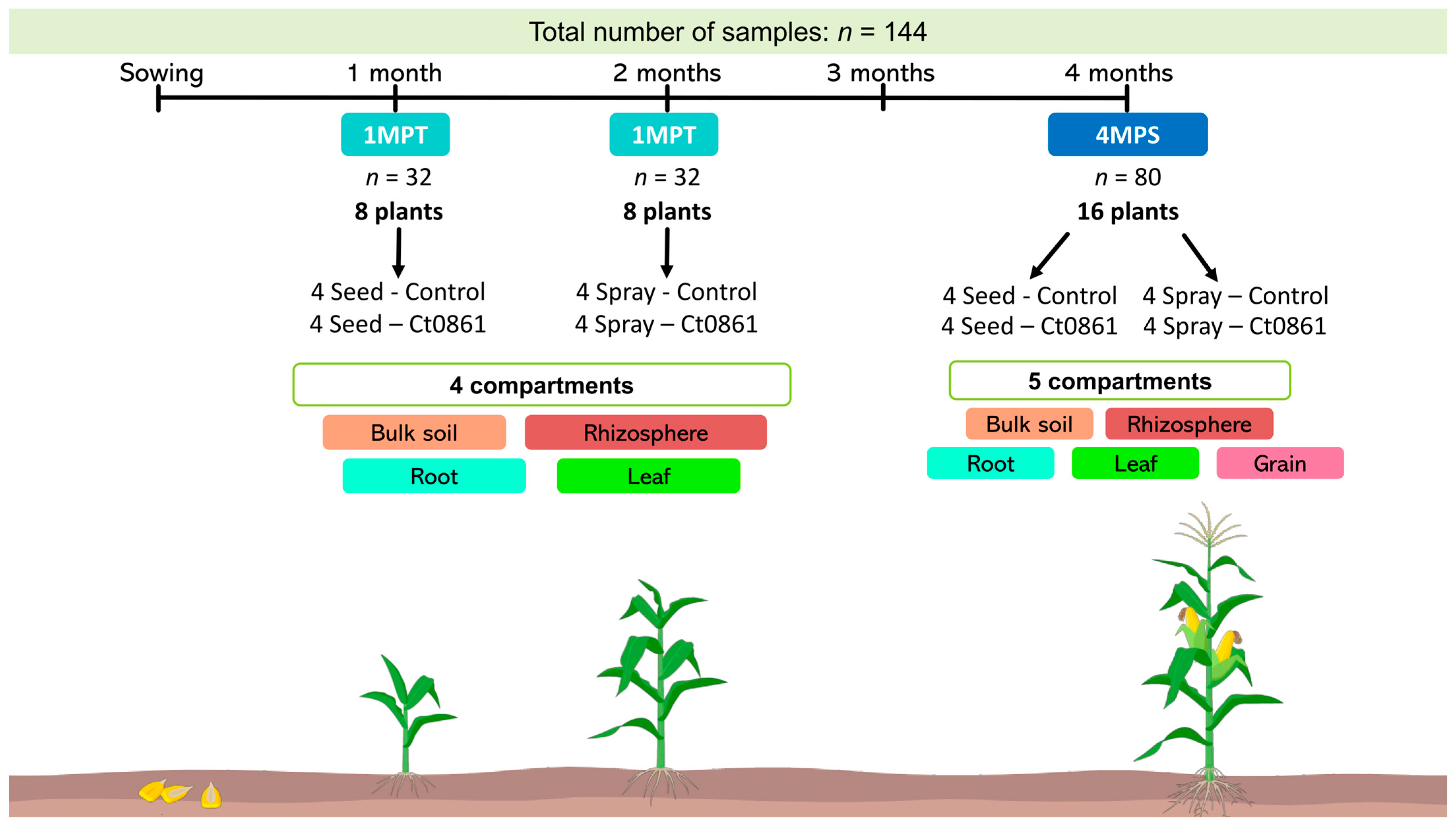
2.5. Sample Collection, Processing, and DNA Extraction for Microbiome Analysis
2.6. Amplicon Generation and Sequencing
2.7. Read Data Processing and Taxonomic Assignment
2.8. Bioinformatic Analysis
2.8.1. Diversity Analysis
2.8.2. Taxonomic Composition
2.8.3. Aspergillus spp. Abundance
2.9. Ct0861 and Aspergillus Biomass Quantification in Grain Samples
2.10. Controlled Greenhouse Infection Assays with A. flavus in Maize Plants
2.11. Ct0861-A. flavus Dual Culture Bioassay
2.12. Statistical Analyses
3. Results
3.1. Ct0861 Seed and Spray Applications Have Comparable Plant Growth and Yield Promotion Effects in Field Trials
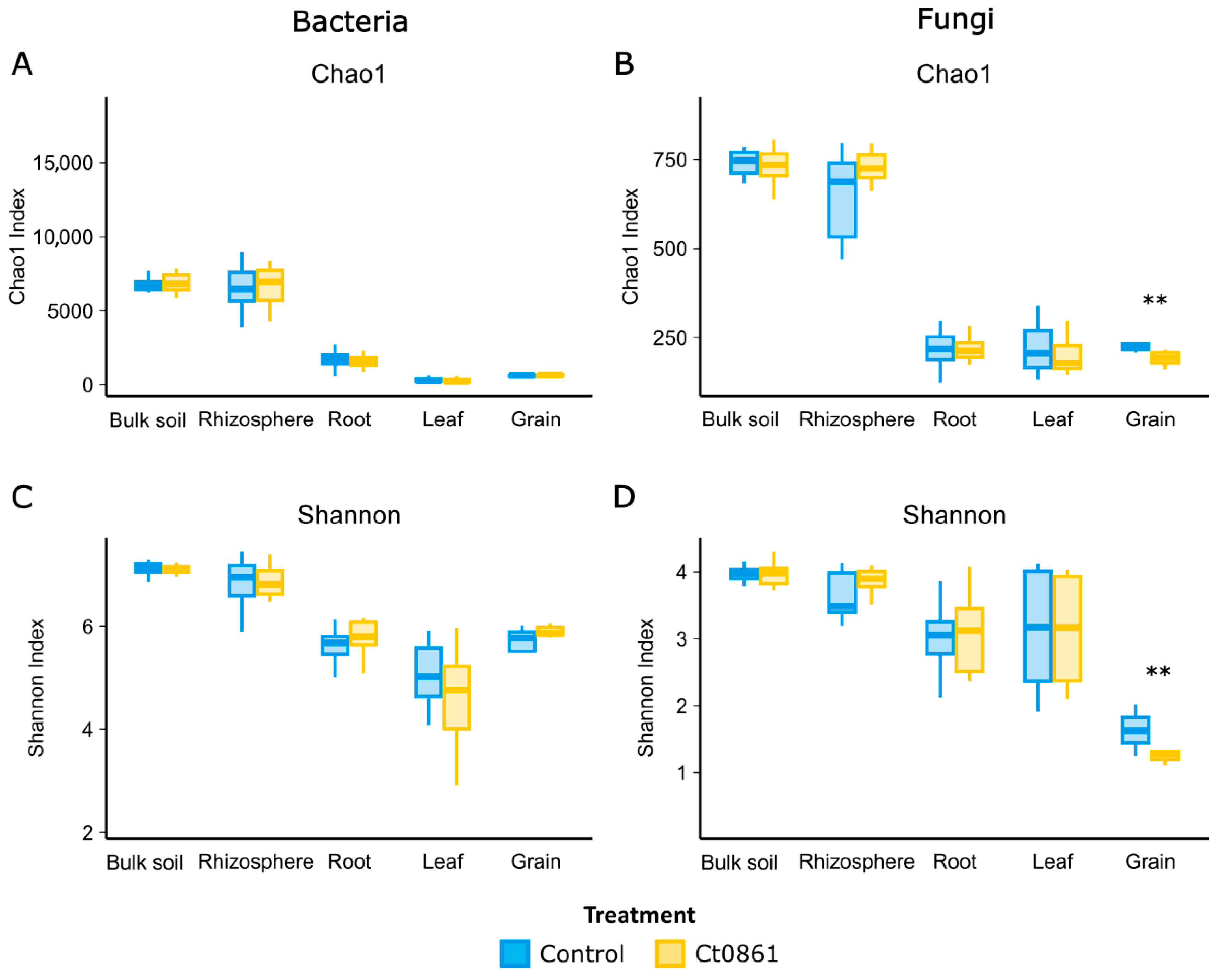
3.2. Ct0861 Treatment Decreased Alpha-Diversity of Fungal Communities in the Grain
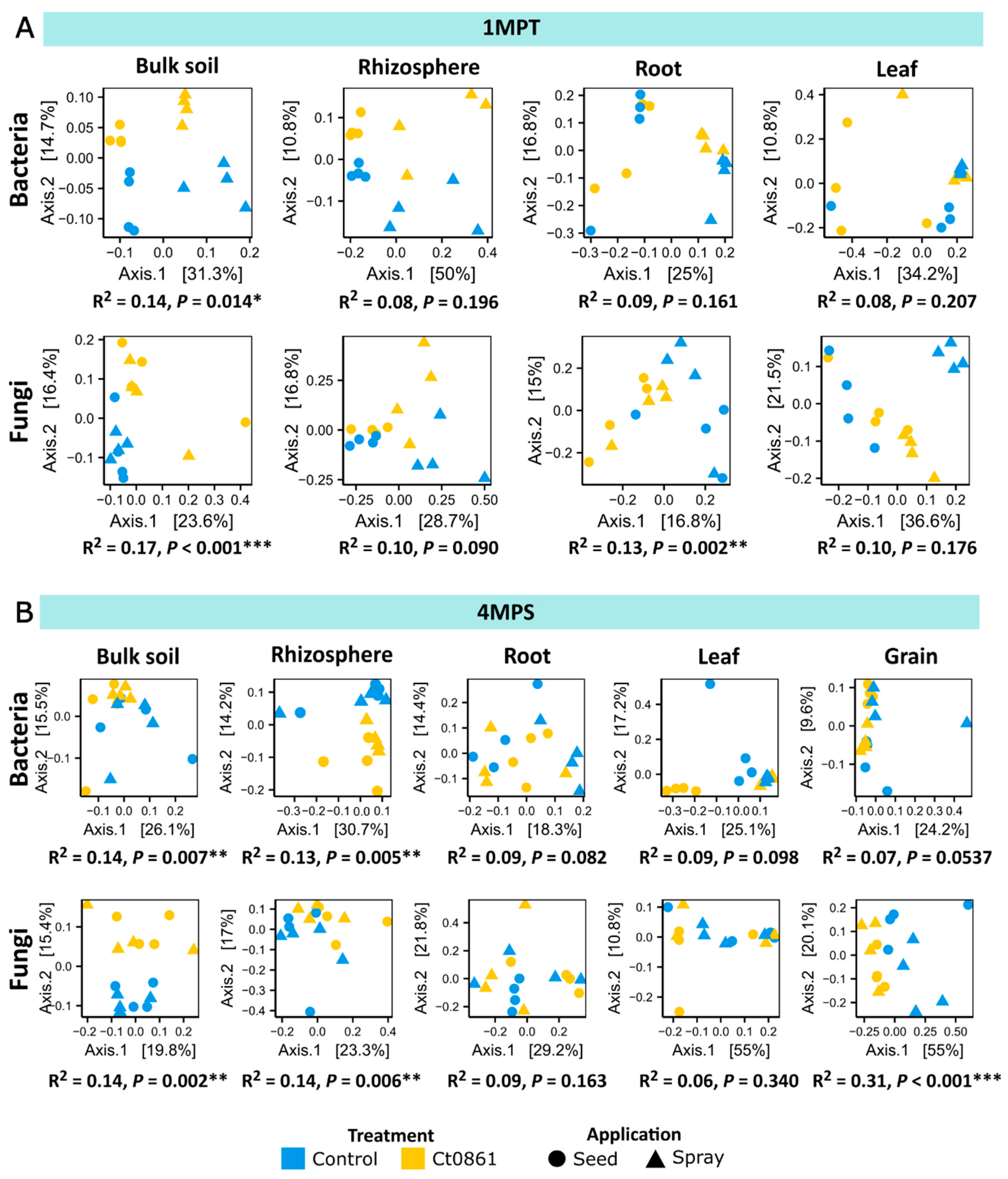
3.3. Ct0861 Application Exerts an Influence on Microbial Beta-Diversity, Mainly in the Soil Compartments and the Grain
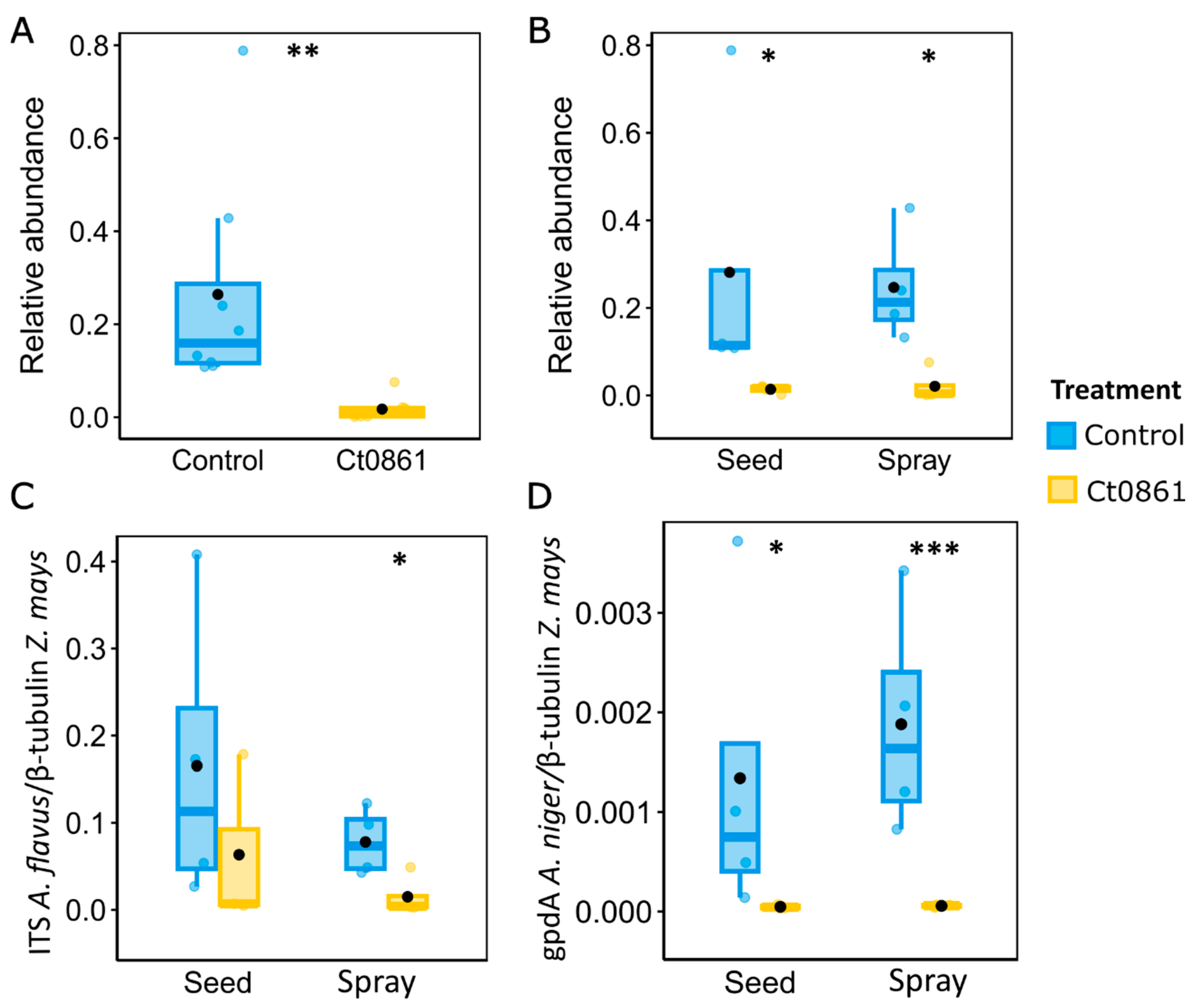
3.4. Ct0861-Treated Plants Showed Reduced Abundances of Aspergillus spp. in the Grain
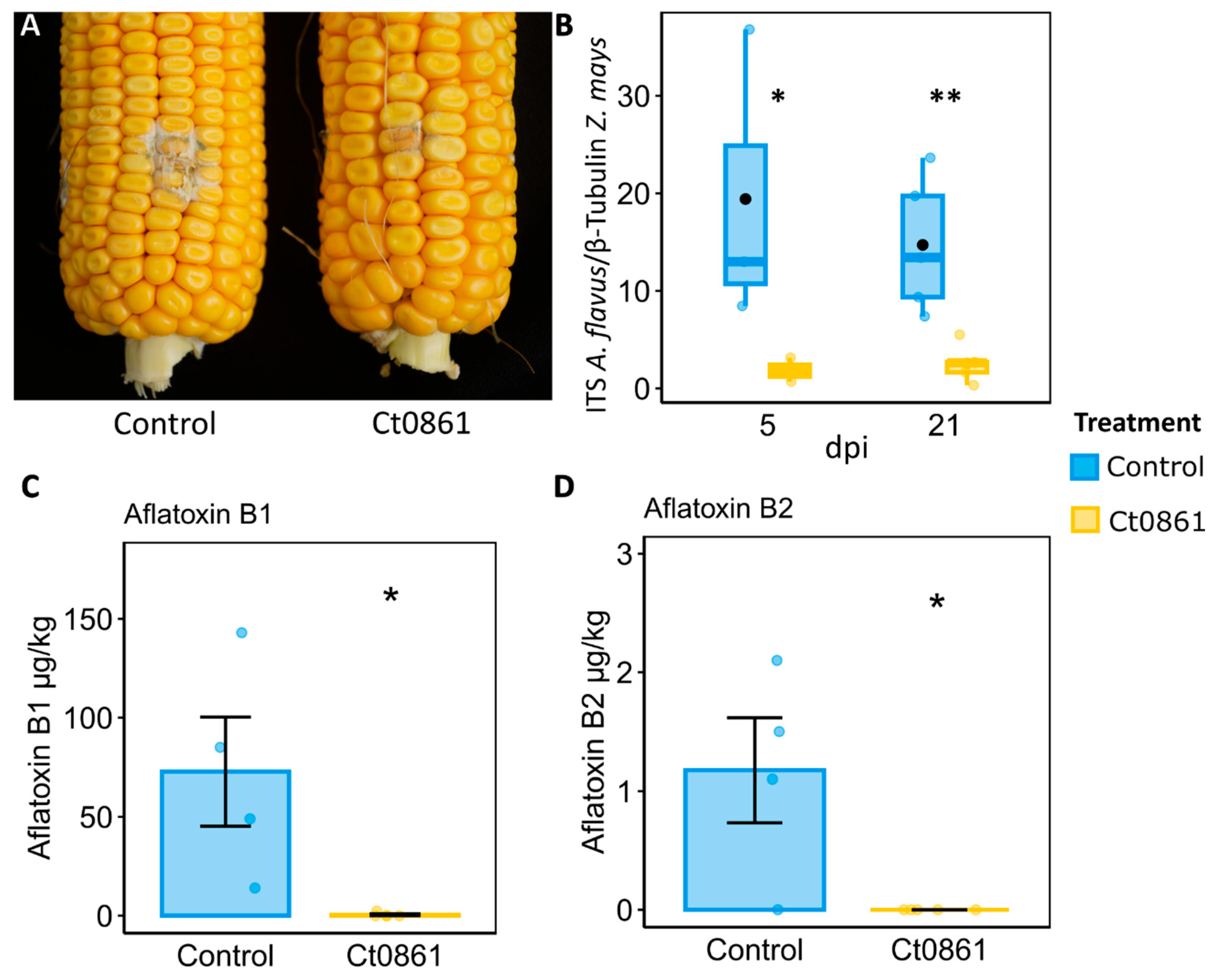
3.5. Ct0861 Treatment Reduces the Growth of Artificially Inoculated A. flavus and Aflatoxins Levels in Maize Grains
3.6. Ct0861 Does Not Have a Direct Antagonistic Effect on A. flavus Growth
4. Discussion
4.1. Ct0861 Enhances Maize Growth and Yield Irrespective of the Application Method
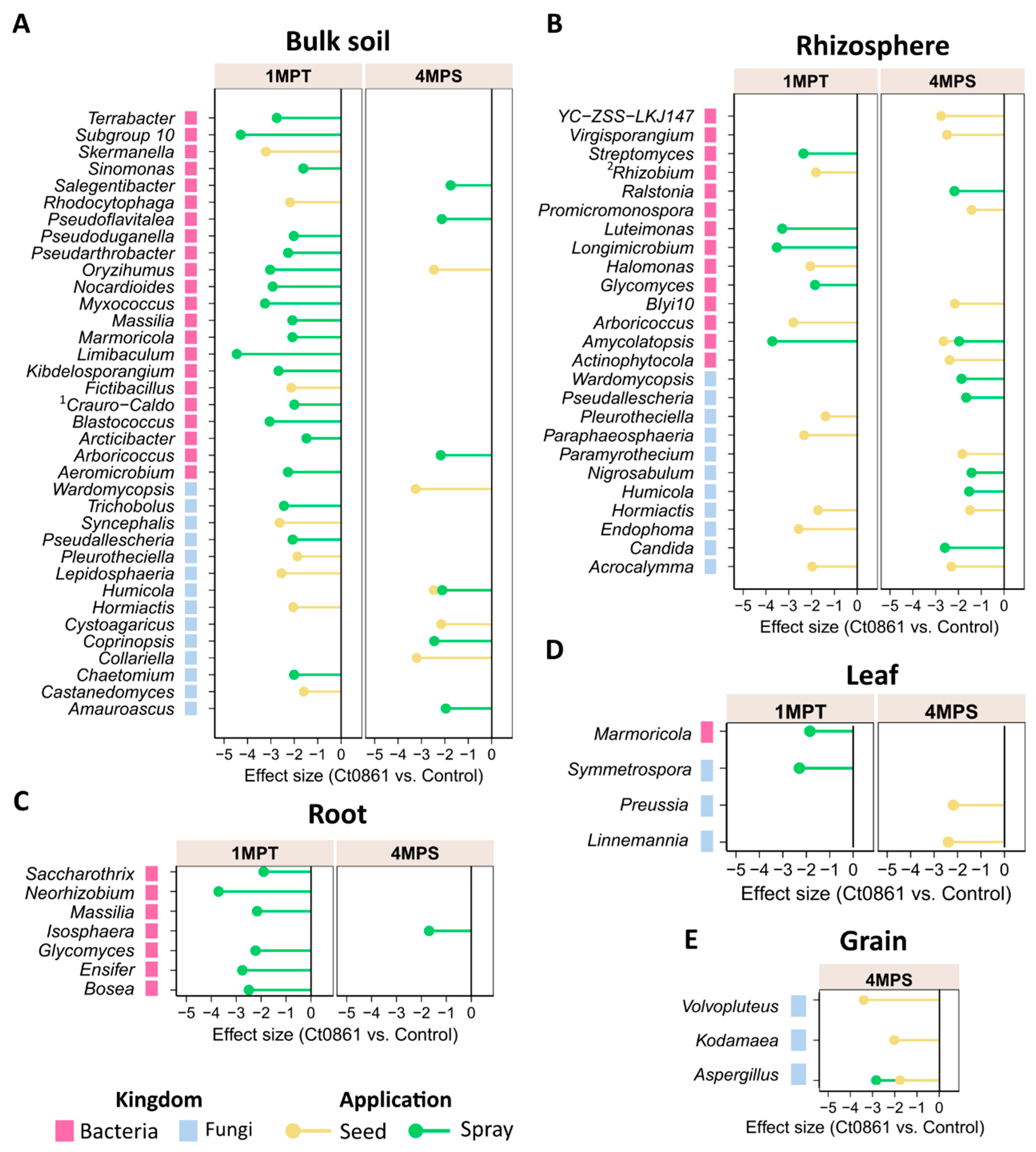
4.2. Ct0861 Induces Subtle Compartment-Specific Shifts in the Maize Microbiome While Preserving Its General Structure
4.3. Ct0861 Treatment Reduces Aspergillus spp. and Mycotoxin Contamination in Maize Grains, Likely Through Induced Resistance
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ranum, P.; Peña-Rosas, J.P.; Garcia-Casal, M.N. Global Maize Production, Utilization, and Consumption. Ann. N. Y Acad. Sci. 2014, 1312, 105–112. [Google Scholar] [CrossRef]
- Wollenberg, E.; Vermeulen, S.J.; Girvetz, E.; Loboguerrero, A.M.; Ramirez-Villegas, J. Reducing Risks to Food Security from Climate Change. Glob. Food Sec. 2016, 11, 34–43. [Google Scholar] [CrossRef]
