A Cryopreservation and Regeneration Protocol for Embryogenic Callus of Larix olgensis
Abstract
1. Introduction
2. Results
2.1. Optimization of 2,3,5-Triphenyltetrazolium Chloride (TTC) Concentration for Cell Viability Assay
2.2. Screening of Optimal Preculture and Cryoprotectant Protocols
2.2.1. Effects of Preculture and Cryoprotectant Protocols on Cell Viability
2.2.2. Effects of Preculture and Cryoprotectant Protocols on Proliferation Rate
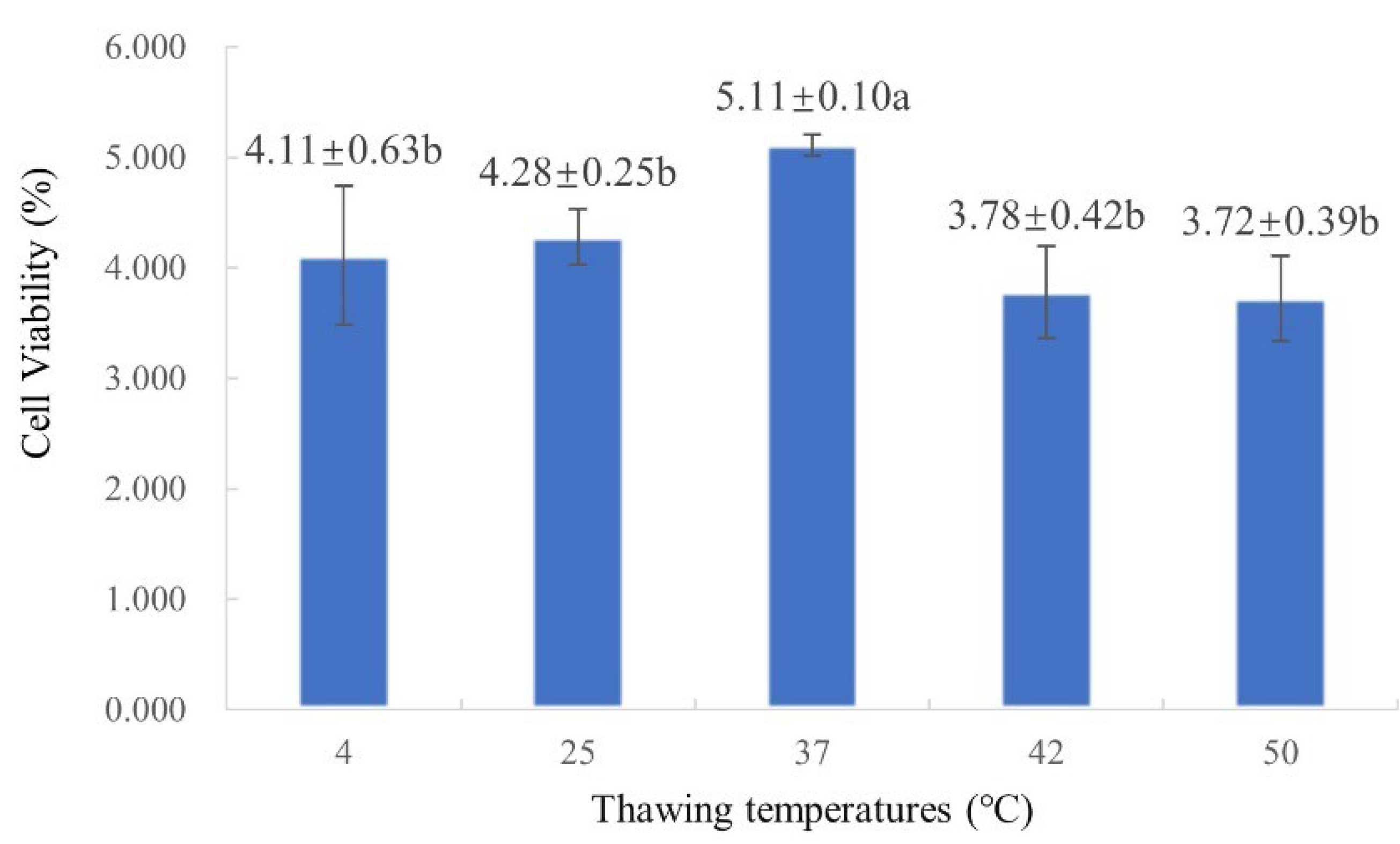
2.3. Screening of Optimal Thawing Temperature
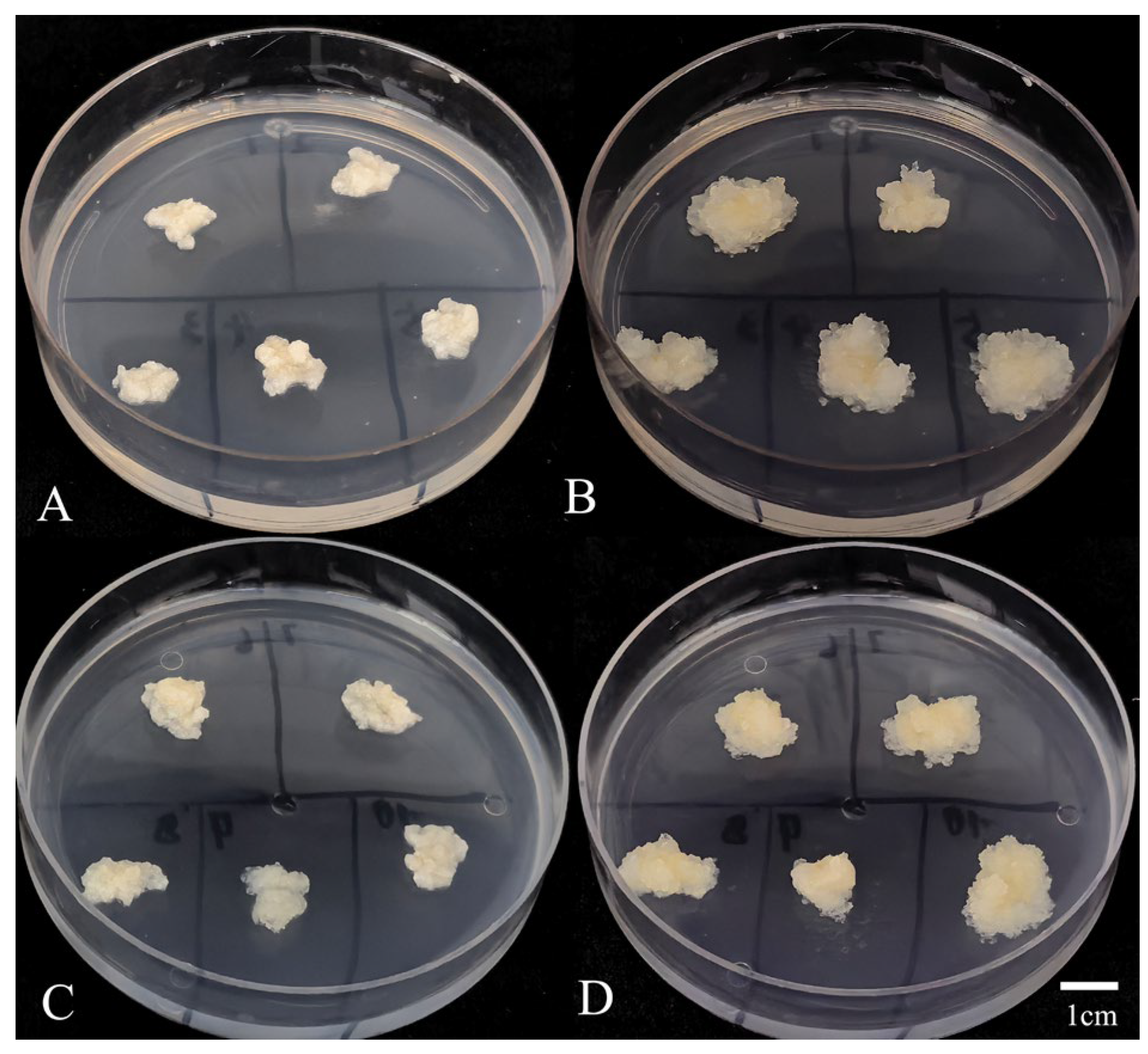
2.4. Recovery Rate Assessment
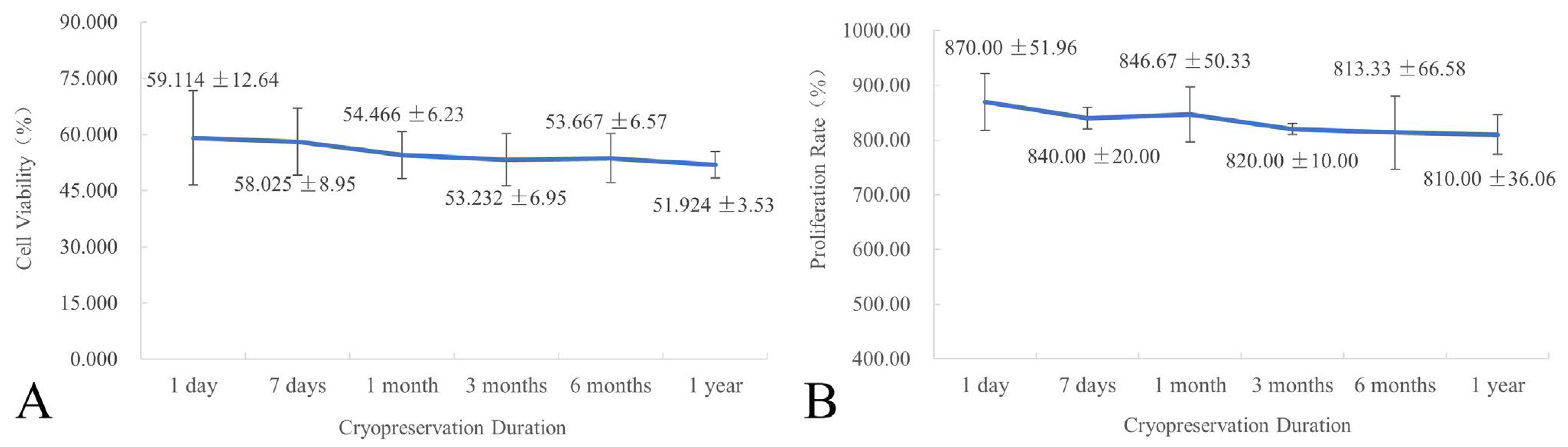
2.5. Effect of Cryopreservation Duration on Cell Viability and Proliferation
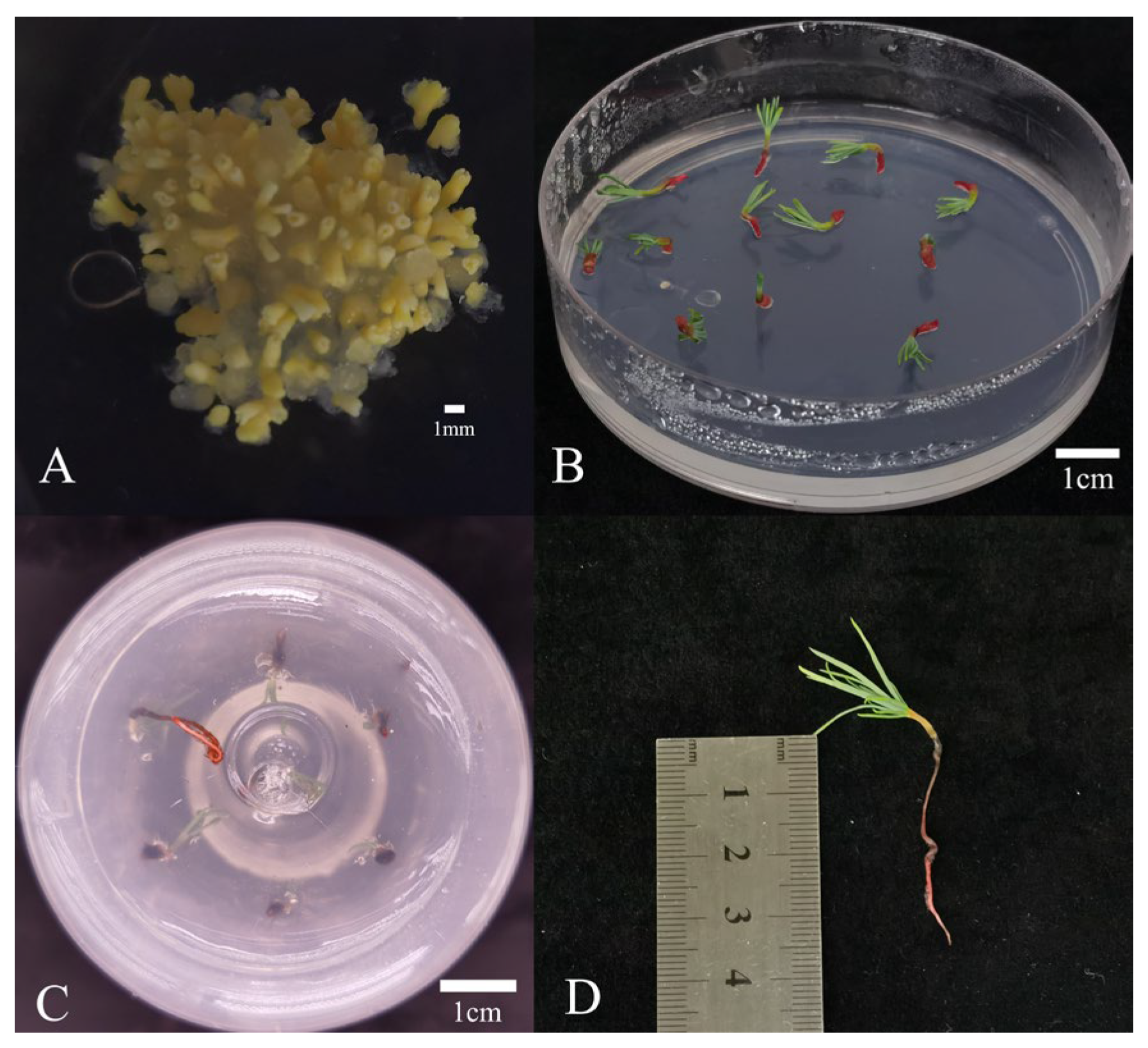
2.6. SE and Germination After Cryopreservation
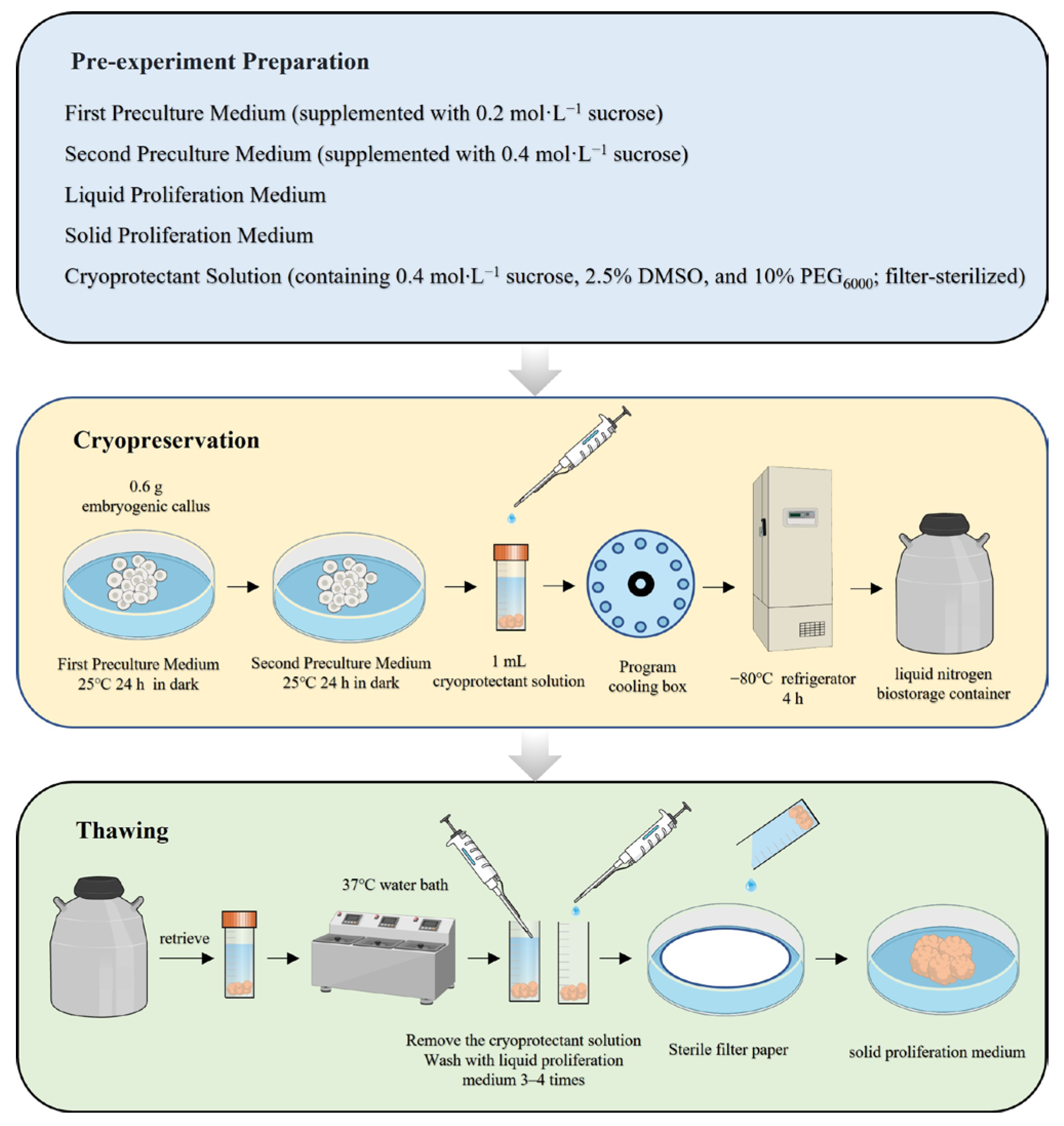
2.7. Flowchart of the Optimized Protocol
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Culture Medium Formulation
4.3. Methods
4.3.1. Optimization of TTC Concentration
4.3.2. Preculture and Cryoprotectant Treatment
4.3.3. Thawing Treatment
4.3.4. Proliferation Rate
4.3.5. Effect of Cryopreservation Duration (Storage Duration in Liquid Nitrogen) on Cell Viability and Proliferation Rate
4.3.6. Assessment of Recovery Rate
4.3.7. Validation of Somatic Embryogenic Potential
4.4. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| EC | Embryogenic callus |
| SE | Somatic embryogenesis |
| DMSO | Dimethyl Sulfoxide |
| PEG6000 | Polyethylene Glycol 6000 |
| TTC | 2,3,5-triphenyltetrazolium chloride |
| OATS | Orthogonal array testing strategy |
References
- An, P.Q.; Cao, Q.; Wang, C.; Wang, J.H.; Zhang, H.G.; Zhang, L. Spatiotemporal Expression and Bioinformatic Analyses of the HD-Zip Transcription Factor Family in Larix olgensis. Plant Mol. Biol. Rep. 2021, 39, 212–225. [Google Scholar] [CrossRef]
- Zhao, R.R.; Qi, S.Z.; Cui, Y.; Gao, Y.; Jiang, S.F.; Zhao, J.; Zhang, J.F.; Kong, L.S. Transcriptomic and physiological analysis identifies a gene network module highly associated with brassinosteroid regulation in hybrid sweetgum tissues differing in the capability of somatic embryogenesis. Hortic. Res. 2022, 9, uhab047. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.H.; Liu, M.; Zhou, R.; Jiang, F.L.; Li, P.; Li, M.Q.; Zhang, M.; Wei, H.Y.; Wu, Z. Construction of ceRNA Networks at Different Stages of Somatic Embryogenesis in Garlic. Int. J. Mol. Sci. 2023, 24, 5311. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.F.; Chen, X.Y.; Gao, Y.; Cui, Y.; Kong, L.S.; Zhao, J.; Zhang, J.F. Plant Regeneration via Somatic Embryogenesis in Larix principis-rupprechtii Mayr. Forests 2021, 12, 1335. [Google Scholar] [CrossRef]
