Improving the Quality of Ylang-Ylang Essential Oils [Cananga odorata (Lam.) Hook.f. &Thomson] Through Microwave-Assisted Extraction Compared to Conventional Extraction Methods
Abstract
1. Introduction
2. Experiment
2.1. Plant Material
2.2. Essential Oil Extraction
2.2.1. Hydrodistillation Apparatus and Procedure
2.2.2. Steam-Water Distillation Apparatus and Procedure
2.2.3. Solvent-Free Microwave Extraction Apparatus and Procedure
2.3. Density and Yield Measurements
2.4. GC-MS/FID Analysis
2.5. Statistical Analysis
3. Results and Discussions
3.1. Extraction Time, Yield, and Density
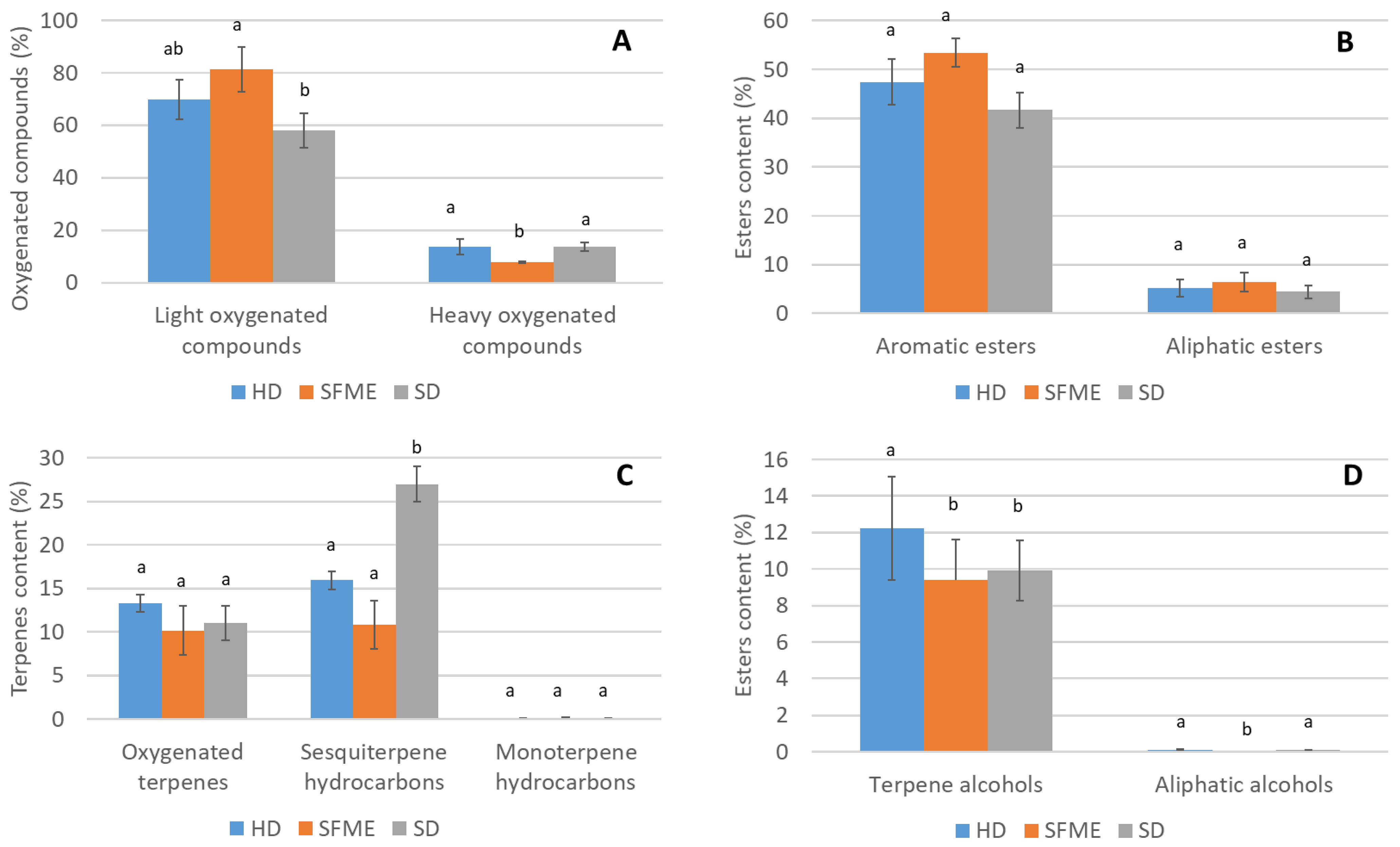
3.2. Chemical Families, Groups, Subgroups, and Oxygenated Compounds
3.3. Diversity of Volatile Compounds in Essential Oils Obtained by HD, SFME, and SD
3.4. Chemical Composition
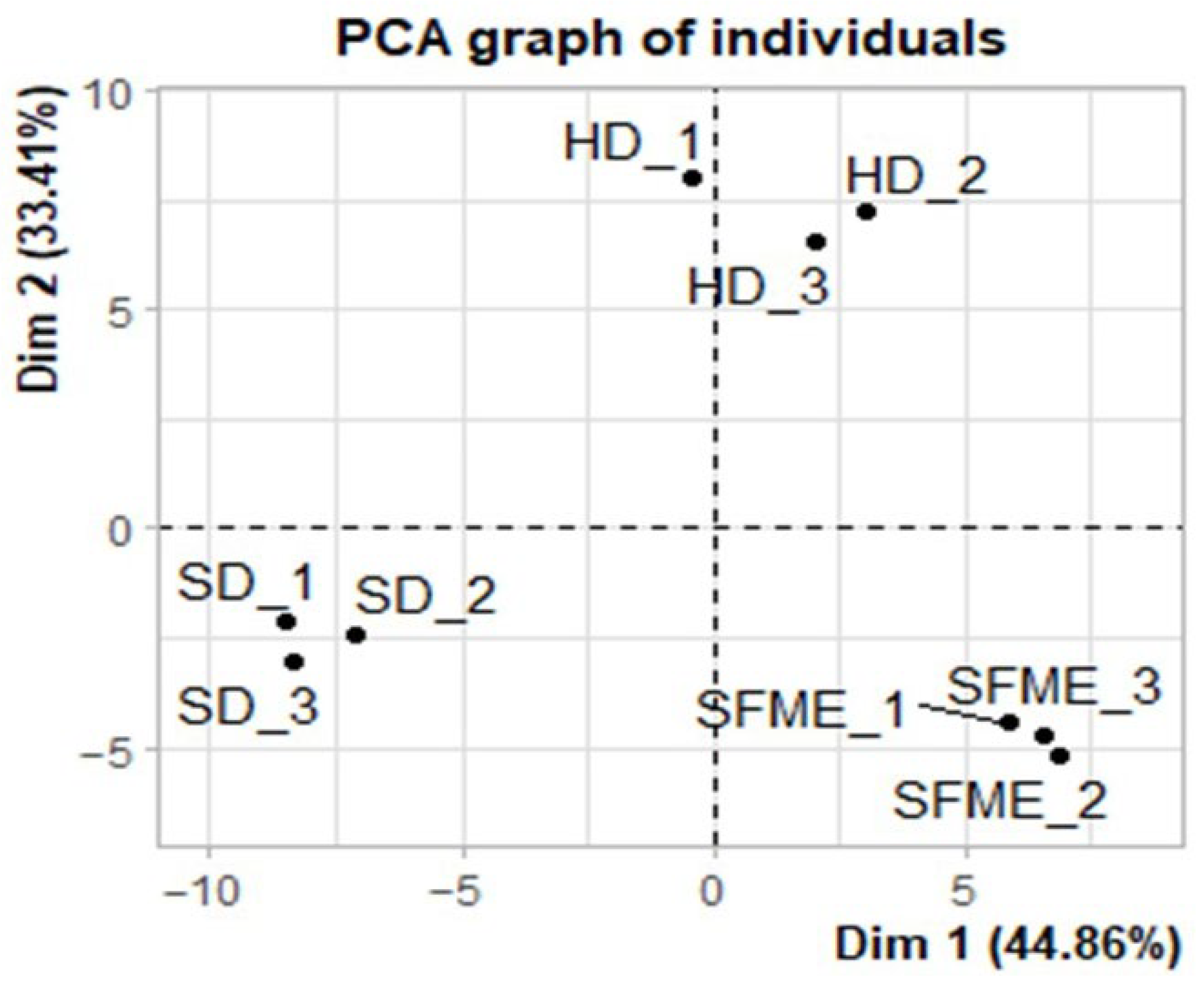
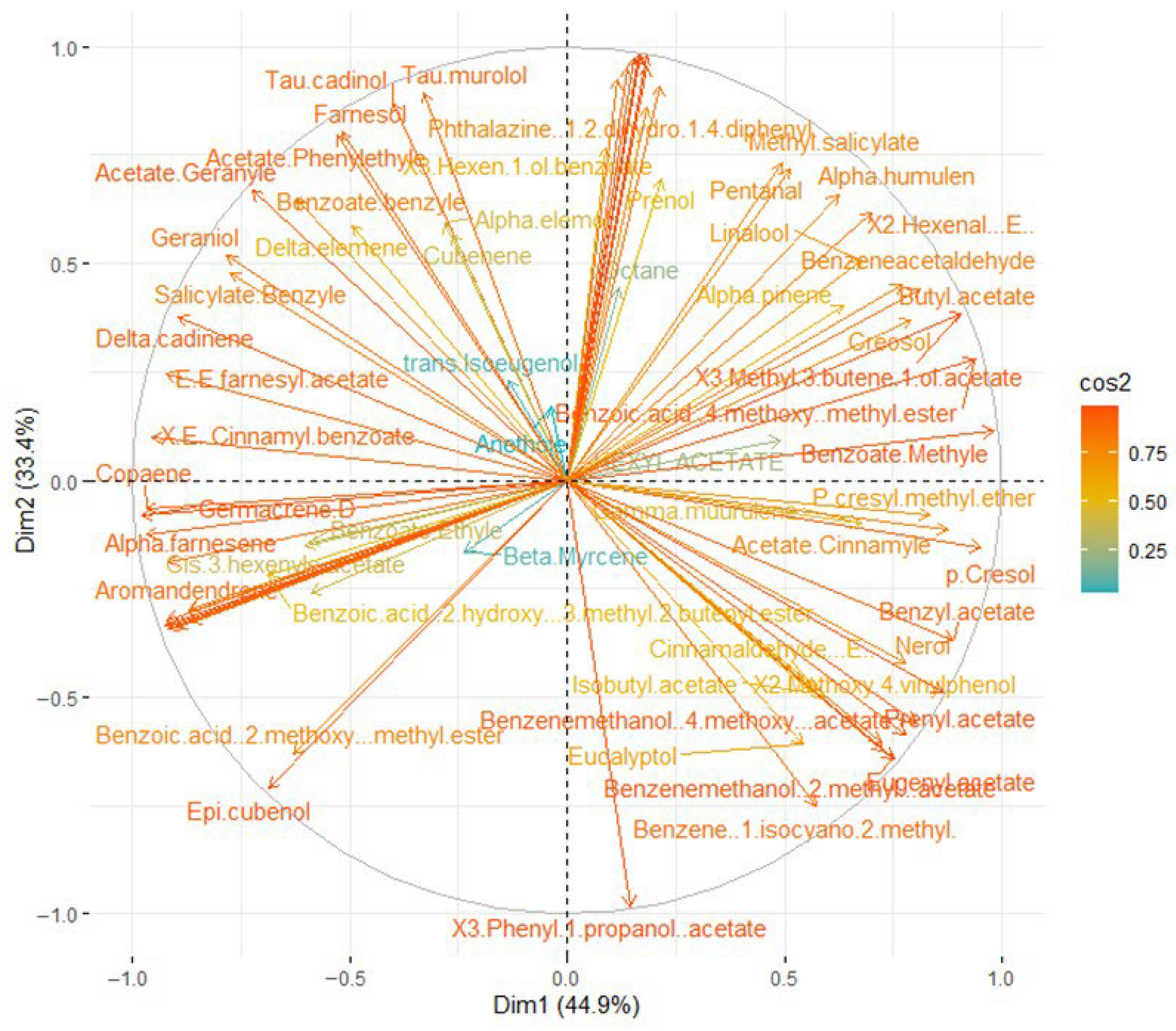
3.5. Principal Component Analysis of Ylang-Ylang Essential Oils Obtained by HD, SFME, and SD
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Parameter | AFNOR Extra-Sup (Comoros) | AFNOR Extra (Comoros/Madagascar) | AFNOR First (Comoros/Madagascar) | AFNOR Second (Comoros/Madagascar) | AFNOR Third (Comoros/Madagascar) | HD Extract | SFME Extract | SD Extract |
|---|---|---|---|---|---|---|---|---|
| Relative density at 20 °C | 0.970–0.990 | 0.955–0.976/0.950–0.965 | 0.938–0.960/0.933–0.949 | 0.925–0.945/0.922–0.942 | 0.906–0.925/0.906–0.925 | 0.985 | 0.988 | 0.959 |
| Components | ||||||||
| prenyl acetate | 1.5–3.2 | 1–2.3/0.6–2.2 | 0.3–1.8/0.2–1 | 0.2–0.9/0.1–0.5 | 0.1–0.2/Traces–0.2 | 2.57 | 4.23 | 2.15 |
| p-cresyl methyl ether | 7–13 | 5–13/7–16 | 3–8.5/5–10 | 2–5/1–4.6 | 0.1–1/0.1–1.4 | 12.62 | 13.54 | 11.23 |
| methyl benzoate | 4.5–8 | 4–6.5/4.5–9 | 1.5–5.5/3–5 | 1–3.5/1–3 | 0.1–0.8/0.1–0.9 | 8.39 | 9.01 | 6.23 |
| linalool | 8–13 | 7–12/15.24 | 3–10/12–19 | 2–6/4–9.5 | 0.1–2/0.6–4 | 8.26 | 7.67 | 6.58 |
| benzyl acetate | 14–20 | 11–17.5/5.5–14 | 6–14/2.8–10 | 4–8.8/0.5–5 | 0.5–3/0.1–2.2 | 20.14 | 28.94 | 15.98 |
| geraniol | 0.1–0.7 | 0.1–0.5/1.3–3 | 0.1–0.3/1.6–2.6 | 0.1–0.3/0.7–2.4 | Traces–0.1/0.2–0.8 | 0.05 | ND | 0.05 |
| geranyl acetate | 2–6 | 2.5–6/7.0–14 | 2–5/8–15 | 1.7–6/5.6–12 | 0.4–3/1–6.6 | 1.02 | 0.69 | 1.01 |
| E-cinnamyl acetate | 4–6 | 3–6.5/0.5–3 | 2.2–5/0.5–2 | 2–4.8/0.4–2.2 | 0.5–2.5/0.1–2 | 7.48 | 7.84 | 7.06 |
| β-caryophyllene | 2–6 | 2.5–8/2.5–8.5 | 4–10/5.5–12 | 4.8–14/10–17 | 5–15/12–19 | ND | ND | ND |
| germacrene D | 9–15 | 14–20/5–15 | 10–24/9.5–18 | 16–28/13–28 | 20–35/15–34 | 5.8 | 3.55 | 12.24 |
| (E,E)-α-farnesene | 2–6 | 6.5–15/1–5 | 7–18/3–8 | 14–21/5–11.5 | 12–29/9–25 | 7.15 | 5.68 | 12.61 |
| (E,E)-farnesol | 0.8–1.5 | 0.8–1.6/0.5–3 | 0.8–2/0.1–2.5 | 0.8–3/1.2–3.5 | 0.8–3/1.2–4 | 1.46 | 0.63 | 1.19 |
| benzyl benzoate | 3–6 | 4–6/3.5–8 | 4.2–9.2/4.5–8 | 4.5–7.8/6–10 | 4–8/4.8–8.5 | 7.43 | 4.6 | 7.08 |
| (E,E)-farnesyl acetate | 1–3 | 1–3/0.5–3 | 1–4/1–2 | 1–3.5/1.2–3.5 | 1.5–5/1.7–5 | 0.76 | 0.35 | 1.08 |
| benzyl salicylate | 1.5–3.5 | 2–3.8/1.2–4 | 2–4/1.6–4 | 2–4/1.8–4 | 2.5–4.8/2–5 | 3.16 | 1.73 | 4.6 |
References
- Lucchesi, M.E.; Chemat, F.; Smadja, J. Solvent-Free Microwave Extraction: An Innovative Tool for Rapid Extraction of Essential Oil from Aromatic Herbs and Spices. J. Microw. Power Electromagn. Energy 2004, 39, 135–139. [Google Scholar] [CrossRef]
