Safety Evaluation of Cyhalofop-Butyl on Agronomic Traits and Antioxidant Enzyme Activities in Foxtail Millet
Abstract
1. Introduction
2. Results
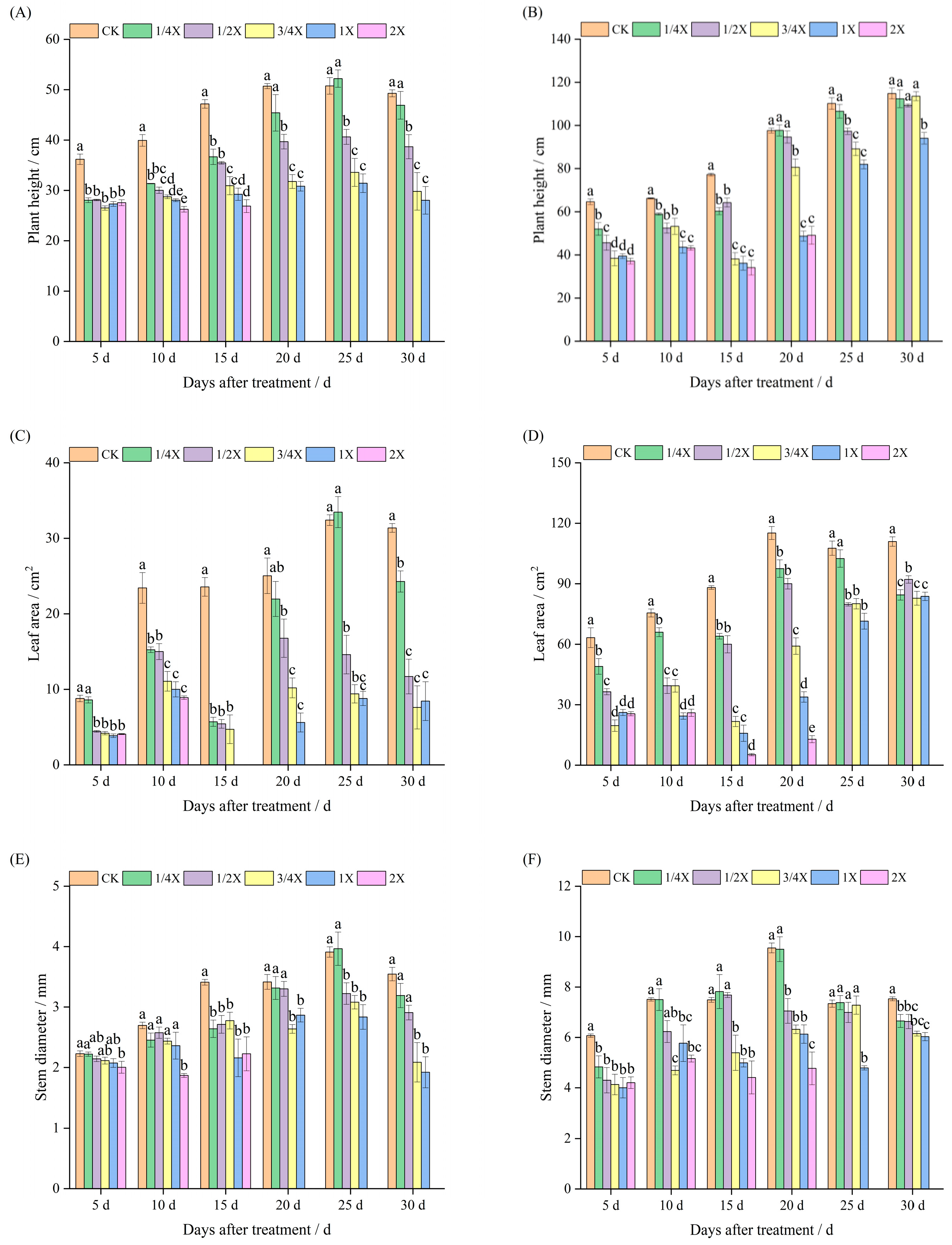
2.1. Effects of Spraying Different Doses of Cyhalofop-Butyl on Agronomic Traits of Foxtail Millet
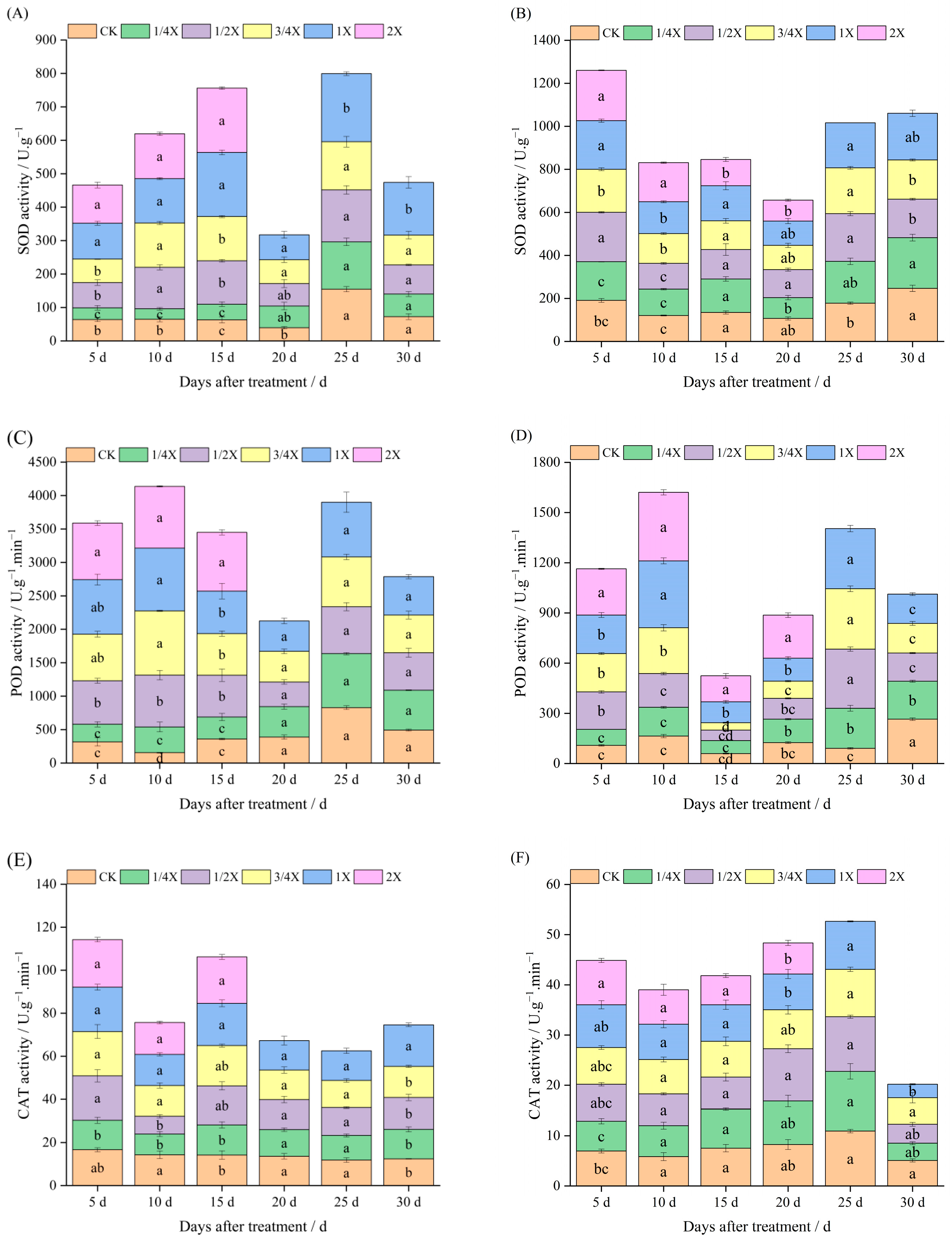
2.2. Effects of Spraying Different Doses of Cyhalofop-Butyl on Antioxidant Enzyme Activity of Foxtail Millet
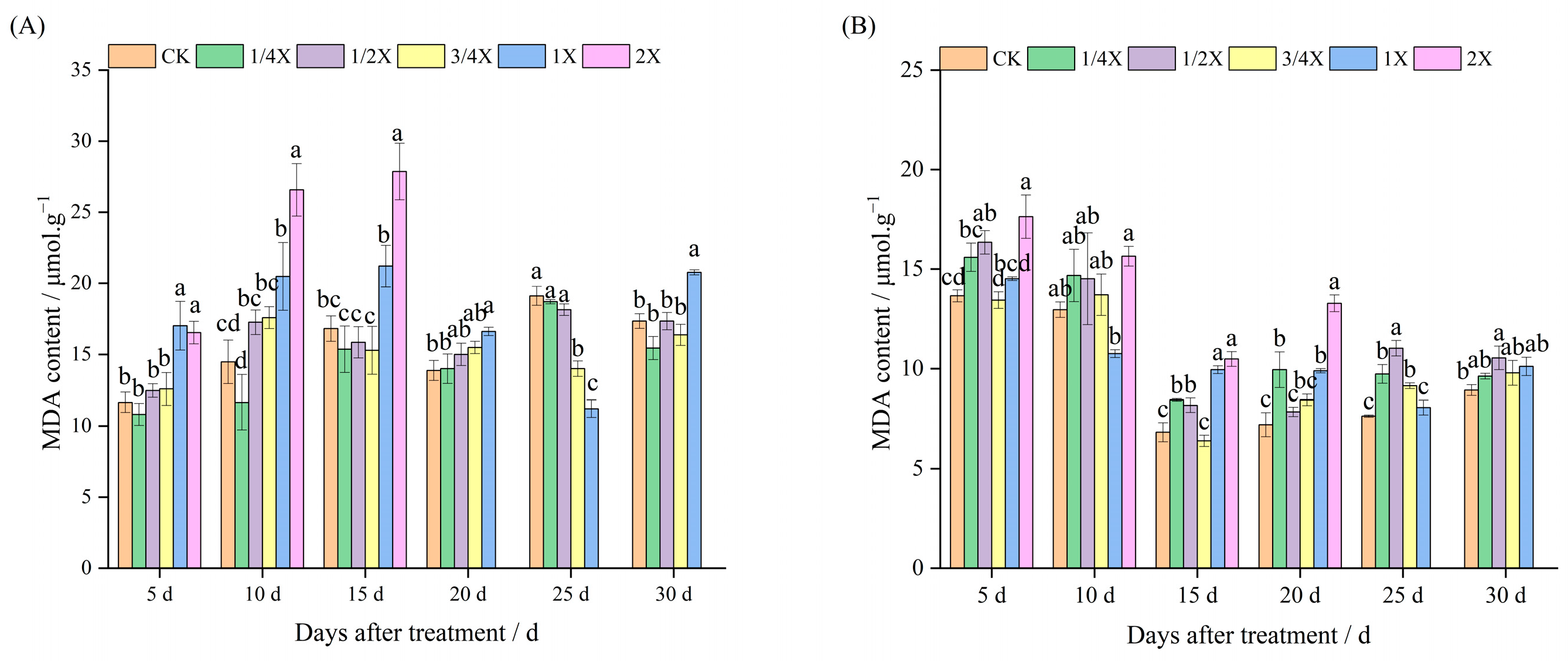
2.3. Effects of Spraying Different Doses of Cyhalofop-Butyl on MDA Content of Foxtail Millet
2.4. Study on the Weed Control Efficacy of Cyhalofop-Butyl in Foxtail Millet Fields
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Experimental Design
4.2.1. Study on the Safety of Different Doses of Cyhalofop-Butyl to Foxtail Millet
4.2.2. Study on the Weed Control Efficacy of Different Doses of Cyhalofop-Butyl in Foxtail Millet Fields
4.3. Analysis of Test Indicators and Methods
4.4. Data Processing
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hwang, J.I.; Norsworthy, J.K.; McElroy, J.S.; Rutland, C.A.; Barber, L.T.; Butts, T.R. Metabolic exploration for cyhalofop-butyl antagonism in Barnyardgrass [Echinochloa crus-galli (L.) P. Beauv.] following pretreatment of malathion. J. Agric. Food Chem. 2023, 71, 6617–6625. [Google Scholar] [CrossRef]
- Wang, L.; Sun, X.; Peng, Y.; Chen, K.; Wu, S.; Guo, Y.; Zhang, J.; Yang, H.; Jin, T.; Wu, L.; et al. Genomic insights into the origin, adaptive evolution, and herbicide resistance of Leptochloa chinensis, a devastating tetraploid weedy grass in rice fields. Mol. Plant 2022, 15, 1045–1058. [Google Scholar] [CrossRef]
- Cutti, L.; Rigon, C.A.G.; Girelli, N.; Angonese, P.S.; Ulguim, A.D.R.; Merotto, A. The safener isoxadifen-ethyl confers fenoxaprop-p-ethyl resistance on a biotype of Echinochloa crus-galli. Pest Manag. Sci. 2022, 78, 2287–2298. [Google Scholar] [CrossRef]