- Song, Y.; Bai, X.M.; Dong, S.W.; Yang, Y.N.; Dong, H.; Wang, N.R.; Zhang, H.G.; Li, S.J. Stable and Efficient Agrobacterium-Mediated Genetic Transformation of Larch Using Embryogenic Callus. Front. Plant Sci. 2020, 11, 584492. [Google Scholar] [CrossRef]
- Caeiro, A.; Jarak, I.; Correia, S.; Canhoto, J.; Carvalho, R. Primary Metabolite Screening Shows Significant Differences between Embryogenic and Non-Embryogenic Callus of Tamarillo (Solanum betaceum Cav.). Plants 2023, 12, 2869. [Google Scholar] [CrossRef]
- Vásquez-Rivera, A.; Sommer, K.K.; Oldenhof, H.; Higgins, A.Z.; Brockbank, K.G.M.; Hilfiker, A.; Wolkers, W.F. Simultaneous monitoring of different vitrification solution components permeating into tissues. Analyst 2018, 143, 420–428. [Google Scholar] [CrossRef]
- Hu, J.W.; Pu, Z.Y.; Hao, C.H.; Yan, H.L.; Qian, S.M.; Zhu, T.Q.; Ma, W.J.; An, S.P.; Kong, L.S.; Wang, J.H. Optimized cryopreservation of embryogenic tissue of Picea abies based on differential scanning calorimetry. Cryobiology 2025, 120, 105278. [Google Scholar] [CrossRef]
- Bettoni, J.C.; Bonnart, R.; Volk, G.M. Challenges in implementing plant shoot tip cryopreservation technologies. Plant Cell Tissue Organ Cult. 2021, 144, 21–34. [Google Scholar] [CrossRef]
- Zhang, A.L.; Wang, M.R.; Li, Z.Y.; Panis, B.; Bettoni, J.C.; Vollmer, R.; Xu, L.; Wang, Q.C. Overcoming Challenges for Shoot Tip Cryopreservation of Root and Tuber Crops. Agronomy 2023, 13, 219. [Google Scholar] [CrossRef]
- Engels, J.M.M.; Ebert, A.W. A Critical Review of the Current Global Ex Situ Conservation System for Plant Agrobiodiversity. I. History of the Development of the Global System in the Context of the Political/Legal Framework and Its Major Conservation Components. Plants 2021, 10, 1557. [Google Scholar] [CrossRef]
- Nagel, M.; Pence, V.; Ballesteros, D.; Lambardi, M.; Popova, E.; Panis, B. Plant Cryopreservation: Principles, Applications, and Challenges of Banking Plant Diversity at Ultralow Temperatures. Annu. Rev. Plant Biol. 2024, 75, 797–824. [Google Scholar] [CrossRef] [PubMed]