- Giacometti, J.; Bursać Kovačević, D.; Putnik, P.; Gabrić, D.; Bilušić, T.; Krešić, G.; Stulić, V.; Barba, F.J.; Chemat, F.; Barbosa-Cánovas, G.; et al. Extraction of Bioactive Compounds and Essential Oils from Mediterranean Herbs by Conventional and Green Innovative Techniques: A Review. Food Res. Int. 2018, 113, 245–262. [Google Scholar] [CrossRef]
- Lucchesi, M.E.; Chemat, F.; Smadja, J. Solvent-Free Microwave Extraction of Essential Oil from Aromatic Herbs: Comparison with Conventional Hydro-Distillation. J. Chromatogr. A 2004, 1043, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Luque de Castro, M.D.; Jiménez-Carmona, M.; Fernández-Pérez, V. Towards More Rational Techniques for the Isolation of Valuable Essential Oils from Plants. TrAC Trends Anal. Chem. 1999, 18, 708–716. [Google Scholar] [CrossRef]
- Pollien, P.; Ott, A.; Fay, L.B.; Maignial, L.; Chaintreau, A. Simultaneous Distillation-Extraction: Preparative Recovery of Volatiles under Mild Conditions in Batch or Continuous Operations. Flavour Fragr. J. 1998, 13, 413–423. [Google Scholar] [CrossRef]
- Fan, Y.; Li, Q. An Efficient Extraction Method for Essential Oil from Angelica sinensis Radix by Natural Deep Eutectic Solvents-Assisted Microwave Hydrodistillation. Sustain. Chem. Pharm. 2022, 29, 100792. [Google Scholar] [CrossRef]
- Reverchon, E. Supercritical Fluid Extraction and Fractionation of Essential Oils and Related Products. J. Supercrit. Fluids 1997, 10, 1–37. [Google Scholar] [CrossRef]
- Vinatoru, M. An Overview of the Ultrasonically Assisted Extraction of Bioactive Principles from Herbs. Ultrason. Sonochem. 2001, 8, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Craveiro, A.A.; Matos, F.J.A.; Alencar, J.W.; Plumel, M.M. Microwave Oven Extraction of an Essential Oil. Flavour Fragr. J. 1989, 4, 43–44. [Google Scholar] [CrossRef]
- Chen, S.S.; Spiro, M. Study of Microwave Extraction of Essential Oil Constituents from Plant Materials. J. Microw. Power Electromagn. Energy 1994, 29, 231–241. [Google Scholar] [CrossRef]
- Paré, J.R.J.; Bélanger, J.M.R. Instrumental Methods in Food Analysis; Elsevier: Amsterdam, The Netherlands, 1997. [Google Scholar]
- Mandal, S.C.; Mandal, V.; Das, A. Essentials of Botanical Extraction—Principles and Applications, 1st ed.; Academic press: Cambridge, MA, USA, 2015; pp. 83–136. [Google Scholar]
- Chouhan, K.B.S.; Tandey, R.; Sen, K.K.; Mehta, R.; Mandal, V. Critical Analysis of Microwave Hydrodiffusion and Gravity as a Green Tool for Extraction of Essential Oils: Time to Replace Traditional Distillation. Trends Food Sci. Technol. 2019, 92, 12–21. [Google Scholar] [CrossRef]
- Kokolakis, A.; Golfinopoulos, S. Microwave-Assisted Techniques (MATs); A Quick Way to Extract a Fragrance: A Review. Nat. Prod. Commun. 2013, 8, 1493–1501. [Google Scholar] [CrossRef]
- Farhat, A.; Fabiano-Tixier, A.S.; Maataoui, M.E.; Maingonnat, J.F.; Romdhane, M.; Chemat, F. Microwave Steam Diffusion for Extraction of Essential Oil from Orange Peel: Kinetic Data, Extract’s Global Yield and Mechanism. Food Chem. 2011, 125, 255–261. [Google Scholar] [CrossRef]
- Sahraoui, N.; Vian, M.A.; Bornard, I.; Boutekedjiret, C.; Chemat, F. Improved Microwave Steam Distillation Apparatus for Isolation of Essential Oils: Comparison with Conventional Steam Distillation. J. Chromatogr. A 2008, 1210, 229–233. [Google Scholar] [CrossRef]
