Alkaloid Profile, Anticholinesterase and Antioxidant Activities, and Sexual Propagation in Hieronymiella peruviana (Amaryllidaceae) †
Abstract
1. Introduction
2. Results and Discussion
2.1. Alkaloid Profile
2.2. Cholinesterase Inhibitory Activity
2.3. Total Phenolic, Flavonoid Contents and Antioxidant Activity
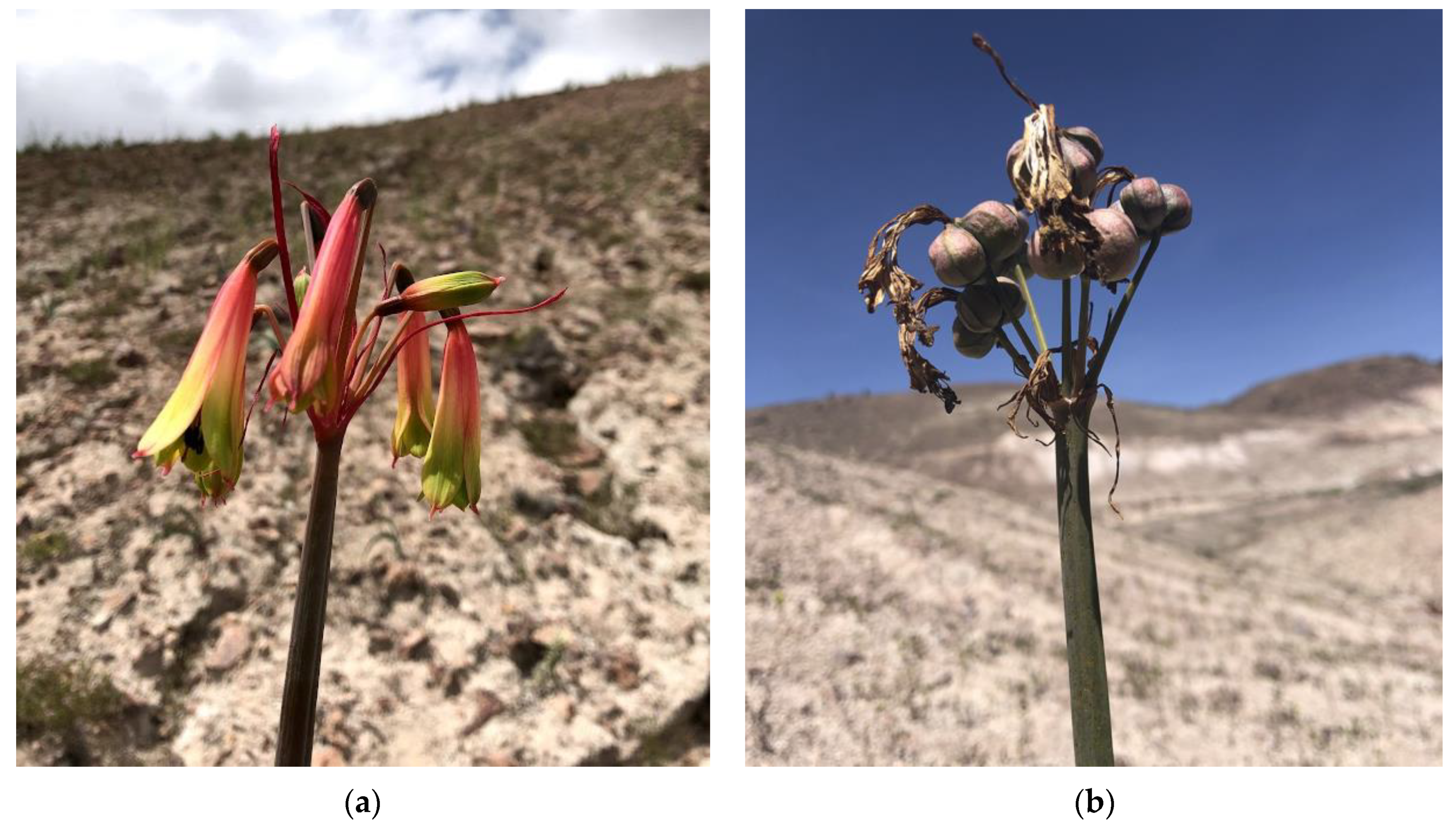
2.4. Seed Propagation
3. Materials and Methods
3.1. Chemicals
3.2. Plant Material and Procedure Extraction
3.3. Gas Chromatography Coupled to Mass Spectrometry (GC-MS) and Ultra Performance Liquid Chromatography (UPLC) Analysis
3.4. Cholinesterase Inhibitory Activities
3.5. Preliminary Toxicological Assessment
3.6. Assessment of Total Phenolic Content (TPC) and Total Flavonoid Content (TFC)
3.7. Antioxidant Assays
3.7.1. DPPH Radical Scavenging Activity
3.7.2. Ferric Reducing Antioxidant Property Assay (FRAP)
3.8. Germination and Seedling Survival
3.9. Statistical Data Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berkov, S.; Osorio, E.; Viladomat, F.; Bastida, J. Chemodiversity, Chemotaxonomy and Chemoecology of Amaryllidaceae Alkaloids. In Alkaloids: Chemistry and Biology; Elsevier Inc.: Amsterdam, The Netherlands, 2020; Volume 83, pp. 113–185. ISBN 9780128209813. [Google Scholar]
- Blotnick-Rubin, E.; Anglister, L. Fine Localization of Acetylcholinesterase in the Synaptic Cleft of the Vertebrate Neuromuscular Junction. Front. Mol. Neurosci. 2018, 11, 123. [Google Scholar] [CrossRef] [PubMed]
- Sattar, Y.; Adnan, M.; Bachu, R.; Patel, N.; Shrestha, S. Neuropharmacology of Alzheimer’s Disease and Dementia. Pharmacotherapy 2018, 106, 553–565. [Google Scholar] [CrossRef]
- Babashpour-Asl, M.; Kaboudi, P.S.; Barez, S.R. Therapeutic and Medicinal Effects of Snowdrop (Galanthus Spp.) in Alzheimer’s Disease: A Review. J. Educ. Health Promot. 2023, 12, 128. [Google Scholar] [CrossRef] [PubMed]