- Ore, A.; Olayinka, E.T. Toxic Mechanisms of Aryloxyphenoxypropionates in Target and Nontarget Organisms. Int. J. Biochem. Res. Rev. 2019, 27, 1–11. [Google Scholar] [CrossRef]
- Zhang, J.; Weaver, S.E.; Hamill, A. Risks and reliability of using herbicides at below-labeled doses. Weed Technol. 2000, 14, 106–115. [Google Scholar] [CrossRef]
- Robinson, A.P.; Simpson, D.M.; Johnson, W.G. Response of glyphosate-tolerant soybean yield components to dicamba exposure. Weed Sci. 2013, 61, 526–536. [Google Scholar] [CrossRef]
- Boulahia, K.; Ould said, C.; Abrous-Belbachir, O. Exogenous application of salicylic acid improve growth and some physio-biochemical parameters in herbicide stressed Phaseolus vulgaris L. Gesunde Pflanzen. 2023, 75, 2301–2318. [Google Scholar] [CrossRef]
- Jiang, L.; Yang, H. Prometryne-induced oxidative stress and impact on antioxidant enzymes in wheat. Ecotoxicol. Environ. Saf. 2009, 72, 1687–1693. [Google Scholar] [CrossRef]
- Jiang, L.; Maa, L.; Sui, Y.; Han, S.Q.; Wu, Z.Y.; Feng, Y.X.; Yang, H. Effect of manure compost on the herbicide prometryne bioavailability to wheat plants. J. Hazard. Mater. 2010, 184, 337–344. [Google Scholar] [CrossRef]
- Singh, G.; Wright, D. Effects of herbicides on nodulation, symbiotic nitrogen fixation, growth and yield of pea (Pisum sativum). J. Agric. Sci. 1999, 133, 21–30. [Google Scholar] [CrossRef]
- Boulahia, K.; Carol, P.; Planchais, S.; Abrous-Belbachir, O. Phaseolus vulgaris L. seedlings exposed to prometryn herbicide contaminated soil trigger an oxidative stress response. J. Agric. Food Chem. 2016, 64, 3150–3160. [Google Scholar] [CrossRef]
- Kehrer, J.P. Free radicals as mediators of tissue injury and disease. Crit. Rev. Toxicol. 1993, 23, 21–48. [Google Scholar] [CrossRef]