- Farhat, A.; Ginies, C.; Romdhane, M.; Chemat, F. Eco-Friendly and Cleaner Process for Isolation of Essential Oil Using Microwave Energy: Experimental and Theoretical Study. J. Chromatogr. A 2009, 1216, 5077–5085. [Google Scholar] [CrossRef]
- Filly, A.; Fernandez, X.; Minuti, M.; Visinoni, F.; Cravotto, G.; Chemat, F. Solvent Free Microwave Extraction of Essential Oil from Aromatic Herbs: From Laboratory to Pilot and Industrial Scale. Food Chem. 2014, 150, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.T.H.; Lee, L.H.; Yin, W.F.; Chan, C.K.; Abdul Kadir, H.; Chan, K.G.; Goh, B.H. Traditional uses, phytochemistry, and bioactivities of Cananga odorata (Ylang-Ylang). Evid. Based Complement. Altern. Medicine. 2015, 1, 896314. [Google Scholar]
- Benini, C.; Danflous, J.-P.; Wathelet, J.-P.; du Jardin, P.; Fauconnier, M.-L. L’Ylang-Ylang [Cananga odorata (Lam.) Hook.f. & Thomson]: Une Plante à Huile Essentielle Méconnue dans une Filière en Danger. Biotechnol. Agron. Soc. Environ. 2010, 14, 693–705. [Google Scholar]
- NF ISO 3063; Huile Essentielle d’Ylang-Ylang [Cananga odorata (Lamarck), J.D. Hooker et Thomson forma genuina]. AFNOR (Association Française de Normalisation): France, Paris, 2005.
- Ferhat, M.A.; Meklati, B.Y.; Smadja, J.; Chemat, F. An Improved Microwave Clevenger Apparatus for Distillation of Essential Oils from Orange Peel. J. Chromatogr. A 2006, 1112, 121–126. [Google Scholar] [CrossRef]
- Lebanov, L.; Chatterjee, S.; Tedone, L.; Chapman, S.C.; Linford, M.R.; Paull, B. Comprehensive Characterisation of Ylang-Ylang Essential Oils According to Distillation Time, Origin, and Chemical Composition Using a Multivariate Approach Applied to Average Mass Spectra and Segmented Average Mass Spectral Data. J. Chromatogr. A 2020, 1618, 460853. [Google Scholar] [CrossRef] [PubMed]
- Chakira, A.; Garcia, C.; Soria, C.; Minier, J.; Chillet, M. Effect of Flower Development Stages on the Dynamics of Volatile Compounds in Ylang-Ylang (Cananga odorata) Essential Oil. Horticulturae 2022, 8, 986. [Google Scholar] [CrossRef]
- Kristiawan, M.; Sobolik, V.; Allaf, K. Yield and Composition of Indonesian Cananga Oil Obtained by Steam Distillation and Organic Solvent Extraction. Int. J. Food Eng. 2012, 8, 1–19. [Google Scholar] [CrossRef]
- Stashenko, E.E.; Prada, N.Q.; Martinez, J.R. HRGC/FID/NPD and HRGC/MSD Study of Colombian Ylang-Ylang (Cananga odorata) Oils Obtained by Different Extraction Techniques. J. High Resolut. Chromatogr. 1996, 19, 353–358. [Google Scholar] [CrossRef]
- AFNOR (Association Française de Normalisation). Norme Française NF ISO 279: Essential oils. Determination of Relative Density at 20 Degrees Celsius. Reference Method; AFNOR: Paris, France, 1998. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 22 May 2025).
- Ragab, T.I.; El Gendy, A.N.G.; Saleh, I.A.; Esawy, M.A. Chemical Composition and Evaluation of Antimicrobial Activity of the Origanum majorana Essential Oil Extracted by Microwave-Assisted Extraction, Conventional Hydro-Distillation and Steam Distillation. J. Essent. Oil Bear. Plants 2019, 22, 563–573. [Google Scholar] [CrossRef]
- Chenni, M.; El Abed, D.; Rakotomanomana, N.; Fernandez, X.; Chemat, F. Comparative Study of Essential Oils Extracted from Egyptian Basil Leaves (Ocimum basilicum L.) Using Hydro-Distillation and Solvent-Free Microwave Extraction. Molecules 2016, 21, 113. [Google Scholar] [CrossRef] [PubMed]
