Elucidating the Mechanistic Role of Exogenous Melatonin in Salt Stress Tolerance of Maize (Zea mays L.) Seedlings: An Integrated Physiological, Metabolomic, and Proteomic Profiling Analysis
Abstract
1. Introduction
2. Results
2.1. Melatonin Alleviates Salt-Induced Stress in Maize Seedlings
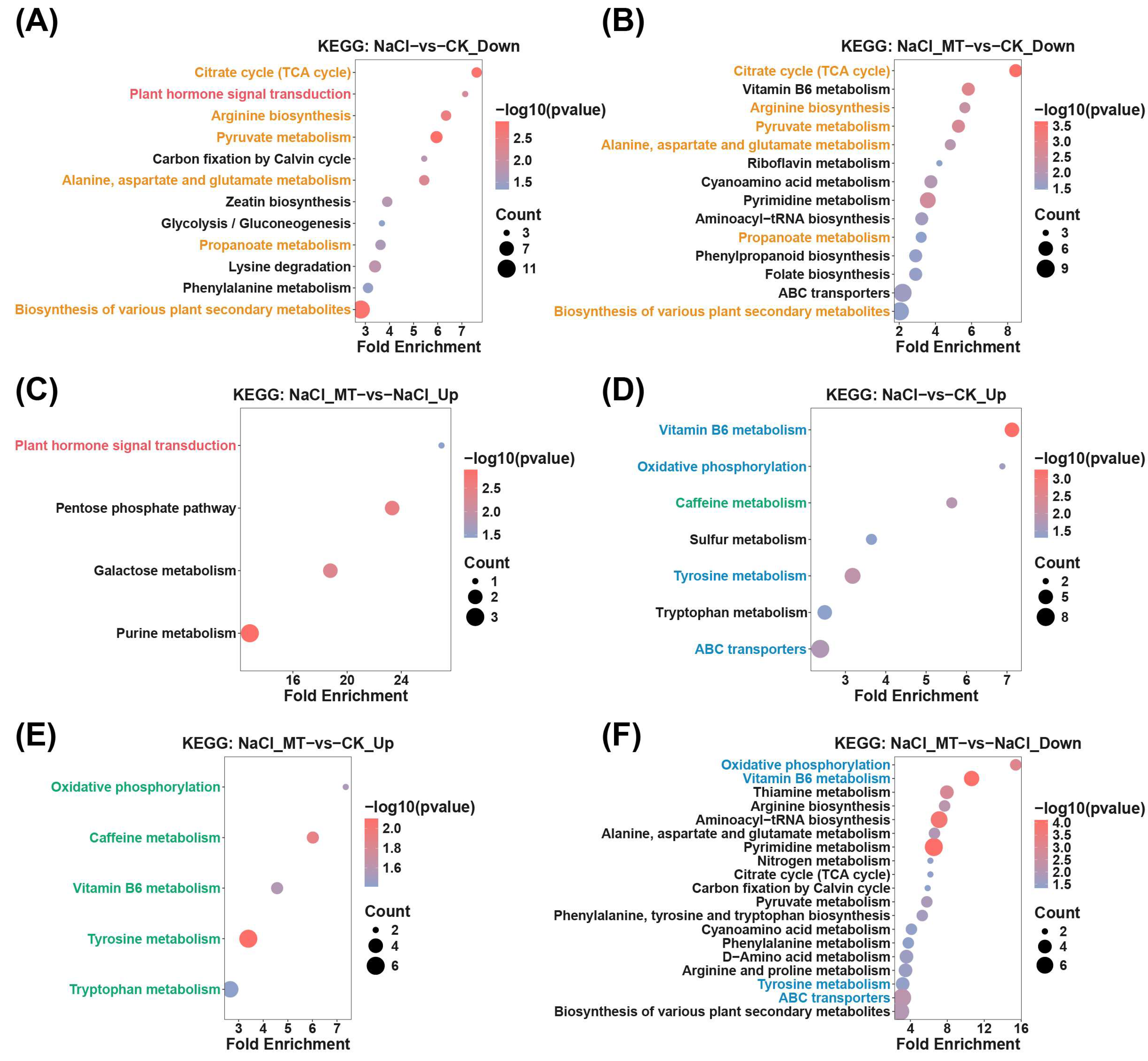
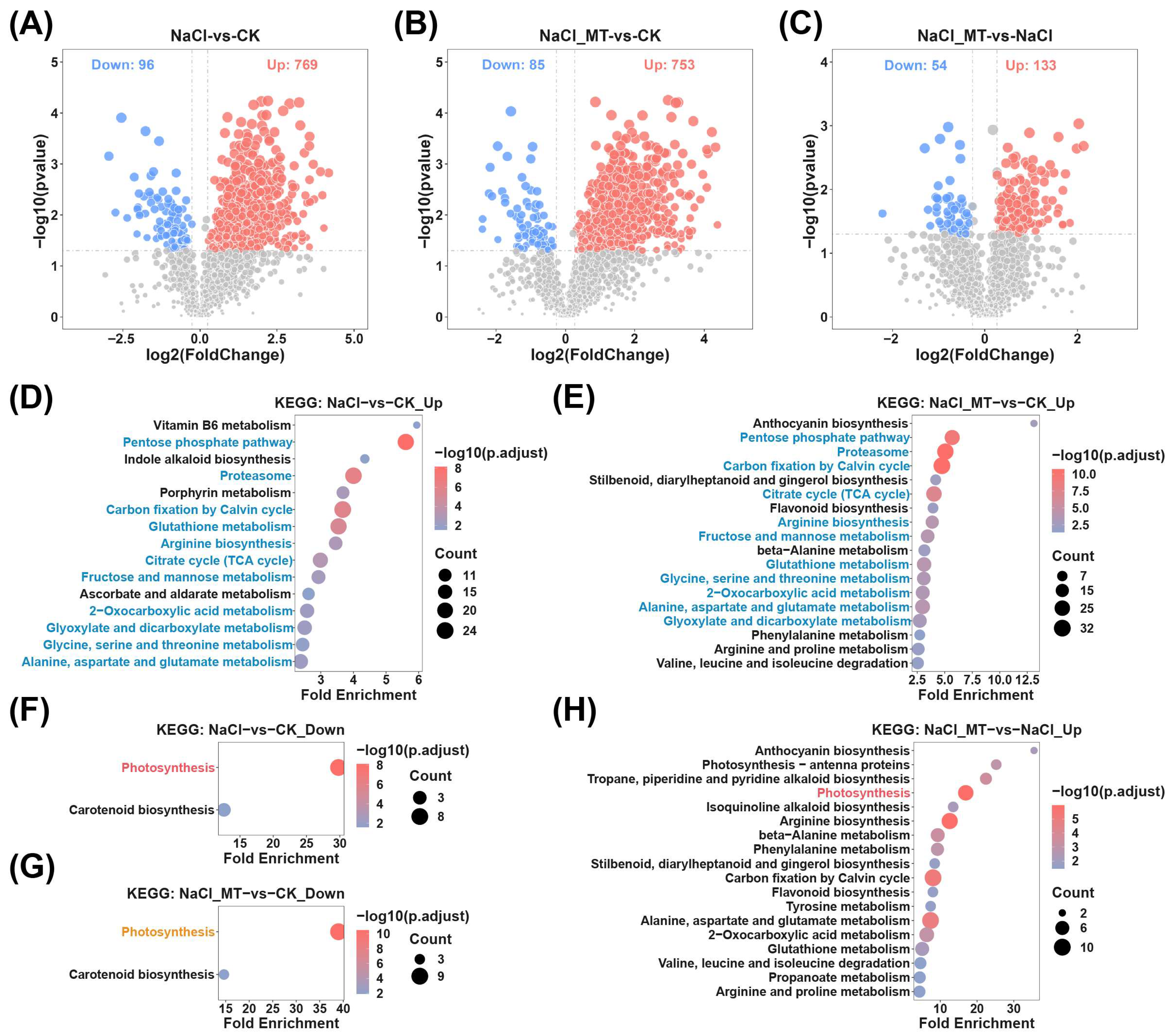
2.2. Identification of Key Metabolites and Metabolic Enrichment Analysis in Melatonin-Mediated Alleviation of Salt Stress
2.3. Proteomic Analysis and Protein-Level Enrichment Analysis
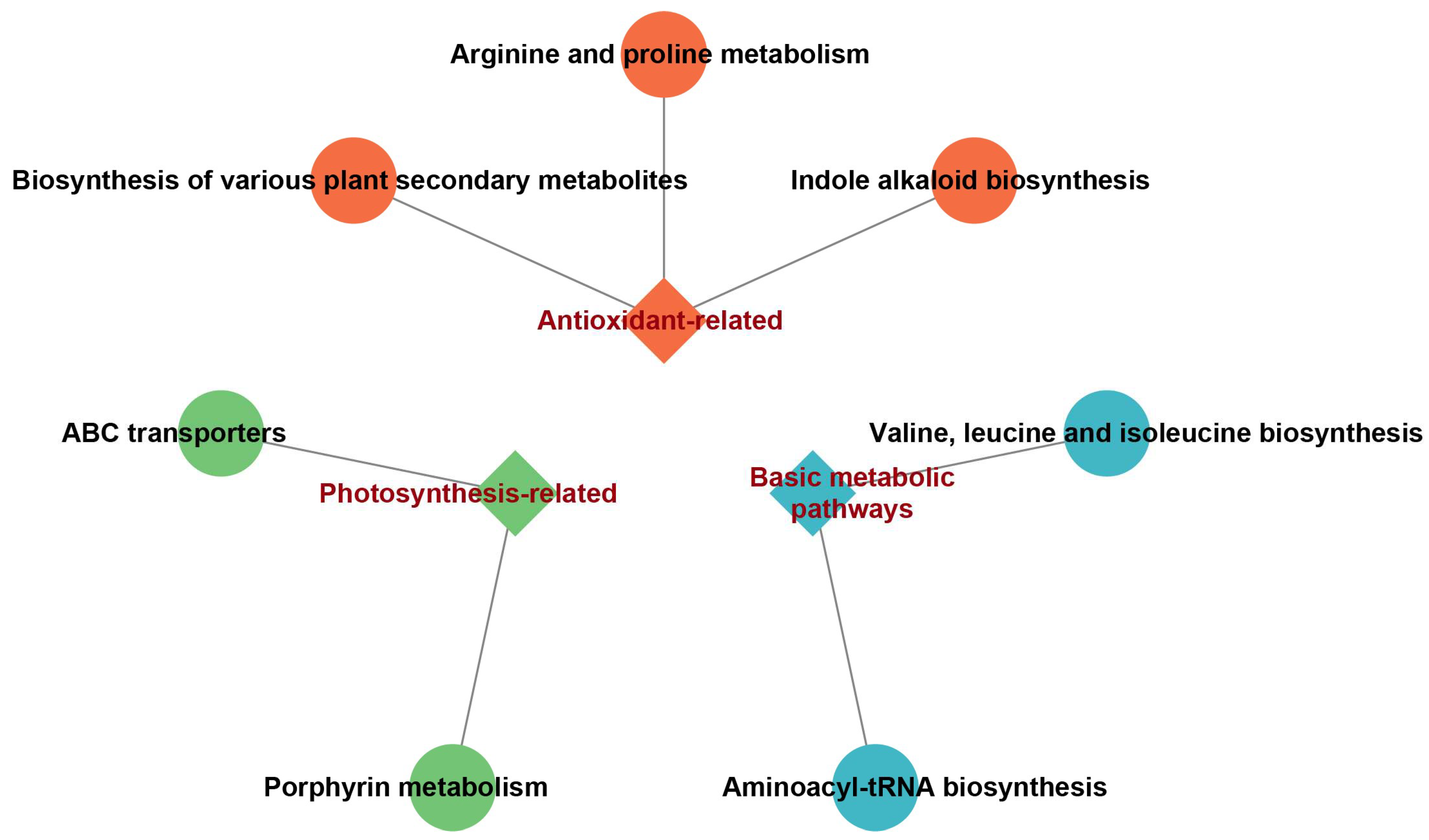
2.4. Integrated Metabolomic and Proteomic Analysis Reveals Key Mechanisms Underlying Melatonin’s Mitigating Effects
2.5. Melatonin Alleviates Drought-like Salt Stress Effects in Maize Seedlings by Enhancing Photosynthesis
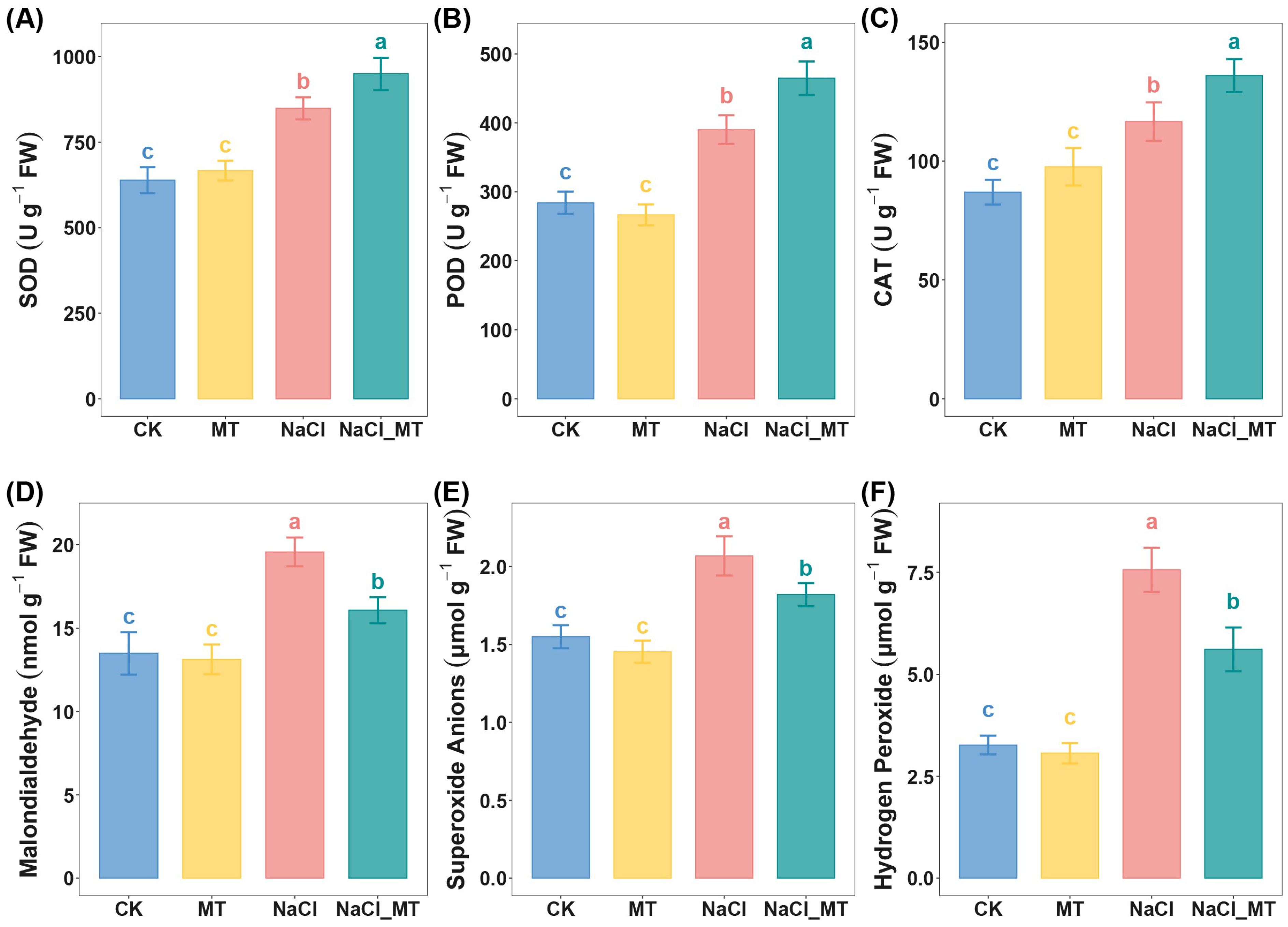
2.6. Melatonin Enhances Antioxidant Defense in Seedlings to Promote Salt Stress Tolerance
3. Discussion
4. Materials and Methods
4.1. Plant Materials, Chemicals, and Experimental Design
4.2. Metabolomic Analysis
4.3. Label-Free Proteomic Analysis
4.4. Identification and Analysis of Metabolites and Proteins
4.5. Chlorophyll Content Determination
4.6. Measurement of Photosynthetic Parameters and Chlorophyll a Fluorescence Parameters
4.7. Chloroplast Ultrastructural Analysis
4.8. DAB Staining for H2O2 Detection
4.9. NBT Staining for O2·− Detection
4.10. SOD Activity Assay
4.11. POD Activity Assay
4.12. CAT Activity Assay
4.13. O2·− Content Assay
4.14. MDA Content Assay
4.15. H2O2 Content Assay
4.16. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| DAB | 3,3′-diaminobenzidine |
| ANOVA | analysis of variance |
| BH | Benjamini–Hochberg |
| CAT | catalase |
| Ca | chlorophyll a |
| Cb | chlorophyll b |
| DEMs | differentially expressed metabolites |
| DEPs | differentially expressed proteins |
| FC | fold change |
| MDA | malondialdehyde |
| MT | melatonin |
| NBT | nitroblue tetrazolium |
| PPP | pentose phosphate pathway |
| POD | peroxidase |
| PS | photosystem |
| PCA | principal component analysis |
| ROS | reactive oxygen species |
| SAH | S-Adenosylhomocysteine |
| SOD | superoxide dismutase |
| TBA | thiobarbituric acid |
| TCA | trichloroacetic acid |
| TEM | transmission electron microscope |
References
- Şavkan, A.N.; Çandar, A. Effects of Salt Stress on Early Seedling Development and Germination in Some Root Vegetables. Anadolu Ege Tarımsal Araştırma Enstitüsü Derg. 2024, 34, 60–69. [Google Scholar] [CrossRef]