- Chen, J.; Shiyab, S.; Han, F.X.; Monts, D.L.; Waggoner, C.A.; Yang, Z.; Su, Y. Bioaccumulation and physiological effects of mercury in Pteris vittata and Nephrolepis exaltata. Ecotoxicology 2009, 18, 110–121. [Google Scholar] [CrossRef]
- Mahaboob Khan, S.; Kour, G. Subacute oral toxicity of chlorpyriphos and protective effect of green tea extract. Pestici. Biochem. Physiol. 2007, 89, 118–123. [Google Scholar] [CrossRef]
- Panfili, I.; Bartucca, M.L.; Marrollo, G.; Povero, G.; Del Buono, D. Application of a plant biostimulant to improve maize (Zea mays) tolerance to metolachlor. J. Agric. Food Chem. 2019, 67, 12164–12171. [Google Scholar] [CrossRef]
- Qu, Q.; Li, Y.; Zhang, Z.Y.; Cui, H.Z.; Zhao, Q.Q.; Liu, W.Y.; Lu, T.; Qian, H.F. Effects of S-metolachlor on wheat (Triticum aestivum L.) seedling root exudates and the rhizosphere microbiome. J. Hazard. Mater. 2021, 411, 125137. [Google Scholar] [CrossRef]
- Bowler, C.; Slooten, L.; Vandenbranden, S.; De Rycke, R.; Botterman, J.; Sybesma, C.; Van Montagu, M.; Inzé, D. Manganese superoxide dismutase can reduce cellular damage mediated by oxygen radicals in transgenic plants. EMBO J. 1991, 10, 1723–1732. [Google Scholar] [CrossRef]
- McKersie, B.; Chen, Y.; Beus, M.; Bowley, S.; Bowler, C.; Inze, D.; D’Halluin, K.; Botterman, J. Superoxide dismutase enhances tolerance of Freezing stress in transgenic alfalfa (Medicao saiva L.). Plant Physiol. 1993, 103, 1155–1163. [Google Scholar] [CrossRef]
- Wang, M.E.; Zhou, Q.X. Effects of herbicide chlorimuron-ethyl on physiological mechanisms in wheat (Triticum aestivum). Ecotoxicol. Environ. Saf. 2006, 64, 190–197. [Google Scholar] [CrossRef]
- Yuan, X.Y.; Guo, P.Y.; Qi, X.; Ning, N.; Wang, H.; Wang, H.F.; Wang, X.; Yang, Y. Safety of herbicide sigma broad on radix isatidis (Isatis indigotica fort.) seedlings and their photosynthetic physiological responses. Pestic. Biochem. Phys. 2013, 106, 45–50. [Google Scholar] [CrossRef]