- Kim, H. Optimizing the Droplet-Vitrification Procedure by Balancing the Cryoprotection and Cytotoxicity of Alternative Plant Vitrification Solutions Based on the Nature of Donor Plant Vigor. Plants 2023, 12, 4040. [Google Scholar] [CrossRef] [PubMed]
- Volk, G.M.; Bonnart, R.M.; Henk, A.D.; Chen, K.Y.; Bettoni, J.C.; Wang, Q.C.; Kreckel, H.D.; Levinger, N.E. Fundamentals of plant cryopreservation: Dormant bud two-step cooling and shoot tip vitrification. In Proceedings of the ISHS Acta Horticulturae 14211, Oslo, Norway, 27 February 2025; pp. 117–124. [Google Scholar]
- Wang, M.; Jing, Y.; Chen, L.; Wang, X.; Meng, C.; Huang, B.; Xu, L.; Li, Z. Applications and Prospects of Cryogenic Technology in the Conservation of Tropical Crop Germplasm Resources. Chin. J. Trop. Crops 2025, 46, 611–628. [Google Scholar]
- Xiang, J.; Mlambo, R.; Shaw, I.; Seid, Y.; Shah, H.M.; He, Y.J.; Kpegah, J.; Tan, S.W.; Zhou, W.H.; He, B.S. Cryopreservation of bioflavonoid-rich plant sources and bioflavonoid-microcapsules: Emerging technologies for preserving bioactivity and enhancing nutraceutical applications. Front. Nutr. 2023, 10, 1232129. [Google Scholar] [CrossRef]
- Hassani, S.B.; Trontin, J.F.; Raschke, J.; Zoglauer, K.; Rupps, A. Constitutive Overexpression of a Conifer WOX2 Homolog Affects Somatic Embryo Development in Pinus pinaster and Promotes Somatic Embryogenesis and Organogenesis in Arabidopsis Seedlings. Front. Plant Sci. 2022, 13, 838421. [Google Scholar] [CrossRef]
- Zeng, L.; He, M.-J.; Chen, K.; Wei, J.-H. Cryopreservation study on seeds and embryos in Dalbergia odorifera. China J. Chin. Mater. Medica 2014, 39, 2263–2266. [Google Scholar]
- Endoh, K.; Hanaoka, S.; Matsushita, M.; Ubukata, M.; Yamada, H. Ex situ conservation of birch trees by cryopreservation of dormant buds adapted to subzero temperatures by extracellular freezing. New For. 2023, 54, 515–523. [Google Scholar] [CrossRef]
- Halmagyi, A.; Valimareanu, S.; Sovarel, G.; Coste, A. Cryo-Technologies for Ex Situ Conservation of Rosa Germplasm. Plants 2022, 11, 1095. [Google Scholar] [CrossRef]
- Zhang, L.; Tian, H.; Liu, J.; Liu, Y. Cryopreservation of Shoot—Tips of Populus euphratica Dormant Buds by Encapsulation—Vitrification. Xinjiang Agric. Sci. 2017, 54, 2232–2238. [Google Scholar]