- Kilgore, M.B.; Augustin, M.M.; Starks, C.M.; O’Neil-Johnson, M.; May, G.D.; Crow, J.A.; Kutchan, T.M. Cloning and Characterization of a Norbelladine 4′-o-Methyltransferase Involved in the Biosynthesis of the Alzheimer’s Drug Galanthamine in Narcissus Sp. Aff. Pseudonarcissus. PLoS ONE 2014, 9, e103223. [Google Scholar] [CrossRef]
- Huaylla, H.; Slanis, A.C.; Llalla-Cordova, O. Hieronymiella Peruviana (Amaryllidaceae, Amaryllidoideae, Eustephieae): A New Species and First Record of the Genus for the Flora of Peru. Darwiniana 2024, 12, 149–155. [Google Scholar] [CrossRef]
- Ortiz, J.E.; Garro, A.; Pigni, N.B.; Agüero, M.B.; Roitman, G.; Slanis, A.; Enriz, R.D.; Feresin, G.E.; Bastida, J.; Tapia, A. Cholinesterase-Inhibitory Effect and in Silico Analysis of Alkaloids from Bulbs of Hieronymiella Species. Phytomedicine 2018, 39, 66–74. [Google Scholar] [CrossRef]
- Ortiz, J.E.; Piñeiro, M.; Martinez-Peinado, N.; Barrera, P.; Sosa, M.; Bastida, J.; Alonso-Padilla, J.; Feresin, G.E. Candimine from Hippeastrum Escoipense (Amaryllidaceae): Anti-Trypanosoma Cruzi Activity and Synergistic Effect with Benznidazole. Phytomedicine 2023, 114, 154788. [Google Scholar] [CrossRef]
- Ibrakaw, A.S.; Akinfenwa, A.O.; Hussein, A.A. A Comprehensive Review of Non-Alkaloidal Metabolites from the Subfamily Amaryllidoideae (Amaryllidaceae). Open Chem. 2023, 21, 20220252. [Google Scholar] [CrossRef]
- Zaragoza-Puchol, D.; Ortiz, J.E.; Orden, A.A.; Sanchez, M.; Palermo, J.; Tapia, A.; Bastida, J.; Feresin, G.E. Alkaloids Analysis of Habranthus Cardenasianus (Amaryllidaceae), Anti-Cholinesterase Activity and Biomass Production by Propagation Strategies. Molecules 2021, 26, 192. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, And, Evolution of Dormancy and Germination; Elsevier Science: Amsterdam, The Netherlands, 2001; ISBN 9780120802630. [Google Scholar]