- Ocwa, A.; Harsanyi, E.; Széles, A.; Holb, I.J.; Szabó, S.; Rátonyi, T.; Mohammed, S. A Bibliographic Review of Climate Change and Fertilization as the Main Drivers of Maize Yield: Implications for Food Security. Agric. Food Secur. 2023, 12, 14. [Google Scholar] [CrossRef]
- Ismaiel, A.; Papenbrock, J. Mycotoxins: Producing Fungi and Mechanisms of Phytotoxicity. Agriculture 2015, 5, 492–537. [Google Scholar] [CrossRef]
- Nji, Q.N.; Babalola, O.O.; Mwanza, M. Aflatoxins in Maize: Can Their Occurrence Be Effectively Managed in Africa in the Face of Climate Change and Food Insecurity? Toxins 2022, 14, 574. [Google Scholar] [CrossRef] [PubMed]
- Stoev, S.D. Foodborne Mycotoxicoses, Risk Assessment and Underestimated Hazard of Masked Mycotoxins and Joint Mycotoxin Effects or Interaction. Env. Toxicol. Pharmacol. 2015, 39, 794–809. [Google Scholar] [CrossRef] [PubMed]
- Omotayo, O.P.; Omotayo, A.O.; Mwanza, M.; Babalola, O.O. Prevalence of Mycotoxins and Their Consequences on Human Health. Toxicol. Res. 2019, 35, 1–7. [Google Scholar] [CrossRef]
- Sammauria, R.; Kumawat, S.; Kumawat, P.; Singh, J.; Jatwa, T.K. Microbial Inoculants: Potential Tool for Sustainability of Agricultural Production Systems. Arch. Microbiol. 2020, 202, 677–693. [Google Scholar] [CrossRef]
- Busby, P.E.; Soman, C.; Wagner, M.R.; Friesen, M.L.; Kremer, J.; Bennett, A.; Morsy, M.; Eisen, J.A.; Leach, J.E.; Dangl, J.L. Research Priorities for Harnessing Plant Microbiomes in Sustainable Agriculture. PLoS Biol. 2017, 15, e2001793. [Google Scholar] [CrossRef]
- Jat, S.L.; Suby, S.B.; Parihar, C.M.; Gambhir, G.; Kumar, N.; Rakshit, S. Microbiome for Sustainable Agriculture: A Review with Special Reference to the Corn Production System. Arch. Microbiol. 2021, 203, 2771–2793. [Google Scholar] [CrossRef] [PubMed]
- Poveda, J.; Eugui, D.; Abril-Urías, P.; Velasco, P. Endophytic Fungi as Direct Plant Growth Promoters for Sustainable Agricultural Production. Symbiosis 2021, 85, 1–19. [Google Scholar] [CrossRef]
- Lugtenberg, B.J.J.; Caradus, J.R.; Johnson, L.J. Fungal Endophytes for Sustainable Crop Production. FEMS Microbiol. Ecol. 2016, 92, fiw194. [Google Scholar] [CrossRef] [PubMed]
