Optimizing Rhizome Quality in Ligusticum chuanxiong Hort. Through High Maltose Concentration
Abstract
1. Introduction
2. Results
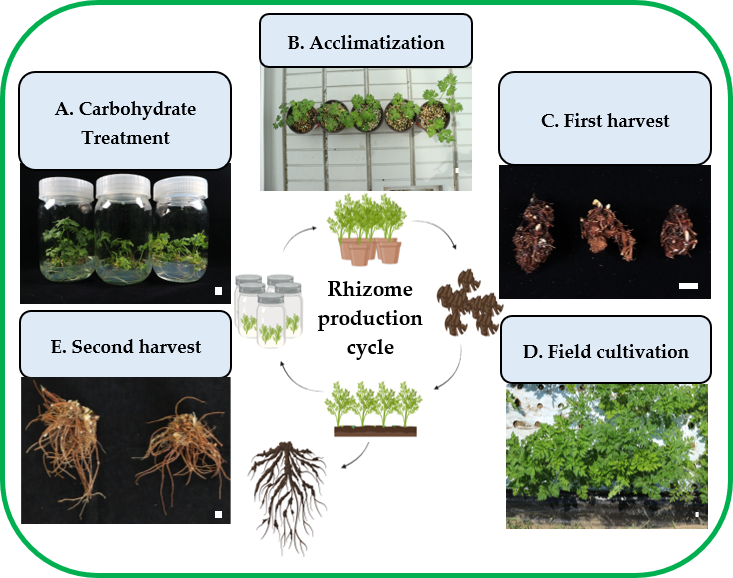
2.1. Carbohydrate Treatment In Vitro
2.2. Acclimatization and Investigation
2.3. First Harvest and Investigation
2.4. Field Growth and Second Harvest
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Carbohydrate Treatment
4.3. In Vitro Culture Conditions and Data Collection
4.4. Acclimatization and Field Growth
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ran, X.; Ma, L.; Peng, C.; Zhang, H.; Qin, L.-P. Ligusticum Chuanxiong Hort: A Review of Chemistry and Pharmacology. Pharm. Biol. 2011, 49, 1180–1189. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, C.; Gao, F.; Fu, Q.; Fu, C.; He, Y.; Zhang, J. A Systematic Review on the Rhizome of Ligusticum Chuanxiong Hort. (Chuanxiong). Food Chem. Toxicol. 2018, 119, 309–325. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, H.; Xie, X.; Li, Y.; Peng, F.; Zhao, Y.; Xu, H. Active Ingredients Biosynthesis and Genic Response of Traditional Chinese Medicine Ligusticum Sinense Cv. Chuanxiong under Different Cadmium Level. Ind. Crops Prod. 2023, 202, 117074. [Google Scholar] [CrossRef]
- Park, C.H.; Lee, M.S.; Namkoong, S.B.; Yu, H.S.; Park, H.W. Embryological Characteristics on Seed Sterility of Ligusticum Chuanxiong Hort. Korean J. Med. Crop Sci. 2004, 12, 209–213. [Google Scholar]
- Zahid, N.A.; Jaafar, H.Z.E.; Hakiman, M. Alterations in Microrhizome Induction, Shoot Multiplication and Rooting of Ginger (Zingiber officinale Roscoe) Var. Bentong with Regards to Sucrose and Plant Growth Regulators Application. Agronomy 2021, 11, 320. [Google Scholar] [CrossRef]
- Jie, E.Y.; Ahn, M.S.; Lee, J.; Cheon, Y.I.; Kim, C.Y.; Kim, S.W. Establishment of a High-Frequency Plant Regeneration System from Rhizome-Derived Embryogenic Cell-Suspension Cultures of Curcuma longa L. Plant Biotechnol. Rep. 2019, 13, 123–129. [Google Scholar] [CrossRef]
- Chithra, M.; Martin, K.P.; Sunandakumari, C.; Madhusoodanan, P.V. Protocol for Rapid Propagation, and to Overcome Delayed Rhizome Formation in Field Established In Vitro Derived Plantlets of Kaempferia galanga L. Sci. Hortic. 2005, 104, 113–120. [Google Scholar] [CrossRef]
- Ondo Ovono, P.; Kevers, C.; Dommes, J. Effects of Reducing Sugar Concentration on In Vitro Tuber Formation and Sprouting in Yam (Dioscorea Cayenensis–D. Rotundata Complex). Plant Cell Tiss. Organ. Cult. 2009, 99, 55–59. [Google Scholar] [CrossRef]
- Momena, K.; Adeba, R.; Mehraj, H.; Jamal Uddin, A.F.M.; Islam, S.; Rahman, L. In Vitro Microtuberization of Potato (Solanum tuberosum L.) Cultivar Through Sucrose and Growth Regulators; Available online: https://papers.ssrn.com/abstract=3610755Social Science Research Network: Rochester, NY, USA, 2014; Volume 2, pp. 76–82. (accessed on 21 August 2025).