- Ozudogru, E.A.; Kirdok, E.; Kaya, E.; Capuana, M.; Benelli, C.; Engelmann, F. Cryopreservation of redweed (Sequoia sempervirens (D.Don) Endl.) in vitro buds using vitrification-based techniques. Cryoletters 2011, 32, 99–110. [Google Scholar]
- Rohini, M.R.; Malik, S.K.; Choudhary, R.; Kaur, S.; Uchoi, A.; Chaudhury, R. Storage behavior and cryopreservation studies in Indian rough lemon (Citrus jambhiri): A promising rootstock for long-term conservation. Turk. J. Agric. For. 2016, 40, 865–873. [Google Scholar] [CrossRef]
- Pu, Z.; Hu, J.; Wang, J.; Xu, Y.; An, S.; Wang, L.; Xu, N.; Zhu, T. Research Progress in Cryopreservation of Forest Trees. World For. Res. 2024, 37, 22–29. [Google Scholar]
- Wasilenczyk, U.; Wawrzyniak, M.K.; Martins, J.P.R.; Kosek, P.; Chmielarz, P. Cryopreservation of sessile oak (Quercus petraea (Matt.) Liebl.) plumules using aluminium cryo-plates: Influence of cryoprotection and drying. Plant Methods 2024, 20, 53. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.X. Somatic Embryo Maturation and Embryogenic Callus Preservation During Somatic Embryogenesis of Pinus koraiensis. Master’s Thesis, Northeast Forestry University, Harbin, China, 2019. [Google Scholar] [CrossRef]
- Chen, X.; Jiang, S.; Dai, J.; Yuan, D.; Kong, L.; Zhang, J.; Zhao, J. Cryopreservation of embryogenic callus for Larix gmelinii var. principis-rupprechtii. J. Beijing For. Univ. 2021, 43, 47–53. [Google Scholar]
- Gao, F.; Chen, S.; Qin, C.; Cai, J.; Wang, C.; Dong, H.; Tao, J. Optimization of somatic embryogenesis system and cryopreservation of Picea koraiensis. J. Nanjing For. Univ. Nat. Sci. Ed. 2021, 45, 100–108. [Google Scholar]
- Shen, L.Y. Somatic Embryogenesis and Cryopreservation for Embryogenic Callus of Nematode-Resistant Pinus massoniana Lamb. Master’s Thesis, Nanjing Forestry University, Nanjing, China, 2019. [Google Scholar] [CrossRef]
- Varis, S.; Ahola, S.; Jaakola, L.; Aronen, T. Reliable and practical methods for cryopreservation of embryogenic cultures and cold storage of somatic embryos of Norway spruce. Cryobiology 2017, 76, 8–17. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, B. Application of Cryopreservation Technology in Biobank. Refrig. Technol. 2020, 40, 66–71. [Google Scholar]
- Fatiha, B.; Carolina, S. Effect of Cryopreservation on Olive (Olea europaea L.) Plant Regeneration via Somatic Embryogenesis. Plants 2020, 10, 34. [Google Scholar] [CrossRef]
