Differential Induction of Resistance Mechanisms by Methyl Jasmonate in Two Vaccinium corymbosum L. Cultivars Under Combined Water Deficit and Aluminum Toxicity
Abstract
1. Introduction
2. Results
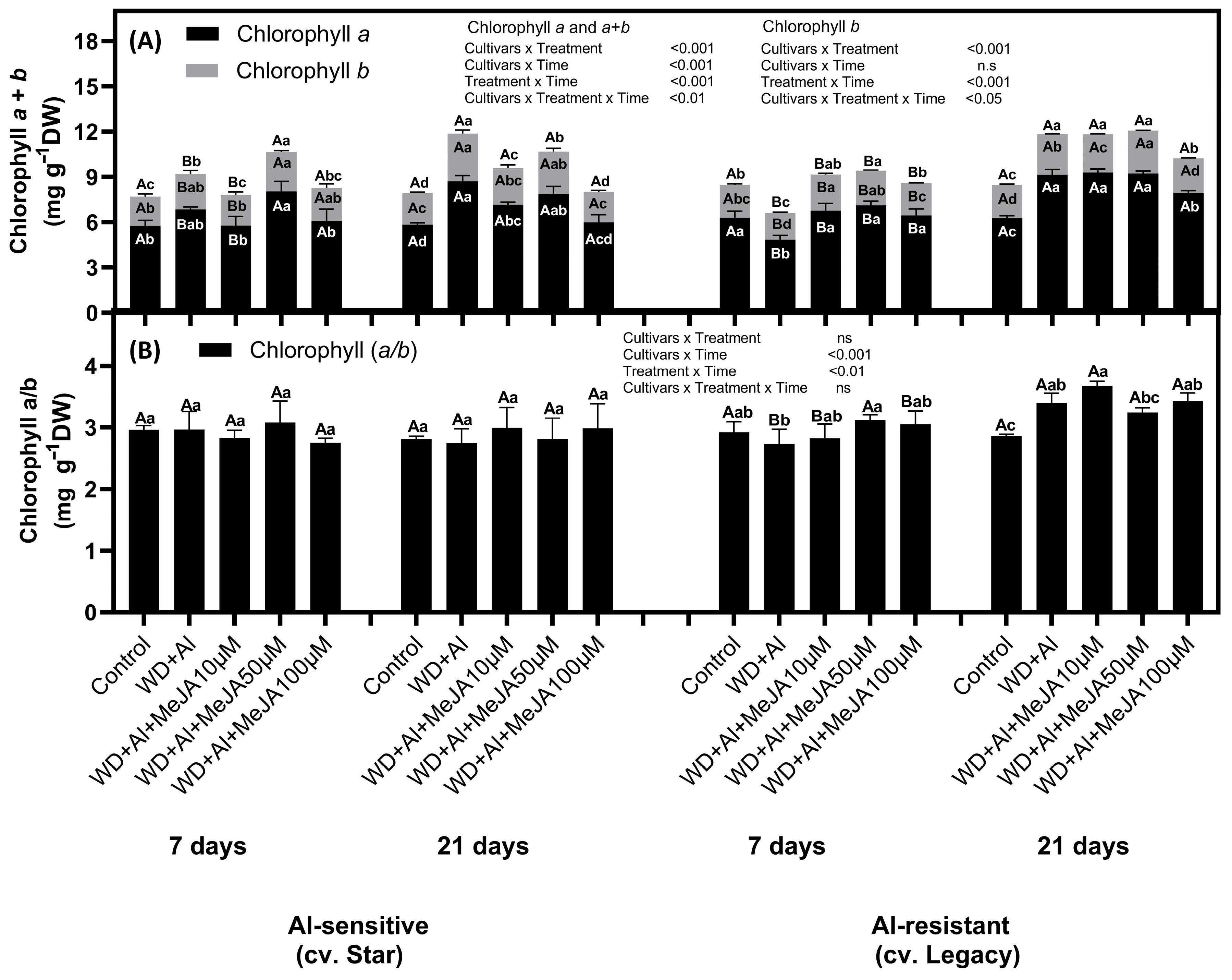
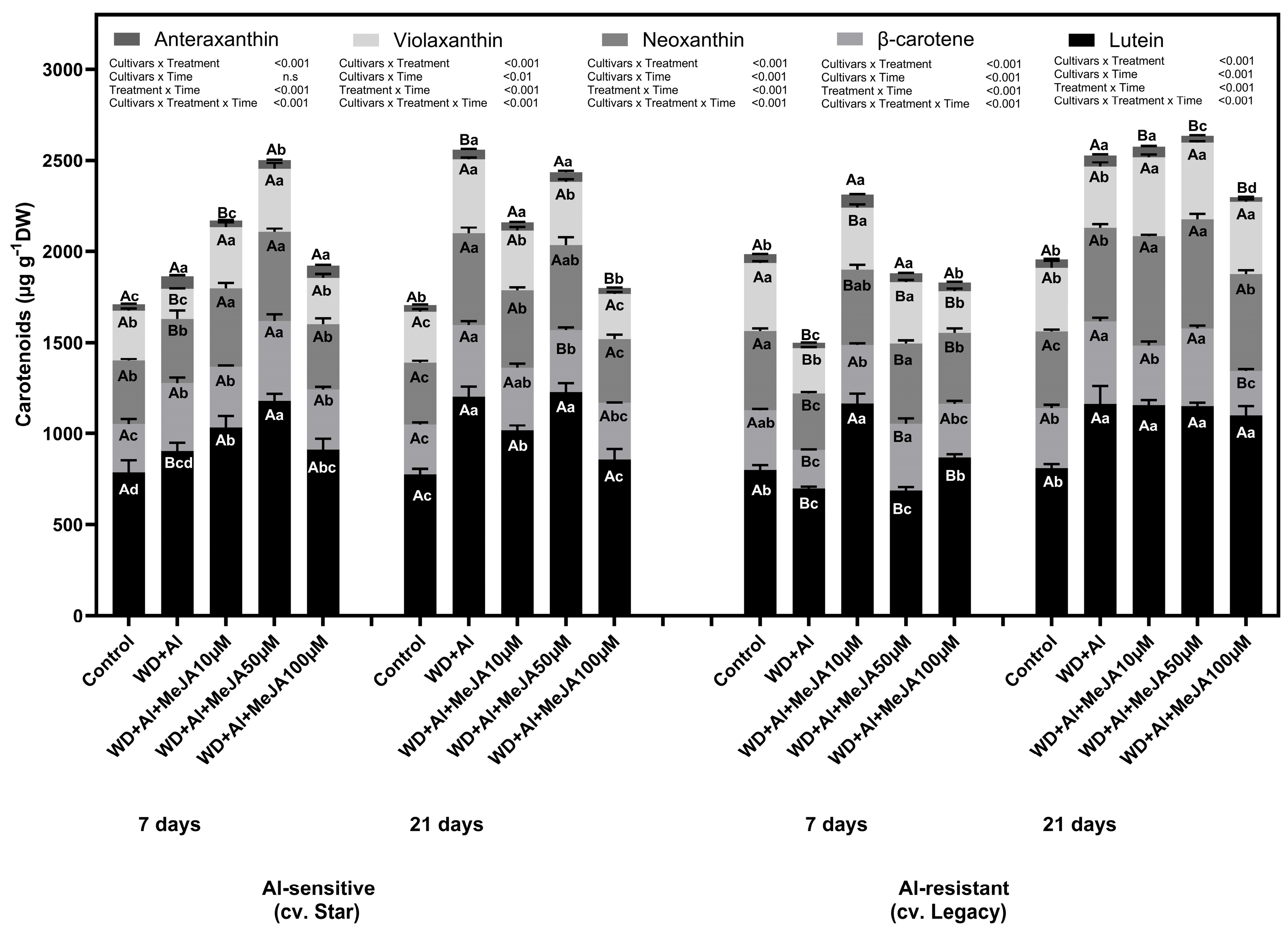
2.1. Effect of MeJA Foliar Application on Photosynthetic Pigment Levels
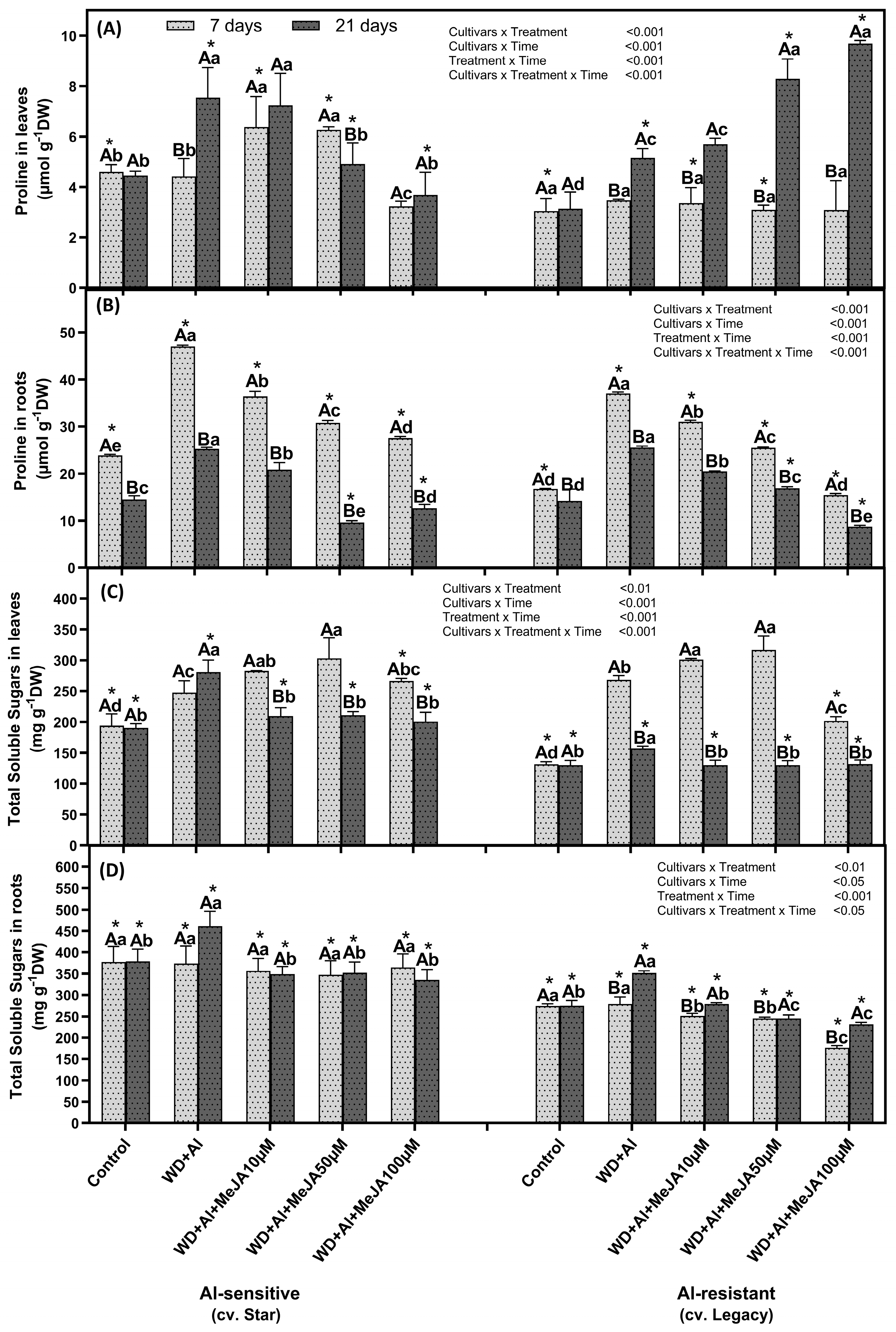
2.2. Foliar MeJA Application Effect on Osmolyte Levels
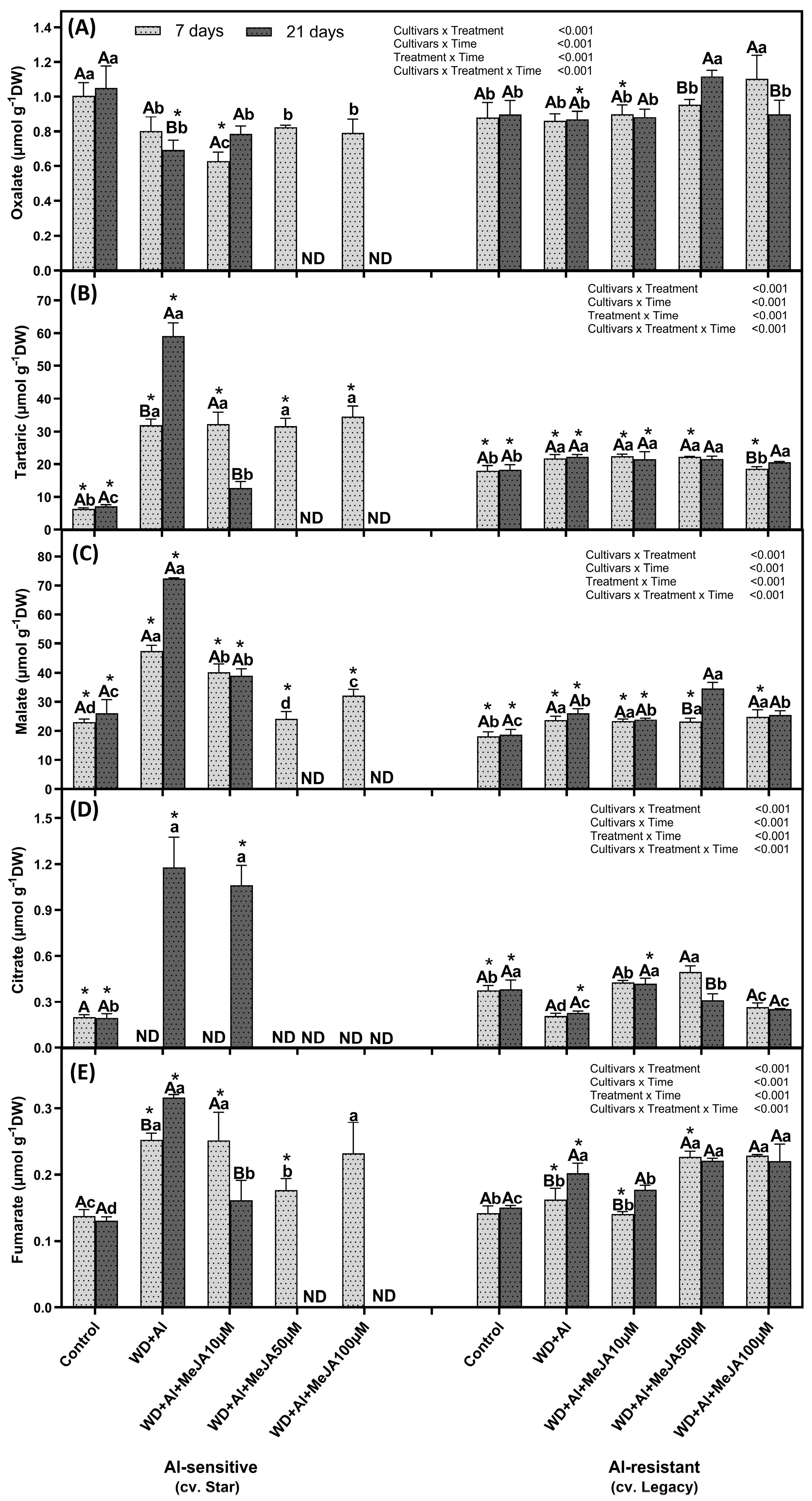
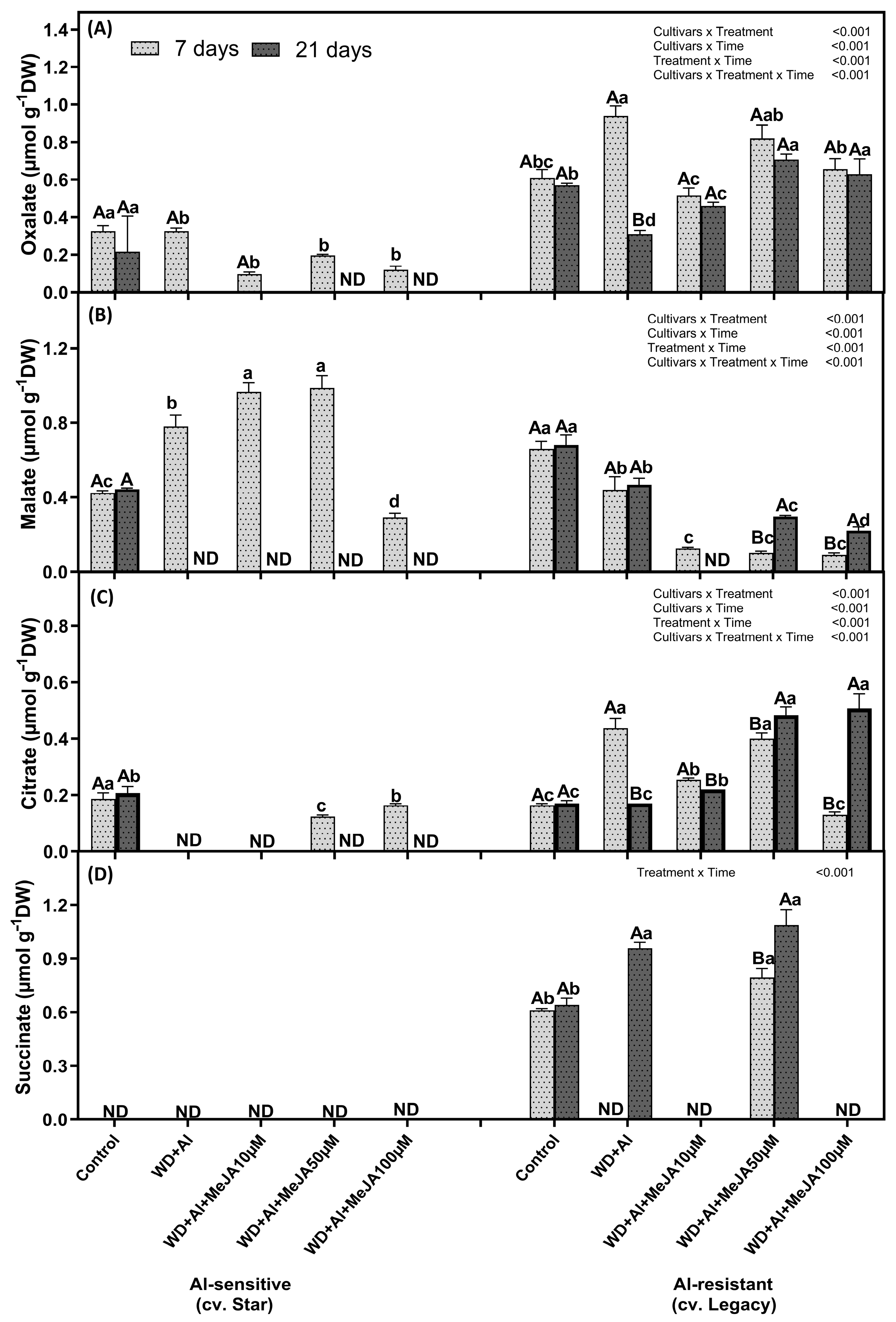
2.3. Effect of MeJA Foliar Application on Organic Acid Levels
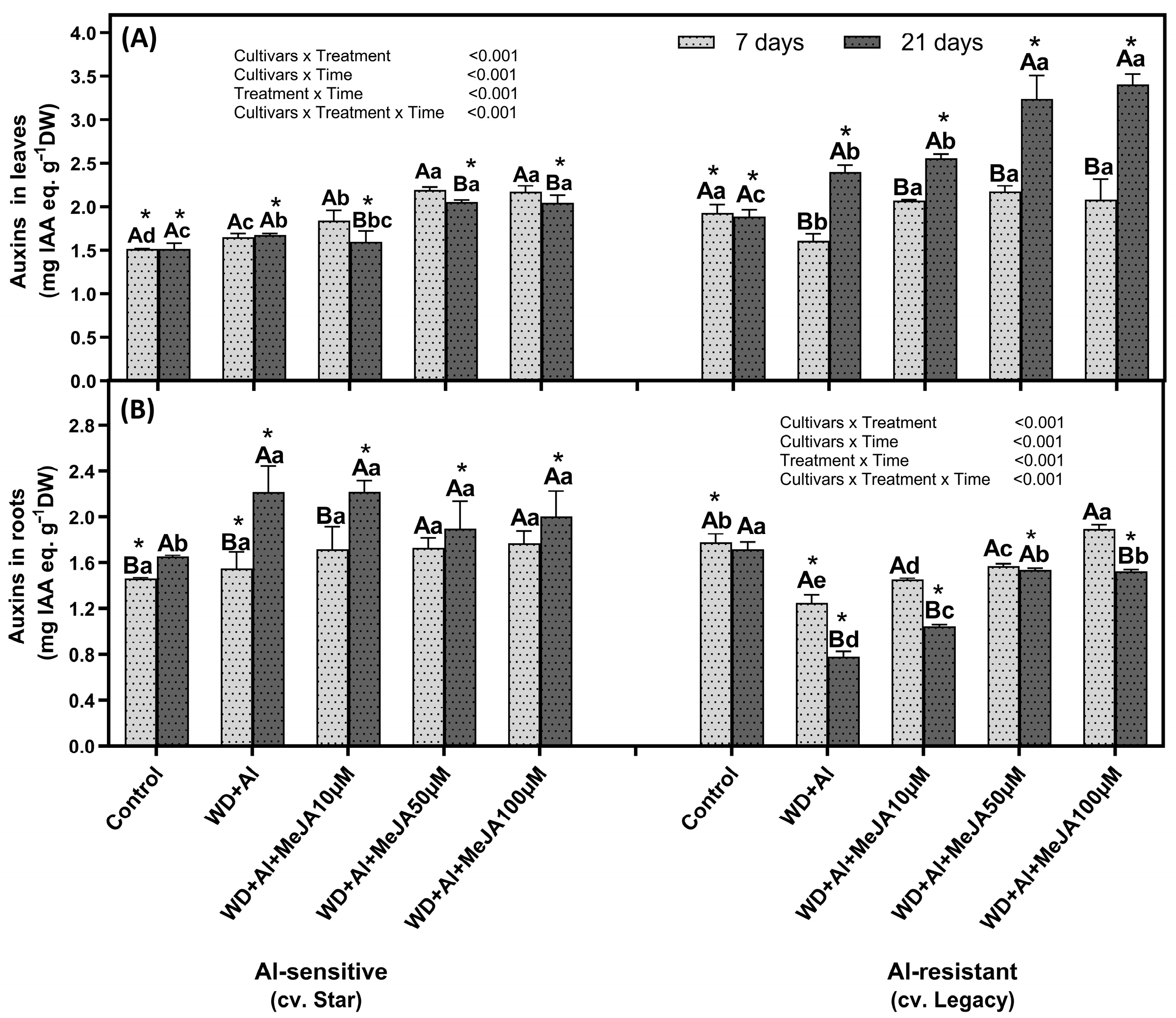
2.4. Impact of Foliar MeJA Application on Auxin Levels in Leaves and Roots
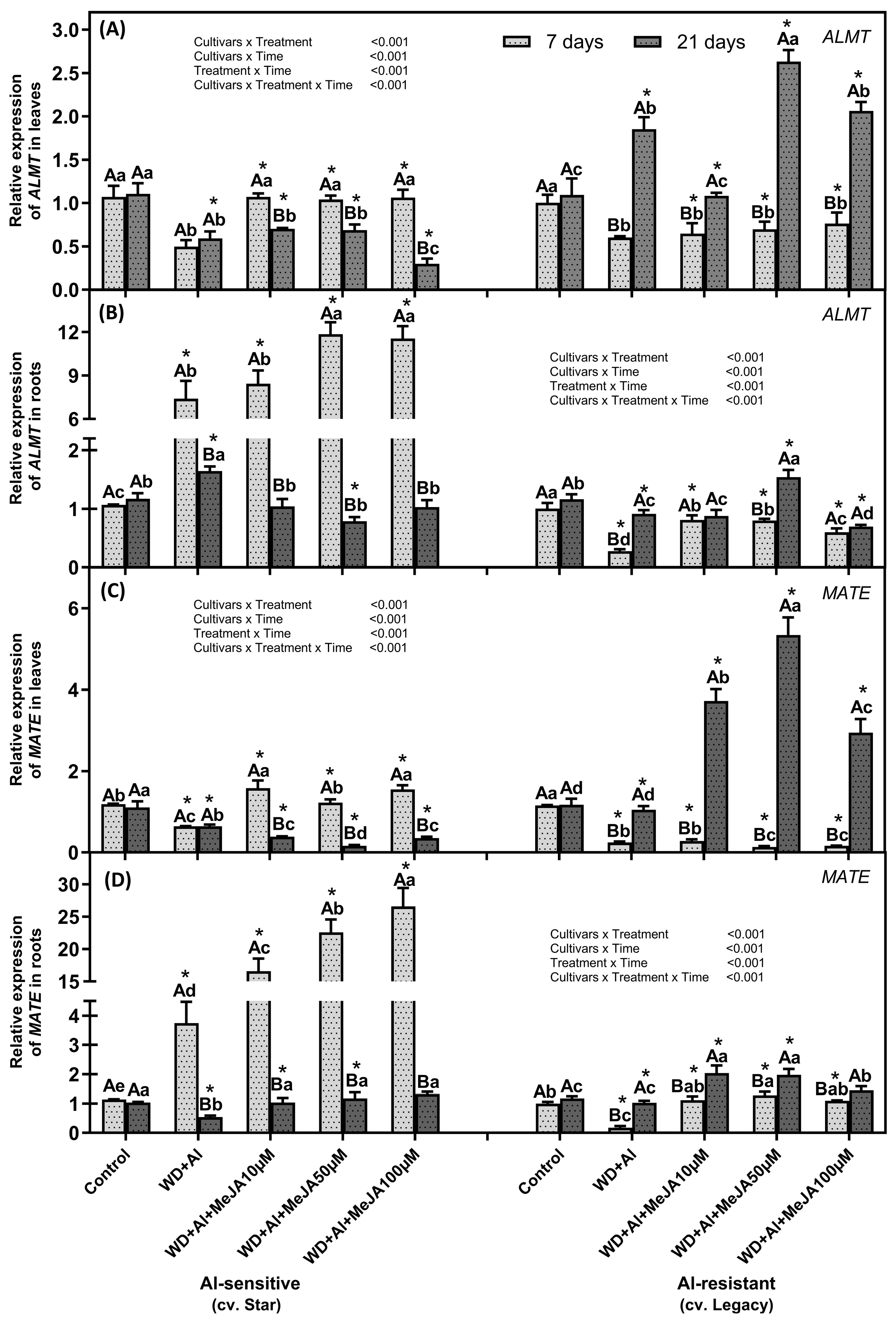
2.5. Foliar MeJA Application Effect on the Gene Expressions Associated with Aluminium Resistance Mechanisms
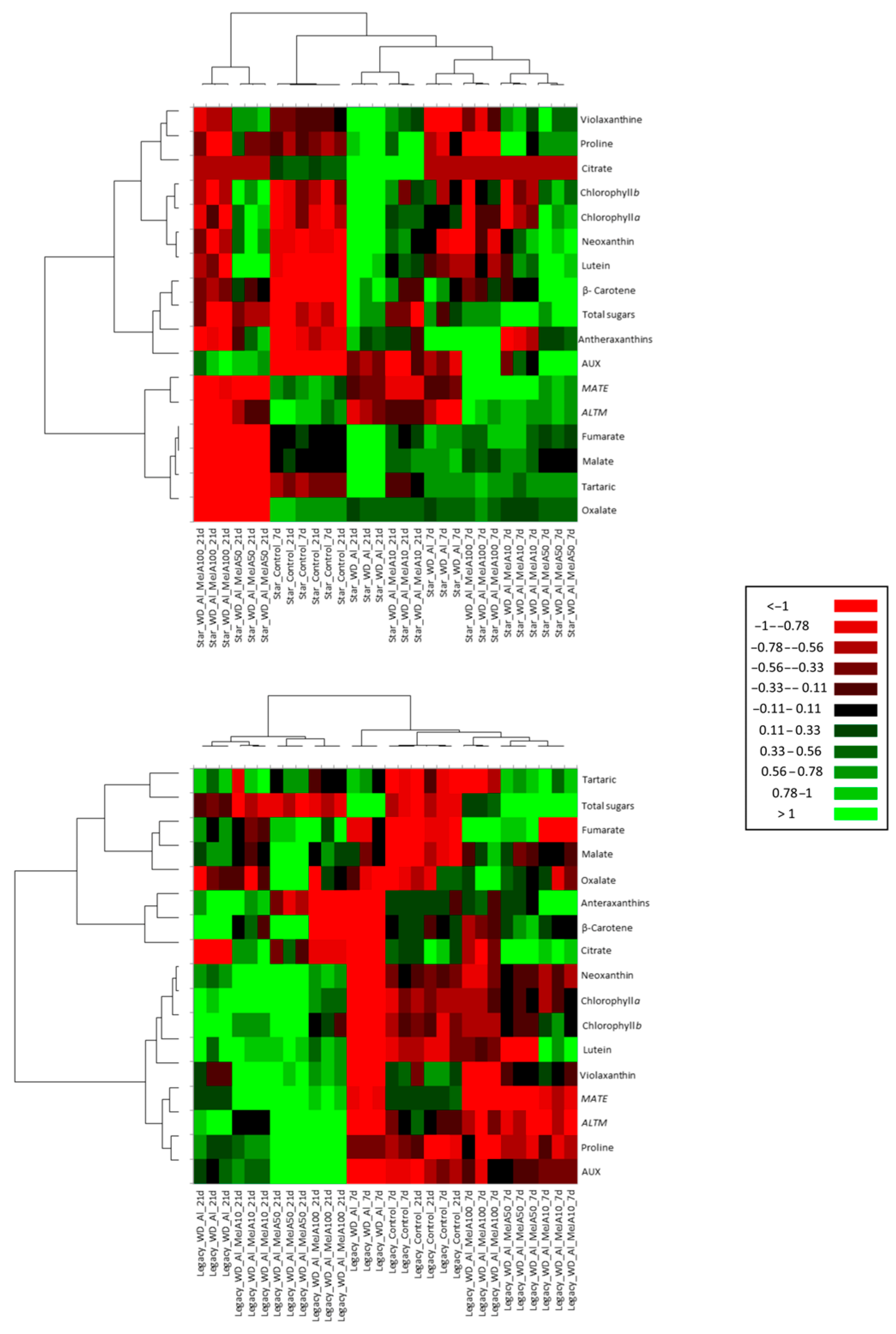
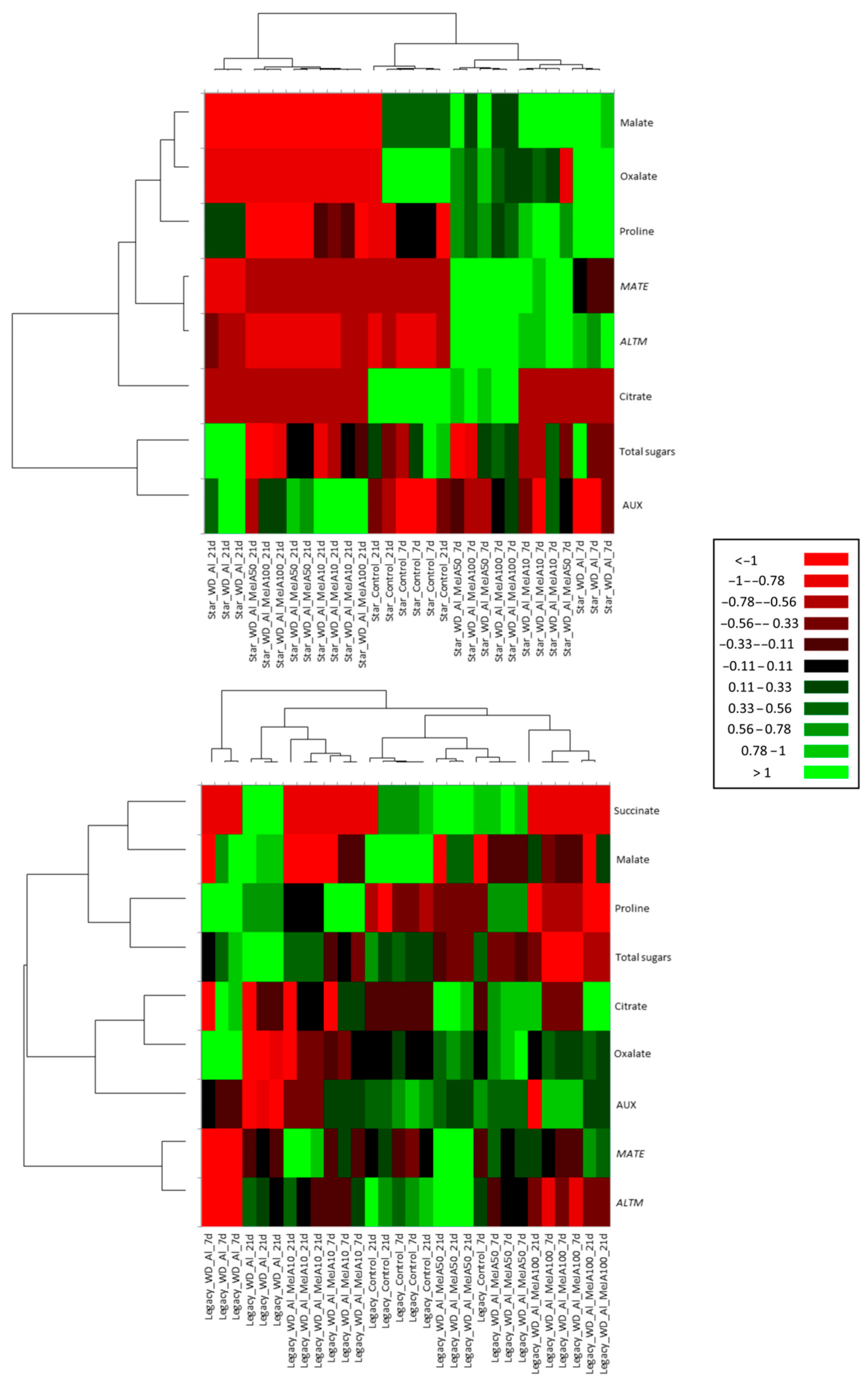
2.6. Multivariate Analysis
3. Discussion
3.1. Exogenous MeJA Enhances Chlorophyll and Carotenoid Accumulation, Improving Resistance to Combined WD + Al Stress in V. corymbosum
3.2. Foliar Application of MeJA Modulates the Osmolytes Levels, Favoring Resistance to WD + Al Stress in V. corymbosum
3.3. MeJA Enhances Differential Accumulation of Organic Acids by Regulating ALMT and MATE Transporters in Response to Combined WD + Al Stress in V. corymbosum
3.4. MeJA-Induced Auxin Accumulation May Be Involved in the Regulation of Organic Acid Transport
3.5. The WD + Al-Sensitive Cultivar Responds Earlier to the Combined WD + Al Stress than the Resistant Cultivar
4. Materials and Methods
4.1. Soil, Plant Material, and Growing Conditions
4.2. Applied Treatments and Experimental Design
4.3. Determination of Pigment Levels
4.4. Osmolytes Determination
4.5. Organic Acid Determinations
4.6. Leaf and Roots Auxins Concentration
4.7. Transcript Analyses by Quantitative Real-Time PCR (qRT-PCR)
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| WD | Water Deficit |
| MeJA | Methyl Jasmonate |