- Aidoo, M.K.; Bdolach, E.; Fait, A.; Lazarovitch, N.; Rachmilevitch, S. Tolerance to high soil temperature in foxtail millet (Setaria italica L.) is related to shoot and root growth and metabolism. Plant Physiol. Bioch. 2016, 106, 73–81. [Google Scholar] [CrossRef]
- Song, N.H.; Yin, X.L.; Chen, G.F.; Yang, H. Biological responses of wheat (Triticum aestivum) plants to the herbicide chlorotoluron in soils. Chemosphere 2007, 68, 1779–1787. [Google Scholar] [CrossRef]
- Liang, L.; Lu, Y.L.; Yang, H. Toxicology of isoproturon to the food crop wheat as affected by salicylic acid. Environ. Sci. Pollt. Res. Int. 2012, 19, 2044–2054. [Google Scholar] [CrossRef]
- Jiang, L.; Yang, Y.; Jia, L.X.; Lin, J.L.; Liu, Y.; Pan, B.; Lin, Y. Biological responses of wheat (Triticum aestivum) plants to the herbicide simetryne in soils. Ecotoxicol. Environ. Saf. 2016, 127, 87–94. [Google Scholar] [CrossRef]
- Fernandes, B.; Soares, C.; Braga, C.; Rebotim, A.; Rafael Ferreira, R.; Ferreira, J.; Fidalgo, F.; Pereira, R.; Cachada, A. Ecotoxicological assessment of a glyphosate-based herbicide in cover plants: Medicago sativa L. as a model species. Appl Sci. 2020, 10, 5098. [Google Scholar] [CrossRef]
- Ma, K.; Zhang, W.; Zhang, L.; He, X.; Fan, Y.; Alam, S.; Yuan, X. Effect of pyrazosulfuron-methyl on the photosynthetic characteristics and antioxidant systems of foxtail millet. Front. Plant Sci. 2021, 12, 696169. [Google Scholar] [CrossRef]
- Jalal, A.; Oliveira Junior, J.C.; Ribeiro, J.S.; Fernandes, G.C.; Mariano, G.G.; Trindade, V.D.R.; Reis, A.R.D. Hormesis in plants: Physiological and biochemical responses. Ecotoxicol. Environ. Saf. 2020, 207, 111225. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Oxygen processing in photosynthesis regulation and signaling. New Phytol. 2000, 146, 359–388. [Google Scholar] [CrossRef]