- Song, Y.; Zhen, C.; Zhang, H.; Li, S. Embryogenic Callus Induction and Somatic Embryogenesis from Immature Zygotic Embryos of Larix olgensis. Sci. Silvae Sin. 2016, 52, 45–54. [Google Scholar]
- Munasinghe, S.; Somaratne, S.; Weerakoon, S.; Ranasinghe, C. Sustainable utilization of Gyrinops walla Gaetner: In Vitro production of sesquiterpenes by chemical and biological elicitation. J. Genet. Eng. Biotechnol. 2021, 19, 134. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Bi, W.L.; Shukla, M.R.; Saxena, P.K. In Vitro Technologies for American Chestnut (Castanea dentata (Marshall) Borkh) Conservation. Plants 2022, 11, 21. [Google Scholar] [CrossRef]
- Jorquera, L.; Vidal, N.; Sánchez, C.; Vieitez, A.M. Optimizing conditions for successful plant regeneration from cryopreserved Castanea sativa shoot tips. In Proceedings of the ISHS Acta Horticulturae 693, Chaves, Portugal, 31 October 2005; pp. 511–518. [Google Scholar] [CrossRef]
- Verleysen, H.; Fernandes, P.; Pinto, I.S.; Van Bockstaele, E.; Debergh, P. Cryopreservation of Robinia pseudoacacia. Plant Cell Tissue Organ Cult. 2005, 81, 193–202. [Google Scholar] [CrossRef]
- Pathirana, R.; McLachlan, A.; Hedderley, D.; Panis, B.; Carimi, F. Pre-treatment with salicylic acid improves plant regeneration after cryopreservation of grapevine (Vitis spp.) by droplet vitrification. Acta Physiol. Plant. 2016, 38, 2002425. [Google Scholar] [CrossRef]
- Chang, T.; Zhao, G. Ice Inhibition for Cryopreservation: Materials, Strategies, and Challenges. Adv. Sci. 2021, 8, 2002425. [Google Scholar] [CrossRef]
- Chen, T.T.; Jia, X.Y.; Yu, C.G.; Yin, Y.L.; Hua, J.F. A simple and efficient protocol for cryopreservation of Taxodium hybrid ‘zhongshanshan’ embryogenic callus. Plant Cell Tissue Organ Cult. 2024, 156, 44. [Google Scholar] [CrossRef]
- Patel, M.; Park, J.K.; Jeong, B. Rediscovery of poly(ethylene glycol)s as a cryoprotectant for mesenchymal stem cells. Biomater. Res. 2023, 27, 104423. [Google Scholar] [CrossRef]
- Bai, G.Y.; Hu, J.H.; Qin, S.J.; Qi, Z.P.; Zhuang, H.N.; Sun, F.D.; Lu, Y.H.; Jin, S.L.; Gao, D.; Wang, J.J. Small-molecule fulvic acid with strong hydration ability for non-vitreous cellular cryopreservation. Iscience 2022, 25, 104423. [Google Scholar] [CrossRef]