- Manouchehri, R.; Saharkhiz, M.J.; Karami, A.; Niakousari, M. Extraction of Essential Oils from Damask Rose Using Green and Conventional Techniques: Microwave and Ohmic Assisted Hydrodistillation versus Hydrodistillation. Sustain. Chem. Pharm. 2018, 8, 76–81. [Google Scholar] [CrossRef]
- Gavahian, M.; Farahnaky, A.; Farhoosh, R.; Javidnia, K.; Shahidi, F. Extraction of Essential Oils from Mentha piperita Using Advanced Techniques: Microwave versus Ohmic Assisted Hydrodistillation. Food Bioprod. Process. 2015, 94, 50–58. [Google Scholar] [CrossRef]
- Araujo, A.R.; Périno, S.; Fernandez, X.; Cunha, C.; Rodrigues, M.; Ribeiro, M.P.; Jordao, L.; Silva, L.A.; Rodilla, J.; Coutinho, P.; et al. Solvent-Free Microwave Extraction of Thymus mastichina Essential Oil: Influence on Their Chemical Composition and on the Antioxidant and Antimicrobial Activities. Pharmaceuticals 2021, 14, 709. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.; Anwar, F.; Ahmad, N. Variation in Physicochemical Composition and Biological Attributes of Common Basil Essential Oils Produced by Hydro-Distillation and Supercritical Fluid Extraction. J. Essent. Oil-Bear. Plants 2017, 20, 95–109. [Google Scholar] [CrossRef]
- Li, Y.; Fabiano-Tixier, A.S.; Vian, M.A.; Chemat, F. Solvent-Free Microwave Extraction of Bioactive Compounds Provides a Tool for Green Analytical Chemistry. TrAC Trends Anal. Chem. 2013, 47, 1–11. [Google Scholar] [CrossRef]
- Asbahani, A.E.; Miladi, K.; Badri, W.; Sala, M.; Addi, E.H.A.; Casabianca, H.; Jordao, L.; Silva, L.A.; Rodilla, J.; Coutinho, P.; et al. Essential Oils: From Extraction to Encapsulation. Int. J. Pharm. 2015, 483, 220–243. [Google Scholar] [CrossRef]
- Jugreet, B.S.; Mahomoodally, M.F. Essential Oils from 9 Exotic and Endemic Medicinal Plants from Mauritius Shows In Vitro Antibacterial and Antibiotic Potentiating Activities. S. Afr. J. Bot. 2020, 132, 355–362. [Google Scholar] [CrossRef]
- da Silva, W.M.F.; Kringel, D.H.; de Souza, E.J.D.; da Rosa Zavareze, E.; Dias, A.R.G. Basil Essential Oil: Methods of Extraction, Chemical Composition, Biological Activities, and Food Applications. Food Bioprocess Technol. 2021, 15, 1–27. [Google Scholar] [CrossRef]
- Brokl, M.; Fauconnier, M.-L.; Benini, C.; Lognay, G.; du Jardin, P.; Focant, J.-F. Improvement of Ylang-Ylang Essential Oil Characterization by GC×GC-TOFMS. Molecules 2013, 18, 1783–1797. [Google Scholar] [CrossRef] [PubMed]
- Raut, J.S.; Karuppayil, S.M. A Status Review on the Medicinal Properties of Essential Oils. Ind. Crops Prod. 2014, 62, 250–264. [Google Scholar] [CrossRef]
- Guenther, E. The Essential Oils, Vol. 5, Individual Essential Oils of the Plant Families; Van Nostrand Company Inc.: New York, NY, USA, 1952. [Google Scholar]
- Brulé, C.; Pecout, W. L’ylang-Ylang: Un Parfum Subtil; Arco-Charbot: Grasse, France; V.F. Aromatique: Paris, France, 1995; pp. 1–16. [Google Scholar]
- Stashenko, E.E.; Tores, W.; Morales, R.M. A Study of the Compositional Variation of the Essential Oil of Ylang-Ylang (Cananga odorata HoK Fil. and Thomson, forma genuina) during Flower Development. J. High Resolut. Chromatogr. 1995, 18, 101–104. [Google Scholar] [CrossRef]
- Lago, S.; Rodríguez, H.; Soto, A.; Arce, A. Deterpenation of Citrus Essential Oil by Liquid–Liquid Extraction with 1-Alkyl-3-methylimidazolium Bis (trifluoromethylsulfonyl) amide Ionic Liquids. J. Chem. Eng. Data 2011, 56, 1273–1281. [Google Scholar] [CrossRef]
