Bryophyte Community Composition and Diversity as Bioindicators of Elevational Zonation in Tropical Rainforests in Hainan Island, China
Abstract
1. Introduction
2. Results
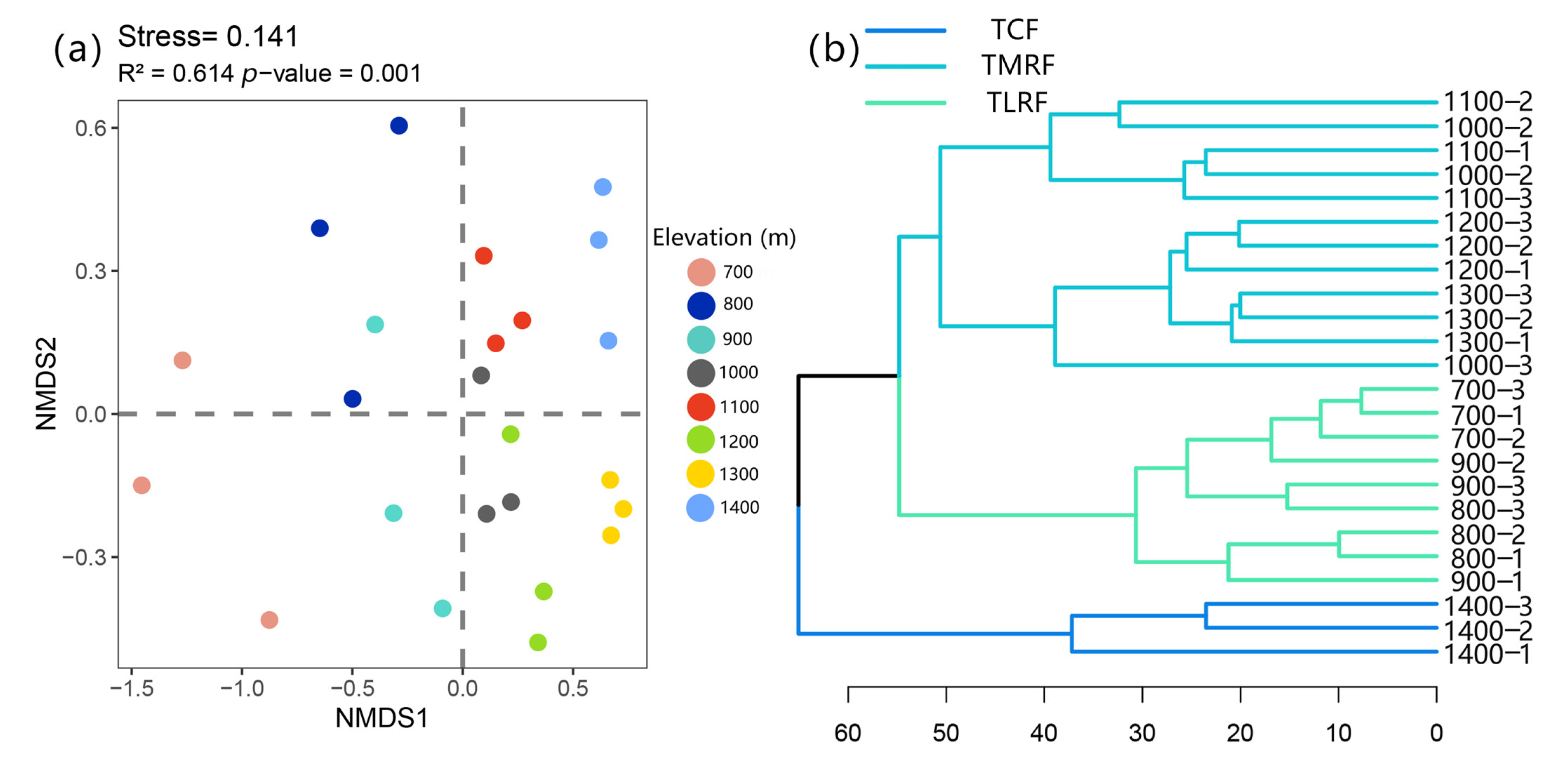
2.1. Variation in Species Diversity Along the Elevation Gradient
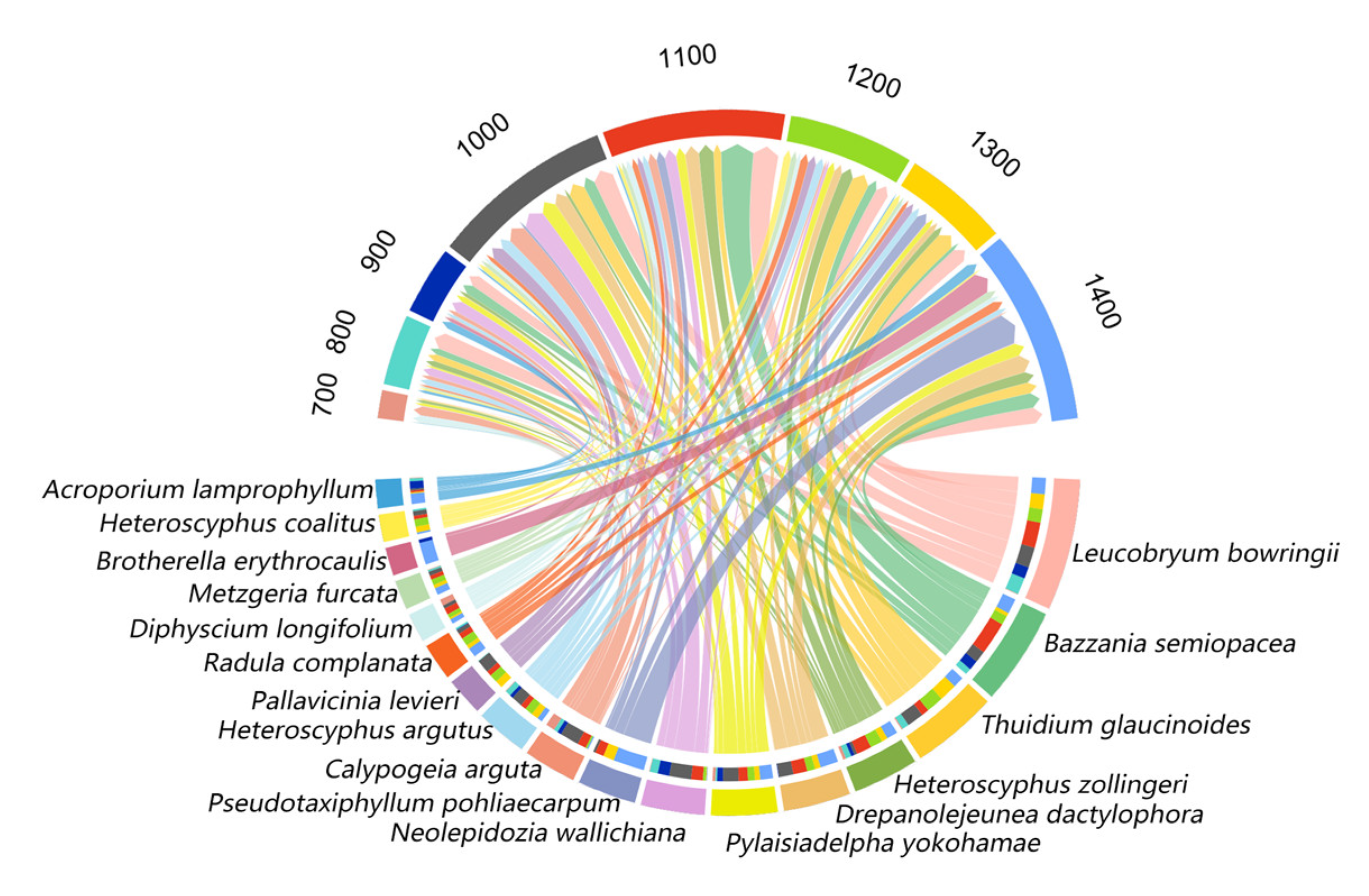
2.2. Community Assemblages Along the Elevation Gradient
2.3. Local Indicator Species for Vegetation Types Along Elevation
2.4. Indicator Species for Vegetation Types Along Elevation
3. Discussion
3.1. Species Diversity and Distribution Patterns of Bryophyte Communities Along the Elevation
3.2. Bryophyte Communities Coupling with Vegetation Types Along Elevation Gradients
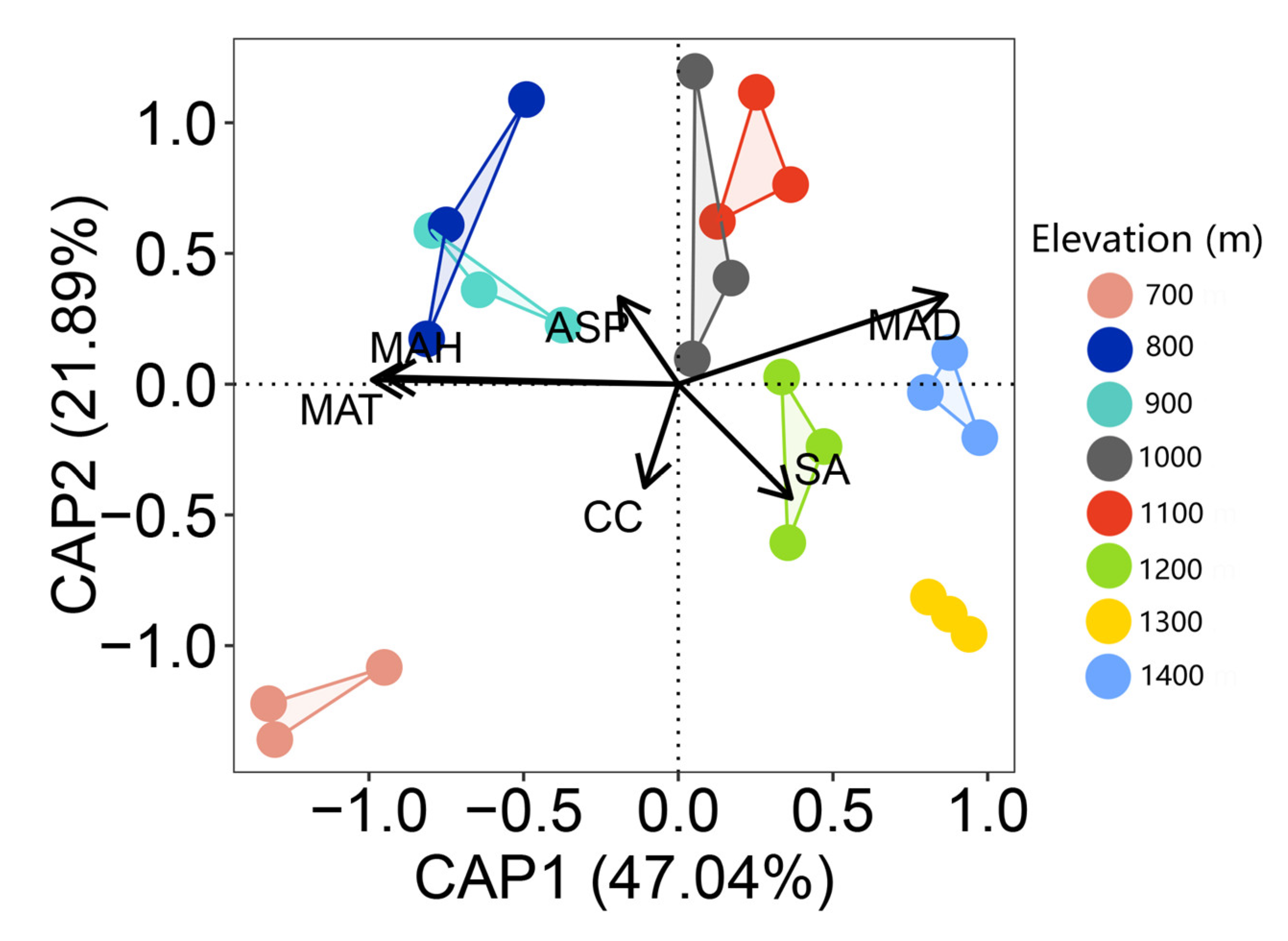
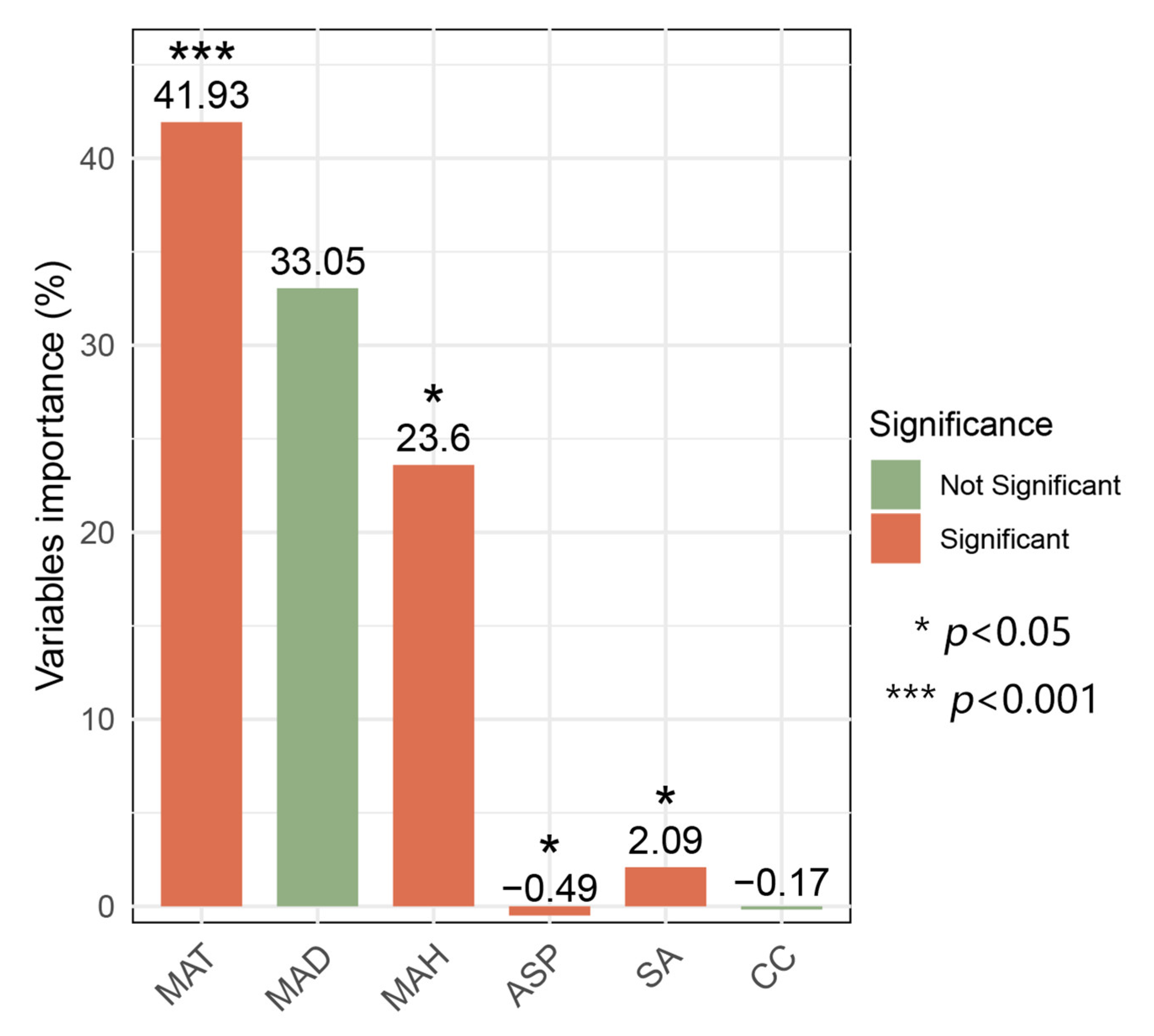
3.3. The Contribution of Environmental Factors to Bryophyte Distribution
4. Materials and Methods
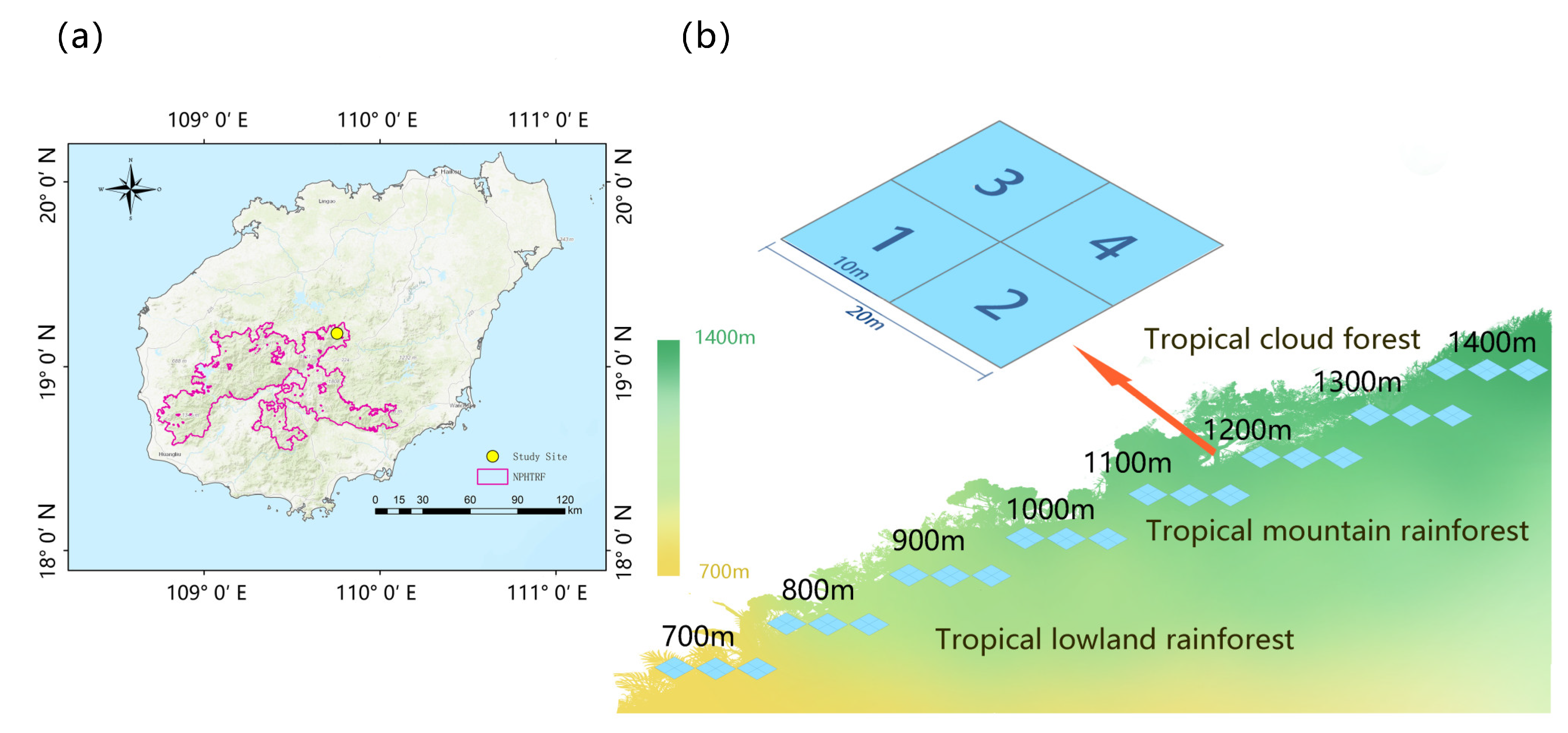
4.1. Study Site
4.2. Bryophyte Sampling Collection and Identification
4.3. Environmental Variables
4.4. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
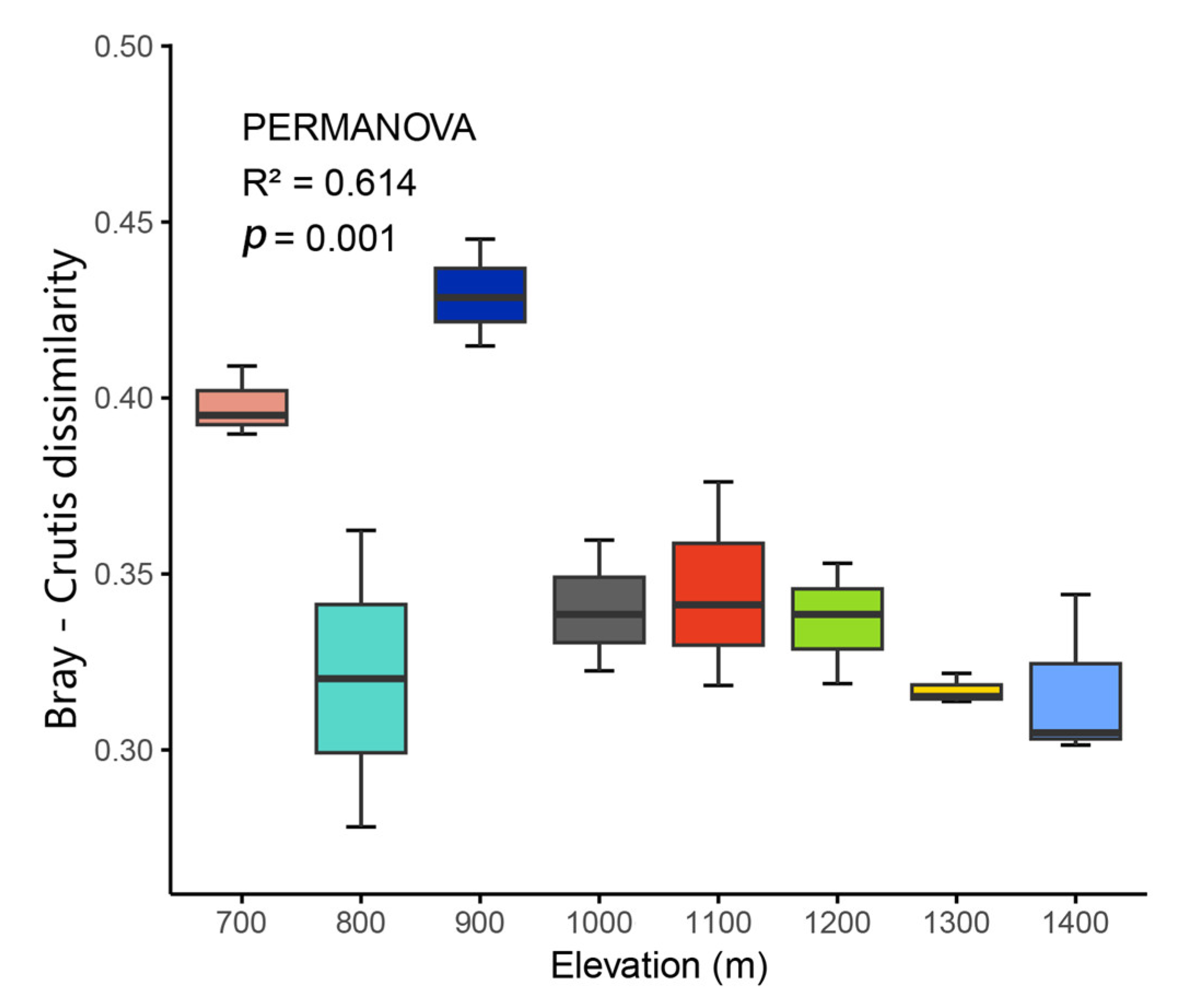
Appendix A.1. Bryophyte Species Beta Diversity on Altitudinal Gradient
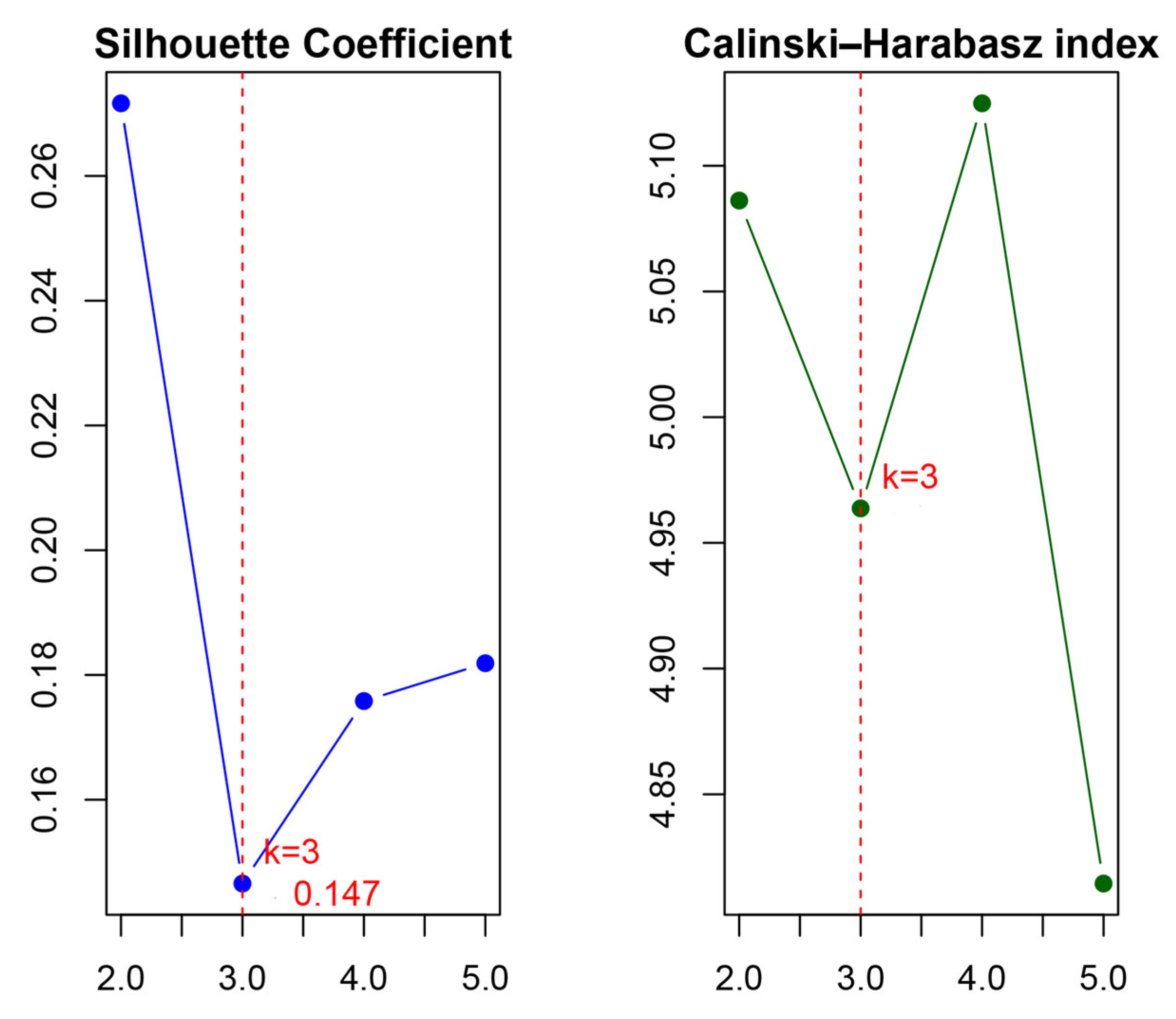
Appendix A.2. Optimal Number of Clusters into Distinct Vegetation Types
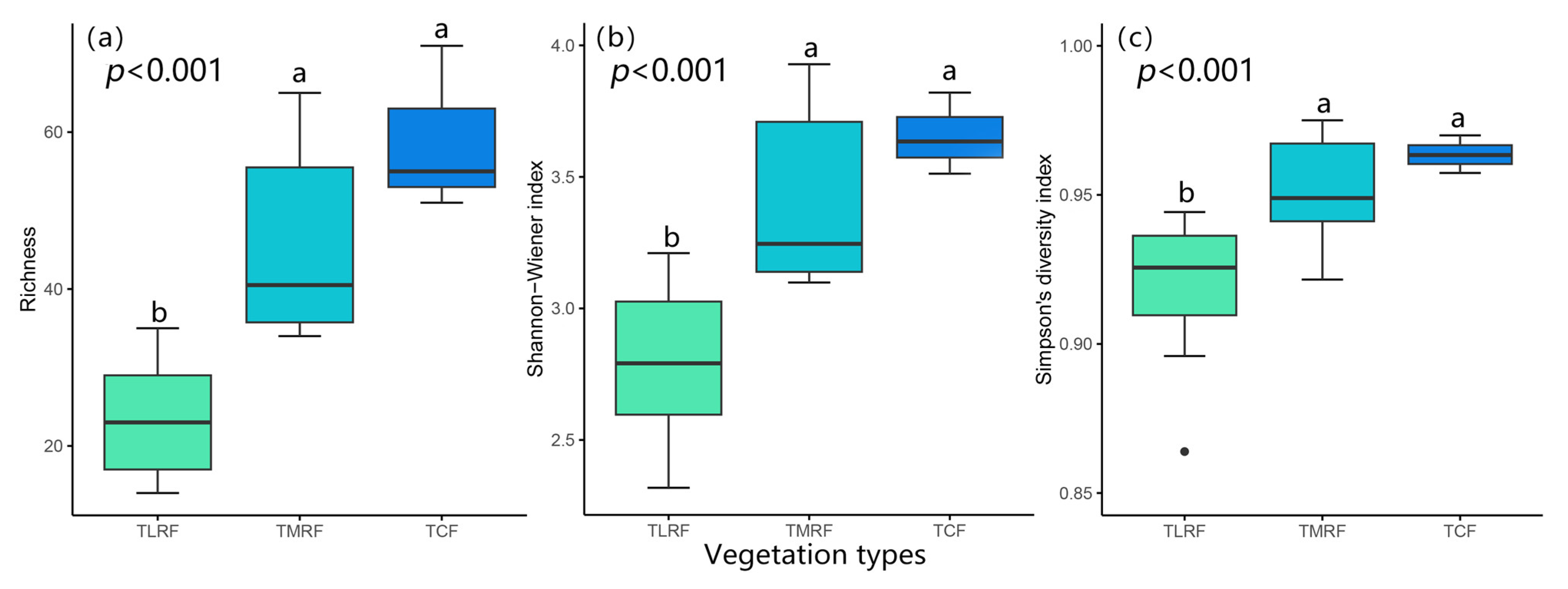
Appendix A.3. Bryophyte Species Alpha Diversity in Three Vegetation Types
References
- Rahbek, C.; Borregaard, M.K.; Antonelli, A.; Colwell, R.K.; Holt, B.G.; Nogues-Bravo, D.; Rasmussen, C.M.Ø.; Richardson, K.; Rosing, M.T.; Whittaker, R.J.; et al. Building Mountain Biodiversity: Geological and Evolutionary Processes. Science 2019, 365, 1114–1119. [Google Scholar] [CrossRef]
- Rahbek, C.; Borregaard, M.K.; Colwell, R.K.; Dalsgaard, B.; Holt, B.G.; Morueta-Holme, N.; Nogues-Bravo, D.; Whittaker, R.J.; Fjeldså, J. Humboldt’s Enigma: What Causes Global Patterns of Mountain Biodiversity? Science 2019, 365, 1108–1113. [Google Scholar] [CrossRef]
- Galván, J.D.; Camarero, J.J.; Gutiérrez, E. Seeing the Trees for the Forest: Drivers of Individual Growth Responses to Climate in Pinus uncinata Mountain. For. J. Ecol. 2014, 102, 1244–1257. [Google Scholar] [CrossRef]
