Electrophysiological Regulation of Nutrient Transport in Mangrove Species Under Salinity Stress: A Comparative Physiological Analysis of Aegiceras corniculatum (L.) Blanco and Kandelia obovata Sheue, H.Y. Liu & J.W.H. Yong
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Experimental Design
2.2. Data Collection
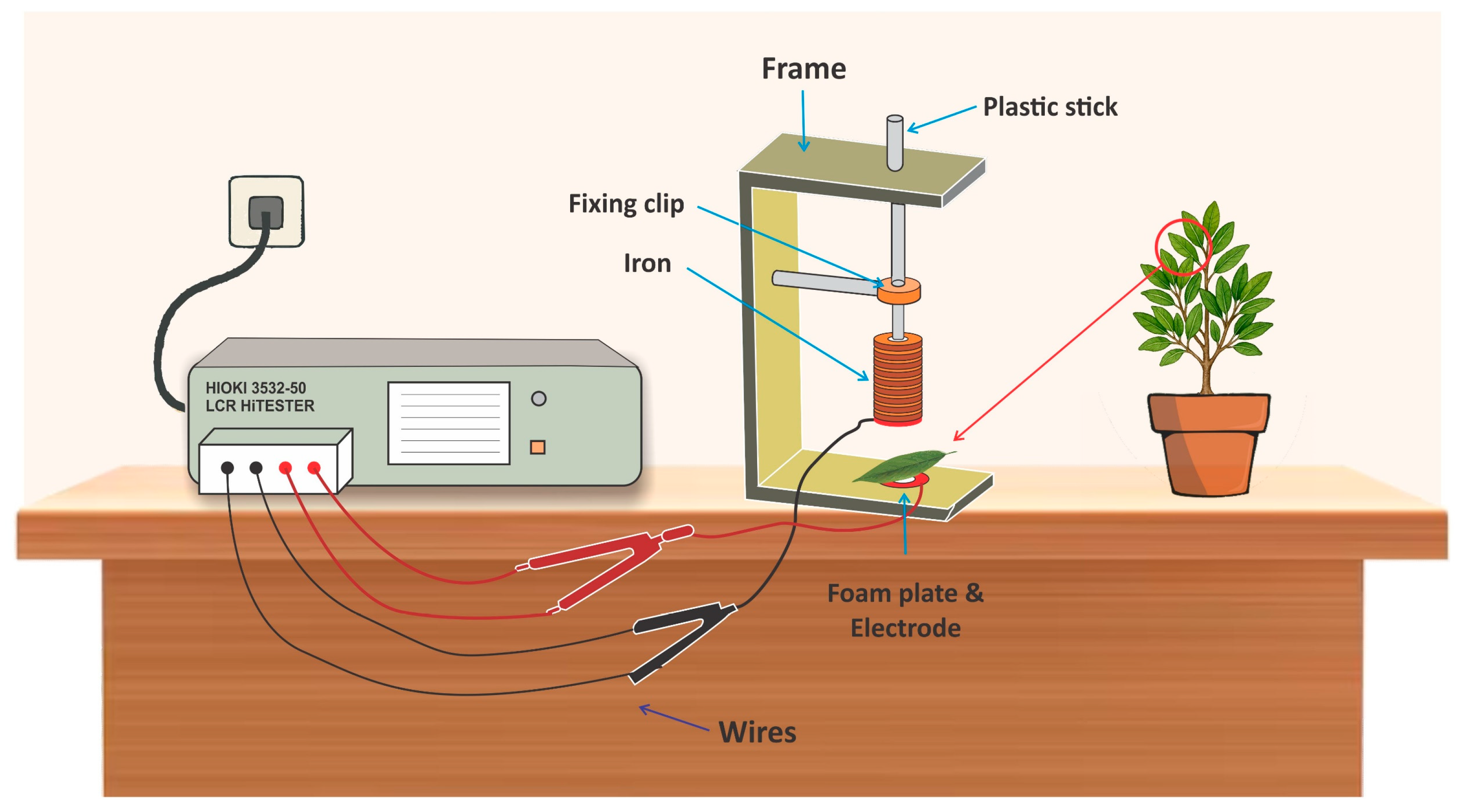
2.3. Determination of Electrophysiological Parameters
2.4. Determination of Inherent Electrophysiological Parameters in Mangrove Leaves
2.5. Nutrient Plunder Capacity of A. corniculatum and K. obavata Species
2.6. Photosynthetic Traits
2.7. Growth Rate
2.8. Statistical Analysis
3. Results
3.1. Inherent Electrophysiological Parameters of Both Mangrove Species
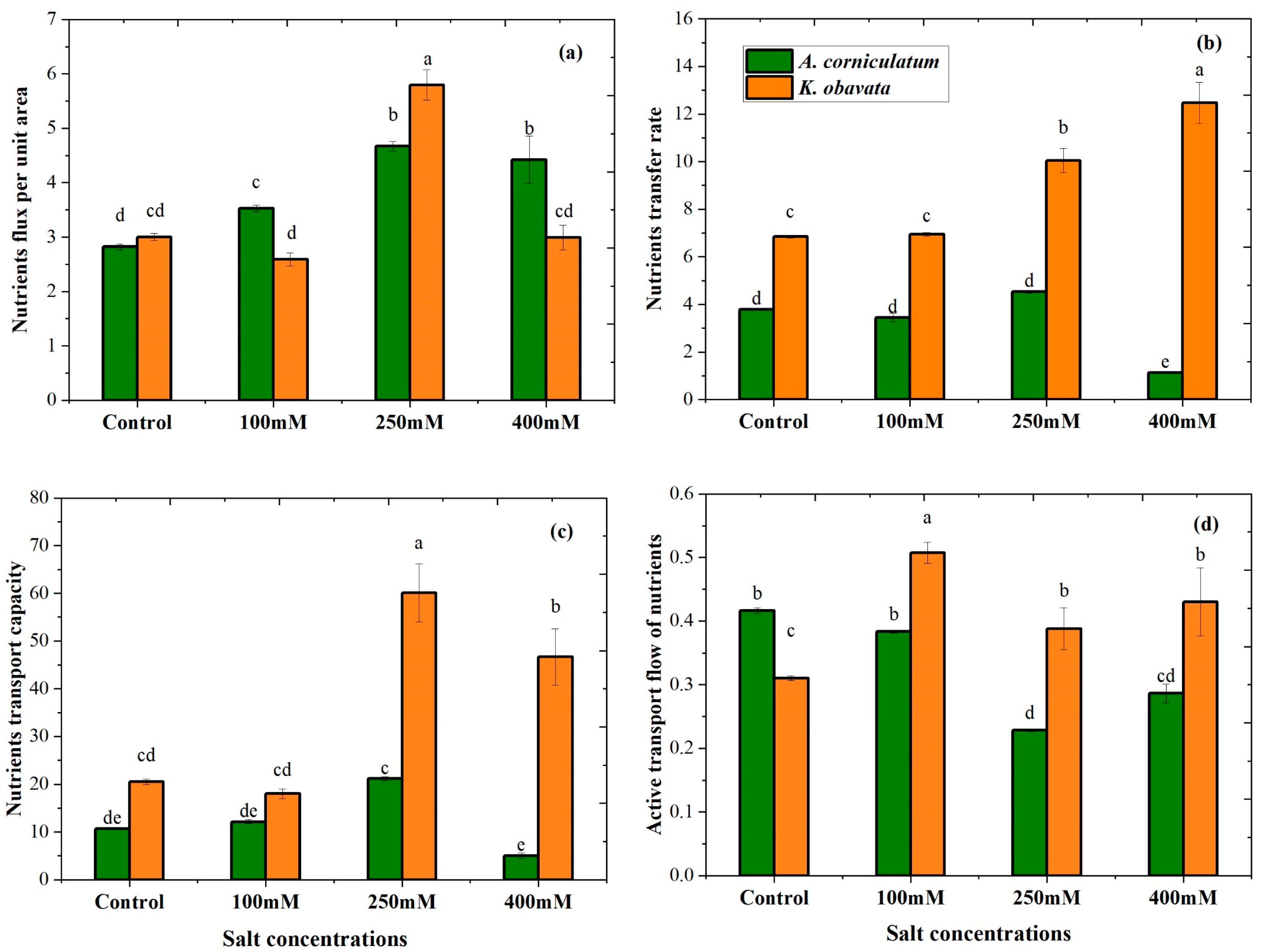
3.2. Nutrients Transport Parameters
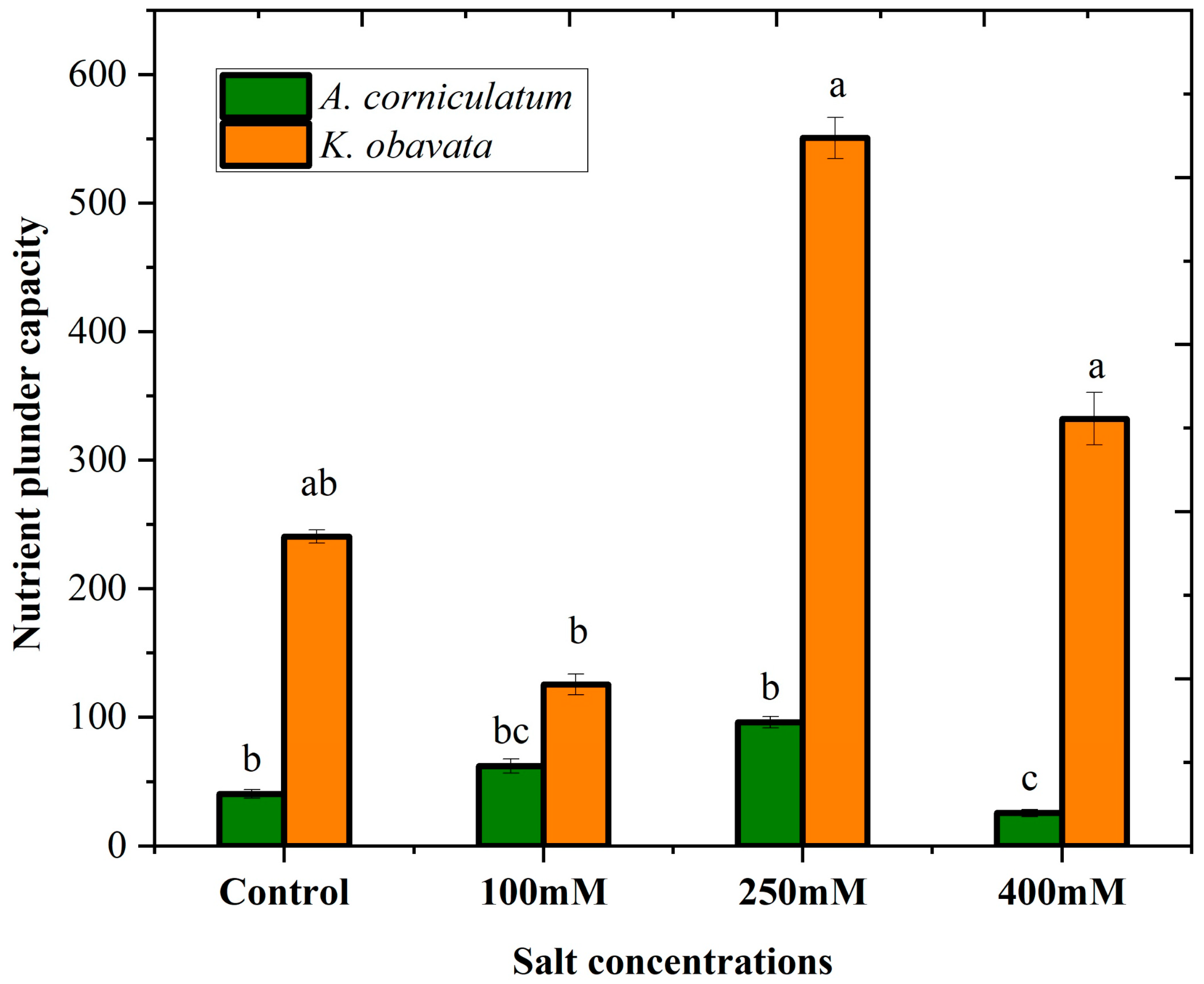
3.3. Nutrients Plunder Capacity
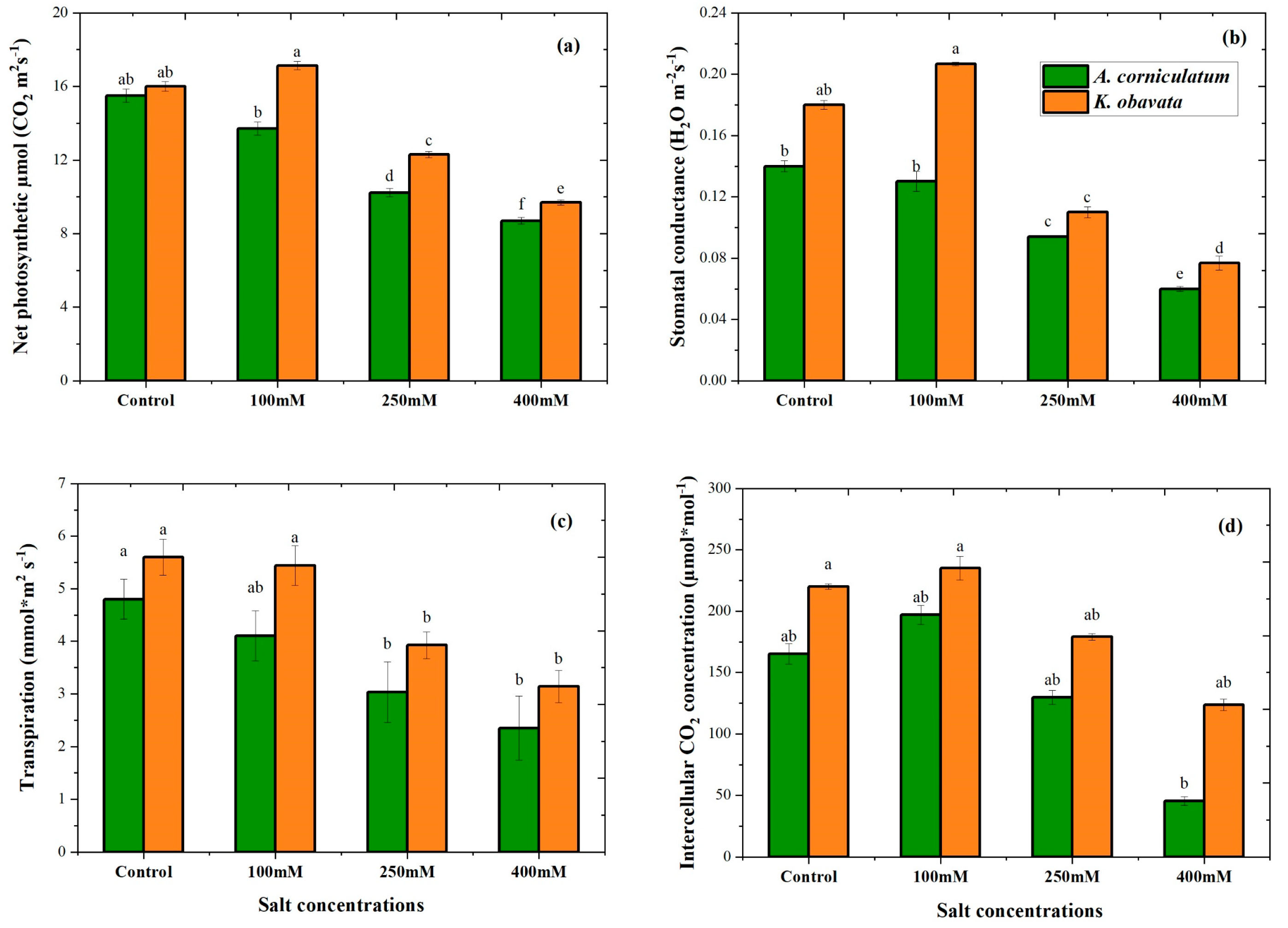
3.4. Photosynthetic Parameters
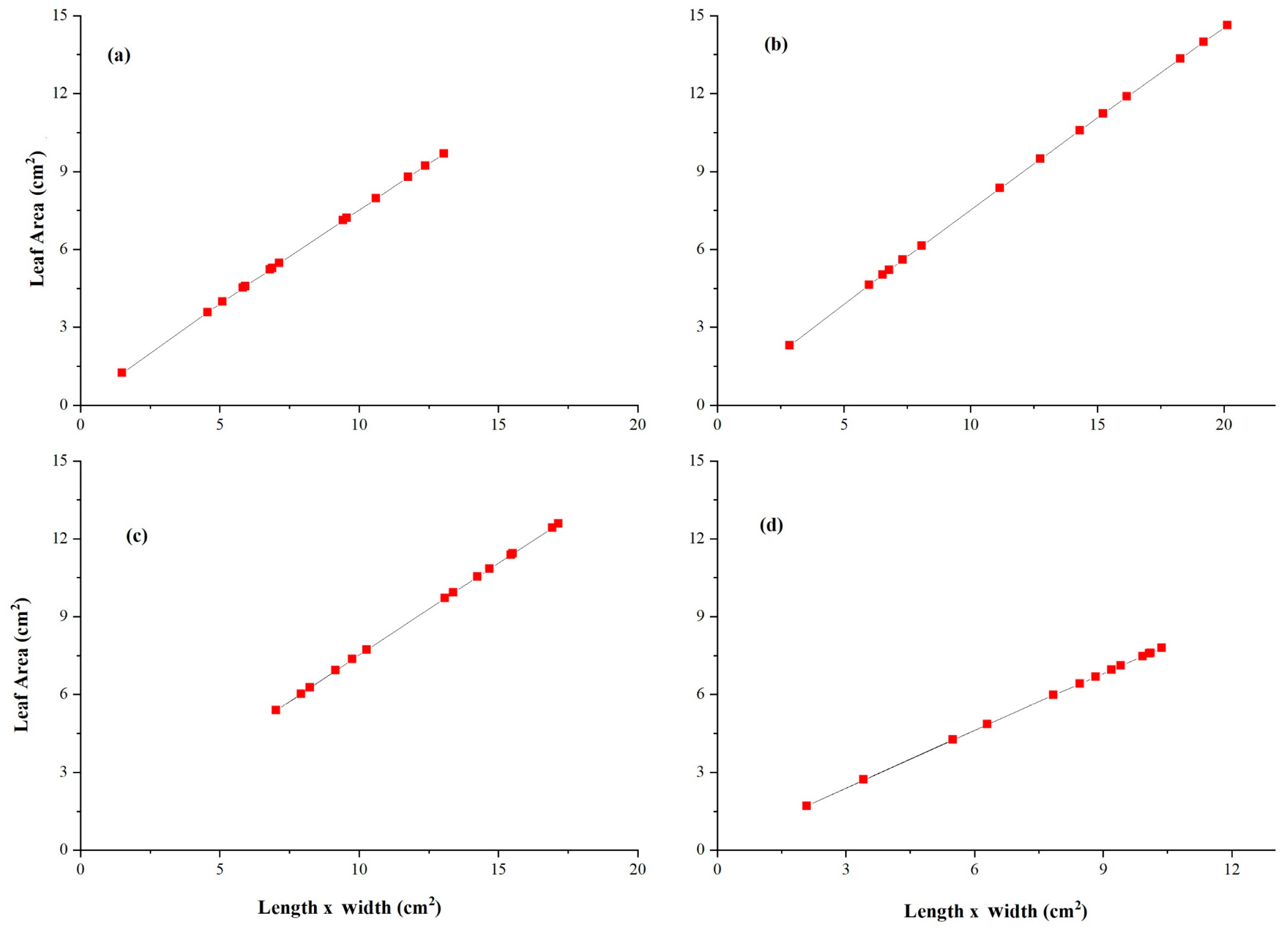
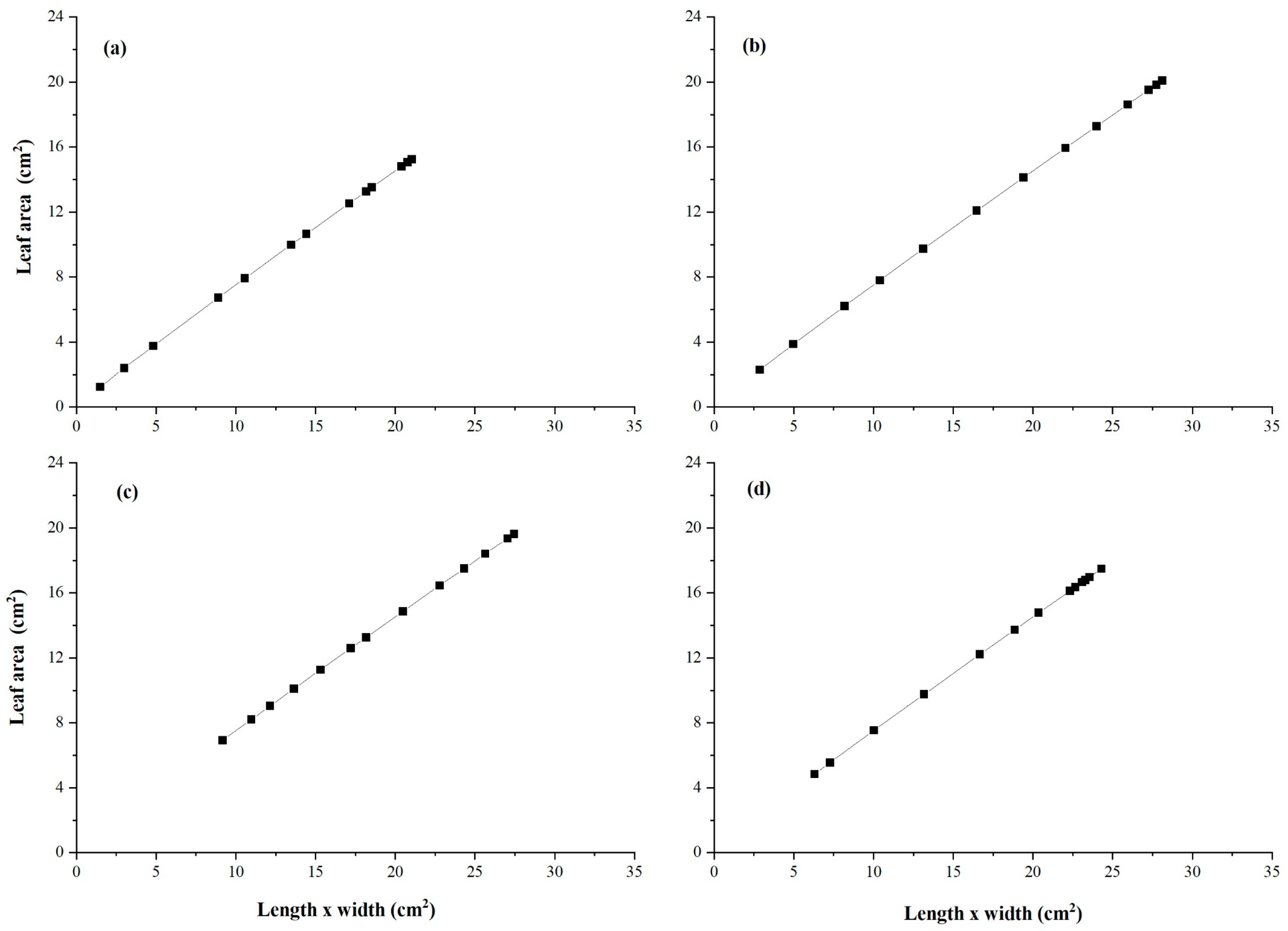
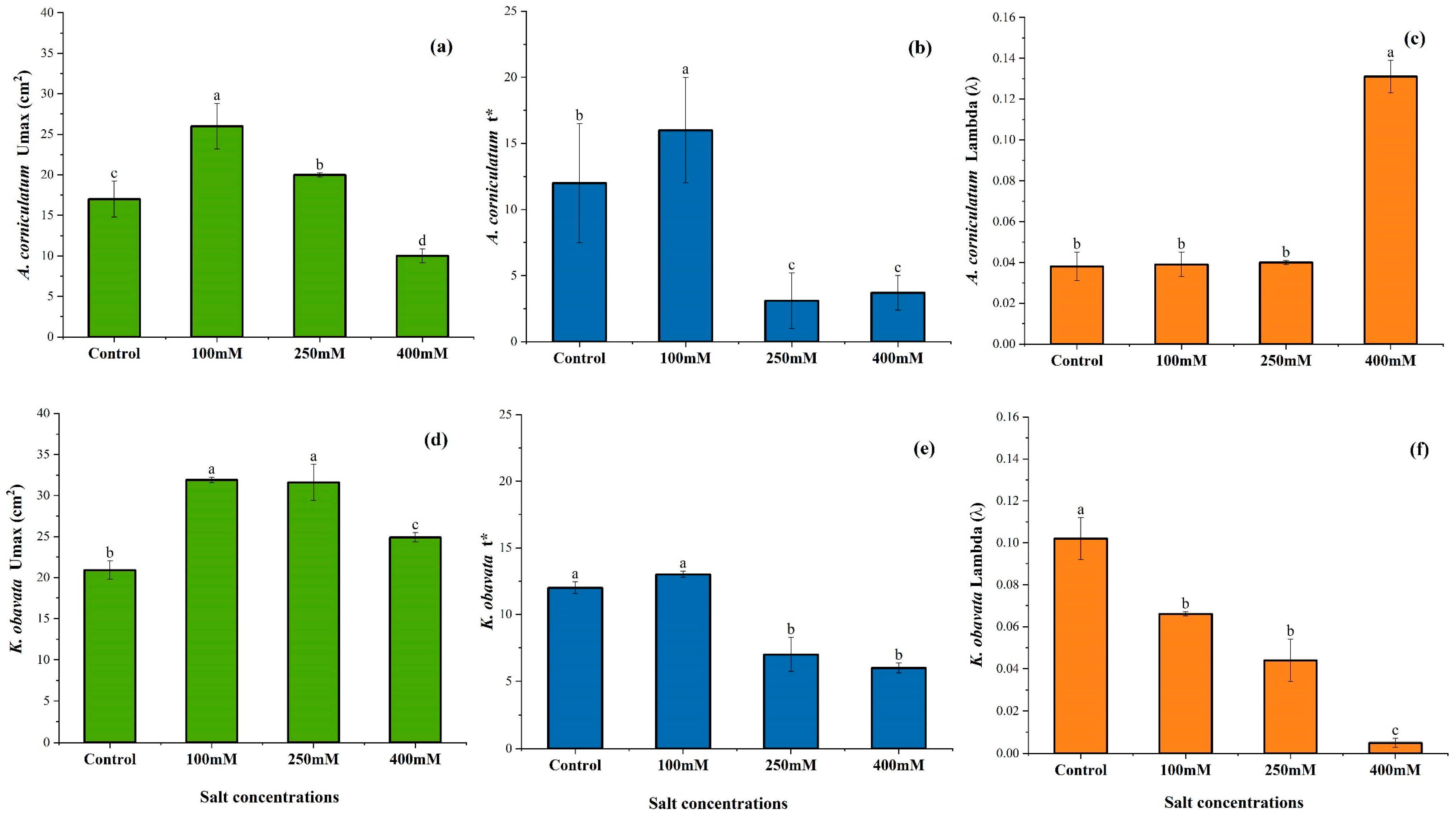
3.5. Allometric Curve Fitting Growth Model
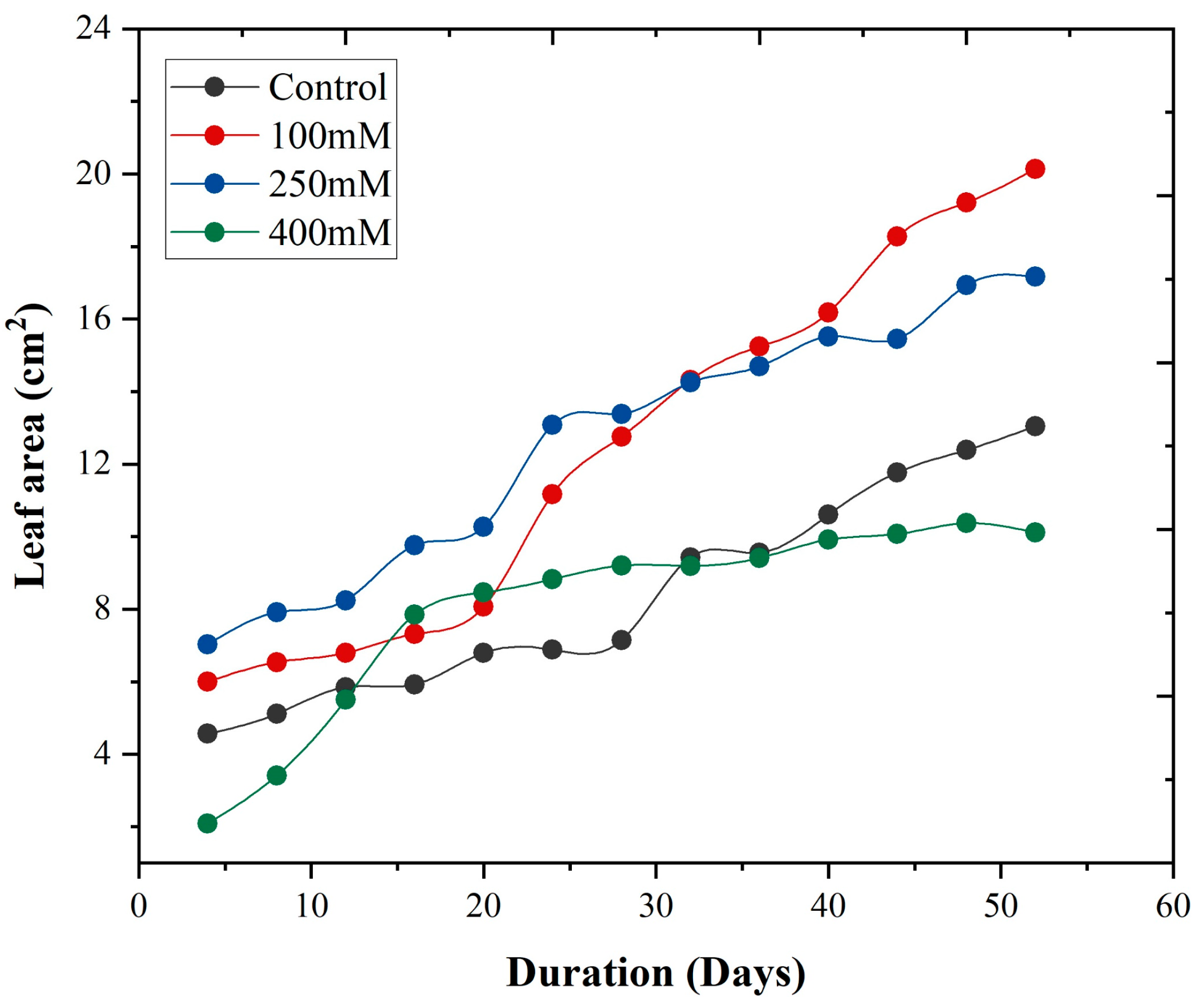
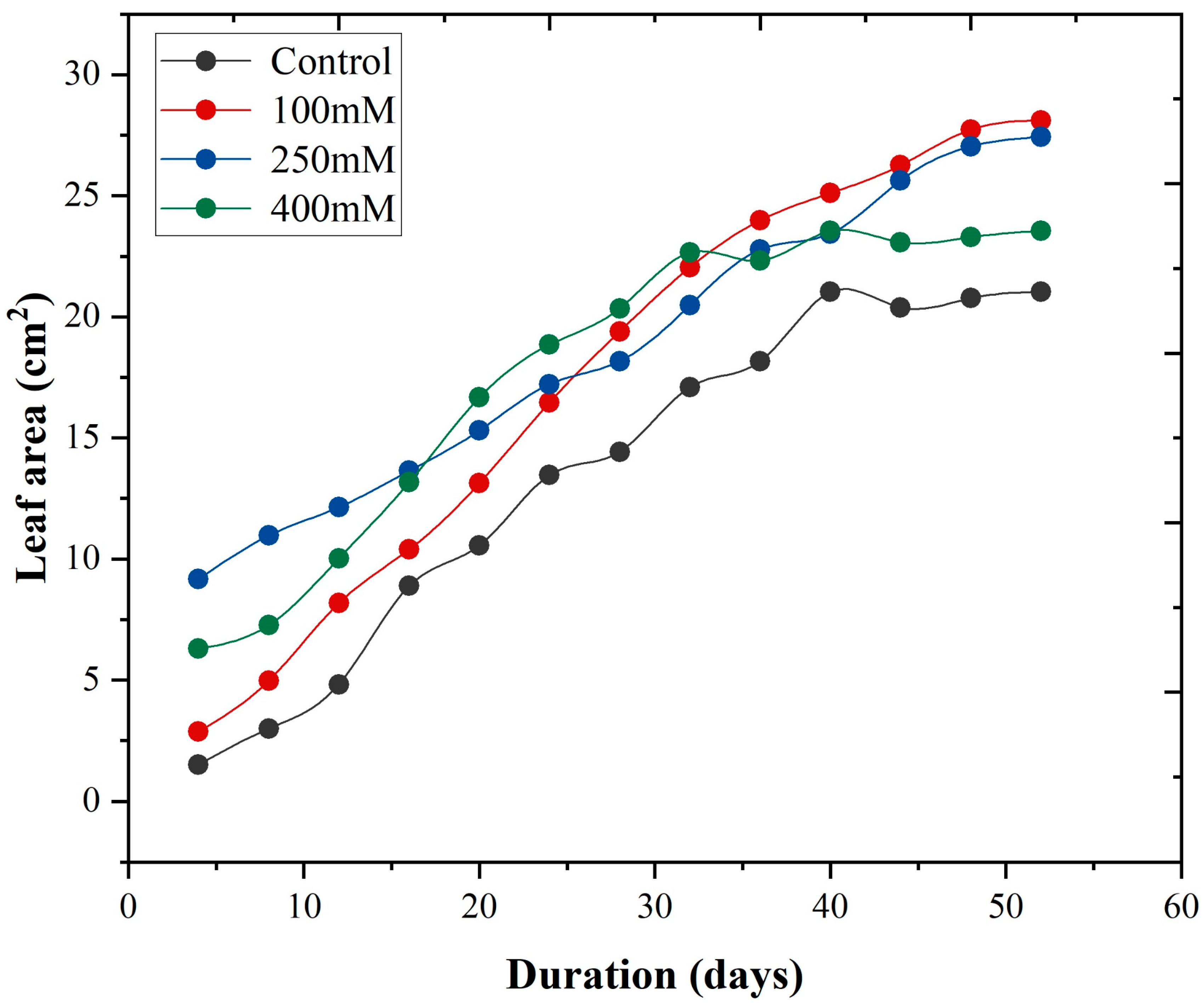
3.6. Leaf Growth Rate
3.7. Three Logistic Growth Parameter Equations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lu, C.; Liu, J.; Jia, M.; Liu, M.; Man, W.; Fu, W.; Zhong, L.; Lin, X.; Su, Y.; Gao, Y. Dynamic Analysis of Mangrove Forests Based on an Optimal Segmentation Scale Model and Multi-Seasonal Images in Quanzhou Bay, China. Remote Sens. 2018, 10, 2020. [Google Scholar] [CrossRef]
- Getzner, M.; Islam, M.S. Ecosystem Services of Mangrove Forests: Results of a Meta-Analysis of Economic Values. Int. J. Environ. Res. Public Health 2020, 17, 5830. [Google Scholar] [CrossRef]