- Wei, T.J.; Li, G.; Cui, Y.R.; Xie, J.; Gao, X.A.; Teng, X.; Zhao, X.Y.; Guan, F.C.; Liang, Z.W. Variation Characteristics of Root Traits of Different Alfalfa Cultivars under Saline-Alkaline Stress and their Relationship with Soil Environmental Factors. Phyton-Int. J. Exp. Bot. 2024, 93, 29–43. [Google Scholar] [CrossRef]
- Shrivastava, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef]
- Acosta-Motos, J.R.; Ortuño, M.F.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.J.; Hernandez, J.A. Plant Responses to Salt Stress: Adaptive Mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef]
- Isayenkov, S.V.; Maathuis, F.J.M. Plant Salinity Stress: Many Unanswered Questions Remain. Front. Plant Sci. 2019, 10, 80. [Google Scholar] [CrossRef]
- Liu, J.H.; Fu, C.C.; Li, G.J.; Khan, M.N.; Wu, H.H. ROS Homeostasis and Plant Salt Tolerance: Plant Nanobiotechnology Updates. Sustainability 2021, 13, 3552. [Google Scholar] [CrossRef]
- Yan, K.; Wu, C.W.; Zhang, L.H.; Chen, X.B. Contrasting photosynthesis and photoinhibition in tetraploid and its autodiploid honeysuckle (Lonicera japonica Thunb.) under salt stress. Front. Plant Sci. 2015, 6, 227. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.B.; Wang, Y.X.; Du, F.P.; Zhang, Y.; Yin, M.; Zhao, X.Q.; Xu, J.L.; Yang, Y.Q.; Wang, W.S.; et al. Transcriptome and Metabolome Analyses Reveal Complex Molecular Mechanisms Involved in the Salt Tolerance of Rice Induced by Exogenous Allantoin. Antioxidants 2022, 11, 2045. [Google Scholar] [CrossRef]
- Zhao, X.Q.; Shi, J.; Niu, Y.N.; Lu, P.N.; Chen, X.J.; Mao, T.T. 24-Epibrassinolide Alleviates Aluminum Toxicity by Improving Leaf Chlorophyll Fluorescence and Photosynthetic Performance and Root Antioxidant-Oxidant Balance and Ascorbate-Glutathione Cycle in Maize. Russ. J. Plant Physiol. 2022, 69, 99. [Google Scholar] [CrossRef]
- Cao, Y.B.; Liang, X.Y.; Yin, P.; Zhang, M.; Jiang, C.F. A domestication-associated reduction in K+-preferring HKT transporter activity underlies maize shoot K+ accumulation and salt tolerance. N. Phytol. 2019, 222, 301–317. [Google Scholar] [CrossRef]
- Nie, M.E.; Ning, N.; Chen, J.; Zhang, Y.Z.; Li, S.S.; Zheng, L.; Zhang, H.P. Melatonin enhances salt tolerance in sorghum by modulating photosynthetic performance, osmoregulation, antioxidant defense, and ion homeostasis. Open Life Sci. 2023, 18, 20220734. [Google Scholar] [CrossRef] [PubMed]