- Jucker, T.; Bongalov, B.; Burslem, D.F.R.P.; Nilus, R.; Dalponte, M.; Lewis, S.L.; Phillips, O.L.; Qie, L.; A Coomes, D. Topography Shapes the Structure, Composition and Function of Tropical Forest Landscapes. Ecol. Lett. 2018, 21, 989–1000. [Google Scholar] [CrossRef]
- Antonelli, A.; Kissling, W.D.; Flantua, S.G.A.; Bermúdez, M.A.; Mulch, A.; Muellner-Riehl, A.N.; Kreft, H.; Linder, H.P.; Badgley, C.; Fjeldså, J.; et al. Geological and Climatic Influences on Mountain Biodiversity. Nat. Geosci. 2018, 11, 718–725. [Google Scholar] [CrossRef]
- Ye, Y.; Lu, D.; Wu, Z.; Liao, K.; Zhou, M.; Jian, K.; Li, D. Vertical Characteristics of Vegetation Distribution in Wuyishan National Park Based on Multi-Source High-Resolution Remotely Sensed Data. Remote Sens. 2023, 15, 5023. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, X.; Zheng, D. Vertical Differentiation of Land Cover in the Central Himalayas. J. Geogr. Sci. 2020, 30, 969–987. [Google Scholar] [CrossRef]
- Yang, X.B.; Chen, Z.Z.; Li, D.H. Classification and Distribution of Vegetation in Hainan, China. Sci. Sin. Vitae 2021, 51, 321–333. [Google Scholar] [CrossRef]
- Marselis, M.S.; Hao, T.; David, A.J.; Kim, C.; Labrière, N.; Ralph, D. Distinguishing Vegetation Types with Airborne Waveform LiDAR Data in a Tropical Forest–Savanna Mosaic: A Case Study in Lopé National Park, Gabon. Remote Sens. Environ. 2018, 216, 626–634. [Google Scholar] [CrossRef]
- Liu, M.; Zhan, Y.; Li, J.; Kang, Y.; Sun, X.; Gu, X.; Wei, X.; Wang, C.; Li, L.; Gao, H.; et al. Validation of Red-Edge Vegetation Indices in Vegetation Classification in Tropical Monsoon Region—A Case Study in Wenchang, Hainan, China. Remote Sens. 2024, 16, 1865. [Google Scholar] [CrossRef]
- Malik, Z.A.; Hussain, A.; Iqbal, K.; Bhatt, A.B. Species richness and diversity along the disturbance gradient in Kedarnath Wildlife Sanctuary and its adjoining areas in Garhwal Himalaya, India. Int. J. Curr. Res. 2014, 6, 10918–10926. [Google Scholar]