- Berkov, S.; Denev, R.; Sidjimova, B.; Zarev, Y.; Shkondrov, A.; Torras-Claveria, L.; Viladomat, F.; Bastida, J. Gas Chromatography–Mass Spectrometry of Some Homolycorine-Type Amaryllidaceae Alkaloids. Rapid Commun. Mass Spectrom. 2023, 37, e9506. [Google Scholar] [CrossRef]
- Katoch, D.; Sharma, U. Simultaneous Quantification and Identification of Amaryllidaceae Alkaloids in Narcissus Tazetta by Ultra Performance Liquid Chromatography-Diode Array Detector-Electrospray Ionisation Tandem Mass Spectrometry. J. Pharm. Biomed. Anal. 2019, 175, 112750. [Google Scholar] [CrossRef] [PubMed]
- Torras-Claveria, L.; Berkov, S.; Viladomat, F.; Bastida, J. QToF Exact Mass and ESI Fragmentation of Bioactive Amaryllidaceae Alkaloids. S. Afr. J. Bot. 2021, 136, 81–90. [Google Scholar] [CrossRef]
- Kong, C.K.; Low, L.E.; Siew, W.S.; Yap, W.H.; Khaw, K.Y.; Ming, L.C.; Mocan, A.; Goh, B.H.; Goh, P.H. Biological Activities of Snowdrop (Galanthus Spp., Family Amaryllidaceae). Front. Pharmacol. 2021, 11, 552453. [Google Scholar] [CrossRef] [PubMed]
- Berkov, S.; Atanasova, M.; Georgiev, B.; Bastida, J.; Doytchinova, I. The Amaryllidaceae Alkaloids: An Untapped Source of Acetylcholinesterase Inhibitors. Phytochem. Rev. 2022, 21, 1415–1443. [Google Scholar] [CrossRef]
- Khalifa, M.; Attia, E.; Fahim, J.; Kamel, M. An Overview on the Chemical and Biological Aspects of Lycorine Alkaloid. J. Adv. Biomed. Pharm. Sci. 2018, 1, 41–49. [Google Scholar] [CrossRef]
- Castañeda, C.; Bravo, K.; Cortés, N.; Bedoya, J.; de S. Borges, W.; Bastida, J.; Osorio, E. Amaryllidaceae Alkaloids in Skin Cancer Management: Photoprotective Effect on Human Keratinocytes and Anti-Proliferative Activity in Melanoma Cells. J. Appl. Biomed. 2023, 21, 36–47. [Google Scholar] [CrossRef]