- Dubbels, R.; Reiter, R.J.; Klenke, E.; Goebel, A.; Schnakenberg, E.; Ehlers, C.; Schiwara, H.W.; Schloot, W. Melatonin in edible plants identified by radioimmunoassay and by high performance liquid chromatography-mass spectrometry. J. Pineal Res. 1995, 18, 28–31. [Google Scholar] [CrossRef]
- Wang, J.; Yan, D.; Lu, Z.; Liu, R.; Hong, Y.; Wang, Y.; Peng, Z.; Yu, C.; Gao, Y.; Liu, Z.; et al. Integration of the Metabolome and Transcriptome Reveals Diurnal Variability in the Effects of Melatonin on Salt Tolerance in Maize Seedlings. J. Plant Growth Regul. 2024, 43, 1672–1688. [Google Scholar] [CrossRef]
- Zhou, R.; Cen, B.J.; Jiang, F.L.; Sun, M.T.; Wen, J.Q.; Cao, X.; Cui, S.Y.; Kong, L.P.; Zhou, N.N.; Wu, Z. Reducing the Halotolerance Gap between Sensitive and Resistant Tomato by Spraying Melatonin. Agronomy 2022, 12, 84. [Google Scholar] [CrossRef]
- Talaat, N.B. Drought Stress Alleviator Melatonin Reconfigures Water-Stressed Barley (Hordeum vulgare L.) Plants’ Photosynthetic Efficiency, Antioxidant Capacity, and Endogenous Phytohormone Profile. Int. J. Mol. Sci. 2023, 24, 16228. [Google Scholar] [CrossRef] [PubMed]
- Sezer, I.; Kiremit, M.S.; Öztürk, E.; Subrata, B.A.G.; Osman, H.M.; Akay, H.; Arslan, H. Role of melatonin in improving leaf mineral content and growth of sweet corn seedlings under different soil salinity levels. Sci. Hortic. 2021, 288, 110376. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.-K. Abiotic stress responses in plants. Nat. Rev. Genet. 2022, 23, 104–119. [Google Scholar] [CrossRef]
- Su, W.; Qiu, J.; Soufan, W.; El Sabagh, A. Synergistic effects of melatonin and glycine betaine on seed germination, seedling growth, and biochemical attributes of maize under salinity stress. Physiol. Plant. 2024, 176, e14514. [Google Scholar] [CrossRef]
- Ren, J.; Ye, J.; Yin, L.; Li, G.; Deng, X.; Wang, S. Exogenous Melatonin Improves Salt Tolerance by Mitigating Osmotic, Ion, and Oxidative Stresses in Maize Seedlings. Agronomy 2020, 10, 663. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Liu, H.; Xie, H.; Liu, J.; Hua, J.; Xiong, M.; Song, H.; Yong, C. Effect of Exogenous Melatonin on Corn Seed Germination and Seedling Salt Damage Mitigation Under NaCl Stress. Plants 2025, 14, 1139. [Google Scholar] [CrossRef]