- Bhat, J.A.; Kumar, M.; Negi, A.K.; Todaria, N.P.; Malik, Z.A.; Pala, N.A.; Kumar, A.; Shukla, G. Species diversity of woody vegetation along altitudinal gradient of the Western Himalayas. Glob. Ecol. Conserv. 2020, 24, e01302. [Google Scholar] [CrossRef]
- Grau, O.; Grytnes, J.A.; Birks, H.J.B. A Comparison of Altitudinal Species Richness Patterns of Bryophytes with Other Plant Groups in Nepal, Central Himalaya. J. Biogeogr. 2007, 34, 1907–1915. [Google Scholar] [CrossRef]
- Glime, J.M. Meet the Bryophytes. In Bryophyte Ecology: Physiological Ecology; Glime, J.M., Ed.; Michigan Technological University: Houghton, MI, USA, 2017; Volume 1, pp. 1–10. [Google Scholar]
- Frego, K.A. Bryophytes as Potential Indicators of Forest Integrity. For. Ecol. Manag. 2007, 242, 65–75. [Google Scholar] [CrossRef]
- Karger, D.N.; Kessler, M.; Lehnert, M.; Jetz, W. Limited Protection and Ongoing Loss of Tropical Cloud Forest Biodiversity and Ecosystems Worldwide. Nat. Ecol. Evol. 2021, 5, 854–862. [Google Scholar] [CrossRef]
- He, X.; He, K.S.; Hyvönen, J. Will Bryophytes Survive in a Warming World? Perspect. Plant Ecol. Evol. Syst. 2016, 19, 49–60. [Google Scholar] [CrossRef]
- Berdugo, M.B.; Quant, J.M.; Wason, J.W.; Dovciak, M. Latitudinal Patterns and Environmental Drivers of Moss Layer Cover in Extratropical Forests. Glob. Ecol. Biogeogr. 2018, 27, 729–739. [Google Scholar] [CrossRef]
- Shen, T.; Corlett, R.T.; Song, L.; Ma, W.; Guo, X.; Song, Y.; Wu, Y. Vertical Gradient in Bryophyte Diversity and Species Composition in Tropical and Subtropical Forests in Yunnan, SW China. J. Veg. Sci. 2018, 29, 1075–1087. [Google Scholar] [CrossRef]
- Gradstein, S.R. Epiphyllous Bryophyte Diversity in Lowland Rain Forest and Lowland Cloud Forest of French Guiana. Cryptogam. Bryol. 2022, 43, 187–193. [Google Scholar] [CrossRef]
- Frahm, J.P.; Gradstein, S.R. An Altitudinal Zonation of Tropical Rain Forests Using Bryophytes. J. Biogeogr. 1991, 18, 669–678. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Fonseca, G.A.B.; Kent, J. Biodiversity Hotspots for Conservation Priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Francisco-Ortega, J.; Wang, Z.; Wang, F.; Xing, F.-W.; Liu, H.; Xu, H.; Xu, W.-X.; Luo, Y.-B.; Song, X.-Q.; Gale, S.; et al. Seed Plant Endemism on Hainan Island: A Framework for Conservation Actions. Bot. Rev. 2010, 76, 346–376. [Google Scholar] [CrossRef]