- Evidente, A. Advances on the Amaryllidacea Alkaloids Collected in South Africa, Andean South America and the Mediterranean Basin. Molecules 2023, 28, 4055. [Google Scholar] [CrossRef]
- Rojas-Vera, J.D.C.; Buitrago-Díaz, A.A.; Possamai, L.M.; Timmers, L.F.S.M.; Tallini, L.R.; Bastida, J. Alkaloid Profile and Cholinesterase Inhibition Activity of Five Species of Amaryllidaceae Family Collected from Mérida State-Venezuela. S. Afr. J. Bot. 2020, 136, 126–136. [Google Scholar] [CrossRef]
- Karimi, E.; Mehrabanjoubani, P.; Homayouni-Tabrizi, M.; Abdolzadeh, A.; Soltani, M. Phytochemical Evaluation, Antioxidant Properties and Antibacterial Activity of Iranian Medicinal Herb Galanthus Transcaucasicus Fomin. J. Food Meas. Charact. 2018, 12, 433–440. [Google Scholar] [CrossRef]
- Boshra, Y.R.; Attia, E.Z.; Darwish, A.G.; Boshra, M.R.; Amin, M.N.; Hamed, A.N.E.; Desoukey, S.Y.; Fahim, J.R. Narcissus Pseudonarcissus L. (Amaryllidaceae) Bulbs Metabolite Profiling and Biological Activities. S. Afr. J. Bot. 2023, 160, 633–644. [Google Scholar] [CrossRef]
- Yagi, S.; Nilofar; Zengin, G.; Yildiztugay, E.; Caprioli, G.; Piatti, D.; Menghini, L.; Ferrante, C.; Di Simone, S.C.; Chiavaroli, A.; et al. Exploring for HPLC-MS/MS Profiles and Biological Activities of Different Extracts from Allium Lycaonicum Siehe Ex Hayek from Turkey Flora. Foods 2023, 12, 4507. [Google Scholar] [CrossRef] [PubMed]
- Leporini, M.; Catinella, G.; Bruno, M.; Falco, T.; Tundis, R.; Loizzo, M.R. Investigating the Antiproliferative and Antioxidant Properties of Pancratium Maritimum L. (Amaryllidaceae) Stems, Flowers, Bulbs, and Fruits Extracts. Evid.-Based Complement. Altern. Med. 2018, 2018, 9301247. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, M.F.; Fahim, J.R.; Allam, A.E.; Shoman, M.E.; El Zawily, A.; Kamel, M.S.; Shimizu, K.; Attia, E.Z. Studies on the Nonalkaloidal Secondary Metabolites of Hippeastrum Vittatum (L’ Her.) Herb. Bulbs. ACS Omega 2023, 8, 26749–26761. [Google Scholar] [CrossRef] [PubMed]