- Kapoor, P.; Rao, I.U. In Vitro Rhizome Induction and Plantlet Formation from Multiple Shoots in Bambusa Bambos Var. Gigantea Bennet and Gaur by Using Growth Regulators and Sucrose. Plant Cell Tiss. Organ. Cult. 2006, 85, 211–217. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, S.; Mohapatra, T. Interaction between macro-and micro-nutrients in plants. Front. Plant Sci. 2021, 12, 665583. [Google Scholar] [CrossRef]
- Nadeem, F.; Hanif, M.A.; Majeed, M.I.; Mushtaq, Z. Role of macronutrients and micronutrients in the growth and development of plants and prevention of deleterious plant diseases-a comprehensive review. Int. J. Chem. Biochem. Sci. 2018, 13, 31–52. [Google Scholar]
- Khuri, S.; Moorby, J. Investigations into the Role of Sucrose in Potato Cv. Estima Microtuber Production in Vitro. Ann. Bot. 1995, 75, 295–303. [Google Scholar] [CrossRef]
- Denaxa, N.-K.; Vemmos, S.N.; Roussos, P.A. The Role of Endogenous Carbohydrates and Seasonal Variation in Rooting Ability of Cuttings of an Easy and a Hard to Root Olive Cultivars (Olea europaea L.). Sci. Hortic. 2012, 143, 19–28. [Google Scholar] [CrossRef]
- Abbas, M.; Aly, U.; Taha, H.; Gaber, E.-S. In vitro production of microrhizomes in ginger (Zingiber officinale rosco). J. Microb. Biotech. Food Sci. 2014, 4, 142–148. [Google Scholar] [CrossRef]
- Yaseen, M.; Ahmad, T.; Sablok, G.; Standardi, A.; Hafiz, I.A. Review: Role of Carbon Sources for In Vitro Plant Growth and Development. Mol. Biol. Rep. 2013, 40, 2837–2849. [Google Scholar] [CrossRef]