- Long, W.; Yao, X.H.; Ren, H.D. A Cryopreservation Protocol for Olea europaea Embryogenic Callus. China Patent CN113854283A, 31 December 2021. [Google Scholar]
- Zhao, W.N.; Liu, Y.; Wang, C.; Ning, Y.J.; Cui, C.P.; Zhang, H.G.; Li, M.; Li, S.J. 5-AzaC Facilitates Somatic Embryogenesis and Germination Across Two Embryogenic Lines in Larix olgensis. Plants 2025, 14, 2818. [Google Scholar] [CrossRef]
- Song, Y. Studies on Somatic Embryogenesis and Genetic Transformation of Larix olgensis. Master’s Thesis, Northeast Forestry University, Harbin, China, 2017. [Google Scholar]
- Granata, A.; Nicoletti, R.; Perego, P.; Iorio, E.; Krishnamachary, B.; Benigni, F.; Ricci, A.; Podo, F.; Bhujwalla, Z.M.; Canevari, S.; et al. Global metabolic profile identifies choline kinase alpha as a key regulator of glutathione-dependent antioxidant cell defense in ovarian carcinoma. Oncotarget 2015, 6, 11216–11230. [Google Scholar] [CrossRef]
- Hawrylak-Nowak, B.; Matraszek-Gawron, R. Difference between Selenite and Selenate in the Regulation of Growth and Physiological Parameters of Nickel-Exposed Lettuce. Biology 2020, 9, 465. [Google Scholar] [CrossRef] [PubMed]

| Treatments | Factors | Cell Viability (%) | |||
|---|---|---|---|---|---|
| Preculture Duration (T) | Preculture Strategy (M) | DMSO (D) | PEG (P) | ||
| 1 | T1 | M1 | D1 | P1 | 3.889 |
| 2 | T3 | M3 | D1 | P3 | 4.500 |
| 3 | T4 | M4 | D1 | P4 | 1.778 |
| 4 | T2 | M2 | D1 | P2 | 4.111 |
| 5 | T2 | M4 | D3 | P1 | 2.667 |
| 6 | T4 | M3 | D2 | P1 | 2.611 |
| 7 | T3 | M2 | D4 | P1 | 2.722 |
| 8 | T1 | M4 | D4 | P3 | 0.889 |
| 9 | T4 | M1 | D4 | P2 | 2.778 |
| 10 | T1 | M3 | D3 | P2 | 0.389 |
| 11 | T2 | M3 | D4 | P4 | 2.333 |
| 12 | T2 | M1 | D2 | P3 | 4.833 |
| 13 | T3 | M1 | D3 | P4 | 4.000 |
| 14 | T3 | M4 | D2 | P2 | 2.444 |
| 15 | T4 | M2 | D3 | P3 | 2.222 |
| 16 | T1 | M2 | D2 | P4 | 2.500 |
| K1 | 23.000 | 46.500 | 42.833 | 35.667 | |
| K2 | 41.833 | 34.667 | 37.167 | 37.167 | |
| K3 | 41.000 | 29.500 | 27.833 | 37.333 | |
| K4 | 28.167 | 23.333 | 26.167 | 31.833 | |
| `X1 | 1.917 | 3.875 | 3.569 | 2.972 | |
| `X2 | 3.486 | 2.889 | 3.097 | 3.097 | |
| `X3 | 3.417 | 2.458 | 2.319 | 3.111 | |
| `X4 | 2.347 | 1.944 | 2.181 | 2.653 | |
| R | 1.569 | 1.931 | 1.389 | 0.458 | |
| Levels | Preculture Duration (h) | Preculture Strategy | DMSO (%) | PEG (%) |
|---|---|---|---|---|
| 1 | 1.917 ± 1.483 B | 3.875 ± 0.951 A | 3.569 ± 1.366 A | 2.972 ± 0.813 |