| ALMT | Aluminium-Activated Malate Transporter |
| MATE | Multidrug and Toxic Compound Extrusion |
| Ψw | Water Potential |
| RWC | Relative Water Content |
| RGR | Relative Growth Rate |
| Pn | Net Photosynthesis |
| gs | Stomatal Conductance |
| E | Transpiration Rate |
| LHC | Light-Harvesting Complexes |
| NPQ | Non-Photochemical quenching |
| OAs | Organic Acids |
| AUXs | Auxins |
References
- Ahmed, I.M.; Nadira, U.A.; Qiu, C.W.; Cao, F.; Zhang, G.; Holford, P.; Wu, F. Tolerance to drought, low pH and Al combined stress in Tibetan wild barley is associated with improvement of ATPase and modulation of antioxidant defense system. Int. J. Mol. Sci. 2018, 19, 3553. [Google Scholar] [CrossRef]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop production under drought and heat stress: Plant responses and management options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef]
- Kochian, L.V.; Piñeros, M.A.; Liu, J.; Magalhaes, J.V. Plant adaptation to acid soils: The molecular basis for crop aluminum resistance. Annu. Rev. Plant Biol. 2015, 66, 571–598. [Google Scholar] [CrossRef]
- Sade, H.; Meriga, B.; Surapu, V.; Gadi, J.; Sunita, M.S.L.; Suravajhala, P.; Kavi Kishor, P.B. Toxicity and tolerance of aluminum in plants: Tailoring plants to suit to acid soils. BioMetals 2016, 29, 187–210. [Google Scholar] [CrossRef] [PubMed]
- Cárcamo-Fincheira, P.; Reyes-Díaz, M.; Omena-Garcia, R.P.; Nunes-Nesi, A.; Inostroza-Blancheteau, C. Physiological and metabolic responses to aluminum toxicity reveal differing resistance mechanisms to long-term exposure in highbush blueberry cultivars. Sci. Hortic. 2023, 309, 111665. [Google Scholar] [CrossRef]
- Ulloa-Inostroza, E.; Córdova, C.; Campos, M.; Reyes-Díaz, M. Methyl jasmonate improves photosynthetic performance in blueberry plants under water deficit. Horticulturae 2024, 10, 259. [Google Scholar] [CrossRef]