- Cortes, N.; Posada-Duque, R.; Cardona-Gómez, G.P.; Bastida, J.; Osorio, E. Amaryllidaceae Alkaloids and Neuronal Cell Protection. In Pathology Oxidative Stress and Dietary Antioxidants; Academic Press: Cambridge, MA, USA, 2020; pp. 135–144. [Google Scholar] [CrossRef]
- Santa Cruz, R.; Tapia, A.; Romero, A.; Quiroga, A. Propagación Por Semilla de Zephyranthes Mesochloa, Especie Nativa Con Potencial Ornamental. Biol. Agron. 2011, 1, 17–23. [Google Scholar]
- Acosta, M.C.; Alcaraz, M.L.; Scaramuzzino, R.L.; Manfreda, V.T. Fisiología de La Germinación de Rhodophiala Bifida. FAVE—Cienc. Agrar. 2021, 19, 159–173. [Google Scholar] [CrossRef]
- Herranz, J.M.; Copete, E.; Copete, M.Á.; Ferrandis, P. Germination Ecology of the Endemic Iberian Daffodil Narcissus Radinganorum (Amaryllidaceae). Dormancy Induction by Cold Stratification or Desiccation in Late Stages of Embryo Growth. For. Syst. 2015, 24, e013. [Google Scholar] [CrossRef][Green Version]
- Oliveira, A.C.S.; Martins, G.N.; Silva, R.F.; Vieira, H.D. Testes de Vigor Em Sementes Baseados No Desempenho de Plântulas. Rev. Cientifica Internac. 2009, 1, 1–21. [Google Scholar]
- Salazar, C.; Landeros, F.; Bustos, E.; Bravo, P.; Pérez, C.; Becerra, J.; Ríos, D.; Uribe, M. Propagation and Bulblet Enhancement of Rhodophiala Pratensis from Seeds Germinated in Vitro. Cienc. Investig. Agrar. 2019, 46, 12–22. [Google Scholar] [CrossRef]
- Pérez-Hernández, I.; Ochoa-Gaona, S.; Vargas-Simón, G.; Mendoza-Carranza, M.; González-Valdivia, N.A. Germination and Survival of Six Native Species in a Tropical Forest of Tabasco, México. Madera Bosques 2011, 17, 71–91. [Google Scholar] [CrossRef]