- Wang, Z.; Ye, W.; Xing, F. Bryophyte Diversity on a Tropical Continental Island (Hainan, China): Potential Vulnerable Species and Environmental Indicators. J. Bryol. 2019, 41, 350–360. [Google Scholar] [CrossRef]
- Rodríguez-Quiel, E.E.; Kluge, J.; Mendieta-Leiva, G.; Bader, M.Y. Elevational Patterns in Tropical Bryophyte Diversity Differ among Substrates: A Case Study on Baru Volcano, Panama. J. Veg. Sci. 2022, 33, e13136. [Google Scholar] [CrossRef]
- Marline, L.; Ah-Peng, C.; Hedderson, T.A.J. Epiphytic Bryophyte Diversity and Range Distributions along an Elevational Gradient in Marojejy, Madagascar. Biotropica 2020, 52, 616–626. [Google Scholar] [CrossRef]
- Maul, K.; Wei, Y.; Iskandar, E.A.P.; Chantanaorrapint, S.; Ho, B.; Quandt, D.; Kessler, M. Liverworts show a globally consistent mid-elevation richness peak. Ecol. Evolut. 2023, 13, e9862. [Google Scholar] [CrossRef]
- Wang, J.; Qian, H.; Dai, Z.; Zhang, J.; Kessler, M. Geographic and Ecological Effects on Species Richness of Liverworts Worldwide. Ecography 2024, 2024, e07277. [Google Scholar] [CrossRef]
- Iskandar, E.A.P.; Stech, M.; De Oliveira, S.M. The Two Faces of Mt Gede, Java—Species Richness, Composition and Zonation of Epiphytic Bryophytes. Cryptogamie Bryol. 2020, 41, 69–81. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Wu, Z.; Li, S.; Dai, W. Identification of Biodiversity Priority Conservation Areas in China by Integrating Genetic, Species and Ecosystem Diversity. Biol. Conserv. 2024, 300, 110854. [Google Scholar] [CrossRef]
- Hernández-Hernández, R.; Borges, P.A.V.; Gabriel, R.; Rigal, F.; Ah-Peng, C.; González-Mancebo, J.M. Scaling α- and β-Diversity: Bryophytes along an Elevational Gradient on a Subtropical Oceanic Island (La Palma, Canary Islands). J. Veg. Sci. 2017, 28, 1209–1219. [Google Scholar] [CrossRef]