- Cáceres, C.; Cazor-Curilef, C.; Delgado-Santibañez, P.; Machado, M.; Delgado, M.; Ribera-Fonseca, A.; Inostroza-Blancheteau, C.; Bravo, L.A.; González-Villagra, J.; Nunes-Nesi, A.; et al. Foliar Methyl Jasmonate Application Activates Antioxidant Mechanisms to Counteract Water Deficits and Aluminum Stress in Vaccinium corymbosum L. Horticulturae 2024, 10, 1172. [Google Scholar] [CrossRef]
- Beigi, A.; Ghooshchi, F.; Moghaddam, H.R.T.; Nasiri, M.; Kasraie, P. Effect of Methyl Jasmonate and Potassium and Their Combined Action on Drought Tolerance of Fodder Corn. Russ. J. Plant Physiol. 2023, 70, 102. [Google Scholar] [CrossRef]
- Raza, A.; Charagh, S.; Zahid, Z.; Mubarik, M.S.; Javed, R.; Siddiqui, M.H.; Hasanuzzaman, M. Jasmonic acid: A key frontier in conferring abiotic stress tolerance in plants. Plant Cell Rep. 2020, 40, 1513–1541. [Google Scholar] [CrossRef]
- Wasternack, C.; Hause, B. Jasmonates: Biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann. Bot. 2013, 111, 1021–1058. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, W.; Zhang, Y.; Zhang, X.; Lang, D.; Zhang, X. The roles of methyl jasmonate to stress in plants. Funct. Plant Biol. 2019, 46, 197–212. [Google Scholar] [CrossRef]
- Đurić, M.; Subotić, A.; Prokić, L.; Trifunović-Momčilov, M.; Milošević, S. Foliar application of methyl jasmonate affects impatiens walleriana growth and leaf physiology under drought stress. Plant Signal. Behav. 2023, 18, 2219936. [Google Scholar] [CrossRef] [PubMed]
- Khan, V.; Princi; Mubashshir, M.; Umar, S.; Iqbal, N. Methyl Jasmonate and Pseudomonas fluorescens synergistically boost antioxidative defense, secondary metabolites, and osmolyte production to enhance drought resilience in mustard. J. Plant Growth Regul. 2025, 44, 198–216. [Google Scholar] [CrossRef]
- Tang, C.-J.; Luo, M.-Z.; Zhang, S.; Jia, G.-Q.; Tang, S.; Jia, Y.-C.; Zhi, H.; Diao, X.-M. Variations in chlorophyll content, stomatal conductance, and photosynthesis in Setaria EMS mutants. Sci. Agric. Sin. 2023, 22, 1618–1630. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Shahzad, B.; Ramakrishnan, M.; Singh Sidhu, G.P.; Bali, A.S.; Handa, N.; Kapoor, D.; Yadav, P.; Khanna, K.; et al. Photosynthetic Response of Plants Under Different Abiotic Stresses: A Review. J. Plant Growth Regul. 2020, 39, 509–531. [Google Scholar] [CrossRef]