- Hardoim, P.R.; van Overbeek, L.S.; Berg, G.; Pirttilä, A.M.; Compant, S.; Campisano, A.; Döring, M.; Sessitsch, A. The Hidden World within Plants: Ecological and Evolutionary Considerations for Defining Functioning of Microbial Endophytes. Microbiol. Mol. Biol. Rev. 2015, 79, 293–320. [Google Scholar] [CrossRef]
- Bamisile, B.S.; Dash, C.K.; Akutse, K.S.; Keppanan, R.; Wang, L. Fungal Endophytes: Beyond Herbivore Management. Front. Microbiol. 2018, 9, 544. [Google Scholar] [CrossRef]
- De Silva, N.I.; Brooks, S.; Lumyong, S.; Hyde, K.D. Use of Endophytes as Biocontrol Agents. Fungal Biol. Rev. 2019, 33, 133–148. [Google Scholar] [CrossRef]
- Poveda, J.; Díaz-González, S.; Díaz-Urbano, M.; Velasco, P.; Sacristán, S. Fungal Endophytes of Brassicaceae: Molecular Interactions and Crop Benefits. Front. Plant Sci. 2022, 13, 932288. [Google Scholar] [CrossRef]
- Saleem, S.; Sekara, A.; Pokluda, R. Serendipita Indica—A Review from Agricultural Point of View. Plants 2022, 11, 3417. [Google Scholar] [CrossRef]
- Vishwakarma, K.; Kumar, N.; Shandilya, C.; Mohapatra, S.; Bhayana, S.; Varma, A. Revisiting Plant–Microbe Interactions and Microbial Consortia Application for Enhancing Sustainable Agriculture: A Review. Front. Microbiol. 2020, 11, 560406. [Google Scholar] [CrossRef]
- Poppeliers, S.W.; Sánchez-Gil, J.J.; de Jonge, R. Microbes to Support Plant Health: Understanding Bioinoculant Success in Complex Conditions. Curr. Opin. Microbiol. 2023, 73, 102286. [Google Scholar] [CrossRef]
- Berg, G.; Kusstatscher, P.; Abdelfattah, A.; Cernava, T.; Smalla, K. Microbiome Modulation—Toward a Better Understanding of Plant Microbiome Response to Microbial Inoculants. Front. Microbiol. 2021, 12, 650610. [Google Scholar] [CrossRef] [PubMed]
- Hassani, M.A.; Durán, P.; Hacquard, S. Microbial Interactions within the Plant Holobiont. Microbiome 2018, 6, 58. [Google Scholar] [CrossRef]
- Mesny, F.; Hacquard, S.; Thomma, B.P. Co-evolution within the Plant Holobiont Drives Host Performance. EMBO Rep. 2023, 24, e57455. [Google Scholar] [CrossRef]
- García, E.; Alonso, Á.; Platas, G.; Sacristán, S. The Endophytic Mycobiota of Arabidopsis Thaliana. Fungal Divers. 2013, 60, 71–89. [Google Scholar] [CrossRef]