- Zhang, J.; Feng, Y.; Maestre, F.T.; Berdugo, M.; Wang, J.; Coleine, C.; Sáez-Sandino, T.; García-Velázquez, L.; Singh, B.K.; Delgado-Baquerizo, M. Water Availability Creates Global Thresholds in Multidimensional Soil Biodiversity and Functions. Nat. Ecol. Evol. 2023, 7, 1002–1011. [Google Scholar] [CrossRef]
- Ali Solangi, K.; Wu, Y.; Chen, Q.; Ahmed Qureshi, W.; Xing, D.; Hussain Tunio, M.; Ali Shaikh, S. The Differential Responses of Aegiceras corniculatum and Kandelia candel under Salt Stress and Re-Watering Phase. A Study of Leaf Electrophysiological and Growth Parameters. J. Plant Interact. 2021, 16, 307–320. [Google Scholar] [CrossRef]
- Sanaullah, S.; Yang, D.; Zhong, R.; Zhao, L.; Shafi, M.; Akbar, A.J. Mangrove Dynamics in Pakistan: A Long-Term Study of Coastal Ecosystem Shifts over More than Three Decades. Ecol. Indic. 2025, 174, 113452. [Google Scholar] [CrossRef]
- Patil, V.; Singh, A.; Naik, N.; Unnikrishnan, S. Estimation of Mangrove Carbon Stocks by Applying Remote Sensing and GIS Techniques. Wetlands 2015, 35, 695–707. [Google Scholar] [CrossRef]
- Shoushtarian, F.; Negahban-Azar, M. Worldwide Regulations and Guidelines for Agricultural Water Reuse: A Critical Review. Water 2020, 12, 971. [Google Scholar] [CrossRef]
- Nobi, E.P.; Dilipan, E.; Thangaradjou, T.; Sivakumar, K.; Kannan, L. Geochemical and Geo-Statistical Assessment of Heavy Metal Concentration in the Sediments of Different Coastal Ecosystems of Andaman Islands, India. Estuar. Coast. Shelf Sci. 2010, 87, 253–264. [Google Scholar] [CrossRef]
- Maúre, E.d.R.; Terauchi, G.; Ishizaka, J.; Clinton, N.; DeWitt, M. Globally Consistent Assessment of Coastal Eutrophication. Nat. Commun. 2021, 12, 6142. [Google Scholar] [CrossRef]
- Spencer, K.L.; Harvey, G.L. Understanding System Disturbance and Ecosystem Services in Restored Saltmarshes: Integrating Physical and Biogeochemical Processes. Estuar. Coast. Shelf Sci. 2012, 106, 23–32. [Google Scholar] [CrossRef]
- Shaham, A. Evaluating a Decade of Mangrove Restorations in Mumbai: Success or Failure? Consilience 2024, 1–24. [Google Scholar] [CrossRef]