- Ah-Peng, C.; Chuah-Petiot, M.; Descamps-Julien, B.; Bardat, J.; Stamenoff, P.; Strasberg, D. Bryophyte Diversity and Distribution along an Altitudinal Gradient on a Lava Flow in La Réunion. Divers. Distrib. 2007, 13, 654–662. [Google Scholar] [CrossRef]
- Bruun, H.H.; Moen, J.; Virtanen, R.; Grytnes, J.A.; Oksanen, L.; Angerbjörn, A. Effects of Altitude and Topography on Species Richness of Vascular Plants, Bryophytes and Lichens in Alpine Communities. J. Veg. Sci. 2006, 17, 37–46. [Google Scholar] [CrossRef]
- Fu, L.; Mei, X.; Xu, P.; Zhao, J.; Gao, D. The Species Richness and Community Composition of Different Growth Forms and Life Forms of Mosses Are Dominated by Different Factors along an Elevational Gradient of China. Glob. Ecol. Conserv. 2023, 47, e02646. [Google Scholar] [CrossRef]
- Tusiime, F.M.; Byarujali, S.M.; Bates, J.W. Diversity and Distribution of Bryophytes in Three Forest Types of Bwindi Impenetrable National Park, Uganda. Afr. J. Ecol. 2007, 45, 79–87. [Google Scholar] [CrossRef]
- Santos, N.D.; Costa, D.P.; Kinoshita, L.S.; Shepherd, G.J. Variations in Bryophyte Communities in a Short Elevational Gradient in Atlantic Forest of Southeastern Brazil. Cryptogam. Bryol. 2017, 38, 191–211. [Google Scholar] [CrossRef]
- Hao, J.; Chu, L. Vegetation Types Attributed to Deforestation and Secondary Succession Drive the Elevational Changes in Diversity and Distribution of Terrestrial Mosses in a Tropical Mountain Forest in Southern China. Forests 2021, 12, 961. [Google Scholar] [CrossRef]
- Ah-Peng, C.; Wilding, N.; Kluge, J.; Descamps-Julien, B.; Bardat, J.; Chuah-Petiot, M.; Strasberg, D.; Hedderson, T.A. Bryophyte Diversity and Range Size Distribution along Two Altitudinal Gradients: Continent vs. Island. Acta Oecol. 2012, 42, 58–65. [Google Scholar] [CrossRef]
- Zotz, G. Altitudinal Changes in Diversity and Abundance of Non-Vascular Epiphytes in the Tropics: An Ecophysiological Explanation. Selbyana 1999, 20, 256–260. Available online: http://www.jstor.org/stable/41760030 (accessed on 4 November 2024).
- Santos, N.D.; Costa, D.P. Altitudinal Zonation of Liverworts in the Atlantic Forest, Southeastern Brazil. Bryologist 2010, 113, 631–645. [Google Scholar] [CrossRef]