- Arora, A.; Sairam, R.K.; Srivastava, G.C. Oxidative stress and antioxidative system in plants. Curr. Sci. 2002, 82, 1227–1238. [Google Scholar]
- Cui, J.; Zhang, R.; Wu, G.L.; Zhu, H.M.; Yang, H. Salicylic acid reduces napropamide toxicity by preventing its accumulation in rapeseed (Brassica napus L.). Arch. Environ. Contam. Toxicol. 2010, 59, 100–108. [Google Scholar] [CrossRef]
- Spormann, S.; Soares, C.; Fidalgo, F. Salicylic acid alleviates glyphosate-induced oxidative stress in Hordeum vulgare L. J. Environ. Manag. 2019, 241, 226–234. [Google Scholar] [CrossRef]
- Ould said, C.; Boulahia, K.; Eid, M.A.M.; Rady, M.M.; Djebbar, R.R.; Abrous-Belbachir, O. Exogenously used proline offers pPotent antioxidative and osmoprotective strategies to re-balance growth and physio-biochemical attributes in herbicide-stressed Trigonella foenum-graecum. J. Soil Sci. Plant Nutr. 2021, 21, 3254–3268. [Google Scholar] [CrossRef]
- Wang, Q.; Que, X.; Zheng, R.; Pang, Z.; Li, C.; Xiao, B. Phytotoxicity assessment of atrazine on growth and physiology of three emergent plants. Environ. Sci. Pollut. Res. 2015, 22, 9646–9657. [Google Scholar] [CrossRef]
- Shopova, E.; Brankova, L.; Katerova, Z.; Dimitrova, L.; Todorova, D.; Sergiev, I.; Talaat, N.B. Salicylic Acid Pretreatment Modulates Wheat Responses to Glyphosate. Crops 2021, 1, 88–96. [Google Scholar] [CrossRef]
- Yu, Q.Q.; Lu, F.F.; Ma, L.Y.; Yang, H.; Song, N.H. Residues of reduced herbicides terbuthylazine, ametryn, and atrazine and toxicology to maize and the environment through salicylic acid. ACS Omega 2021, 6, 27396–27404. [Google Scholar] [CrossRef]
- Li, X.; Riaz, M.; Song, B.; Liang, X.; Liu, H. Exogenous salicylic acid alleviates fomesafen toxicity by improving photosynthetic characteristics and antioxidant defense system in sugar beet. Ecotoxicol. Environ. Saf. 2022, 238, 113587. [Google Scholar] [CrossRef]