- Li, W.Q.; Li, J.Y.; Bi, S.J.; Jin, J.Y.; Fan, Z.L.; Shang, Z.L.; Zhang, Y.F.; Wang, Y.J. Melatonin Enhances Maize Germination, Growth, and Salt Tolerance by Regulating Reactive Oxygen Species Accumulation and Antioxidant Systems. Plants 2025, 14, 296. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.E.; Mao, J.J.; Sun, L.Q.; Huang, B.; Ding, C.B.; Gu, Y.; Liao, J.Q.; Hu, C.; Zhang, Z.W.; Yuan, S.; et al. Exogenous melatonin enhances salt stress tolerance in maize seedlings by improving antioxidant and photosynthetic capacity. Physiol. Plant. 2018, 164, 349–363. [Google Scholar] [CrossRef]
- Wang, J.; Yan, D.; Liu, R.; Wang, T.; Lian, Y.; Lu, Z.; Hong, Y.; Wang, Y.; Li, R. The Physiological and Molecular Mechanisms of Exogenous Melatonin Promote the Seed Germination of Maize (Zea mays L.) under Salt Stress. Plants 2024, 13, 2142. [Google Scholar] [CrossRef]
- Altelaar, A.F.; Munoz, J.; Heck, A.J. Next-generation proteomics: Towards an integrative view of proteome dynamics. Nat. Rev. Genet. 2013, 14, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Song, H.E.; Lee, H.Y.; Yoo, H.J. Mass Spectrometry-based Metabolomics in Translational Research. Adv. Exp. Med. Biol. 2021, 1310, 509–531. [Google Scholar] [CrossRef]
- Weng, X.; Li, H.; Ren, C.; Zhou, Y.; Zhu, W.; Zhang, S.; Liu, L. Calcium Regulates Growth and Nutrient Absorption in Poplar Seedlings. Front. Plant Sci. 2022, 13, 887098. [Google Scholar] [CrossRef]
- Singh, V.P.; Srivastava, P.K.; Prasad, S.M. Nitric oxide alleviates arsenic-induced toxic effects in ridged Luffa seedlings. Plant Physiol. Biochem. 2013, 71, 155–163. [Google Scholar] [CrossRef]
- Mochizuki, S.; Fukumoto, T.; Ohara, T.; Ohtani, K.; Yoshihara, A.; Shigematsu, Y.; Tanaka, K.; Ebihara, K.; Tajima, S.; Gomi, K.; et al. The rare sugar d-tagatose protects plants from downy mildews and is a safe fungicidal agrochemical. Commun. Biol. 2020, 3, 423. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Verbeek, L. Inhibition of carotenoid synthesis during nitrogen deficiency. Planta 1973, 112, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.; Woo, E.R.; Lee, D.G. Isoquercitrin, isolated from Aster yomena triggers ROS-mediated apoptosis in Candida albicans. J. Funct. Foods 2016, 22, 347–357. [Google Scholar] [CrossRef]
- Backlund, P.S., Jr.; Carotti, D.; Cantoni, G.L. Effects of the S-adenosylhomocysteine hydrolase inhibitors 3-deazaadenosine and 3-deazaaristeromycin on RNA methylation and synthesis. Eur. J. Biochem. 1986, 160, 245–251. [Google Scholar] [CrossRef]