- Giné-Bordonaba, J.; Terry, L.A. Effect of deficit irrigation and methyl jasmonate application on the composition of strawberry (Fragaria x ananassa) fruit and leaves. Sci. Hortic. 2016, 199, 63–70. [Google Scholar] [CrossRef]
- Lopes, A.S.; Dias, T.J.; Henschel, J.M.; da Silva, J.H.B.; de Oliveira Sousa, V.F.; Targino, V.A.; da Silva Leal, M.P.; da Silva Gomes, D.; de Albuquerque, M.B.; Batista, D.S. Methyl Jasmonate Mitigates Drought Stress in Purple Basil by Enhancing Photosynthesis and Secondary Metabolism. J. Plant Growth Regul. 2025, 44, 233–246. [Google Scholar] [CrossRef]
- Mohamed, H.I.; Latif, H.H. Improvement of drought tolerance of soybean plants by using methyl jasmonate. Physiol. Mol. Biol. Plants 2017, 23, 545–556. [Google Scholar] [CrossRef]
- Lei, Y.; Yin, C.; Li, C. Adaptive responses of Populus przewalskii to drought stress and SNP application. Acta Physiol. Plant. 2007, 29, 519–526. [Google Scholar] [CrossRef]
- Gani, U.; Sharma, P.; Tiwari, H.; Nautiyal, A.K.; Kundan, M.; Wajid, M.A.; Kesari, R.; Nargotra, A.; Misra, P. Comprehensive genome-wide identification, characterization, and expression profiling of MATE gene family in Nicotiana tabacum. Gene 2021, 783, 145554. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, L.; Su, H.; Guo, L.; Zhang, J.; Li, Y.; Xu, J.; Zhang, X.; Guo, Y.-D.; Zhang, N. Jasmonate and aluminum crosstalk in tomato: Identification and expression analysis of WRKYs and ALMTs during JA/Al-regulated root growth. Plant Physiol. Biochem. 2020, 154, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, D.K.; Yadav, V.; Vaculík, M.; Gassmann, W.; Pike, S.; Arif, N.; Singh, V.P.; Deshmukh, R.; Sahi, S.; Tripathi, D.K. Aluminum toxicity and aluminum stress-induced physiological tolerance responses in higher plants. Crit. Rev. Biotechnol. 2021, 41, 715–730. [Google Scholar] [CrossRef]