- Rodríguez-Escobar, M.L.; Tallini, L.R.; Lisa-Molina, J.; Berkov, S.; Viladomat, F.; Meerow, A.; Bastida, J.; Torras-Claveria, L. Chemical and Biological Aspects of Different Species of the Genus Clinanthus Herb. (Amaryllidaceae) from South America. Molecules 2023, 28, 5408. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.J.; Feather-Stone, R.M. A New and Rapid Colorimetric Determination of Acetylcholinesterase Activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.; Kemmler, E.; Dunkel, M.; Preissner, R. ProTox 3.0: A Webserver for the Prediction of Toxicity of Chemicals. Nucleic Acids Res 2024, 52, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Helrich, K. Official Methods of Analysis of the Association of Official Analytical Chemists; Association of Official Analytical Chemists: Rockville, MD, USA, 1990. [Google Scholar]
- Ismail, H.I.; Chan, K.W.; Mariod, A.A.; Ismail, M. Phenolic Content and Antioxidant Activity of Cantaloupe (Cucumis Melo) Methanolic Extracts. Food Chem. 2010, 119, 643–647. [Google Scholar] [CrossRef]
- Luna, L.C.; Pigni, N.B.; Torras-Claveria, L.; Monferran, M.V.; Maestri, D.; Wunderlin, D.A.; Feresin, G.E.; Bastida, J.; Tapia, A. Ramorinoa Girolae Speg (Fabaceae) Seeds, an Argentinean Traditional Indigenous Food: Nutrient Composition and Antioxidant Activity. J. Food Compos. Anal. 2013, 31, 120–128. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Luna, L.; Simirgiotis, M.J.; Lima, B.; Bórquez, J.; Feresin, G.E.; Tapia, A. UHPLC-MS Metabolome Fingerprinting: The Isolation of Main Compounds and Antioxidant Activity of the Andean Species Tetraglochin Ameghinoi (Speg.) Speg. Molecules 2018, 23, 793. [Google Scholar] [CrossRef]
- Maguire, J.D. Speed of Germination—Aid In Selection And Evaluation for Seedling Emergence And Vigor 1. Crop Sci. 1962, 2, 176–177. [Google Scholar] [CrossRef]
- Ferreira, A.G. Interpretação de Resultados de Germinação. In Germinação: Do Básico ao Aplicado; Artmed: Porto Alegre, Brazil, 2014; pp. 209–222. [Google Scholar]