- Suyadi; Basyuni, M.; Amukti, R.; Damayanti, C.; Stokes, D.; Mubaraq, A.; Rahmila, Y.I. Ecological Features of Mangroves as Indicators of Seawater Intrusion. Glob. J. Environ. Sci. Manag. 2025, 11, 609–630. [Google Scholar] [CrossRef]
- Hwang, J.H.; Park, J.S.; Han, Y.S.; Yarish, C.; Kim, J.K. Seasonal Variations in Biomass, Height, Photosynthetic Efficiency, and Carbon and Nitrogen Contents of Suaeda Japonica in Incheon Salt Marshes (Korea). Front. Plant Sci. 2025, 16, 1513624. [Google Scholar] [CrossRef]
- Sheue, C.-R.; Liu, H.-Y.; Yong, J.W.H. Kandelia obovata (Rhizophoraceae), a New Mangrove Species from Eastern Asia. Taxon 2003, 52, 287–294. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Zhang, Y.; Xiang, J.; Yang, Z.; Gu, F.; Wu, M. Evaluating the Physiological and Biochemical Responses of Different Mangrove Species to Upwelling. Front. Mar. Sci. 2022, 9, 989055. [Google Scholar] [CrossRef]
- Qiu, D.L.; Lin, P.; Su, J.W. Relationship of Leaf Ultrastructure of Mangrove Kandelia candel (L.) Druce to Salt Tolerance. J. For. Sci. 2005, 51, 476–480. [Google Scholar] [CrossRef]
- Parida, A.K.; Jha, B. Salt Tolerance Mechanisms in Mangroves: A Review. Trees Struct. Funct. 2010, 24, 199–217. [Google Scholar] [CrossRef]
- Sudhir, S.; Arunprasath, A.; Vel, V.S. A Critical Review on Adaptations, and Biological Activities of the Mangroves. J. Nat. Pestic. Res. 2022, 1, 100006. [Google Scholar] [CrossRef]
- Pachú, J.K.S.; Macedo, F.C.O.; Malaquias, J.B.; Ramalho, F.S.; Oliveira, R.F.; Godoy, W.A.C.; Salustino, A.S. Electrical Signalling and Plant Response to Herbivory: A Short Review. Plant Signal. Behav. 2023, 18, 2277578. [Google Scholar] [CrossRef]
- Dolfi, M.; Dini, C.; Morosi, S.; Comparini, D.; Masi, E.; Pandolfi, C.; Mancuso, S. Electrical Signaling Related to Water Stress Acclimation. Sens. Bio-Sens. Res. 2021, 32, 100420. [Google Scholar] [CrossRef]