- Lee, J.E.; Cho, Y.U.; Kim, K.H.; Lee, D.Y. Distinctive metabolomic responses of Chlamydomonas reinhardtii to the chemical elicitation by methyl jasmonate and salicylic acid. Process Biochem. 2016, 51, 1147–1154. [Google Scholar] [CrossRef]
- Cai, Z.-T.; Xu, P.; Zhao, P.-X.; Liu, R.; Yu, L.-H.; Xiang, C.-B. Arabidopsis ERF109 mediates cross-talk between jasmonic acid and auxin biosynthesis during lateral root formation. Nat. Commun. 2014, 5, 5833. [Google Scholar] [CrossRef]
- Han, D.; Oh, Y.; Park, W.J. Methyl jasmonate increases activities of aldehyde oxidase and auxin contents in maize (Zea mays). J. Anim. Plant Sci. 2015, 25, 314–317. [Google Scholar]
- Mao, J.; Niu, C.; Chen, S.; Xu, Y.; Khan, A.; Zuo, Q.; Wang, C.; Han, M.; Bao, L.; Zhang, D. Effects of exogenous methyl-jasmonate on the morphology, hormone status, and gene expression of developing lateral roots in Malus hupehensis. Sci. Hortic. 2021, 289, 110419. [Google Scholar] [CrossRef]
- Ranjan, A.; Sinha, R.; Sharma, T.R.; Pattanayak, A.; Singh, A.K. Alleviating aluminum toxicity in plants: Implications of reactive oxygen species signaling and crosstalk with other signaling pathways. Physiol. Plant. 2021, 173, 1765–1784. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Q.L.; Geng, M.J.; Guo, Z.H.; Zhao, Z. Effect of indole-3-acetic acid on aluminum-induced efflux of malic acid from wheat (Triticum aestivum L.). Plant Soil 2011, 346, 215–230. [Google Scholar] [CrossRef]
- Mohi-Ud-Din, M.; Tahery, M.H.; Nazran, A.; Talukder, D.; Hasan, M.M.; Al-Meraj, S.M.Z.; Ahmed, S.F. Synergistic effects of methyl jasmonate and salicylic acid enhances photosynthetic efficiency in drought-stressed French beans. S. Afr. J. Bot. 2025, 177, 630–642. [Google Scholar] [CrossRef]
- Ulloa-Inostroza, E.M.; Alberdi, M.; Ivanov, A.G.; Reyes-Díaz, M. Protective Effect of Methyl Jasmonate on Photosynthetic Performance and Its Association with Antioxidants in Contrasting Aluminum-Resistant Blueberry Cultivars Exposed to Aluminum. J. Soil Sci. Plant Nutr. 2019, 9, 203–216. [Google Scholar] [CrossRef]