- Farooq, M.; Basra, S.M.A.; Ahmad, N.; Hafeez, K. Thermal Hardening: A New Seed Vigor Enhancement Tool in Rice. J. Integr. Plant Biol. 2005, 47, 187–193. [Google Scholar] [CrossRef]
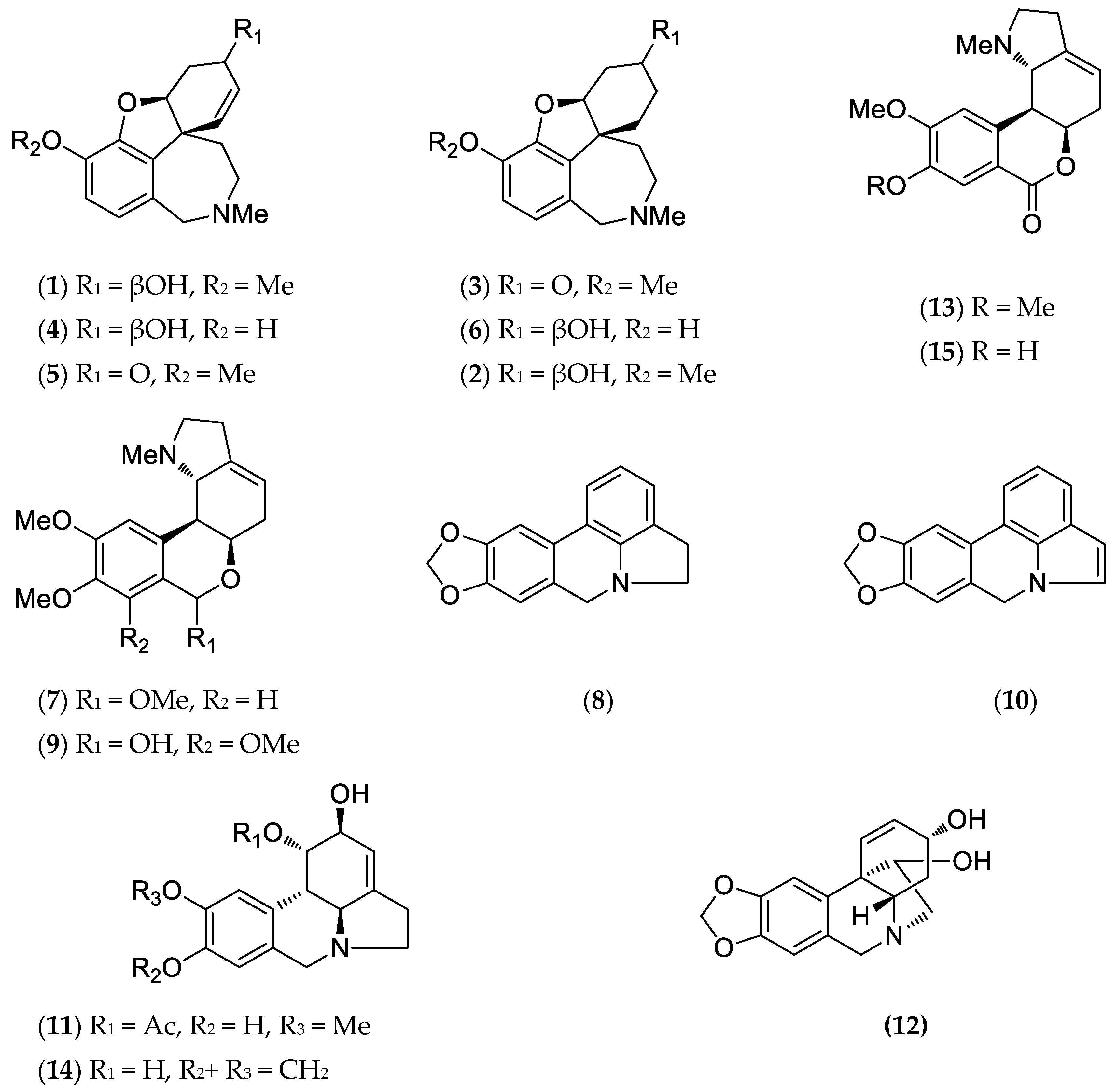
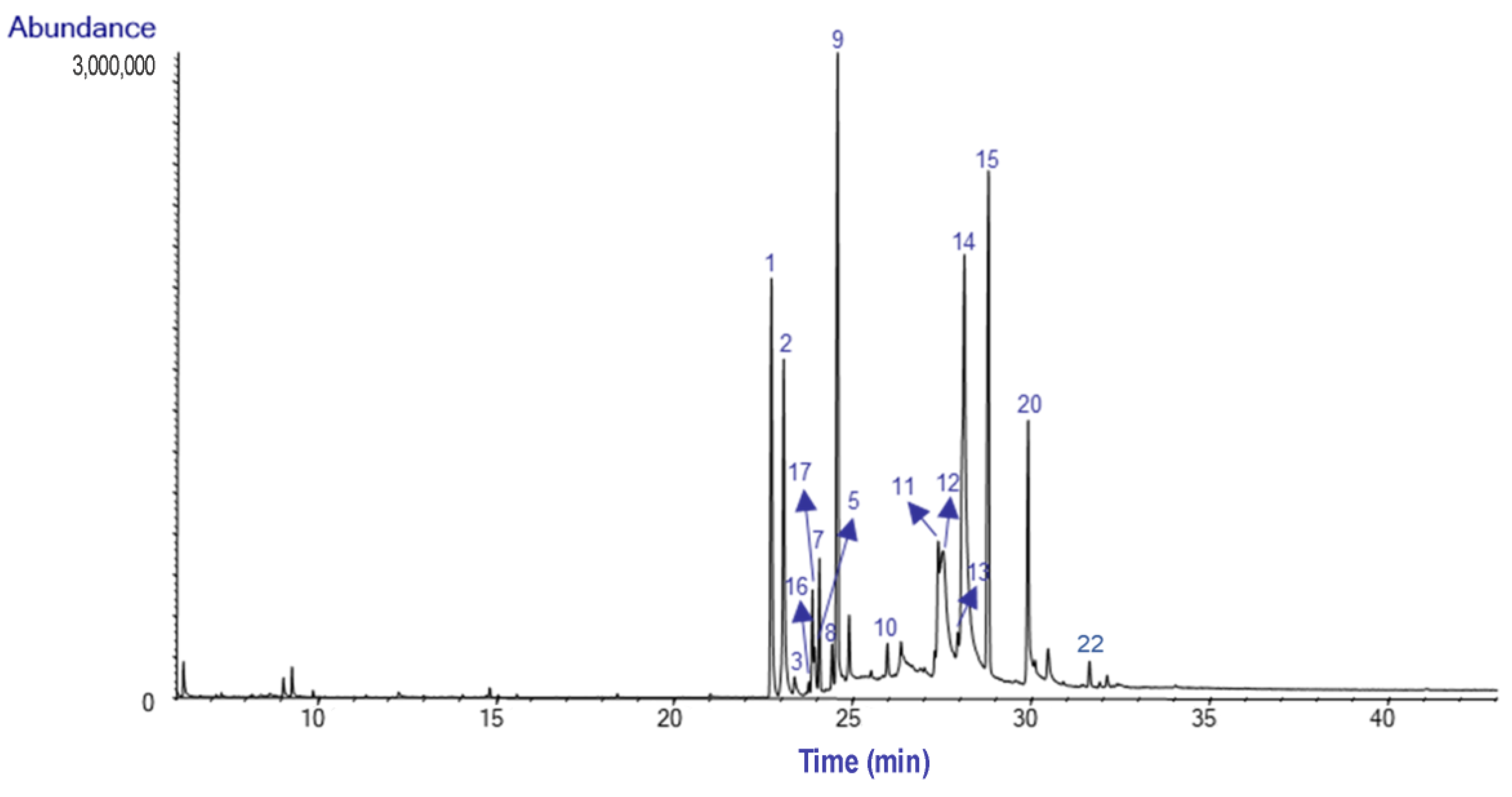
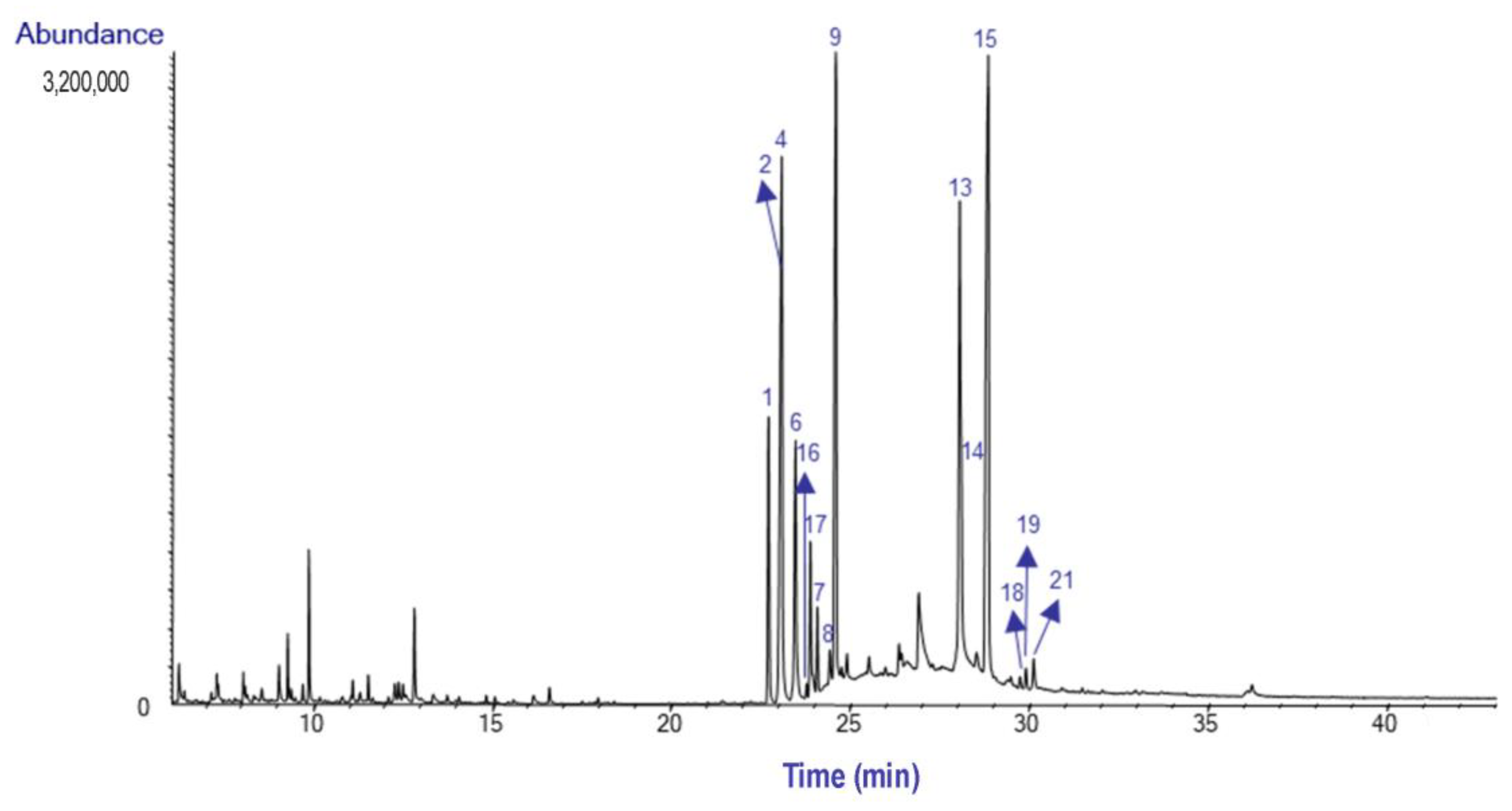


| Alkaloids (Alkaloid-Type) | M+ | RI | Bulbs | Leaves | |
|---|---|---|---|---|---|
| (1) | Galanthamine (Gal-T) | 287 | 2397.6 | 10.0 | 5.6 |
| (2) | Lycoramine (Gal-T) | 289 | 2419.6 | 9.8 | 12.3 |
| (3) | Lycoraminone (Gal-T) | 287 | 2439.2 | 0.3 | - |
| (4) | Sanguinine (Gal-T) | 273 | 2420.9 | - | 14.9 |
| (5) | Narwedine (Gal-T) | 285 | 2475.7 | 0.8 | - |
| (6) | O-Demethyllycoramine (Gal-T) | 275 | 2477.4 | - | 6.4 |
| (7) | O-Methyllycorenine (Hom-T) | 331 | 2484.1 | 2.4 | 1.3 |
| (8) | Anhydrolycorine (Lyc-T) | 251 | 2507.2 | 1.1 | 0.7 |
| (9) | Nerinine (Hom-T) | 347 | 2517.6 | 19.9 | 17.6 |
| (10) | 11,12-dehydroanhydrolycorine (Lyc-T) | 249 | 2610.4 | 0.9 | - |
| (11) | Sternbergine (Lyc-T) | 331 | 2707.7 | 3.2 | - |
| (12) | Hamayne (Hae-T) | 331 | 2715.1 | 1.1 | - |
| (13) | Homolycorine (Hom-T) | 315 | 2755.1 | 2.0 | 8.3 |
| (14) | Lycorine (Lyc-T) | 287 | 2759.1 | 21.1 | 4.6 |
| (15) | 8-O-Demethylhomolycorine (Hom-T) | 301 | 2838.2 | 16.9 | 24.4 |
| (16) | Not identified (Hom-T*a) ‘ | 301*b | 2464.8 | 0.2 | 0.2 |
| (17) | Not identified (Hom-T*a) ‘ | 329*b | 2471.6 | 2.0 | 2.8 |
| (18) | Not identified (Hom-T*a) ‘ | 345*b | 2850.4 | - | 0.2 |
| (19) | Not identified | 281*b | 2865.3 | - | 0.4 |
| (20) | Not identified | 279*b | 2875.4 | 7.7 | - |
| (21) | Not identified | 343*b | 2876.2 | - | 0.4 |
| (22) | Not identified | 293*b | 3017.8 | 0.6 | - |
| TPC a (mg GAE/g) | TFC b (mg QE/g) | DPPH c (%) | FRAP d (mg TE/g) |