- Ntanos, D.A.; Koutroubas, S.D.; Mavrotas, C. Barnyardgrass (Echinochloa crus-galli) control in water-seeded rice (Oryza sativa) with cyhalofopbutyl. Weed Technol. 2000, 14, 383–388. [Google Scholar] [CrossRef]
- Deng, W.; Cai, J.; Zhang, J.; Chen, Y.; Chen, Y.; Di, Y.; Yuan, S. Molecular basis of resistance to ACCase-inhibiting herbicide cyhalofop-butyl in Chinese sprangletop (Leptochloa chinensis (L.) Nees) from China. Pestic. Biochem. Physiol. 2019, 158, 143–148. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Chance, B.; Maehly, A.C. Assay of catalases and peroxidases. Methods Enzymol. 1955, 2, 764–775. [Google Scholar]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef]
| Days After Treatment | Treatment | E. crus-galli | D. sanguinalis | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Number of Plants/m2 | Plant Control Effect/% | Fresh Weight/g | Fresh Weight Control Effect/% | Number of Plants/m2 | Plant Control Effect/% | Fresh Weight/g | Fresh Weight Control Effect/% | ||
| 5 d | CK | 7.75 a | / | 41.85 a | / | 7.33 a | / | 2.08 a | / |
| 1/4X | 2.50 b | 67.74 b | 6.59 b | 84.25 b | 2.00 b | 72.73 a | 0.32 b | 84.77 a | |
| 1/2X | 0.50 b | 93.55 ab | 1.59 b | 96.21 ab | 1.75 b | 76.14 a | 0.28 b | 86.45 a | |
| 3/4X | 0.50 b | 93.55 ab | 1.64 b | 96.09 ab | 1.50 b | 79.55 a | 0.03 b | 98.38 a | |
| 1X | 0.00 b | 100.00 a | 0.00 b | 100.00 a | 1.25 b | 82.95 a | 0.03 b | 98.64 a | |
| 2X | 0.25 b | 96.78 a | 0.59 b | 98.59 ab | 1.00 b | 86.36 a | 0.04 b | 98.28 a | |
| 10 d | CK | 6.50 a | / | 98.66 a | / | 2.00 a | / | 16.00 a | / |
| 1/4X | 4.00 b | 38.46 c | 39.61 b | 59.85 b | 1.00 b | 57.14 b | 4.53 b | 71.72 a | |
| 1/2X | 2.50 bc | 61.54 b | 24.27 b | 75.40 ab | 0.25 bc | 89.29 ab | 3.14 b | 80.37 a | |
| 3/4X | 1.75 cd | 73.08 ab | 12.98 b | 86.85 a | 0.50 bc | 78.57 ab | 4.00 b | 75.00 a | |
| 1X | 0.50 d | 92.31 a | 5.11 b | 94.82 a | 0.00 c | 100.00 a | 0.00 b | 100.00 a | |