- Javadipour, Z.; Balouchi, H.; Movahhedi Dehnavi, M.; Yadavi, A. Physiological Responses of Bread Wheat (Triticum aestivum) Cultivars to Drought Stress and Exogenous Methyl Jasmonate. J. Plant Growth Regul. 2022, 41, 3433–3448. [Google Scholar] [CrossRef]
- Esmaielzadeh, S.; Fallah, H.; Niknejad, Y.; Mahmoudi, M.; Tari, D.B. Methyl jasmonate increases aluminum tolerance in rice by augmenting the antioxidant defense system, maintaining ion homeostasis, and increasing nonprotein thiol compounds. Environ. Sci. Pollut. Res. 2022, 29, 46708–46720. [Google Scholar] [CrossRef]
- Wilhelm, C.; Selmar, D. Energy dissipation is an essential mechanism to sustain the viability of plants: The physiological limits of improved photosynthesis. J. Plant Physiol. 2011, 168, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Slugeňová, K.; Ditmarová, L.; Kurjak, D.; Válka, J. Drought and aluminium as stress factors in Norway spruce (Picea abies [L.] Karst) seedlings. J. For. Sci. 2011, 57, 547–554. [Google Scholar] [CrossRef]
- Fugate, K.K.; Lafta, A.M.; Eide, J.D.; Li, G.; Lulai, E.C.; Olson, L.L.; Deckard, E.L.; Khan, M.F.R.; Finger, F.L. Methyl jasmonate alleviates drought stress in young sugar beet (Beta vulgaris L.) plants. J. Agron. Crop Sci. 2018, 204, 566–576. [Google Scholar] [CrossRef]
- Zivcak, M.; Brestic, M.; Sytar, O. Osmotic Adjustment and Plant Adaptation to Drought Stress. In Drought Stress Tolerance in Plants; Hossain, M., Wani, S., Bhattacharjee, S., Burritt, D., Tran, L.S., Eds.; Springer: Cham, Switzerland, 2016; Volume 1. [Google Scholar] [CrossRef]
- He, H.; He, L.F. Regulation of gaseous signaling molecules on proline metabolism in plants. Plant Cell Rep. 2018, 37, 387–392. [Google Scholar] [CrossRef]
- Lehmann, S.; Funck, D.; Szabados, L.; Rentsch, D. Proline metabolism and transport in plant development. Amino Acids 2010, 39, 949–962. [Google Scholar] [CrossRef]
- Ruan, Y.-L. Sucrose Metabolism: Gateway to Diverse Carbon Use and Sugar Signaling. Annu. Rev. Plant Biol. 2014, 65, 33–67. [Google Scholar] [CrossRef]