- Hacquard, S.; Kracher, B.; Hiruma, K.; Münch, P.C.; Garrido-Oter, R.; Thon, M.R.; Weimann, A.; Damm, U.; Dallery, J.F.; Hainaut, M.; et al. Survival Trade-Offs in Plant Roots during Colonization by Closely Related Beneficial and Pathogenic Fungi. Nat. Commun. 2016, 7, 11362. [Google Scholar] [CrossRef]
- Hiruma, K.; Gerlach, N.; Sacristán, S.; Nakano, R.T.; Hacquard, S.; Kracher, B.; Neumann, U.; Ramírez, D.; Bucher, M.; O’Connell, R.J.; et al. Root Endophyte Colletotrichum Tofieldiae Confers Plant Fitness Benefits That Are Phosphate Status Dependent. Cell 2016, 165, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Díaz-González, S.; Marín, P.; Sánchez, R.; Arribas, C.; Kruse, J.; González-Melendi, P.; Brunner, F.; Sacristán, S. Mutualistic Fungal Endophyte Colletotrichum Tofieldiae Ct0861 Colonizes and Increases Growth and Yield of Maize and Tomato Plants. Agronomy 2020, 10, 1493. [Google Scholar] [CrossRef]
- Wicklow, D.T.; Shotwell, O.L.; Adams, G.L. Use of Aflatoxin-Producing Ability Medium to Distinguish Aflatoxin-Producing Strains of Aspergillus Flavus. Appl. Env. Microbiol. 1981, 41, 697–699. [Google Scholar] [CrossRef]
- Díaz-González, S.; González-Bodí, S.; González-Sanz, C.; Marín, P.; Brunner, F.; Sacristán, S. Maize Associated Bacterial and Fungal Microbiomes Show Contrasting Conformation Patterns Dependent on Plant Compartment and Water Availability. BMC Plant Biol. 2025, 25, 448. [Google Scholar] [CrossRef]
- AEMET [Agencia Estatal de Meteorología (Ministerio de Medio Ambiente y Medio Rural y Marino)]. Iberian Climate Atlas. Available online: https://www.aemet.es/documentos/es/conocermas/recursos_en_linea/publicaciones_y_estudios/publicaciones/Atlas-climatologico/Atlas.pdf (accessed on 25 October 2019).
- AEMET [Agencia Estatal de Meteorología (Ministerio de Medio Ambiente y Medio Rural y Marino)]. Servicios Climáticos: Datos Climatológicos Normales de La Estación de San Javier Aeropuerto (Murcia). Available online: http://www.aemet.es/es/serviciosclimaticos/datosclimatologicos/valoresclimatologicos?l=7031&k=undefined (accessed on 25 October 2019).