- Salha, G.B.; Díaz, R.H.; Labidi, J.; Abderrabba, M. Deterpenation of Origanum majorana L. Essential Oil by Reduced Pressure Steam Distillation. Ind. Crops Prod. 2017, 109, 116–122. [Google Scholar] [CrossRef]
- Putri, D.K.Y.; Dewi, I.E.P.; Kusuma, H.S.; Mahfud, M. Extraction of an Essential Oil from Fresh Cananga Flowers (Cananga odorata) Using Solvent-Free Microwave Method. J. Chem. Technol. Metall. 2019, 54, 793–802. [Google Scholar]
- Gaydou, E.M.; Randriamiharisoa, R.; Bianchini, J.P. Composition of the Essential Oil of Ylang-Ylang (Cananga odorata Hook Fil. and Thomson forma genuina) from Madagascar. J. Agric. Food Chem. 1986, 34, 481–487. [Google Scholar] [CrossRef]
- Gaydou, E.M.; Randriamiharisoa, R.P.; Bianchini, J.P.; Llinas, J.R. Multidimensional Data Analysis of Essential Oils. Application to Ylang-Ylang (Cananga odorata Hook Fil. and Thomson, Forma genuina) Grades Classification. J. Agric. Food Chem. 1988, 36, 574–579. [Google Scholar] [CrossRef]
- Bendahou, M.; Muselli, A.; Grignon-Dubois, M.; Benyoucef, M.; Desjobert, J.M.; Bernardini, A.F.; Costa, J. Antimicrobial Activity and Chemical Composition of Origanum glandulosum Desf. Essential Oil and Extract Obtained by Microwave Extraction: Comparison with Hydrodistillation. Food Chem. 2008, 106, 132–139. [Google Scholar] [CrossRef]
- Shiwakoti, S.; Saleh, O.; Poudyal, S.; Barka, A.; Qian, Y.; Zheljazkov, V.D. Yield, Composition, and Antioxidant Capacity of the Essential Oil of Sweet Basil and Holy Basil as Influenced by Distillation Methods. Chem. Biodivers. 2017, 14, e1600417. [Google Scholar] [CrossRef]
- Drinić, Z.; Pljevljakušić, D.; Živković, J.; Bigović, D.; Šavikin, K. Microwave-Assisted Extraction of O. vulgare L. spp. hirtum Essential Oil: Comparison with Conventional Hydro-Distillation. Food Bioprod. Process. 2020, 120, 158–165. [Google Scholar] [CrossRef]
- PAFR (Projet d’Appui aux Filières de Rentes). Perspectives D’avenir de L’ylang-Ylang aux Comores Selon les Applications Dans la Parfumerie: Rapport Final; PAFR: Moroni, Comores, 1998. [Google Scholar]
- Villa, C.; Robustelli Della Cuna, F.S.; Russo, E.; Ibrahim, M.F.; Grignani, E.; Preda, S. Microwave-Assisted and Conventional Extractions of Volatile Compounds from Rosa × damascena Mill. Fresh Petals for Cosmetic Applications. Molecules 2022, 27, 3963. [Google Scholar] [CrossRef] [PubMed]
- Périno-Issartier, S.; Ginies, C.; Cravotto, G.; Chemat, F. A Comparison of Essential Oils Obtained from Lavandin via Different Extraction Processes: Ultrasound, Microwave, Turbohydrodistillation, Steam and Hydrodistillation. J. Chromatogr. A 2013, 1305, 41–47. [Google Scholar] [CrossRef] [PubMed]
- de Vasconcelos Silva, M.G.; de Abreu Matos, F.J.; Oliveira Lopes, P.R.; Oliveira Silva, F.; Tavares Holanda, M. Composition of essential oils from three Ocimum species obtained by steam and microwave distillation and supercritical CO2 extraction. Arkivoc 2004, 2004, 66–71. [Google Scholar] [CrossRef]
- Kusuma, H.S.; Putri, D.K.Y.; Dewi, I.E.P.; Mahfud, M. Solvent-free microwave extraction of essential oil from dried basil (Ocimum basilicum L.) leaves. Chem. Chem. Technol. 2018, 12, 543–548. [Google Scholar] [CrossRef]