- Kim, H.-E.; Han, J.-E.; Lee, H.; Murthy, H.N.; Kwon, H.-J.; Lee, G.-M.; Park, S.-Y. Establishment of an Efficient In Vitro Propagation of Cnidium Officinale Makino and Selection of Superior Clones through Flow Cytometric Assessment of DNA Content. Genes 2022, 13, 1815. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.S.; Leung, D.W.M. Relative Importance of Maltose and Sucrose Supplied during a 2-Step Potato Microtuberization Process. Acta Physiol. Plant 2004, 26, 47–52. [Google Scholar] [CrossRef]
- Ngaha, M.; Omokolo, N.D. Effect of carbon source on minituberization of cocoyam (Xanthosoma sagittifolium L. Schott): Analysis of soluble sugars and amino acids contents. Curr. Res. Microbiol. Biotechnol. 2014, 2, 519–526. [Google Scholar]
- Karimi, M.; Ebrahimi, A.; Sahraroo, A.; Moosavi, S.-A.; Moosavi, F.; Bihamta, M.-R. Callus formation and shoot organogenesis in Moshgak (Ducrosia flabellifolia Boiss.) from cotyledon. J. Food Agric. Environ. 2009, 7, 441–445. [Google Scholar]
- Panathula, C.S.; Mahadev, M.; Naidu, C. Effect of different carbohydrates on in vitro plant regeneration of Centella asiatica (L.)-an important antijaundice medicinal plant. Int. J. Med. Aromat. Plants 2014, 4, 41–47. [Google Scholar]
- Gibson, S.I. Control of Plant Development and Gene Expression by Sugar Signaling. Curr. Opin. Plant Biol. 2005, 8, 93–102. [Google Scholar] [CrossRef]
- Smith, A.M.; Stitt, M. Coordination of Carbon Supply and Plant Growth. Plant Cell Environ. 2007, 30, 1126–1149. [Google Scholar] [CrossRef]
- Niittylä, T.; Messerli, G.; Trevisan, M.; Chen, J.; Smith, A.M.; Zeeman, S.C. A Previously Unknown Maltose Transporter Essential for Starch Degradation in Leaves. Science 2004, 303, 87–89. [Google Scholar] [CrossRef]