- Fromm, J.; Lautner, S. Electrical Signals and Their Physiological Significance in Plants. Plant. Cell Environ. 2007, 30, 249–257. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, Y.; Su, Y.; Li, H.; Fang, L.; Xing, D. Plant’s Electrophysiological Information Manifests the Composition and Nutrient Transport Characteristics of Membrane Proteins. Plant Signal. Behav. 2021, 16, 1918867. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.T.; Kurenda, A.; Stolz, S.; Chételat, A.; Farmer, E.E. Identification of Cell Populations Necessary for Leaf-Toleaf Electrical Signaling in a Wounded Plant. Proc. Natl. Acad. Sci. USA 2018, 115, 10178–10183. [Google Scholar] [CrossRef]
- Ali Solangi, K.; Wu, Y.; Xing, D.; Ahmed Qureshi, W.; Hussain Tunio, M.; Ali Sheikh, S.; Shabbir, A. Can Electrophysiological Information Reflect the Response of Mangrove Species to Salt Stress? A Case Study of Rewatering and Sodium Nitroprusside Application. Plant Signal. Behav. 2022, 17, 2073420. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Yan, H.; Zhang, C.; Wang, G.; Joe, S. Modeling Evapotranspiration for Cucumber Plants Based on the Shuttleworth-Wallace Model in a Venlo-Type Greenhouse. Agric. Water Manag. 2019, 228, 105861. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Circ.-Calif. Agric. Exp. Stn. 1950, 347, 29–31. [Google Scholar]
- Zhang, C.; Wu, Y.; Su, Y.; Xing, D.; Dai, Y.; Wu, Y.; Fang, L. A Plant’s Electrical Parameters Indicate Its Physiological State: A Study of Intracellular Water Metabolism. Plants 2020, 9, 1025. [Google Scholar] [CrossRef]
- Chen, D.; Wang, S.; Cao, B.; Cao, D.; Leng, G.; Li, H.; Yin, L.; Shan, L.; Deng, X. Genotypic Variation in Growth and Physiological Response to Drought Stress and Re-Watering Reveals the Critical Role of Recovery in Drought Adaptation in Maize Seedlings. Front. Plant Sci. 2016, 6, 1241. [Google Scholar] [CrossRef] [PubMed]