| 2X | 0.50 d | 92.31 a | 4.29 b | 95.65 a | 0.00 c | 100.00 a | 0.00 b | 100.00 a | |
| 15 d | CK | 8.00 a | / | 315.46 a | / | 4.50 a | / | 44.18 a | / |
| 1/4X | 4.75 ab | 40.63 b | 96.54 ab | 69.40 b | 0.50 b | 88.89 a | 1.28 b | 97.10 a | |
| 1/2X | 4.00 ab | 50.00 ab | 93.63 ab | 70.32 b | 0.25 b | 94.44 a | 1.72 b | 96.11 a | |
| 3/4X | 2.25 b | 71.88 ab | 42.41 b | 86.56 ab | 0.00 b | 100.00 a | 0.00 b | 100.00 a | |
| 1X | 0.25 b | 96.88 a | 0.30 b | 99.90 a | 0.00 b | 100.00 a | 0.00 b | 100.00 a | |
| 2X | 0.25 b | 96.88 a | 20.75 b | 93.42 a | 0.00 b | 100.00 a | 0.00 b | 100.00 a | |
| 20 d | CK | 5.00 a | / | 202.27 a | / | 3.33 a | / | 3.57 a | / |
| 1/4X | 4.00 ab | 46.67 b | 155.37 ab | 76.19 b | 1.25 b | 62.50 b | 0.59 b | 83.55 b | |
| 1/2X | 2.75 abc | 45.00 b | 47.01 bc | 76.76 b | 1.25 b | 62.50 b | 0.27 b | 92.37 ab | |
| 3/4X | 1.75 bcd | 65.00 ab | 10.50 bc | 94.81 a | 1.00 b | 70.00 ab | 0.37 b | 89.65 ab | |
| 1X | 0.50 cd | 90.00 a | 9.00 bc | 95.55 a | 0.25 b | 92.50 ab | 0.14 b | 96.22 ab | |
| 2X | 0.00 d | 100.00 a | 0.00 c | 100.00 a | 0.00 b | 100.00 a | 0.00 b | 100.00 a | |
| 25 d | CK | 6.75 a | / | 405.25 a | / | 3.33 a | / | 35.67 a | / |
| 1/4X | 2.75 b | 59.26 b | 100.25 b | 75.26 b | 1.00 b | 70.00 a | 3.25 b | 90.89 a | |
| 1/2X | 2.50 b | 62.96 b | 99.25 b | 75.51 b | 0.75 b | 77.50 a | 1.42 b | 96.03 a | |
| 3/4X | 2.50 b | 62.96 b | 53.25 b | 86.86 ab | 0.75 b | 77.50 a | 1.50 b | 95.79 a | |
| 1X | 0.75 b | 88.89 a | 39.50 b | 90.25 ab | 0.00 b | 100.00 a | 0.00 b | 100.00 a | |
| 2X | 0.75 b | 88.89 a | 15.50 b | 96.18 a | 0.00 b | 100.00 a | 0.00 b | 100.00 a | |
| 30 d | CK | 7.50 a | / | 485.25 a | / | 3.67 a | / | 58.67 a | / |
| 1/4X | 3.00 b | 60.00 b | 156.25 b | 67.80 c | 2.50 ab | 31.82 c | 29.75 bc | 49.29 b | |
| 1/2X | 3.00 b | 60.00 b | 139.50 b | 71.25 bc | 1.25 bc | 65.91 b | 16.75 bc | 71.45 ab | |