- Baier, M.; Hemmann, G.; Holman, R.; Corke, F.; Card, R.; Smith, C.; Rook, F.; Bevan, M.W. Characterization of Mutants in Arabidopsis Showing Increased Sugar-Specific Gene Expression, Growth, and Developmental Responses. Plant Physiol. 2004, 134, 81–91. [Google Scholar] [CrossRef]
- Singh, P.; Choudhary, K.K.; Chaudhary, N.; Gupta, S.; Sahu, M.; Tejaswini, B.; Sarkar, S. Salt Stress Resilience in Plants Mediated through Osmolyte Accumulation and Its Crosstalk Mechanism with Phytohormones. Front. Plant Sci. 2022, 13, 1006617. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Sharkey, T.D. Cellular and Organ Level Localization of Maltose in Maltose-Excess Arabidopsis Mutants. Planta 2006, 224, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-Y.; Huh, J.-H.; Yu, Y.-C.; Ho, L.-H.; Chen, L.-Q.; Tholl, D.; Frommer, W.B.; Guo, W.-J. The Arabidopsis Vacuolar Sugar Transporter SWEET2 Limits Carbon Sequestration from Roots and Restricts Pythium Infection. Plant J. 2015, 83, 1046–1058. [Google Scholar] [CrossRef]
- Ruedell, C.M.; de Almeida, M.R.; Fett-Neto, A.G. Concerted Transcription of Auxin and Carbohydrate Homeosta-sis-Related Genes Underlies Improved Adventitious Rooting of Microcuttings Derived from Far-Red Treated Eucalyptus Globulus Labill Mother Plants. Plant Physiol. Biochem. 2015, 97, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.A.; Abdellatif, Y.M.R. Effect of Maltose and Trehalose on Growth, Yield and Some Biochemical Components of Wheat Plant under Water Stress. Ann. Agric. Sci. 2016, 61, 267–274. [Google Scholar] [CrossRef]