- Gupta, K.; Garg, R. Unravelling Differential DNA Methylation Patterns in Genotype Dependent Manner under Salinity Stress Response in Chickpea. Int. J. Mol. Sci. 2023, 24, 1863. [Google Scholar] [CrossRef]
- Tian, L.; Wu, L.; Zhong, X.F.; Ma, L.H.; Du, G.Y. Genome-Wide Characterization of ABC Transporter Genes and Expression Profiles in Red Macroalga Pyropia yezoensis Expose to Low-Temperature. Mar. Biotechnol. 2024, 26, 1179–1193. [Google Scholar] [CrossRef] [PubMed]
- Davidson, A.L.; Dassa, E.; Orelle, C.; Chen, J. Structure, function, and evolution of bacterial ATP-binding cassette systems. Microbiol. Mol. Biol. Rev. 2008, 72, 317–364. [Google Scholar] [CrossRef] [PubMed]
- Henikoff, S.; Greene, E.A.; Pietrokovski, S.; Bork, P.; Attwood, T.K.; Hood, L. Gene families: The taxonomy of protein paralogs and chimeras. Science 1997, 278, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Theodoulou, F.L.; Kerr, I.D. ABC transporter research: Going strong 40 years on. Biochem. Soc. Trans. 2015, 43, 1033–1040. [Google Scholar] [CrossRef]
- Tamizhselvan, P.; Madhavan, S.; Constan-Aguilar, C.; Elrefaay, E.R.; Liu, J.; Pencík, A.; Novák, O.; Cairó, A.; Hrtyan, M.; Geisler, M.; et al. Chloroplast Auxin Efflux Mediated by ABCB28 and ABCB29 Fine-Tunes Salt and Drought Stress Responses in Arabidopsis. Plants 2024, 13, 7. [Google Scholar] [CrossRef]
- Adjei, M.O.; Luo, J.H.; Li, X.; Du, J.; Luan, A.P.; Li, S.J.; Ma, J. Function of ALA Content in Porphyrin Metabolism Regulation of Ananas comosus var. bracteatus. Int. J. Mol. Sci. 2023, 24, 5274. [Google Scholar] [CrossRef]
- Li, H.; Chang, J.J.; Chen, H.J.; Wang, Z.Y.; Gu, X.R.; Wei, C.H.; Zhang, Y.; Ma, J.X.; Yang, J.Q.; Zhang, X. Exogenous Melatonin Confers Salt Stress Tolerance to Watermelon by Improving Photosynthesis and Redox Homeostasis. Front. Plant Sci. 2017, 8, 295. [Google Scholar] [CrossRef]
- Chen, T.H.H.; Murata, N. Enhancement of tolerance of abiotic stress by metabolic engineering of betaines and other compatible solutes. Curr. Opin. Plant Biol. 2002, 5, 250–257. [Google Scholar] [CrossRef]
- Renzetti, M.; Funck, D.; Trovato, M. Proline and ROS: A Unified Mechanism in Plant Development and Stress Response? Plants 2025, 14, 2. [Google Scholar] [CrossRef]
- Cui, G.C.; Zhang, Y.; Zhang, W.J.; Lang, D.Y.; Zhang, X.J.; Li, Z.X.; Zhang, X.H. Response of Carbon and Nitrogen Metabolism and Secondary Metabolites to Drought Stress and Salt Stress in Plants. J. Plant Biol. 2019, 62, 387–399. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Yang, Y.X. Anthocyanin-mediated arsenic tolerance in plants. Environ. Pollut. 2022, 292, 118475. [Google Scholar] [CrossRef]