- Wood, A.J. The Nature and Distribution of Vegetative Desiccation-Tolerance in Hornworts, Liverworts and Mosses. Bryologist 2007, 110, 163–177. [Google Scholar] [CrossRef]
- Gradstein, S.R.; Pócs, T. Bryophytes. In Ecosystems of the World: Tropical Rain Forest Ecosystems; Lieth, H., Werger, M.J.A., Eds.; Elsevier: Amsterdam, The Netherlands, 1989; pp. 311–325. [Google Scholar] [CrossRef]
- Bubb, P.; May, I.; Miles, L.; Sayer, J. Cloud Forest Agenda; UNEP-WCMC: Cambridge, UK, 2004. [Google Scholar]
- Zang, R.; Ding, Y.; Zhang, Z.; Deng, F.; Mao, P. Ecological Foundation of Conservation and Restoration for the Major Functional Groups in Tropical Natural Forests on Hainan Island; Science Press: Beijing, China, 2010. [Google Scholar]
- Callaghan, D.A.; Ashton, P.A. Bryophyte Distribution and Environment across an Oceanic Temperate Landscape. J. Bryol. 2008, 30, 23–35. [Google Scholar] [CrossRef]
- Kentjens, W.; Glenny, D.; Curran, T.J.; Sullivan, J.J. Bryophyte Community Composition Is Influenced by Microhabitat and Cover of Vascular Plants and Lichens in New Zealand Montane Forest. N. Z. J. Bot. 2023, 63, 573–596. [Google Scholar] [CrossRef]
- Kutnar, L.; Kermavnar, J.; Sabovljević, M.S. Bryophyte Diversity, Composition and Functional Traits in Relation to Bedrock and Tree Species Composition in Close-to-Nature Managed Forests. Eur. J. For. Res. 2023, 142, 865–882. [Google Scholar] [CrossRef]
- Yao, X.; Zhou, L.; Wu, T.; Yang, X.; Ren, M. Ecosystem Services in National Park of Hainan Tropical Rainforest of China: Spatiotemporal Dynamics and Conservation Implications. J. Nat. Conserv. 2024, 80, 126649. [Google Scholar] [CrossRef]
- Číhal, L.; Kaláb, O.; Plášek, V. Modeling the Distribution of Rare and Interesting Moss Species of the Family Orthotrichaceae (Bryophyta) in Tajikistan and Kyrgyzstan. Acta Soc. Bot. Pol. 2017, 86, 15. [Google Scholar] [CrossRef]
- Stuiver, B.M.; Wardle, D.A.; Gundale, M.J.; Nilsson, M.C. The Impact of Moss Species and Biomass on the Growth of Pinus sylvestris Tree Seedlings at Different Precipitation Frequencies. Forests 2014, 5, 1931–1951. [Google Scholar] [CrossRef]