| 2 | 3.486 ± 1.375 A | 2.889 ± 0.941 B | 3.097 ± 1.240 A | 2.431 ± 1.508 |
| 3 | 3.417 ± 1.086 A | 2.458 ± 1.719 BC | 2.320 ± 1.433 B | 3.111 ± 1.849 |
| 4 | 2.347 ± 0.694 B | 1.945 ± 0.903 C | 2.181 ± 0.941 B | 2.653 ± 1.043 |
| Treatments | Factors | Cell Viability (%) | |||
|---|---|---|---|---|---|
| Preculture Duration (T) | Preculture Strategy (M) | DMSO (D) | PEG (P) | ||
| 1 | T1 | M1 | D1 | P1 | 3.889 ± 0.59 AB |
| 2 | T3 | M3 | D3 | P3 | 4.500 ± 1.17 A |
| 3 | T4 | M4 | D4 | P4 | 1.778 ± 0.67 CDE |
| 4 | T2 | M2 | D2 | P2 | 4.111 ± 1.18 A |
| 5 | T2 | M2 | D2 | P2 | 2.667 ± 0.76 BC |
| 6 | T4 | M4 | D4 | P4 | 2.611 ± 1.00 BC |
| 7 | T3 | M3 | D3 | P3 | 2.722 ± 0.10 BC |
| 8 | T1 | M1 | D1 | P1 | 0.889 ± 0.48 EF |
| 9 | T4 | M4 | D4 | P4 | 2.778 ± 0.19 BC |
| 10 | T1 | M1 | D1 | P1 | 0.389 ± 0.25 F |
| 11 | T2 | M2 | D2 | P2 | 2.333 ± 1.04 CD |
| 12 | T2 | M2 | D2 | P2 | 4.833 ± 1.01 A |
| 13 | T3 | M3 | D3 | P3 | 4.000 ± 0.6 A |
| 14 | T3 | M3 | D3 | P3 | 2.444 ± 0.59 C |
| 15 | T4 | M4 | D4 | P4 | 2.222 ± 0.51 CD |
| 16 | T1 | M1 | D1 | P1 | 2.500 ± 0.17 C |
| control | - | - | - | - | 1.111 ± 0.42 DEF |
| optimized protocol | T2 | M1 | D1 | P3 | 5.111 ± 0.1 A |
| Treatments | Factors | Proliferation Rate (%) | |||
|---|---|---|---|---|---|
| Preculture Duration (T) | Preculture Strategy (M) | DMSO (D) | PEG (P) | ||
| 1 | T1 | M1 | D1 | P1 | 0.00 ± 0.00 F |
| 2 | T3 | M3 | D3 | P3 | 370.00 ± 27.00 C |
| 3 | T4 | M4 | D4 | P4 | 0.00 ± 0.00 F |
| 4 | T2 | M2 | D2 | P2 | 340.33 ± 4.04 C |
| 5 | T2 | M2 | D2 | P2 | 30.00 ± 17.32 |
| 6 | T4 | M4 | D4 | P4 | 66.67 ± 32.15 E |
| 7 | T3 | M3 | D3 | P3 | 0.00 ± 0.00 F |
| 8 | T1 | M1 | D1 | P1 | 0.00 ± 0.00 F |
| 9 | T4 | M4 | D4 | P4 | 0.00 ± 0.00 F |
| 10 | T1 | M1 | D1 | P1 | 0.00 ± 0.00 F |
| 11 | T2 | M2 | D2 | P2 | 0.00 ± 0.00 F |
| 12 | T2 | M2 | D2 | P2 | 480.00 ± 30.00 B |
| 13 | T3 | M3 | D3 | P3 | 276.67 ± 30.55 D |
| 14 | T3 | M3 | D3 | P3 | 0.00 ± 0.00 F |
| 15 | T4 | M4 | D4 | P4 | 0.00 ± 0.00 F |
| 16 | T1 | M1 | D1 | P1 | 0.00 ± 0.00 F |
| control | - | - | - | - | 0.00 ± 0.00 F |
| optimized protocol | T2 | M1 | D1 | P3 | 713.33 ± 11.55 A |
| Names | Formulations |
|---|---|
| Preculture medium | BM supplemented with 0.15 mg·L−1 2,4-D, 0.05 mg·L−1 6-BA, 0.05 mg·L−1 KT, 1 g·L−1 L-glutamine, 1 g·L−1 inositol, 0.5 g·L−1 casein acids hydrolysate, 5.2 g·L−1 agar and 0.2 mol·L−1/0.4 mol·L−1 sucrose/sorbitol, pH of 6.0 |