- Song, Z.P.; Wang, P.; Chen, X.L.; Peng, Y.F.; Cai, B.; Song, J.Y.; Yin, G.T.; Jia, S.W.; Zhang, H.Y. Melatonin alleviates cadmium toxicity and abiotic stress by promoting glandular trichome development and antioxidant capacity in Nicotiana tabacum. Ecotoxicol. Environ. Saf. 2022, 236, 113437. [Google Scholar] [CrossRef]
- Islam, S.; Mohammad, F.; Shakeel, A.; Corpas, F.J. Glycine betaine: A multifaceted protectant against salt stress in Indian mustard through ionic homeostasis, ROS scavenging and osmotic regulation. Physiol. Plant. 2024, 176, e14530. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Raihan, M.R.H.; Masud, A.A.C.; Rahman, K.; Nowroz, F.; Rahman, M.; Nahar, K.; Fujita, M. Regulation of Reactive Oxygen Species and Antioxidant Defense in Plants under Salinity. Int. J. Mol. Sci. 2021, 22, 9326. [Google Scholar] [CrossRef]
- Luqman, S.; Rizvi, S.I. Protection of lipid peroxidation and carbonyl formation in proteins by capsaicin in human erythrocytes subjected to oxidative stress. Phytother. Res. 2006, 20, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, Y.; Kishimoto, C.; Takada, H.; Kurokawa, M.; Ochiai, H.; Shiraki, K.; Sasayama, S. Role of oxygen derived free radicals in the pathogenesis of coxsackievirus B3 myocarditis in mice. Cardiovasc. Res. 1993, 27, 957–961. [Google Scholar] [CrossRef]
- Thangudu, S.; Su, C.H. Peroxidase Mimetic Nanozymes in Cancer Phototherapy: Progress and Perspectives. Biomolecules 2021, 11, 1015. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Wang, D.; Zhang, X.; Li, R.; Li, Z.; Ma, Y.; Zhang, Y.; Li, N.; Chen, W.; Fan, Z.; et al. Investigation and Analysis of Crop Germplasm Resources in Coastal Areas of Shandong Province. J. Plant Genet. Resour. 2013, 14, 367–372. [Google Scholar]
- Anderson, J.M.; Melis, A. Localization of different photosystems in separate regions of chloroplast membranes. Proc. Natl. Acad. Sci. USA 1983, 80, 745–749. [Google Scholar] [CrossRef]
- Cuello, J.; Quiles, M.J. Fractionation of thylakoid membranes into grana and stroma thylakoids. Photosynth. Res. Protoc. 2004, 274, 1–9. [Google Scholar] [CrossRef]