- Kumar, A.; Sood, A.; Palni, L.M.S.; Gupta, A.K. In Vitro Propagation of Gladiolus Hybridus Hort.: Synergistic Effect of Heat Shock and Sucrose on Morphogenesis. Plant Cell Tissue Organ. Cult. 1999, 57, 105–112. [Google Scholar] [CrossRef]
- Molassiotis, A.N.; Sotiropoulos, T.; Tanou, G.; Kofidis, G.; Diamantidis, G.; Therios, E. Antioxidant and Anatomical Responses in Shoot Culture of the Apple Rootstock MM 106 Treated with NaCl, KCl, Mannitol or Sorbitol. Biol. Plant 2006, 50, 331–338. [Google Scholar] [CrossRef]
- Stoop, J.M.H.; Pharr, D.M. Effect of Different Carbon Sources on Relative Growth Rate, Internal Carbohydrates, and Mannitol 1-Oxidoreductase Activity in Celery Suspension Cultures. Plant Physiol. 1993, 103, 1001–1008. [Google Scholar] [CrossRef]
- Darko, E.; Végh, B.; Khalil, R.; Marček, T.; Szalai, G.; Pál, M.; Janda, T. Metabolic responses of wheat seedlings to osmotic stress induced by various osmolytes under iso-osmotic conditions. PLoS ONE 2019, 14, e0226151. [Google Scholar] [CrossRef]
- Rostami-Balan, S.; Dianati Daylami, S.; Karimi, S. In Vitro Tuber Induction and Growth Improvement in Dactylorhiza Umbrosa by Osmotic Pretreatments. J. Hortic. Sci. Biotechnol. 2022, 97, 739–746. [Google Scholar] [CrossRef]
- Ming, N.J.; Binte Mostafiz, S.; Johon, N.S.; Abdullah Zulkifli, N.S.; Wagiran, A. Combination of plant growth regulators, maltose, and partial desiccation treatment enhance somatic embryogenesis in selected Malaysian rice cultivar. Plants 2019, 8, 144. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, G.; Talavera, C.; Oropeza, C.; Desjardins, Y.; Santamaria, J.M. Exogenous Sucrose Can Decrease In Vitro Photosynthesis but Improve Field Survival and Growth of Coconut (Cocos nucifera L.) in Vitro Plantlets. Vitr. Cell. Dev. Biol.—Plant 2005, 41, 69–76. [Google Scholar] [CrossRef]