- IMIDA—Instituto Murciano de Investigación y Desarrollo Agrario y Alimentario. Sistema de Información Agrometeorológica de La Región de Murcia (SIAM). Available online: http://siam.imida.es/apex/f?p=101:46:1821222586473642 (accessed on 1 April 2018).
- FAO Cropwat. A Computer Program for Irrigation Planning and Management; FAO Irrigation and Drainage Publications: Rome, Italy, 1992; Volume 46. [Google Scholar]
- Nleya, T.; Kleinjan, J.; Chungu, C. Corn Growth and Development: Climate Matters. IGrow Corn: Best. Manag. Pract. 2019, 1, 5–8. [Google Scholar]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global Patterns of 16S RRNA Diversity at a Depth of Millions of Sequences per Sample. Proc. Natl. Acad. Sci. USA 2011, 108, 4516–4522. [Google Scholar] [CrossRef] [PubMed]
- Gardes, M.; Bruns, T.D. ITS Primers with Enhanced Specificity for Basidiomycetes—Application to the Identification of Mycorrhizae and Rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt Removes Adapter Sequences from High-Throughput Sequencing Reads. EMBnet J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Yilmaz, P.; Parfrey, L.W.; Yarza, P.; Gerken, J.; Pruesse, E.; Quast, C.; Schweer, T.; Peplies, J.; Ludwig, W.; Glöckner, F.O. The SILVA and “All-Species Living Tree Project (LTP)” Taxonomic Frameworks. Nucleic Acids Res. 2014, 42, D643–D648. [Google Scholar] [CrossRef]
- Nilsson, R.H.; Larsson, K.H.; Taylor, A.F.S.; Bengtsson-Palme, J.; Jeppesen, T.S.; Schigel, D.; Kennedy, P.; Picard, K.; Glöckner, F.O.; Tedersoo, L.; et al. The UNITE Database for Molecular Identification of Fungi: Handling Dark Taxa and Parallel Taxonomic Classifications. Nucleic Acids Res. 2019, 47, D259–D264. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. 2023. Available online: https://cran.r-project.org (accessed on 10 September 2025).
- Oksanen, J.; Simpson, G.L.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; et al. Vegan: Community Ecology Package. 2023. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 10 September 2025).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Russel, J. MicEco: Various Functions for Microbial Community Data. 2024. Available online: https://github.com/Russel88/MicEco (accessed on 10 September 2025).