- Saddhe, A.A.; Manuka, R.; Penna, S. Plant sugars: Homeostasis and transport under abiotic stress in plants. Physiol. Plant. 2021, 171, 739–755. [Google Scholar] [CrossRef]
- Rahman, R.; Upadhyaya, H. Aluminium Toxicity and Its Tolerance in Plant: A Review. J. Plant Biol. 2021, 64, 101–121. [Google Scholar] [CrossRef]
- Ahmed, I.M.; Nadira, U.A.; Cao, F.; He, X.; Zhang, G.; Wu, F. Physiological and molecular analysis on root growth associated with the tolerance to aluminum and drought individual and combined in Tibetan wild and cultivated barley. Planta 2016, 243, 973–985. [Google Scholar] [CrossRef] [PubMed]
- CIREN. Estudio Agrológico IX Región: Descripciones de Suelos, Materiales y Símbolos; No. 122; CIREN: Santiago, Chile, 2002. [Google Scholar]
- Brischke, C.; Wegener, F.L. Impact of water holding capacity and moisture content of soil substrates on the moisture content of wood in terrestrial microcosms. Forests 2019, 10, 485. [Google Scholar] [CrossRef]
- Almutairi, K.F.; Bryla, D.R.; Strik, B.C. Potential of Deficit Irrigation, Irrigation Cutoffs, and Crop Thinning to Maintain Yield and Fruit Quality with Less Water in Northern Highbush Blueberry. HortScience 2017, 52, 625–633. [Google Scholar] [CrossRef]
- Chen, X.; Qiu, L.; Guo, H.; Wang, Y.; Yuan, H.; Yan, D.; Zheng, B. Spermidine induces physiological and biochemical changes in southern highbush blueberry under drought stress. Braz. J. Bot. 2017, 40, 841–851. [Google Scholar] [CrossRef]
- García-Plazaola, J.I.; Becerril, J.M. A rapid high-performance liquid chromatography method to measure lipophilic antioxidants in stressed plants: Simultaneous determination of carotenoids and tocopherols. Phytochem. Anal. 1999, 10, 307–313. [Google Scholar] [CrossRef]
- Carillo, P.; Gibon, Y. PROTOCOL: Extraction and Determination of Proline. Prometheus. Wiki. 2011. Available online: https://www.researchgate.net/publication/211353600_PROTOCOL_Extraction_and_determination_of_proline (accessed on 9 June 2025).