- Aragón, G.; Abuja, L.; Belinchón, R.; Martínez, I. Edge Type Determines the Intensity of Forest Edge Effect on Epiphytic Communities. Eur. J. For. Res. 2015, 134, 443–451. [Google Scholar] [CrossRef]
- Turner, P.A.M.; Kirkpatrick, J.B.; Pharo, E.J. Bryophyte Relationships with Environmental and Structural Variables in Tasmanian Old-Growth Mixed Eucalypt Forest. Aust. J. Bot. 2006, 54, 239–247. [Google Scholar] [CrossRef]
- Ma, X.Y.; Xu, H.; Cao, Z.Y.; Shu, L.; Zhu, R.L. Will Climate Change Cause the Global Peatland to Expand or Contract? Evidence from the Habitat Shift Pattern of Sphagnum Mosses. Glob. Chang. Biol. 2022, 28, 6419–6432. [Google Scholar] [CrossRef]
- Ding, Y.; Zang, R.; Liu, S.; He, F.; Letcher, S.G. Recovery of Woody Plant Diversity in Tropical Rain Forests in Southern China after Logging and Shifting Cultivation. J. Veg. Sci. 2012, 23, 404–415. [Google Scholar] [CrossRef]
- Roberts, D.W. Ordination on the basis of fuzzy set theory. Vegetatio 1986, 66, 123–131. [Google Scholar] [CrossRef]
- Mandl, N.A.; Kessler, M.; Gradstein, S.R. Effects of Environmental Heterogeneity on Species Diversity and Composition of Terrestrial Bryophyte Assemblages in Tropical Montane Forests of Southern Ecuador. Plant Ecol. Divers. 2009, 2, 313–321. [Google Scholar] [CrossRef]
- Miyamoto, S.; Suzuki, S.; Takumi, S. Clustering in Tweets Using a Fuzzy Neighborhood Model. In Proceedings of the IEEE International Conference on Fuzzy Systems, Brisbane, Australia, 10–15 June 2012; pp. 1–6. [Google Scholar] [CrossRef]
- Dixon, P. VEGAN, a Package of R Functions for Community Ecology. J. Veg. Sci. 2003, 14, 927–930. [Google Scholar] [CrossRef]
- Dufrêne, M.; Legendre, P. Species Assemblages and Indicator Species: The Need for a Flexible Asymmetrical Approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar] [CrossRef]
- Roberts, D.W. labdsv: Ordination and Multivariate Analysis for Ecology. R Package Version 2.0–1. Comprehensive R Archive Network (CRAN). 2019. Available online: https://CRAN.R-project.org/package=labdsv (accessed on 29 October 2024).