- Oktavianawati, I. Essential oil extraction of Cananga odorata flowers using hydrodistillation and steam-water distillation processes. IOP Conf. Ser. Mater. Sci. Eng. 2020, 833, 012032. [Google Scholar] [CrossRef]
- Gavahian, M.; Mathad, G.N.; Pandiselvam, R.; Lin, J.; Sun, D.W. Emerging technologies to obtain pectin from food processing by-products: A strategy for enhancing resource efficiency. Trends Food Sci. Technol. 2021, 115, 42–54. [Google Scholar] [CrossRef]
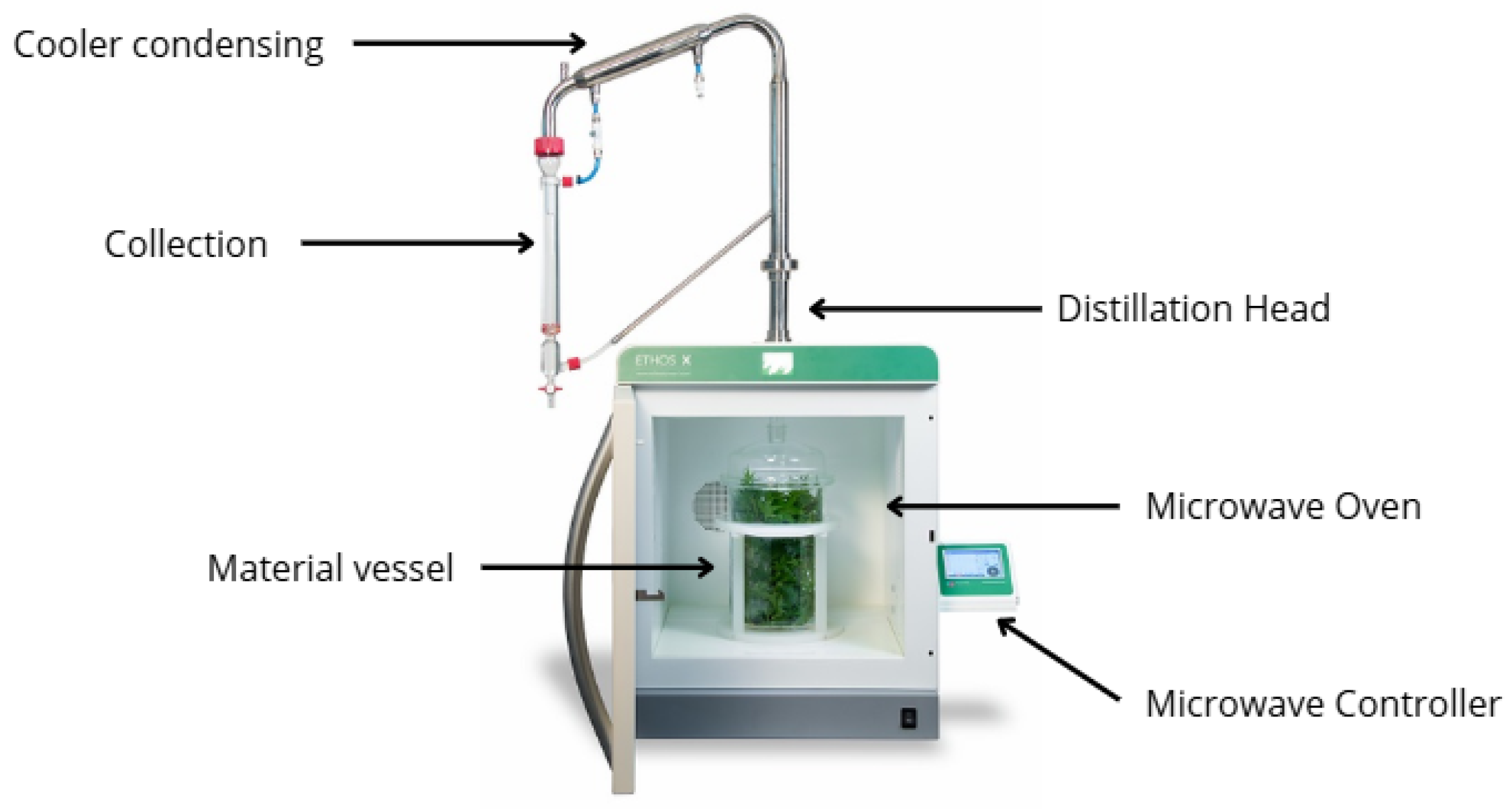

| Extraction Method | Processing Time (min) | Yield (%) | Density |
|---|---|---|---|
| SFME | 60 (20 + 40) ± 0 | 0.956 ± 0.06 a | 0.988 ± 0.002 a |
| HD | 232 (52 + 180) ± 2 | 0.80 ± 0.01 b | 0.985 ± 0.004 a |
| SD | 226 (46 + 180) ± 1 | 0.463 ± 0.02 c | 0.959 ± 0.003 c |
| Chemical Family | Chemical Group | RT [min] | RI | Component Name | Extraction Method | ||
|---|---|---|---|---|---|---|---|
| HD | SFME | SD | |||||
| Total aromatic compounds | 65.01 ± 4.61 a | 72.34 ± 6.07 a | 57.29 ± 3.85 a | ||||
| ester | 19.07 | 1262 | benzyl acetate | 20.14 ± 1.79 a | 28.94 ± 2.44 b | 15.98 ± 1.59 a | |
| ester | 28.02 | 1852 | benzyl benzoate | 7.43 ± 1.36 a | 4.6 ± 0.29 b | 7.08 ± 0.98 a | |
| ester | 29.47 | 1952 | benzyl salicylate | 3.16 ± 0.77 a | 1.73 ± 0.22 b | 4.6 ± 0.29 a | |
| ester | 18.21 | 1210 | methyl benzoate | 8.39 ± 0.63 a | 9.01 ± 0.06 a | 6.23 ± 0.17 b | |
| ester | 23.18 | 1521 | (E,E)-cinnamyl acetate | 7.48 ± 0.39 a | 7.84 ± 0.05 a | 7.06 ± 0.14 a | |
| ester | 19.27 | 1275 | ethyl benzoate | 0.07 ± 0.02 a | 0.06 ± 0.01 a | 0.1 ± 0.04 a | |
| ester | 19.72 | 1302 | methyl salicylate | 0.34 ± 0.04 ab | 0.29 ± 0.01 bc | 0.26 ± 0.01 c | |
| ester | 23.20 | 1522 | phenol, 4-(2-propenyl), acetate | - | 0.05 ± 0.01 a | - | |
| ester | 20.39 | 1344 | phenyl ethyl acetate | 0.32 ± 0.05 a | - | 0.24 ± 0.02 c | |
| ester | 22.24 | 1460 | benzoic acid, 2-methoxy, methyl ester | - | 0.04 ± 0.01 b | 0.07 ± 0.02 c | |
| ester | 20.70 | 1363 | benzoic acid, 4-methoxy, methyl ester | 0.09 ± 0.01 a | 0.09 ± 0.01 a | - | |
| ester | 20.96 | 1380 | benzene methanol, 2-methyl, acetate | - | 0.57 ± 0.03 a | - | |
| ester | 23.26 | 1526 | benzene methanol, 4-methoxy, acetate | 0.02 ± 0.03 ac | 0.15 ± 0.01 b | - | |
| ester | 22.07 | 1449 | 3-phenyl-1-propanol, acetate | - | 0.03 ± 0 b | 0.02 ± 0 c | |
| ester | 31.97 | 2110 | (E)-cinnamyl benzoate | 0.01 ± 0 a | - | 0.02 ± 0.01 c | |
| ester | 24.97 | 1640 | 3-hexen-1-ol, benzoate, (Z) | 0.03 ± 0.02 a | - | - | |
| phenol | 23.29 | 1528 | isoeugenol | 3.25 ± 0.3 a | 3.14 ± 0.25 a | 3.19 ± 0.06 a | |
| phenol | 17.69 | 1178 | p-cresol | 0.4 ± 0.08 a | 0.61 ± 0.04 b | 0.17 ± 0.02 c | |
| phenol | 19.54 | 1291 | creosol | 0.03 ± 0.01 a | 0.03 ± 0.01 a | 0.01 ± 0 b | |
| phenol | 25.74 | 1692 | acetyl eugenol | - | 0.27 ± 0.02 a | - | |
| phenol | 21.98 | 1444 | 2-methoxy-4-vinylphenol | - | 0.01 ± 0.01 a | - | |
| ether-oxide | 17.13 | 1143 | p-cresyl methyl ether | 12.62 ± 1.46 a | 13.54 ± 0.24 a | 11.23 ± 0.93 a | |
| ether-oxide | 20.96 | 1380 | anethole | 0.52 ± 0.11 a | 0.48 ± 0.05 a | 0.51 ± 0.07 a | |
| ether-oxide | 18.34 | 1218 | dimethoxy-1,2-benzene | 0.02 ± 0.01 a | - | - | |
| nitrogen compound | 21.24 | 1397 | 2-phenyl nitroethane | 0.37 ± 0.03 a | 0.58 ± 0.04 b | 0.37 ± 0.02 a | |
| nitrogen compound | 18.82 | 1247 | benzene, 1-isocyano-2-methyl | 0.01 ± 0.0 a | 0.02 ± 0 b | 0.01 ± 0 a | |