- Chen, H. VennDiagram: Generate High-Resolution Venn and Euler Plots. 2022. Available online: https://cran.r-project.org/web/packages/VennDiagram/index.html (accessed on 10 September 2025).
- Gloor, G.B.; Macklaim, J.M.; Fernandes, A.D. Displaying Variation in Large Datasets: Plotting a Visual Summary of Effect Sizes. J. Comput. Graph. Stat. 2016, 25, 971–979. [Google Scholar] [CrossRef]
- Fernandes, A.D.; Reid, J.N.S.; Macklaim, J.M.; McMurrough, T.A.; Edgell, D.R.; Gloor, G.B. Unifying the Analysis of High-Throughput Sequencing Datasets: Characterizing RNA-Seq, 16S RRNA Gene Sequencing and Selective Growth Experiments by Compositional Data Analysis. Microbiome 2014, 2, 15. [Google Scholar] [CrossRef]
- Fernandes, A.D.; Macklaim, J.M.; Linn, T.G.; Reid, G.; Gloor, G.B. ANOVA-Like Differential Expression (ALDEx) Analysis for Mixed Population RNA-Seq. PLoS ONE 2013, 8, e67019. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. A Rapid DNA Isolation Procedure for Small Quantities of Fresh Leaf Tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Mideros, S.X.; Windham, G.L.; Williams, W.P.; Nelson, R.J. Aspergillus Flavus Biomass in Maize Estimated by Quantitative Real-Time Polymerase Chain Reaction Is Strongly Correlated with Aflatoxin Concentration. Plant Dis. 2009, 93, 1163–1170. [Google Scholar] [CrossRef]
- Benoit-Gelber, I.; Gruntjes, T.; Vinck, A.; van Veluw, J.G.; Wösten, H.A.B.; Boeren, S.; Vervoort, J.J.M.; de Vries, R.P. Mixed Colonies of Aspergillus Niger and Aspergillus Oryzae Cooperatively Degrading Wheat Bran. Fungal Genet. Biol. 2017, 102, 31–37. [Google Scholar] [CrossRef]
- Liu, H.; Wu, H.; Wang, Y.; Wang, H.; Chen, S.; Yin, Z. Comparative Transcriptome Profiling and Co-Expression Network Analysis Uncover the Key Genes Associated Withearly-Stage Resistance to Aspergillus Flavus in Maize. BMC Plant Biol. 2021, 21, 216. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Gavíra, A.; Diánez, F.; Sánchez-Montesinos, B.; Santos, M. Biocontrol Effects of Paecilomyces Variotii against Fungal Plant Diseases. J. Fungi 2021, 7, 415. [Google Scholar] [CrossRef]
- Hothorn, T.; Hornik, K.; Van De Wiel, M.A.; Zeileis, A. A Lego System for Conditional Inference. Am. Stat. 2006, 60, 257–263. [Google Scholar] [CrossRef]
- Hothorn, T.; Van De Wiel, M.A.; Hornik, K.; Zeileis, A. Implementing a Class of Permutation Tests: The Coin Package. J. Stat. Softw. 2008, 28, 1–23. [Google Scholar] [CrossRef]
- Ministerio de Agricultura Pesca y Alimentación Superficies y Producciones de Cultivos: Cereales de Grano—Maíz. Available online: https://www.mapa.gob.es/es/estadistica/temas/publicaciones/anuario-de-estadistica/2019/default.aspx?parte=3&capitulo=07&grupo=1&seccion=8 (accessed on 15 March 2021).
- Pfliegler, W.P.; Pócsi, I.; Győri, Z.; Pusztahelyi, T. The Aspergilli and Their Mycotoxins: Metabolic Interactions With Plants and the Soil Biota. Front. Microbiol. 2020, 10, 2921. [Google Scholar] [CrossRef] [PubMed]
- Alshannaq, A.; Yu, J.-H. Occurrence, Toxicity, and Analysis of Major Mycotoxins in Food. Int. J. Env. Res. Public. Health 2017, 14, 632. [Google Scholar] [CrossRef]
- Frisvad, J.C.; Smedsgaard, J.; Samson, R.A.; Larsen, T.O.; Thrane, U. Fumonisin B 2 Production by Aspergillus Niger. J. Agric. Food Chem. 2007, 55, 9727–9732. [Google Scholar] [CrossRef]
- Nielsen, K.F.; Mogensen, J.M.; Johansen, M.; Larsen, T.O.; Frisvad, J.C. Review of Secondary Metabolites and Mycotoxins from the Aspergillus Niger Group. Anal. Bioanal. Chem. 2009, 395, 1225–1242. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.A. Incidence and Significance of Black Aspergilli in Agricultural Commodities: A Review, with a Key to All Species Accepted to-Date. Eur. J. Biol. Res. 2017, 7, 207–222. [Google Scholar] [CrossRef]
- Gil-Serna, J.; García-Díaz, M.; Vázquez, C.; González-Jaén, M.T.; Patiño, B. Significance of Aspergillus Niger Aggregate Species as Contaminants of Food Products in Spain Regarding Their Occurrence and Their Ability to Produce Mycotoxins. Food Microbiol. 2019, 82, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Xiong, C.; Singh, B.K.; He, J.Z.; Han, Y.L.; Li, P.P.; Wan, L.H.; Meng, G.Z.; Liu, S.Y.; Wang, J.T.; Wu, C.F.; et al. Plant Developmental Stage Drives the Differentiation in Ecological Role of the Maize Microbiome. Microbiome 2021, 9, 171. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, Y.N.; Wang, X.; Liao, K.; He, S.; Zhao, X.; Guo, H.; Zhao, D.; Wei, H.L. Dynamics of Rice Microbiomes Reveal Core Vertically Transmitted Seed Endophytes. Microbiome 2022, 10, 216. [Google Scholar] [CrossRef]
- He, C.; Zeng, Q.; Chen, Y.; Chen, C.; Wang, W.; Hou, J.; Li, X. Colonization by Dark Septate Endophytes Improves the Growth and Rhizosphere Soil Microbiome of Licorice Plants under Different Water Treatments. Appl. Soil. Ecol. 2021, 166, 103993. [Google Scholar] [CrossRef]
- Yurgel, S.N.; Ajeethan, N.; Smertenko, A. Response of Plant-Associated Microbiome to Plant Root Colonization by Exogenous Bacterial Endophyte in Perennial Crops. Front. Microbiol. 2022, 13, 863946. [Google Scholar] [CrossRef]
- Sylla, J.; Alsanius, B.W.; Krüger, E.; Reineke, A.; Strohmeier, S.; Wohanka, W. Leaf Microbiota of Strawberries as Affected by Biological Control Agents. Phytopathology 2013, 103, 1001–1011. [Google Scholar] [CrossRef]
- Roberts, E.L.; Ferraro, A. Rhizosphere Microbiome Selection by Epichloë Endophytes of Festuca Arundinacea. Plant Soil. 2015, 396, 229–239. [Google Scholar] [CrossRef]