- Morosinotto, T.; Segalla, A.; Giacometti, G.M.; Bassi, R. Purification of structurally intact grana from plants thylakoids membranes. J. Bioenerg. Biomembr. 2010, 42, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Albanese, P.; Nield, J.; Tabares, J.A.M.; Chiodoni, A.; Manfredi, M.; Gosetti, F.; Marengo, E.; Saracco, G.; Barber, J.; Pagliano, C. Isolation of novel PSII-LHCII megacomplexes from pea plants characterized by a combination of proteomics and electron microscopy. Photosynth. Res. 2016, 130, 19–31. [Google Scholar] [CrossRef]
- Farmer, E.E.; Mueller, M.J. ROS-mediated lipid peroxidation and RES-activated signaling. Annu. Rev. Plant Biol. 2013, 64, 429–450. [Google Scholar] [CrossRef] [PubMed]
- Itri, R.; Junqueira, H.C.; Mertins, O.; Baptista, M.S. Membrane changes under oxidative stress: The impact of oxidized lipids. Biophys. Rev. 2014, 6, 47–61. [Google Scholar] [CrossRef]
- Yasar, F.; Ellialtioglu, S.; Yildiz, K. Effect of Salt Stress on Antioxidant Defense Systems, Lipid Peroxidation, and Chlorophyll Content in Green Bean. Russ. J. Plant Physiol. 2008, 55, 782–786. [Google Scholar] [CrossRef]
- Rahoutei, J.; García-Luque, I.; Barón, M. Inhibition of photosynthesis by viral infection:: Effect on PSII structure and function. Physiol. Plant. 2000, 110, 286–292. [Google Scholar] [CrossRef]
- Jiang, C.Q.; Cui, Q.R.; Feng, K.; Xu, D.F.; Li, C.F.; Zheng, Q.S. Melatonin improves antioxidant capacity and ion homeostasis and enhances salt tolerance in maize seedlings. Acta Physiol. Plant. 2016, 38, 82. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. Omics 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K. [34] Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1987; Volume 148, pp. 350–382. [Google Scholar]
- Leakey, A.D.B.; Ferguson, J.N.; Pignon, C.P.; Wu, A.; Jin, Z.; Hammer, G.L.; Lobell, D.B. Water Use Efficiency as a Constraint and Target for Improving the Resilience and Productivity of C3 and C4 Crops. Annu. Rev. Plant Biol. 2019, 70, 781–808. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Hou, J.; Gao, W.; Tong, X.; Li, M.; Chu, X.; Chen, G. Exogenous spermidine alleviates the adverse effects of aluminum toxicity on photosystem II through improved antioxidant system and endogenous polyamine contents. Ecotoxicol. Environ. Saf. 2021, 207, 111265. [Google Scholar] [CrossRef] [PubMed]
- Sako, K.; Nagashima, R.; Tamoi, M.; Seki, M. Exogenous ethanol treatment alleviates oxidative damage of Arabidopsis thaliana under conditions of high-light stress. Plant Biotechnol. 2021, 38, 339–344. [Google Scholar] [CrossRef]