- Analuddin, K.; Sharma, S.; Suwa, R.; Hagihara, A. Crown Foliage Dynamics of Mangrove Kandelia obovata in Manko Wetland, Okinawa Island, Japan. J. Oceanogr. 2009, 65, 121–127. [Google Scholar] [CrossRef]
- Naidoo, G. Salt Secretion in the Mangrove Avicennia marina: Effects of Hypersalinity. Physiol. Plant. 2025, 177, e70105. [Google Scholar] [CrossRef]
- Parida, A.K.; Das, A.B.; Sanada, Y.; Mohanty, P. Effects of Salinity on Biochemical Components of the Mangrove, Aegiceras corniculatum. Aquat. Bot. 2004, 80, 77–87. [Google Scholar] [CrossRef]
- Sreeshan, A.; Meera, S.P.; Augustine, A. A Review on Transporters in Salt Tolerant Mangroves. Trees Struct. Funct. 2014, 28, 957–960. [Google Scholar] [CrossRef]
- Xu, H.M.; Tam, N.F.Y.; Zan, Q.J.; Bai, M.; Shin, P.K.S.; Vrijmoed, L.L.P.; Cheung, S.G.; Liao, W.B. Effects of Salinity on Anatomical Features and Physiology of a Semi-Mangrove Plant Myoporum bontioides. Mar. Pollut. Bull. 2014, 85, 738–746. [Google Scholar] [CrossRef]
- Wu, S.; Gu, X.; Peng, X.; Chen, L. Comparative Analysis of Water Use Strategies in Three Subtropical Mangrove Species: A Study of Sap Flow and Gas Exchange Monitoring. Tree Physiol. 2024, 44, tpae102. [Google Scholar] [CrossRef] [PubMed]
- Noya, I.; González-García, S.; Bacenetti, J.; Fiala, M.; Moreira, M.T. Environmental Impacts of the Cultivation-Phase Associated with Agricultural Crops for Feed Production. J. Clean. Prod. 2018, 172, 3721–3733. [Google Scholar] [CrossRef]
- Shiau, Y.; Lee, S.; Chen, T.; Tian, G.; Chiu, C. Water Salinity Effects on Growth and Nitrogen Assimilation Rate of Mangrove (Kandelia candel) Seedlings. Aquat. Bot. 2016, 137, 50–55. [Google Scholar] [CrossRef]
- Wang, S.M.; Wang, Y.S.; Su, B.Y.; Zhou, Y.Y.; Chang, L.F.; Ma, X.Y.; Li, X.M. Ecophysiological Responses of Five Mangrove Species (Bruguiera gymnorrhiza, Rhizophora stylosa, Aegiceras corniculatum, Avicennia marina, and Kandelia obovata) to Chilling Stress. Front. Mar. Sci. 2022, 9, 846566. [Google Scholar] [CrossRef]