| nitrogen compound | 32.61 | 2145 | 1-benzylindole | - | - | 0.02 ± 0.01 b | |
| aldehyde | 17.49 | 1165 | benzeneacetaldehyde | 0.28 ± 0.07 a | 0.24 ± 0.04 a | 0.12 ± 0.02 b | |
| aldehyde | 20.31 | 1339 | cinnamaldehyde, (E) | - | 0.02 ± 0.01 b | - | |
| alkene | 21.89 | 1438 | benzene, 1-propenyl | 0.03 ± 0 a | - | - | |
| Total terpenes | 29.37± 1.90 abc | 21.11± 1.57 b | 38.1± 2.90 c | ||||
| monoterpene hydrocarbon | 16.04 | 1076 | α-pinene | 0.12 ± 0 a | 0.10 ± 0.03 a | 0.09 ± 0.01 a | |
| monoterpene hydrocarbon | 16.43 | 1100 | β-myrcene | 0.03 ± 0.01 a | 0.03 ± 0 a | 0.04 ± 0.02 a | |
| sesquiterpene hydrocarbon | 22.51 | 1477 | α-copaene | 0.19 ± 0.03 a | 0.14 ± 0.02 a | 0.34 ± 0.04 b | |
| sesquiterpene hydrocarbon | 21.72 | 1427 | δ-elemene | 0.15 ± 0.01 a | 0.12 ± 0.01 a | 0.15 ± 0.02 a | |
| sesquiterpene hydrocarbon | 23.32 | 1530 | α -humulene | 0.29 ± 0.02 a | 0.16 ± 0.11 a | - | |
| sesquiterpene hydrocarbon | 23.88 | 1567 | α-farnesene | 7.15 ± 0.45 a | 5.68 ± 0.94 a | 12.61 ± 1.2 b | |
| sesquiterpene hydrocarbon | 24.07 | 1579 | germacrene-D | 5.8 ± 0.38 a | 3.55 ± 1.14 b | 12.24 ± 0.93 c | |
| sesquiterpene hydrocarbon | 24.46 | 1605 | δ-cadinene | 0.87 ± 0.06 a | 0.45 ± 0.16 b | 1.07 ± 0.02 a | |
| sesquiterpene hydrocarbon | 24.75 | 1625 | α-cubenene | 0.09 ± 0.01 a | 0.04 ± 0.02 a | 0.07 ± 0.06 a | |
| sesquiterpene hydrocarbon | 24.89 | 1634 | α-cadinene | 0.01 ± 0.01 a | 0.01 ± 0.01 a | 0.03 ± 0.01 a | |
| sesquiterpene hydrocarbon | 22.29 | 1463 | aromadendrene | 0.24 ± 0.05 a | 0.2 ± 0.04 a | - | |
| sesquiterpene hydrocarbon | 22.59 | 1482 | alloaromadendrene | - | - | 0.48 ± 0.09 c | |
| sesquiterpene hydrocarbon | 23.91 | 1569 | α-muurolene | 0.84 ± 0.1 a | - | - | |
| sesquiterpene hydrocarbon | 24.02 | 1576 | γ-muurolene | 0.24 ± 0.02 a | 0.47 ± 0.39 a | - | |
| sesquiterpene hydrocarbon | 24.28 | 1593 | zonarene | 0.06 ± 0.01 a | - | - | |
| sesquiterpenic alcohol | 18.09 | 1202 | linalool | 8.26 ± 1.04 ab | 7.67 ± 0.13 bc | 6.58 ± 0.23 c | |
| sesquiterpenic alcohol | 20.25 | 1335 | geraniol | 0.05 ± 0.01 a | - | 0.05 ± 0.02 a | |
| sesquiterpenic alcohol | 26.92 | 1774 | farnesol | 1.46 ± 0.27 a | 0.63 ± 0.02 b | 1.19 ± 0.17 a | |
| sesquiterpenic alcohol | 26.36 | 1735 | τ-cadinol | 0.85 ± 0.19 a | 0.32 ± 0.04 b | 0.61 ± 0.06 a | |
| sesquiterpenic alcohol | 26.55 | 1748 | τ-muurolol | 0.93 ± 0.2 a | 0.37 ± 0.02 b | 0.63 ± 0.06 a | |
| sesquiterpenic alcohol | 25.69 | 1689 | guaiol | 0.22 ± 0.02 a | 0.22 ± 0.02 a | 0.35 ± 0.03 b | |
| sesquiterpenic alcohol | 26.19 | 1723 | epi-cubenol | - | 0.07 ± 0.02 b | 0.16 ± 0.01 c | |
| sesquiterpenic alcohol | 25.88 | 1701 | junenol | 0.15 ± 0.03 a | - | - | |
| sesquiterpenic alcohol | 25.08 | 1647 | α-elemol | 0.05 ± 0.01 a | 0.05 ± 0.01 a | 0.05 ± 0 a | |
| sesquiterpenic alcohol | 25.5 | 1676 | germacrene-D-4-ol | - | - | 0.22 ± 0.04 c | |
| sesquiterpenic alcohol | 25.74 | 1692 | caryophyllene alcohol | 0.1 ± 0.01 a | - | - | |
| sesquiterpenic alcohol | 25.17 | 1653 | 10-epi- γ -eudesmol | 0.07 ± 0.03 a | - | - | |
| sesquiterpenic alcohol | 26.71 | 1759 | agarospirol | - | - | 0.08 ± 0.02 c | |
| sesquiterpenic alcohol | 26.59 | 1751 | bulnesol | 0.08 ± 0.02 a | - | - | |
| sesquiterpenic alcohol | 27.03 | 1137 | β-acorenol | - | 0.05 ± 0.01 a | - | |
| sesquiterpenic alcohol | 27.15 | 1790 | cubebol | 0.01 ± 0.01 a | - | - | |