|---|---|---|---|
| 12.89 ± 0.27 | 0.28 ± 0.03 | 16.36 ± 0.20 | 58.31 ± 13.70 |
| Temperature | GP (%) | GE (%) | GSI | MGT (days) | T50 (days) |
|---|---|---|---|---|---|
| 15 °C | 92 ± 10.4 a | 65 ± 12.7 b | 3.88 ± 0.55 b | 5.13 ± 0.28 b | 4.31 ± 0.32 b |
| 20 °C | 94 ± 5.48 a | 92 ± 5.70 a | 5.61 ± 0.29 a | 3.55 ± 0.21 c | 2.95 ± 0.22 bc |
| 25 °C | 93 ± 5.70 a | 88 ± 5.70 a | 5.97 ± 0.42 a | 3.41 ± 0.18 c | 2.71 ± 0.13 c |
| 30 °C | 82 ± 4.47 a | 39 ± 8.94 c | 3.19 ± 0.22 b | 6.67 ± 1.03 a | 5.92 ± 1.63 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Llalla-Cordova, O.; Ortiz, J.E.; Tallini, L.R.; Torras-Claveria, L.; Bastida, J.; Luna, L.C.; Feresin, G.E. Alkaloid Profile, Anticholinesterase and Antioxidant Activities, and Sexual Propagation in Hieronymiella peruviana (Amaryllidaceae). Plants 2025, 14, 281. https://doi.org/10.3390/plants14020281
Llalla-Cordova O, Ortiz JE, Tallini LR, Torras-Claveria L, Bastida J, Luna LC, Feresin GE. Alkaloid Profile, Anticholinesterase and Antioxidant Activities, and Sexual Propagation in Hieronymiella peruviana (Amaryllidaceae). Plants. 2025; 14(2):281. https://doi.org/10.3390/plants14020281
Chicago/Turabian StyleLlalla-Cordova, Olimpia, Javier E. Ortiz, Luciana R. Tallini, Laura Torras-Claveria, Jaume Bastida, Lorena Celina Luna, and Gabriela E. Feresin. 2025. "Alkaloid Profile, Anticholinesterase and Antioxidant Activities, and Sexual Propagation in Hieronymiella peruviana (Amaryllidaceae)" Plants 14, no. 2: 281. https://doi.org/10.3390/plants14020281
APA StyleLlalla-Cordova, O., Ortiz, J. E., Tallini, L. R., Torras-Claveria, L., Bastida, J., Luna, L. C., & Feresin, G. E. (2025). Alkaloid Profile, Anticholinesterase and Antioxidant Activities, and Sexual Propagation in Hieronymiella peruviana (Amaryllidaceae). Plants, 14(2), 281. https://doi.org/10.3390/plants14020281