| Liquid proliferation medium | BM supplemented with 0.15 mg·L−1 2,4-D, 0.05 mg·L−1 6-BA, 0.05 mg·L−1 KT, 1 g·L−1 L-glutamine, 1 g·L−1 inositol, 0.5 g·L−1 casein acids hydrolysate and 25 g·L−1 sucrose, pH of 6.0 |
| Solid proliferation medium | BM supplemented with 0.15 mg·L−1 2,4-D, 0.05 mg·L−1 6-BA, 0.05 mg·L−1 KT, 1 g·L−1 L-glutamine, 1 g·L−1 inositol, 0.5 g·L−1 casein acids hydrolysate, 5.2 g·L−1 agar and 25 g·L−1 sucrose, pH of 6.0 |
| Pre-maturation medium | 1/4 BM supplemented with 1 g·L−1 L-glutamine, 10 g·L−1 inositol, 0.5 g·L−1 casein acids hydrolysate, 5.2 g·L−1 agar and 60 g·L−1 sucrose, pH of 6.0 |
| Somatic embryo maturation medium | BM supplemented with 20 mg·L−1 abscisic acid, 80 g·L−1 PEG6000, 0.5 g·L−1 L-glutamine, 0.5 g·L−1 inositol, 0.25 g·L−1 casein acids hydrolysate, 2 g·L−1 GelzanTM CM and 60 g·L−1 sucrose, pH of 6.0 |
| Germination medium | 1/2 MS supplemented with sucrose 30 g·L−1 and agar 6.5 g·L−1, pH of 5.8 |
| Levels | Preculture Duration (T) (h) | Preculture Strategy (M) | DMSO (D) (%) | PEG (P) (%) |
|---|---|---|---|---|
| 1 | 12 | 0.2 mol·L−1 + 0.4 mol·L−1 sucrose | 2.5 | 0 |
| 2 | 24 | 0.2 mol·L−1 + 0.4 mol·L−1 sorbitol | 5 | 5 |
| 3 | 36 | 0.4 mol·L−1 sucrose | 10 | 10 |
| 4 | 48 | 0.4 mol·L−1 sorbitol | 15 | 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Zhao, W.; Liu, Y.; Dong, H.; Ning, Y.; Cui, C.; Zhang, H.; Li, M.; Li, S. A Cryopreservation and Regeneration Protocol for Embryogenic Callus of Larix olgensis. Plants 2025, 14, 3127. https://doi.org/10.3390/plants14203127
Wang C, Zhao W, Liu Y, Dong H, Ning Y, Cui C, Zhang H, Li M, Li S. A Cryopreservation and Regeneration Protocol for Embryogenic Callus of Larix olgensis. Plants. 2025; 14(20):3127. https://doi.org/10.3390/plants14203127
Chicago/Turabian StyleWang, Chen, Wenna Zhao, Yu Liu, Hao Dong, Yajing Ning, Chengpeng Cui, Hanguo Zhang, Meng Li, and Shujuan Li. 2025. "A Cryopreservation and Regeneration Protocol for Embryogenic Callus of Larix olgensis" Plants 14, no. 20: 3127. https://doi.org/10.3390/plants14203127
APA StyleWang, C., Zhao, W., Liu, Y., Dong, H., Ning, Y., Cui, C., Zhang, H., Li, M., & Li, S. (2025). A Cryopreservation and Regeneration Protocol for Embryogenic Callus of Larix olgensis. Plants, 14(20), 3127. https://doi.org/10.3390/plants14203127