| 3/4X | 2.25 bc | 70.00 ab | 41.75 b | 91.40 ab | 1.00 bc | 72.73 ab | 11.19 bc | 80.92 a | |
| 1X | 0.50 bc | 93.33 a | 2.00 b | 99.59 a | 0.00 c | 100.00 a | 0.00 c | 100.00 a | |
| 2X | 0.25 c | 96.67 a | 0.75 b | 99.85 a | 0.00 c | 100.00 a | 0.00 c | 100.00 a | |
| Years | pH | Available K mg/kg | Olsen P mg/kg | Available N mg/kg | Total N g/kg | Total P g/kg | Total K g/kg | Organic g/kg |
|---|---|---|---|---|---|---|---|---|
| 2021 | 8.20 | 291.09 | 23.62 | 51.84 | 1.04 | 1.12 | 18.75 | 23.36 |
| Treatment | 1/4X | 1/2X | 3/4X | 1X | 2X | CK |
|---|---|---|---|---|---|---|
| Spraying amount | 22.5 | 45 | 67.5 | 90 | 180 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, C.; Chen, T.; Zhai, X.; Yuan, J.; Shang, S.; Wen, Y.; Song, X.; Zhao, J.; Cao, H.; Dong, S. Safety Evaluation of Cyhalofop-Butyl on Agronomic Traits and Antioxidant Enzyme Activities in Foxtail Millet. Plants 2025, 14, 3170. https://doi.org/10.3390/plants14203170
Hu C, Chen T, Zhai X, Yuan J, Shang S, Wen Y, Song X, Zhao J, Cao H, Dong S. Safety Evaluation of Cyhalofop-Butyl on Agronomic Traits and Antioxidant Enzyme Activities in Foxtail Millet. Plants. 2025; 14(20):3170. https://doi.org/10.3390/plants14203170
Chicago/Turabian StyleHu, Chunyan, Tingting Chen, Xutao Zhai, Jingtao Yuan, Suqi Shang, Yinyuan Wen, Xi’e Song, Juan Zhao, Hui Cao, and Shuqi Dong. 2025. "Safety Evaluation of Cyhalofop-Butyl on Agronomic Traits and Antioxidant Enzyme Activities in Foxtail Millet" Plants 14, no. 20: 3170. https://doi.org/10.3390/plants14203170
APA StyleHu, C., Chen, T., Zhai, X., Yuan, J., Shang, S., Wen, Y., Song, X., Zhao, J., Cao, H., & Dong, S. (2025). Safety Evaluation of Cyhalofop-Butyl on Agronomic Traits and Antioxidant Enzyme Activities in Foxtail Millet. Plants, 14(20), 3170. https://doi.org/10.3390/plants14203170