- Lai, J.; Zou, Y.; Zhang, J.; Peres-Neto, P.R. Generalizing Hierarchical and Variation Partitioning in Multiple Regression and Canonical Analyses Using the rdacca.hp R Package. Methods Ecol. Evol. 2022, 13, 782–788. [Google Scholar] [CrossRef]
- Lai, J.; Zou, Y.; Zhang, S.; Zhang, X.; Mao, L. glmm.hp: An R Package for Computing Individual Effect of Predictors in Generalized Linear Mixed Models. J. Plant Ecol. 2022, 15, 1302–1307. [Google Scholar] [CrossRef]
- Lai, J.; Zhu, W.; Cui, D.; Mao, L. Extension of the glmm.hp Package to Zero-Inflated Generalized Linear Mixed Models and Multiple Regression. J. Plant Ecol. 2023, 16, rtad038. [Google Scholar] [CrossRef]
| Vegetation Type | Indicator Species | % IndVal | p Value |
|---|---|---|---|
| TLRF | Isocladiella surcularis (Dixon) B.C.Tan & Mohamed | 68.13% | 0.005 |
| Leucobryum chlorophyllosum Müll.Hal. | 66.40% | 0.012 | |
| TMRF | Riccardia plumosa (Mitt.) E.O.Campb. | 79.67% | 0.002 |
| Pallavicinia levieri Schiffn. | 73.34% | 0.019 | |
| Heteroscyphus coalitus (Hook.) Schiffn. | 63.99% | 0.009 | |
| Distichophyllum mittenii Bosch & Sande Lac. | 62.50% | 0.032 | |
| TCF | Plagiochila peculiaris Schiffn. | 100.00% | 0.002 |
| Brotherella fauriei (Cardot) Broth. | 100.00% | 0.002 | |
| Brotherella erythrocaulis (Mitt.) M.Fleisch. | 95.00% | 0.001 | |
| Leucobryum scabrum Sande Lac. | 87.42% | 0.001 | |
| Plagiochila trabeculata Steph. | 86.02% | 0.001 | |
| Pseudotaxiphyllum pohliaecarpum (Sull. & Lesq.) Z.Iwats. | 85.04% | 0.001 | |
| Leucoloma molle (Müll. Hal.) Mitt. | 82.76% | 0.003 | |
| Lejeunea alata Gottsche | 81.99% | 0.001 | |
| Sematophyllum subpinnatum (Brid.) E.Britton | 80.00% | 0.002 | |
| Leucobryum boninense (Müll.Hal.) A.Jaeger | 78.05% | 0.004 | |
| Pyrrhobryum latifolium Mitt. | 71.49% | 0.007 | |
| Thuidium pristocalyx (Müll.Hal.) A.Jaeger | 68.97% | 0.005 | |
| Acroporium lamprophyllum Mitt. | 68.35% | 0.017 | |
| Hookeriopsis utacamundiana (Mont.) Broth. | 66.67% | 0.01 | |
| Radula cavifolia Hampe | 66.67% | 0.01 | |
| Frullania linii S.Hatt. | 66.67% | 0.013 | |
| Cololejeunea ceratilobula (P.C.Chen) R.M Schust. | 63.16% | 0.004 | |
| Dicranoloma dicarpum (Nees) Paris | 62.75% | 0.008 | |
| Drepanolejeunea dactylophora (Gottsche, Lindenb. & Nees) J.B.Jack & Steph. | 62.52% | 0.025 | |
| Metalejeunea cucullata (Reinw., Blume & Nees) Grolle | 62.07% | 0.022 | |
| Cololejeunea longifolia (Mitt.) Benedix | 61.54% | 0.013 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, X.; Qi, T.; Li, Y.; Wang, W.; Li, D.; Yang, X.; Hao, J. Bryophyte Community Composition and Diversity as Bioindicators of Elevational Zonation in Tropical Rainforests in Hainan Island, China. Plants 2025, 14, 3209. https://doi.org/10.3390/plants14203209
Su X, Qi T, Li Y, Wang W, Li D, Yang X, Hao J. Bryophyte Community Composition and Diversity as Bioindicators of Elevational Zonation in Tropical Rainforests in Hainan Island, China. Plants. 2025; 14(20):3209. https://doi.org/10.3390/plants14203209
Chicago/Turabian StyleSu, Xin, Tianyun Qi, Yuanling Li, Wenjuan Wang, Donghai Li, Xiaobo Yang, and Jiewei Hao. 2025. "Bryophyte Community Composition and Diversity as Bioindicators of Elevational Zonation in Tropical Rainforests in Hainan Island, China" Plants 14, no. 20: 3209. https://doi.org/10.3390/plants14203209
APA StyleSu, X., Qi, T., Li, Y., Wang, W., Li, D., Yang, X., & Hao, J. (2025). Bryophyte Community Composition and Diversity as Bioindicators of Elevational Zonation in Tropical Rainforests in Hainan Island, China. Plants, 14(20), 3209. https://doi.org/10.3390/plants14203209