| sesquiterpenic alcohol | 20.76 | 1367 | nerol | - | 0.02 ± 0.01 a | - | |
| monoterpene ester | 20.14 | 1329 | linalyl acetate | - | - | 0.01 ± 0.01 c | |
| monoterpene ester | 21.95 | 1442 | geranyl Acetate | 1.02 ± 0.03 a | 0.69 ± 0.07 b | 1.01 ± 0.05 a | |
| monoterpene oxide | 17.30 | 1154 | eucalyptol | 0.04 ± 0.01 a | 0.07 ± 0.01 a | 0.04 ± 0.02 a | |
| Total aliphatic derivatives | 5.5± 0.72 a | 6.52± 1.13 a | 4.57± 0.61 a | ||||
| ester | 28.32 | 1873 | (E,E)-farnesyl acetate | 0.76 ± 0.2 a | 0.35 ± 0.02 b | 1.08 ± 0.2 a | |
| ester | 15.04 | 1015 | 3-methyl-3-butene-1-ol acetate | 1.27 ± 0.14 a | 1.22 ± 0.02 a | 0.61 ± 0.06 b | |
| ester | 15.51 | 1044 | prenyl acetate | 2.57 ± 0.29 a | 4.23 ± 0.06 b | 2.15 ± 0.11 a | |
| ester | 16.53 | 1106 | cis-3-hexenyl-acetate | 0.04 ± 0 a | 0.04 ± 0.01 a | 0.06 ± 0.03 a | |
| ester | 14.17 | 960 | isobutyl acetate | - | 0.01 ± 0.01 a | - | |
| ester | 13.94 | 945 | butyl acetate | 0.09 ± 0.03 a | 0.07 ± 0.03 a | - | |
| ester | 16.72 | 1118 | hexyl-acetate | 0.49 ± 0.1 a | 0.49 ± 0.03 a | 0.44 ± 0.05 a | |
| ester | 14.77 | 999 | 1-butanol, 3-methyl, acetate | - | - | 0.11 ± 0.03 c | |
| alcohol | 12.42 | 837 | 3-buten-2-ol, 2-methyl | 0.09 ± 0.02 a | - | - | |
| alcohol | 29.00 | 1920 | 1-hexadecanol | - | - | 0.06 ± 0.01 c | |
| alcohol | 13.35 | 907 | prenol | 0.03 ± 0.03 a | - | - | |
| alcohol | 19.90 | 1314 | citronellol | - | - | 0.03 ± 0.01 c | |
| aldehyde | 14.12 | 957 | 2-hexenal, (E) | 0.14 ± 0.03 a | 0.1 ± 0.03 a | 0.03 ± 0.01 b | |
| aldehyde | 12.70 | 859 | pentanal | 0.02 ± 0.01 ab | 0.01 ± 0.01 bc | - | |
| Total (%) | 99.52 | 99.97 | 99.96 | ||||
| Extraction Method | Light Oxygenated Compounds | Heavy Oxygenated Compounds | Terpene Hydrocarbons | Aromatics |
|---|---|---|---|---|
| HD | 69.94 ± 7.53 abc | 13.69 ± 2.90 ac | 16.1 ± 1.04 ab | 47.45 ± 4.7 abc |
| SFME | 81.23 ± 8.49 b | 7.75 ± 0.42 b | 10.95 ± 2.71 b | 53.4 ± 2.90 b |
| SD | 57.98 ± 6.62 c | 13.68 ± 1.72 c | 27.12 ± 2.05 c | 41.66 ± 3.6 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chakira, A.; Soria, C.; Lallemand, L.; Mares, G.; Chillet, M.; Garcia, C. Improving the Quality of Ylang-Ylang Essential Oils [Cananga odorata (Lam.) Hook.f. &Thomson] Through Microwave-Assisted Extraction Compared to Conventional Extraction Methods. Plants 2025, 14, 3217. https://doi.org/10.3390/plants14203217
Chakira A, Soria C, Lallemand L, Mares G, Chillet M, Garcia C. Improving the Quality of Ylang-Ylang Essential Oils [Cananga odorata (Lam.) Hook.f. &Thomson] Through Microwave-Assisted Extraction Compared to Conventional Extraction Methods. Plants. 2025; 14(20):3217. https://doi.org/10.3390/plants14203217
Chicago/Turabian StyleChakira, Abacar, Christian Soria, Laura Lallemand, Gary Mares, Marc Chillet, and Cyrielle Garcia. 2025. "Improving the Quality of Ylang-Ylang Essential Oils [Cananga odorata (Lam.) Hook.f. &Thomson] Through Microwave-Assisted Extraction Compared to Conventional Extraction Methods" Plants 14, no. 20: 3217. https://doi.org/10.3390/plants14203217
APA StyleChakira, A., Soria, C., Lallemand, L., Mares, G., Chillet, M., & Garcia, C. (2025). Improving the Quality of Ylang-Ylang Essential Oils [Cananga odorata (Lam.) Hook.f. &Thomson] Through Microwave-Assisted Extraction Compared to Conventional Extraction Methods. Plants, 14(20), 3217. https://doi.org/10.3390/plants14203217