- Roe, J.H. A colorimetric method for the determination of fructose in blood and urine. J. Biol. Chem. 1934, 107, 15–22. [Google Scholar] [CrossRef]
- Reyes-Díaz, M.; Alberdi, M.; Piper, F.; Bravo, L.A.; Corcuera, L.J. Low temperature responses of Nothofagus dombeyi and Nothofagus nitida, two evergreen species from south central Chile. Tree Physiol. 2005, 25, 1389–1398. [Google Scholar] [CrossRef]
- Cárcamo, M.P.; Reyes-Díaz, M.; Rengel, Z.; Alberdi, M.; Omena-Garcia, R.P.; Nunes-Nesi, A.; Inostroza-Blancheteau, C. Aluminum stress differentially affects physiological performance and metabolic compounds in cultivars of highbush blueberry. Sci. Rep. 2019, 2, 11275. [Google Scholar] [CrossRef] [PubMed]
- Millaleo, R.; Alvear, M.; Aguilera, P.; González-Villagra, J.; Mora, M.L.; Alberdi, M.; Reyes-Díaz, M. Mn Toxicity Differentially Affects Physiological and Biochemical Features in Highbush Blueberry (Vaccinium corymbosum L.) Cultivars. J. Soil Sci. Plant Nutr. 2020, 20, 795–805. [Google Scholar] [CrossRef]
- Liu, G.; Zhou, N.; Zhang, M.; Li, S.; Tian, Q.; Chen, J.; Chen, B.; Wu, Y.; Yao, S. Hydrophobic solvent induced phase transition extraction to extract drugs from plasma for high performance liquid chromatography-mass spectrometric analysis. J. Chromatogr. A 2010, 1217, 243–249. [Google Scholar] [CrossRef]
- Sarwar, M.; Kremer, R.J. Determination of bacterially derived auxins using a microplate method. Lett. Appl. Microbiol. 1995, 20, 282–285. [Google Scholar] [CrossRef]
- Glickmann, E.; Dessaux, Y. A critical examination of the specificity of the salkowski reagent for indolic compounds produced by phytopathogenic bacteria. Appl. Environ. Microbiol. 1995, 61, 793–796. [Google Scholar] [CrossRef]
- Jaakola, L.; Pirttilä, A.M.; Halonen, M.; Hohtola, A. Isolation of high quality RNA from bilberry (Vaccinium myrtillus L.) fruit. Mol. Biotechnol. 2001, 19, 201–203. [Google Scholar] [CrossRef]
- Naik, D.; Dhanaraj, A.L.; Arora, R.; Rowland, L.J. Identification of genes associated with cold acclimation in blueberry (Vaccinium corymbosum L.) using a subtractive hybridization approach. Plant Sci. 2007, 173, 213–222. [Google Scholar] [CrossRef]
- Zifkin, M.; Jin, A.; Ozga, J.A.; Zaharia, L.I.; Schernthaner, J.P.; Gesell, A.; Abrams, S.R.; Kennedy, J.A.; Constabel, C.P. Gene expression and metabolite profiling of developing highbush blueberry fruit indicates transcriptional regulation of flavonoid metabolism and activation of abscisic acid metabolism. Plant Physiol. 2012, 158, 200–224. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
| Target Gene | Primer Name | Forward/Reverse |
|---|---|---|
| MET | Metallothionein | 5′-ACC CTG ACA TGA GCT TCT CG-3′ |
| 5′-ACC CAA ATC TCT GCT TGC TG-3′ | ||
| ALMT | Aluminum-activated malate transporter | 5′-GAG TTT GTG GCA AGG CTT CA-3′ |
| 5′-ATT GCC CCT CTT TCT TCC CA-3′ | ||
| MATE | Aluminum-activated citrate transporter | 5′-TCA TGC TGG GAA TGG CTA GT-3′ |
| 5′-GCT ATG TCT TCT GCT TGC CC-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cáceres, C.; Cazor-Curilef, C.; Delgado-Santibañez, P.; González-Villagra, J.; Cárcamo-Fincheira, P.; Delgado, M.; Ribera-Fonseca, A.; Inostroza-Blancheteau, C.; Bravo, L.A.; Nunes-Nesi, A.; et al. Differential Induction of Resistance Mechanisms by Methyl Jasmonate in Two Vaccinium corymbosum L. Cultivars Under Combined Water Deficit and Aluminum Toxicity. Plants 2025, 14, 3202. https://doi.org/10.3390/plants14203202
Cáceres C, Cazor-Curilef C, Delgado-Santibañez P, González-Villagra J, Cárcamo-Fincheira P, Delgado M, Ribera-Fonseca A, Inostroza-Blancheteau C, Bravo LA, Nunes-Nesi A, et al. Differential Induction of Resistance Mechanisms by Methyl Jasmonate in Two Vaccinium corymbosum L. Cultivars Under Combined Water Deficit and Aluminum Toxicity. Plants. 2025; 14(20):3202. https://doi.org/10.3390/plants14203202
Chicago/Turabian StyleCáceres, Cristina, Crystal Cazor-Curilef, Patricio Delgado-Santibañez, Jorge González-Villagra, Paz Cárcamo-Fincheira, Mabel Delgado, Alejandra Ribera-Fonseca, Claudio Inostroza-Blancheteau, Leon A. Bravo, Adriano Nunes-Nesi, and et al. 2025. "Differential Induction of Resistance Mechanisms by Methyl Jasmonate in Two Vaccinium corymbosum L. Cultivars Under Combined Water Deficit and Aluminum Toxicity" Plants 14, no. 20: 3202. https://doi.org/10.3390/plants14203202
APA StyleCáceres, C., Cazor-Curilef, C., Delgado-Santibañez, P., González-Villagra, J., Cárcamo-Fincheira, P., Delgado, M., Ribera-Fonseca, A., Inostroza-Blancheteau, C., Bravo, L. A., Nunes-Nesi, A., & Reyes-Díaz, M. (2025). Differential Induction of Resistance Mechanisms by Methyl Jasmonate in Two Vaccinium corymbosum L. Cultivars Under Combined Water Deficit and Aluminum Toxicity. Plants, 14(20), 3202. https://doi.org/10.3390/plants14203202