- Kramer, G.F.; Norman, H.A.; Krizek, D.T.; Mirecki, R.M. influence of UV-B radiation on polyamines, lipid peroxidation and membrane lipids in cucumber. Phytochemistry 1991, 30, 2101–2108. [Google Scholar] [CrossRef]



| Parameters | CK | MT | NaCl | NaCl_MT |
|---|---|---|---|---|
| Fv/Fm | 0.800 ± 0.00637 (a) | 0.804 ± 0.00615 (a) | 0.766 ± 0.00854 (c) | 0.785 ± 0.00751 (b) |
| Fv/Fo | 3.99 ± 0.162 (a) | 4.02 ± 0.142 (a) | 3.27 ± 0.191 (c) | 3.60 ± 0.185 (b) |
| φEo | 0.431 ± 0.0142 (a) | 0.446 ± 0.0113 (a) | 0.347 ± 0.0176 (c) | 0.386 ± 0.0149 (b) |
| ψEo | 0.535 ± 0.0174 (a) | 0.524 ± 0.0118 (a) | 0.431 ± 0.0179 (c) | 0.482 ± 0.0110 (b) |
| Vj | 0.466 ± 0.0234 (b) | 0.474 ± 0.0129 (b) | 0.558 ± 0.0217 (a) | 0.495 ± 0.0202 (b) |
| Vi | 0.826 ± 0.0175 (b) | 0.833 ± 0.0106 (b) | 0.893 ± 0.0299 (a) | 0.850 ± 0.0208 (b) |
| ABS/RC | 2.78 ± 0.122 (b) | 2.92 ± 0.237 (b) | 3.71 ± 0.216 (a) | 3.02 ± 0.141 (b) |
| DIo/RC | 0.558 ± 0.0239 (c) | 0.569 ± 0.0438 (bc) | 0.852 ± 0.0306 (a) | 0.618 ± 0.0197 (b) |
| TRo/RC | 2.22 ± 0.112 (b) | 2.31 ± 0.199 (b) | 2.81 ± 0.158 (a) | 2.40 ± 0.124 (b) |
| ETo/RC | 1.19 ± 0.0686 (a) | 1.20 ± 0.111 (a) | 1.26 ± 0.0769 (a) | 1.21 ± 0.0435 (a) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Zong, L.; Cai, Q.; Fu, Y.; Gao, Z.; Chen, G. Elucidating the Mechanistic Role of Exogenous Melatonin in Salt Stress Tolerance of Maize (Zea mays L.) Seedlings: An Integrated Physiological, Metabolomic, and Proteomic Profiling Analysis. Plants 2025, 14, 3129. https://doi.org/10.3390/plants14203129
Wang Z, Zong L, Cai Q, Fu Y, Gao Z, Chen G. Elucidating the Mechanistic Role of Exogenous Melatonin in Salt Stress Tolerance of Maize (Zea mays L.) Seedlings: An Integrated Physiological, Metabolomic, and Proteomic Profiling Analysis. Plants. 2025; 14(20):3129. https://doi.org/10.3390/plants14203129
Chicago/Turabian StyleWang, Zhichao, Linhao Zong, Qiqi Cai, Yinjie Fu, Zhiping Gao, and Guoxiang Chen. 2025. "Elucidating the Mechanistic Role of Exogenous Melatonin in Salt Stress Tolerance of Maize (Zea mays L.) Seedlings: An Integrated Physiological, Metabolomic, and Proteomic Profiling Analysis" Plants 14, no. 20: 3129. https://doi.org/10.3390/plants14203129
APA StyleWang, Z., Zong, L., Cai, Q., Fu, Y., Gao, Z., & Chen, G. (2025). Elucidating the Mechanistic Role of Exogenous Melatonin in Salt Stress Tolerance of Maize (Zea mays L.) Seedlings: An Integrated Physiological, Metabolomic, and Proteomic Profiling Analysis. Plants, 14(20), 3129. https://doi.org/10.3390/plants14203129