- Analuddin, K.; Suwa, R.; Hagihara, A. The Self-Thinning Process in Mangrove Kandelia obovata Stands. J. Plant Res. 2009, 122, 53–59. [Google Scholar] [CrossRef]
- Sudhir, P.; Murthy, S.D.S. Effects of Salt Stress on Basic Processes of Photosynthesis. Photosynthetica 2004, 42, 481–486. [Google Scholar] [CrossRef]
- Tavakkoli, E.; Fatehi, F.; Coventry, S.; Rengasamy, P.; McDonald, G.K. Additive Effects of Na+ and Cl− Ions on Barley Growth under Salinity Stress. J. Exp. Bot. 2011, 62, 2189–2203. [Google Scholar] [CrossRef]
- Ma, L.; Yang, S. Growth and Physiological Response of Kandelia obovata and Bruguiera sexangula Seedlings to Aluminum Stress. Environ. Sci. Pollut. Res. 2022, 29, 43251–43266. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.H.; Chen, S.C. Anatomical Responses in Kandelia candel (L.) Druce Seedlings Growing in the Presence of Different Concentrations of NaCl. Bot. Bull. Acad. Sin. 1995, 36, 181–188. [Google Scholar]
| Aegiceras corniculatum | |||||||||
| Re-F | XC-F | XL-F | |||||||
| Branch Leaf | Y0/k1/b1 | R2 | p< | Y0/k2/b2 | R2 | p< | Y0/k3/b3 | R2 | p< |
| 1-1 | 0.5/1.62/0.49 | 0.999 | 0.0001 | 0.17/0.71/0.45 | 0.999 | 0.0001 | 0.66/2.06/0.49 | 0.999 | 0.0001 |
| 1-2 | 0.4/2.20/0.62 | 0.997 | 0.0001 | 0.17/0.91/0.52 | 0.998 | 0.0001 | 0.58/2.73/0.59 | 0.997 | 0.0001 |
| 1-3 | 0.8/1.23/0.44 | 0.999 | 0.0001 | 0.31/1.01/0.47 | 0.998 | 0.0001 | 1.06/1.91/0.46 | 0.999 | 0.0001 |
| 3-1 | 0.2/2.14/0.65 | 0.999 | 0.0001 | 0.11/0.89/0.51 | 0.999 | 0.0001 | 0.36/2.66/0.61 | 0.998 | 0.0001 |
| 3-2 | 0.5/3.61/0.66 | 0.999 | 0.0001 | 0.19/1.10/0.55 | 0.994 | 0.0001 | 0.64/4.19/0.64 | 0.992 | 0.0001 |
| 3-3 | 0.7/3.71/0.47 | 0.999 | 0.0001 | 0.26/1.15/0.40 | 0.998 | 0.0001 | 0.88/4.35/0.46 | 0.996 | 0.0001 |