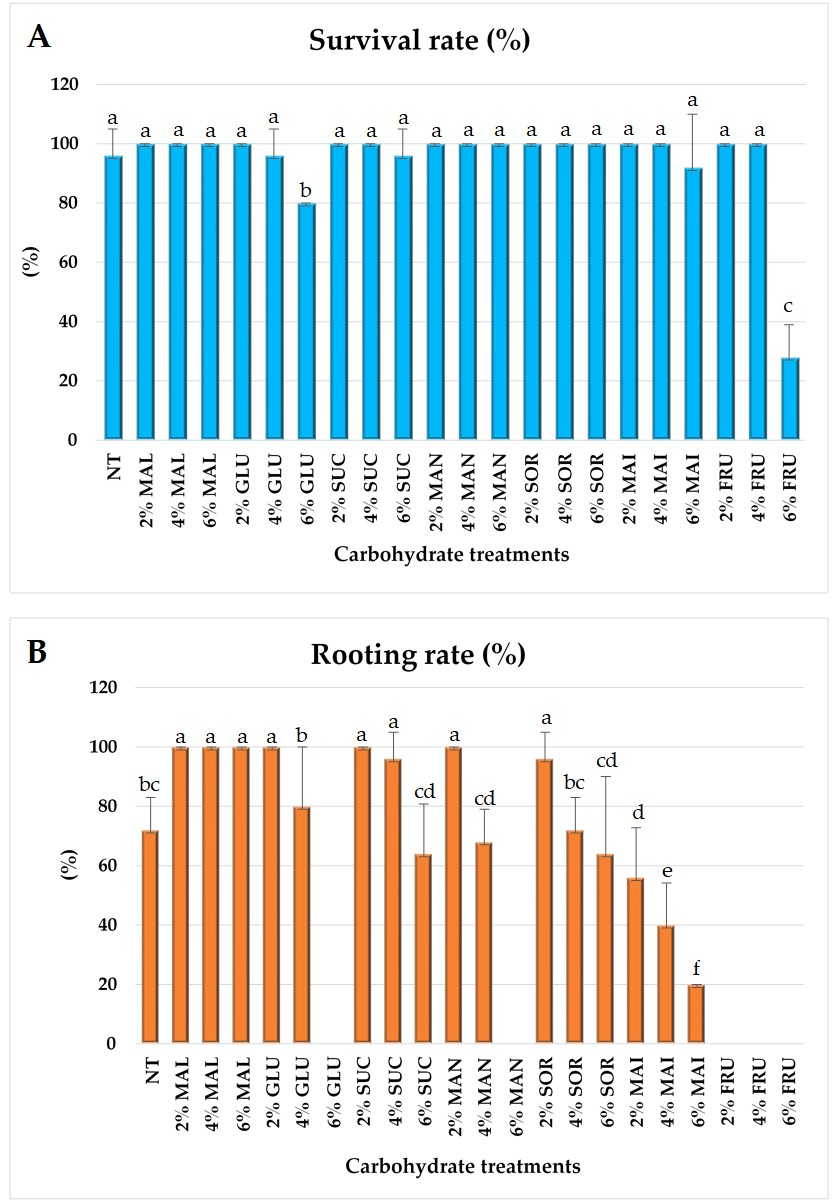

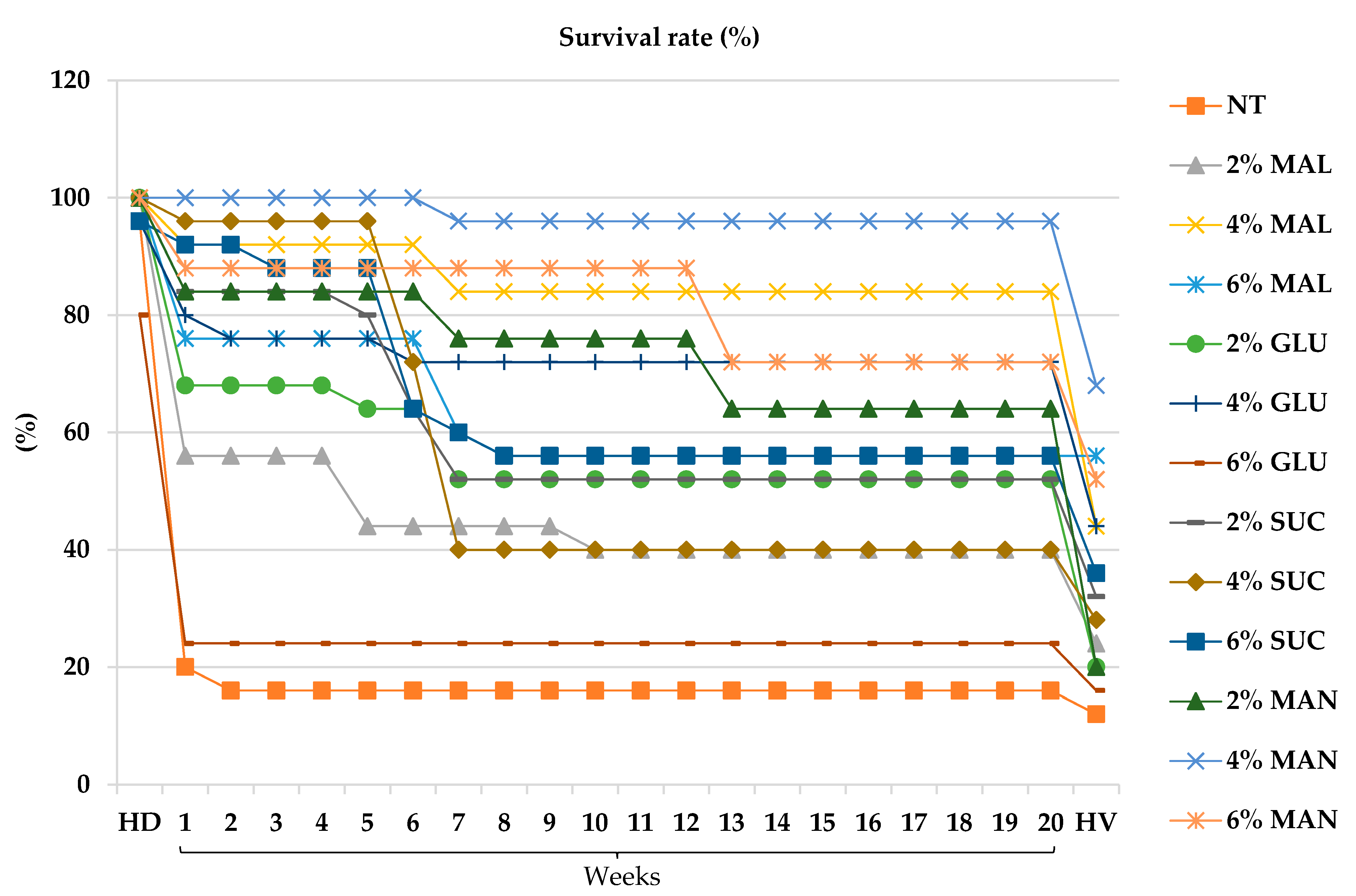

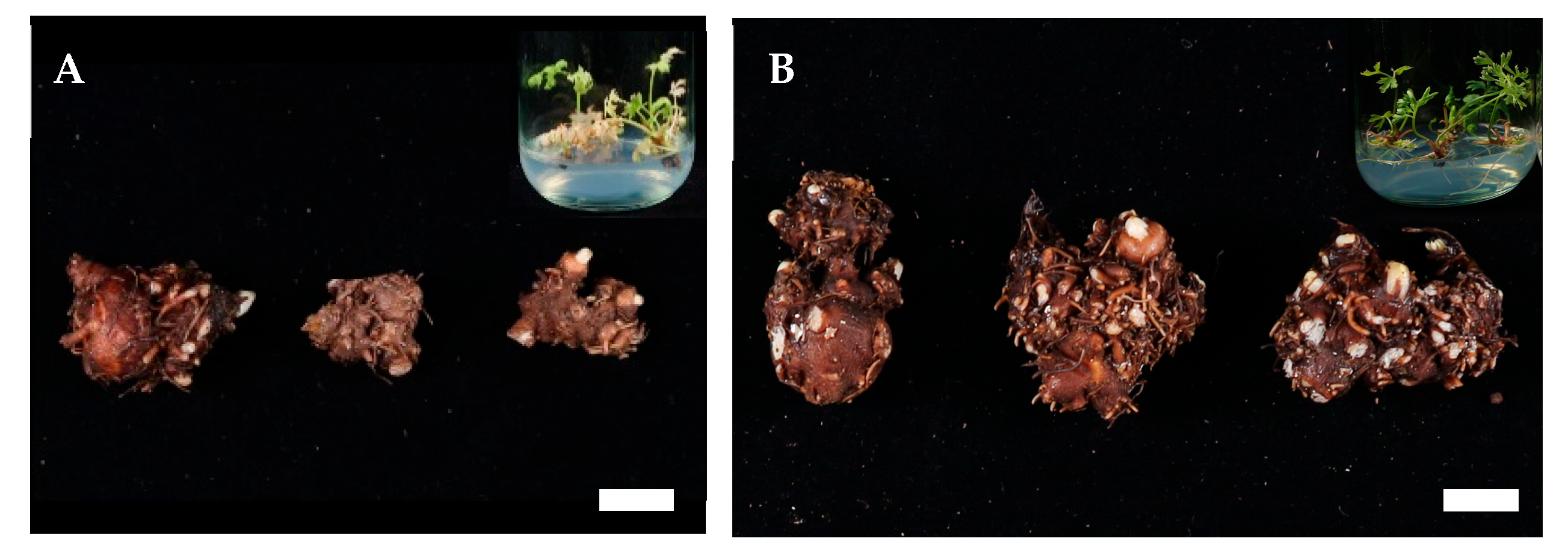


| Carbohydrate Type | Carbohydrate Conc. (%) | Shoot Length (cm) | No. of Roots/Explant | Root Length (cm) |
|---|---|---|---|---|
| Control | - | 2.44 ± 0.49 def | 1.96 ± 0.48 ghi | 1.56 ± 0.47 hi |
| Maltose | 2 | 4.64 ± 0.69 a | 8.28 ± 1.58 b | 8.07 ± 1.63 a |
| 4 | 3.08 ± 0.62 bc | 10.92 ± 1.24 a | 8.52 ± 2.47 a | |
| 6 | 2.15 ± 0.39 efgh | 5.48 ± 0.81 f | 4.75 ± 1.05 cd | |
| Glucose | 2 | 4.26 ± 0.45 a | 7.16 ± 0.82 cd | 6.90 ± 0.82 b |
| 4 | 1.98 ± 0.26 fghij | 2.24 ± 0.36 gh | 2.28 ± 0.66 gh | |
| 6 | 1.36 ± 0.10 klm | - | - | |
| Sucrose | 2 | 2.86 ± 0.63 cd | 7.60 ± 0.58 bc | 5.25 ± 0.46 c |
| 4 | 1.64 ± 0.22 hijk | 5.68 ± 0.70 ef | 3.75 ± 1.23 def | |
| 6 | 1.67 ± 0.41 ghijk | 2.00 ± 0.60 ghi | 3.39 ± 0.57 ef | |
| Mannose | 2 | 3.50 ± 0.51 b | 6.44 ± 0.82 de | 4.41 ± 1.04 cde |
| 4 | 2.06 ± 0.29 efghi | 2.32 ± 0.63 gh | 1.46 ± 0.29 hi | |
| 6 | 1.31 ± 0.13 klm | - | - | |
| Sorbitol | 2 | 2.18 ± 0.45 efg | 2.84 ± 0.67 g | 3.20 ± 0.51 fg |
| 4 | 1.17 ± 0.19 klm | 1.28 ± 0.39 ijk | 1.44 ± 0.29 hi | |
| 6 | 1.13 ± 0.09 klm | 1.04 ± 0.68 jkl | 1.00 ± 0.53 ij | |
| Mannitol | 2 | 1.49 ± 0.20 jkl | 1.80 ± 0.42 hij | 0.84 ± 0.39 ij |
| 4 | 1.02 ± 0.08 lm | 0.60 ± 0.32 klm | 0.26 ± 0.12 j | |
| 6 | 0.92 ± 0.17 mn | 0.24 ± 0.09 lm | 0.10 ± 0.02 j | |
| Fructose | 2 | 2.54 ± 0.46 de | - | - |