- Ju, Y.; Zhong, R.; Christensen, M.J.; Zhang, X. Effects of Epichloë Gansuensis Endophyte on the Root and Rhizosphere Soil Bacteria of Achnatherum Inebrians Under Different Moisture Conditions. Front. Microbiol. 2020, 11, 747. [Google Scholar] [CrossRef]
- Mahmud, K.; Lee, K.; Hill, N.S.; Mergoum, A.; Missaoui, A. Influence of Tall Fescue Epichloë Endophytes on Rhizosphere Soil Microbiome. Microorganisms 2021, 9, 1843. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z.; Chen, Z.; White, J.F.; Malik, K.; Chen, T.; Li, C. Inoculation of Barley (Hordeum vulgare) with the Endophyte Epichloë Bromicola Affects Plant Growth, and the Microbial Community in Roots and Rhizosphere Soil. J. Fungi 2022, 8, 172. [Google Scholar] [CrossRef]
- Sarrocco, S.; Vannacci, G. Preharvest Application of Beneficial Fungi as a Strategy to Prevent Postharvest Mycotoxin Contamination: A Review. Crop Prot. 2018, 110, 160–170. [Google Scholar] [CrossRef]
- Ayofemi Olalekan Adeyeye, S. Aflatoxigenic Fungi and Mycotoxins in Food: A Review. Crit. Rev. Food Sci. Nutr. 2020, 60, 709–721. [Google Scholar] [CrossRef]
- Agriopoulou, S.; Stamatelopoulou, E.; Varzakas, T. Advances in Occurrence, Importance, and Mycotoxin Control Strategies: Prevention and Detoxification in Foods. Foods 2020, 9, 137. [Google Scholar] [CrossRef]
- Persons, K.; Raines, J.M.; Rodriguez, J.M. Antagonistic Effects of Saccharomyces Cerevisiae on the Growth of Aspergillus Flavus and Aspergillus Parasiticus at Varying Temperatures. Mycology 2013, 4, 38–43. [Google Scholar] [CrossRef]
- Ren, X.; Branà, M.T.; Haidukowski, M.; Gallo, A.; Zhang, Q.; Logrieco, A.F.; Li, P.; Zhao, S.; Altomare, C. Potential of Trichoderma Spp. for Biocontrol of Aflatoxin-Producing Aspergillus Flavus. Toxins 2022, 14, 86. [Google Scholar] [CrossRef]
- Malik, M.A.; Ahmad, N.; Bhat, M.Y. The Green Shield: Trichoderma’s Role in Sustainable Agriculture against Soil-Borne Fungal Threats. Curr. Res. Microb. Sci. 2024, 7, 100313. [Google Scholar] [CrossRef] [PubMed]
- Giannelli, G.; Del Vecchio, L.; Cirlini, M.; Gozzi, M.; Gazza, L.; Galaverna, G.; Potestio, S.; Visioli, G. Exploring the Rhizosphere of Perennial Wheat: Potential for Plant Growth Promotion and Biocontrol Applications. Sci. Rep. 2024, 14, 22792. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wang, Z.; Yang, G.; Huang, S.; Liao, S.; Chen, K.; Du, M.; Zalán, Z.; Hegyi, F.; Kan, J. Biocontrol Potential of 1-Pentanal Emitted from Lactic Acid Bacteria Strains against Aspergillus Flavus in Red Pepper (Capsicum annuum L.). Food Control 2022, 142, 109261. [Google Scholar] [CrossRef]
- Tejero, P.; Martín, A.; Rodríguez, A.; Galván, A.I.; Ruiz-Moyano, S.; Hernández, A. In Vitro Biological Control of Aspergillus Flavus by Hanseniaspora Opuntiae L479 and Hanseniaspora Uvarum L793, Producers of Antifungal Volatile Organic Compounds. Toxins 2021, 13, 663. [Google Scholar] [CrossRef]
- Farbo, M.G.; Urgeghe, P.P.; Fiori, S.; Marcello, A.; Oggiano, S.; Balmas, V.; Hassan, Z.U.; Jaoua, S.; Migheli, Q. Effect of Yeast Volatile Organic Compounds on Ochratoxin A-Producing Aspergillus Carbonarius and A. Ochraceus. Int. J. Food Microbiol. 2018, 284, 1–10. [Google Scholar] [CrossRef]
- Tilocca, B.; Balmas, V.; Hassan, Z.U.; Jaoua, S.; Migheli, Q. A Proteomic Investigation of Aspergillus Carbonarius Exposed to Yeast Volatilome or to Its Major Component 2-Phenylethanol Reveals Major Shifts in Fungal Metabolism. Int. J. Food Microbiol. 2019, 306, 108265. [Google Scholar] [CrossRef] [PubMed]
- Podgórska-Kryszczuk, I. Biological Control of Aspergillus Flavus by the Yeast Aureobasidium Pullulans In Vitro and on Tomato Fruit. Plants 2023, 12, 236. [Google Scholar] [CrossRef]
- Ehrlich, K.C. Non-Aflatoxigenic Aspergillus Flavus to Prevent Aflatoxin Contamination in Crops: Advantages and Limitations. Front. Microbiol. 2014, 5, 50. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Ghazali, F.M.; Mahyudin, N.A.; Samsudin, N.I.P. Biocontrol of Aflatoxins Using Non-Aflatoxigenic Aspergillus Flavus: A Literature Review. J. Fungi 2021, 7, 381. [Google Scholar] [CrossRef]
- Moore, G.G. Practical Considerations Will Ensure the Continued Success of Pre-Harvest Biocontrol Using Non-Aflatoxigenic Aspergillus Flavus Strains. Crit. Rev. Food Sci. Nutr. 2022, 62, 4208–4225. [Google Scholar] [CrossRef] [PubMed]
- Augusto, J.; Atehnkeng, J.; Ortega-Beltran, A.; Cotty, P.J.; Bandyopadhyay, R. Keeping Toxigenic Aspergillus Section Flavi and Aflatoxin Contamination at Bay by Deploying Atoxigenic-Based Biocontrol Products during Production of Groundnut and Maize in Mozambique. Front. Microbiol. 2024, 15, 1501924. [Google Scholar] [CrossRef] [PubMed]
- Bonkoungou, S.; Dagno, K.; Basso, A.; Ekanao, T.; Atehnkeng, J.; Agbetiameh, D.; Neya, A.; Toure, M.; Tiendrebeogo, A.; Konate, M.; et al. Mitigation of Aflatoxin Contamination of Maize, Groundnut, and Sorghum by Commercial Biocontrol Products in Farmers’ Fields across Burkina Faso, Mali, Niger, and Togo. CABI Agric. Biosci. 2024, 5, 1–21. [Google Scholar] [CrossRef]
- Shoresh, M.; Harman, G.E.; Mastouri, F. Induced Systemic Resistance and Plant Responses to Fungal Biocontrol Agents. Annu. Rev. Phytopathol. 2010, 48, 21–43. [Google Scholar] [CrossRef]
- Pieterse, C.M.J.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Van Wees, S.C.M.; Bakker, P.A.H.M. Induced Systemic Resistance by Beneficial Microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef]