| 5-1 | 1.1/2.96/0.27 | 0.998 | 0.0001 | 0.32/1.28/0.40 | 0.996 | 0.0001 | 1.31/3.68/0.30 | 0.998 | 0.0001 |
| 5-2 | 1.7/6.37/0.53 | 0.996 | 0.0001 | 0.44/1.56/0.48 | 0.993 | 0.0001 | 2.02/7.21/0.52 | 0.995 | 0.0001 |
| 5-3 | 1.6/8.65/0.67 | 0.998 | 0.0001 | 0.37/1.78/0.52 | 0.995 | 0.0001 | 1.85/9.59/0.65 | 0.989 | 0.0001 |
| Kandelia obovata | |||||||||
| 1-1 | 0.09/1.09/0.63 | 0.992 | 0.0001 | 0.09/0.51/0.53 | 0.995 | 0.0001 | 0.16/1.38/0.59 | 0.993 | 0.0001 |
| 1-2 | 0.04/0.38/0.62 | 0.998 | 0.0001 | 0.06/0.55/0.59 | 0.997 | 0.0001 | 0.09/0.79/0.60 | 0.998 | 0.0001 |
| 1-3 | 0.02/0.61/0.35 | 0.999 | 0.0001 | 0.04/0.63/0.39 | 0.997 | 0.0001 | 0.05/1.05/0.37 | 0.999 | 0.0001 |
| 3-1 | 0.05/0.46/0.75 | 0.994 | 0.0001 | 0.07/0.98/0.87 | 0.996 | 0.0001 | 0.17/1.34/0.67 | 0.992 | 0.0001 |
| 3-2 | 0.10/0.88/0.71 | 0.990 | 0.0001 | 0.10/0.70/0.64 | 0.994 | 0.0001 | 0.18/0.99/0.60 | 0.992 | 0.0001 |
| 3-3 | 0.10/0.56/0.65 | 0.990 | 0.0001 | 0.11/0.60/0.56 | 0.994 | 0.0001 | 0.11/1.22/0.82 | 0.995 | 0.0001 |
| 5-1 | 0.63/4.58/0.53 | 0.999 | 0.0001 | 0.21/1.29/0.51 | 0.996 | 0.0001 | 0.75/5.19/0.52 | 0.998 | 0.0001 |
| 5-2 | 0.62/3.11/1.12 | 0.998 | 0.0001 | 0.17/3.22/0.57 | 0.999 | 0.0001 | 0.76/29.6/1.03 | 0.998 | 0.0001 |
| 5-3 | 0.19/3.46/0.97 | 0.990 | 0.0001 | 0.10/0.89/0.64 | 0.993 | 0.0001 | 0.26/3.75/0.88 | 0.990 | 0.0001 |
| Aegiceras corniculatum | |||||
| Salt Concentrations (mM) | Ire (MΩ) | IXC (MΩ) | IXL (MΩ) | IZ (MΩ) | IC (pF) |
| Control | 3.43 ± 0.06 de | 1.72 ± 0.05 c | 4.13 ± 0.03 d | 1.46 ± 0.01 c | 30.8 ± 0.10 d |
| 100 | 4.11 ± 0.04 cd | 1.61 ± 0.01 cd | 4.21 ± 0.05 d | 1.67 ± 0.09 b | 32.8 ± 0.24 cd |
| 250 | 5.43 ± 0.08 b | 1.43 ± 0.005 cd | 6.26 ± 0.02 b | 1.34 ± 0.01 d | 37.2 ± 0.14 bc |
| 400 | 11.64 ± 0.79 a | 3.41 ± 0.15 a | 11.9 ± 0.09 a | 3.47 ± 0.002 a | 15.6 ± 0.67 f |
| Kandelia obavata | |||||
| Control | 3.01 ± 0.05 e | 1.31 ± 0.006 d | 4.22 ± 0.06 d | 0.93 ± 0.006 e | 40.4 ± 0.17 b |