| 4 | 1.53 ± 0.21 ijkl | - | - | |
| 6 | 0.47 ± 0.10 n | - | - |
| Carbohydrate | Survival Rate (%) | Rhizome Characteristics | |||
|---|---|---|---|---|---|
| Type | Conc. (%) | Length (cm) | Diameter (cm) | Fresh Weight (g) | |
| Control | - | 12 | 1.8 ± 0.5 cde | 1.4 ± 0.2 abc | 0.7 ± 0.1 f |
| Maltose | 2 | 24 | 1.9 ± 0.1 cde | 1.9 ± 0.5 abc | 2.3 ± 0.4 bcd |
| 4 | 44 | 2.8 ± 0.8 ab | 1.7 ± 0.3 abc | 2.6 ± 0.6 b | |
| 6 | 56 | 2.0 ± 0.3 bcde | 1.9 ± 0.4 ab | 3.3 ± 0.2 a | |
| Glucose | 2 | 24 | 1.3 ± 0.2 e | 1.1 ± 0.1 c | 0.6 ± 0.1 f |
| 4 | 44 | 1.6 ± 0.4 de | 1.6 ± 0.8 abc | 1.1 ± 0.5 ef | |
| 6 | 16 | 1.8 ± 0.2 cde | 1.4 ± 0.2 abc | 1.0 ± 0.2 f | |
| Sucrose | 2 | 32 | 2.2 ± 0.4 bcd | 1.5 ± 0.6 abc | 1.7 ± 0.2 de |
| 4 | 28 | 2.5 ± 0.4 abc | 1.3 ± 0.4 bc | 1.9 ± 0.3 cd | |
| 6 | 36 | 2.1 ± 0.2 bcd | 2.0 ± 0.4 ab | 1.7 ± 0.1 de | |
| Mannose | 2 | 20 | 3.0 ± 0.3 a | 1.8 ± 0.2 abc | 3.2 ± 0.3 a |
| 4 | 68 | 2.0 ± 0.2 cde | 1.6 ± 0.2 abc | 2.4 ± 0.3 bc | |
| 6 | 52 | 2.5 ± 0.5 abc | 1.8 ± 0.3 abc | 2.1 ± 0.3 bcd | |
| Sorbitol | 2 | - | - | - | - |
| 4 | - | - | - | - | |
| 6 | - | - | - | - | |
| Mannitol | 2 | - | - | - | - |
| 4 | - | - | - | - | |
| 6 | - | - | - | - | |
| Fructose | 2 | 36 | 2.5 ± 0.3 abc | 1.6 ± 0.2 abc | 1.9 ± 0.3 cd |
| 4 | 28 | 2.1 ± 0.3 bcde | 2.1 ± 0.5 a | 1.9 ± 0.6 cd | |
| 6 | - | - | - | - | |
| Type | Conc. (%) | Survival Rate (%) | Plant Height | Fresh Weight (g) |
|---|---|---|---|---|
| Control | - | 100 | 28.7 ± 7.5 bc | 41.0 ± 15.2 cd |
| Maltose | 2 | 100 | 29.8 ± 0.9 bc | 88.5 ± 17.4 b |
| 4 | 100 | 30.6 ± 1.4 b | 61.7 ± 4.1 c | |
| 6 | 100 | 37.4 ± 1.6 a | 160.8 ± 22.2 a | |