- Sharma, S.; Chen, C.; Navathe, S.; Chand, R.; Pandey, S.P. A Halotolerant Growth Promoting Rhizobacteria Triggers Induced Systemic Resistance in Plants and Defends against Fungal Infection. Sci. Rep. 2019, 9, 4054. [Google Scholar] [CrossRef]
- Mahapatra, R.; Jampala, S.S.M.; Patel, D.R. Induction of Systemic Acquired Resistance in Zea mays L. by Aspergillus Flavus and A. Parasiticus Derived Elicitors. Arch. Phytopathol. Plant Prot. 2015, 48, 120–134. [Google Scholar] [CrossRef]
- Gkizi, D.; Poulaki, E.G.; Tjamos, S.E. Towards Biological Control of Aspergillus Carbonarius and Botrytis Cinerea in Grapevine Berries and Transcriptomic Changes of Genes Encoding Pathogenesis-Related (PR) Proteins. Plants 2021, 10, 970. [Google Scholar] [CrossRef]
- Frerigmann, H.; Piotrowski, M.; Lemke, R.; Bednarek, P.; Schulze-Lefert, P. A Network of Phosphate Starvation and Immune-Related Signaling and Metabolic Pathways Controls the Interaction between Arabidopsis Thaliana and the Beneficial Fungus Colletotrichum Tofieldiae. Mol. Plant-Microbe Interact. ® 2020, 34, 560–570. [Google Scholar] [CrossRef]
- Tang, J.D.; Perkins, A.; Williams, W.P.; Warburton, M.L. Using Genome-Wide Associations to Identify Metabolic Pathways Involved in Maize Aflatoxin Accumulation Resistance. BMC Genom. 2015, 16, 673. [Google Scholar] [CrossRef]
- Warburton, M.L.; Jeffers, D.; Smith, J.S.; Scapim, C.; Uhdre, R.; Thrash, A.; Williams, W.P. Comparative Analysis of Multiple GWAS Results Identifies Metabolic Pathways Associated with Resistance to A. Flavus Infection and Aflatoxin Accumulation in Maize. Toxins 2022, 14, 738. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, L.K.; Mylroie, J.E.; Oliveira, D.A.; Smith, J.S.; Ozkan, S.; Windham, G.L.; Williams, W.P.; Warburton, M.L. Characterization of the Maize Chitinase Genes and Their Effect on Aspergillus Flavus and Aflatoxin Accumulation Resistance. PLoS ONE 2015, 10, e0126185. [Google Scholar] [CrossRef] [PubMed]
- Lozovaya, V.V.; Waranyuwat, A.; Widholm, J.M. β-l,3-Glucanase and Resistance to Aspergillus Flavus Infection in Maize. Crop Sci. 1998, 38, 1255–1260. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Brown, R.L.; Damann, K.E.; Cleveland, T.E. Identification of Maize Kernel Endosperm Proteins Associated with Resistance to Aflatoxin Contamination by Aspergillus Flavus. Phytopathology 2007, 97, 1094–1103. [Google Scholar] [CrossRef]
- Luo, M.; Brown, R.L.; Chen, Z.-Y.; Menkir, A.; Yu, J.; Bhatnagar, D. Transcriptional Profiles Uncover Aspergillus Flavus-Induced Resistance in Maize Kernels. Toxins 2011, 3, 766–786. [Google Scholar] [CrossRef]
| Target Region | Primer ID | Sequence (5′-3′) | Annealing T (°C) | Reference |
|---|---|---|---|---|
| 16S-V4 | 515f | GTGCCAGCMGCCGCGGTAA | 52 | [34] |
| 806r | GGACTACHVGGGTWTCTAAT | |||
| ITS1 | ITS1-F | CTTGGTCATTTAGAGGAAGTAA | 50 | [35] |
| ITS2 | GCTGCGTTCTTCATCGATGC | [36] |
| Fungal Species | Primers ID | Forward Sequence | Reverse Sequence | Annealing T (°C) | Ref. |
|---|---|---|---|---|---|
| C. tofieldiae | PRB110_00602#3 | CTCGTGTGACTGCGTTGTTG | TGGGTTGTGCGGGATTCAG | 60 | [26] |
| A. flavus | Af2 | ATCATTACCGAGTGTAGGGTTCCT | GCCGAAGCAACTAAGGTACAGTAAA | 60 | [51] |
| A. niger | gpdA | TCAAACCGACATTGCGAGAA | TCGCCGTGGTTGATGCT | 60 | [52] |
| Treatments | Stem Height (m) | Shoot Fresh Weight (g) | Root Dry Weight (g) | No. of Cobs per Plant | Cob Weight per Plant (g) | 1000 Grains Weight (g) | Yield per Plant (g) | Yield (t/ha) |
|---|---|---|---|---|---|---|---|---|
| Seed application 1 | ||||||||
| Control | 2.47 ± 0.24 b | 619 ± 124 a | 16.6 ± 5 a | 1.25 ± 0.45 a | 198.2 ± 30 a | 376 ± 22 a | 169 ± 28.8 b | 12.5 ± 2.1 b |
| Ct0861 | 2.63 ± 0.28 a | 634 ± 139 a | 18.5 ± 7 a | 1.31 ± 0.5 a | 237.3 ± 30.4 a | 399 ± 3 a | 206.8 ± 23.3 a | 15.2 ± 1.7 a |
| Spray application | ||||||||
| Control | 2.46 ± 0.24 b | 580 ± 153 b | 14.5 ± 6 b | 1.19 ± 0.4 a | 193.8 ± 12.7 b | 371 ± 5 b | 170 ± 26.6 a | 12.5 ± 2 a |
| Ct0861 | 2.7 ± 0.24 a | 728 ± 144 a | 25.5 ± 10 a | 1.38 ± 0.5 a | 235.1 ± 25.2 a | 393 ± 8 a | 204.5 ± 25.4 a | 15.1 ± 1.9 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-González, S.; González-Sanz, C.; González-Bodí, S.; Marín, P.; Brunner, F.; Sacristán, S. Microbiome Analysis Reveals Biocontrol of Aspergillus and Mycotoxin Mitigation in Maize by the Growth-Promoting Fungal Endophyte Colletotrichum tofieldiae Ct0861. Plants 2025, 14, 3236. https://doi.org/10.3390/plants14213236
Díaz-González S, González-Sanz C, González-Bodí S, Marín P, Brunner F, Sacristán S. Microbiome Analysis Reveals Biocontrol of Aspergillus and Mycotoxin Mitigation in Maize by the Growth-Promoting Fungal Endophyte Colletotrichum tofieldiae Ct0861. Plants. 2025; 14(21):3236. https://doi.org/10.3390/plants14213236
Chicago/Turabian StyleDíaz-González, Sandra, Carlos González-Sanz, Sara González-Bodí, Patricia Marín, Frédéric Brunner, and Soledad Sacristán. 2025. "Microbiome Analysis Reveals Biocontrol of Aspergillus and Mycotoxin Mitigation in Maize by the Growth-Promoting Fungal Endophyte Colletotrichum tofieldiae Ct0861" Plants 14, no. 21: 3236. https://doi.org/10.3390/plants14213236
APA StyleDíaz-González, S., González-Sanz, C., González-Bodí, S., Marín, P., Brunner, F., & Sacristán, S. (2025). Microbiome Analysis Reveals Biocontrol of Aspergillus and Mycotoxin Mitigation in Maize by the Growth-Promoting Fungal Endophyte Colletotrichum tofieldiae Ct0861. Plants, 14(21), 3236. https://doi.org/10.3390/plants14213236