| 100 | 2.61 ± 0.10 e | 1.52 ± 0.04 cd | 3.01 ± 0.01 e | 0.85 ± 0.005 e | 34.8 ± 1.02 bc |
| 250 | 3.96 ± 0.03 cd | 0.95 ± 0.07 e | 2.47 ± 0.02 f | 0.62 ± 0.005 f | 56.1 ± 3.95 a |
| 400 | 5.54 ± 0.03 c | 2.21 ± 0.27 b | 5.14 ± 0.03 c | 0.32 ± 0.005 g | 24.6 ± 2.93 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solangi, K.A.; Wang, Y.; Wu, Y.; Tunio, M.H.; Solangi, F.; Abbas, I.; Zhang, J.; Song, X. Electrophysiological Regulation of Nutrient Transport in Mangrove Species Under Salinity Stress: A Comparative Physiological Analysis of Aegiceras corniculatum (L.) Blanco and Kandelia obovata Sheue, H.Y. Liu & J.W.H. Yong. Plants 2025, 14, 3228. https://doi.org/10.3390/plants14203228
Solangi KA, Wang Y, Wu Y, Tunio MH, Solangi F, Abbas I, Zhang J, Song X. Electrophysiological Regulation of Nutrient Transport in Mangrove Species Under Salinity Stress: A Comparative Physiological Analysis of Aegiceras corniculatum (L.) Blanco and Kandelia obovata Sheue, H.Y. Liu & J.W.H. Yong. Plants. 2025; 14(20):3228. https://doi.org/10.3390/plants14203228
Chicago/Turabian StyleSolangi, Kashif Ali, Yun Wang, Yanyou Wu, Mazhar Hussain Tunio, Farheen Solangi, Irfan Abbas, Jinling Zhang, and Xiqiang Song. 2025. "Electrophysiological Regulation of Nutrient Transport in Mangrove Species Under Salinity Stress: A Comparative Physiological Analysis of Aegiceras corniculatum (L.) Blanco and Kandelia obovata Sheue, H.Y. Liu & J.W.H. Yong" Plants 14, no. 20: 3228. https://doi.org/10.3390/plants14203228
APA StyleSolangi, K. A., Wang, Y., Wu, Y., Tunio, M. H., Solangi, F., Abbas, I., Zhang, J., & Song, X. (2025). Electrophysiological Regulation of Nutrient Transport in Mangrove Species Under Salinity Stress: A Comparative Physiological Analysis of Aegiceras corniculatum (L.) Blanco and Kandelia obovata Sheue, H.Y. Liu & J.W.H. Yong. Plants, 14(20), 3228. https://doi.org/10.3390/plants14203228