| Glucose | 2 | 100 | 30.1 ± 0.8 bc | 30.1 ± 5.5 d |
| 4 | 100 | 26.3 ± 1.4 bc | 54.4 ± 24.7 cd | |
| 6 | 100 | 29.9 ± 1.2 bc | 42.1 ± 6.6 cd | |
| Sucrose | 2 | 100 | 18.7 ± 1.9 e | 46.1 ± 1.0 cd |
| 4 | 100 | 23.5 ± 2.7 cde | 49.8 ± 10.2 cd | |
| 6 | 100 | 19.6 ± 2.7 de | 62.6 ± 27.3 c | |
| Mannose | 2 | 100 | 25.1 ± 7.4 bcd | 53.3 ± 7.0 cd |
| 4 | 89 | 27.6 ± 5.4 bc | 58.6 ± 2.6 c | |
| 6 | 85 | 25.4 ± 0.4 bcd | 63.2 ± 15.3 c | |
| Sorbitol | 2 | - | - | - |
| 4 | - | - | - | |
| 6 | - | - | - | |
| Mannitol | 2 | - | - | - |
| 4 | - | - | - | |
| 6 | - | - | - | |
| Fructose | 2 | 89 | 26.6 ± 1.9 bc | 45.5 ± 2.5 cd |
| 4 | 86 | 29.9 ± 3.1 bc | 35.8 ± 12.9 cd | |
| 6 | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, H.-Y.; Son, H.-J.; Kang, J.-W.; Kim, J.-A. Optimizing Rhizome Quality in Ligusticum chuanxiong Hort. Through High Maltose Concentration. Plants 2025, 14, 3125. https://doi.org/10.3390/plants14203125
Jeong H-Y, Son H-J, Kang J-W, Kim J-A. Optimizing Rhizome Quality in Ligusticum chuanxiong Hort. Through High Maltose Concentration. Plants. 2025; 14(20):3125. https://doi.org/10.3390/plants14203125
Chicago/Turabian StyleJeong, Hui-Yeong, Ho-Jun Son, Jun-Won Kang, and Ji-Ah Kim. 2025. "Optimizing Rhizome Quality in Ligusticum chuanxiong Hort. Through High Maltose Concentration" Plants 14, no. 20: 3125. https://doi.org/10.3390/plants14203125
APA StyleJeong, H.-Y., Son, H.-J., Kang, J.-W., & Kim, J.-A. (2025). Optimizing Rhizome Quality in Ligusticum chuanxiong Hort. Through High Maltose Concentration. Plants, 14(20), 3125. https://doi.org/10.3390/plants14203125






