2.1. Leaf Gas Exchange and Chlorophyll a Fluorescence
All leaf gas exchange variables showed a significant effect for the interaction between treatments and lettuce cultivars (
p ≤ 0.001) (
Table 1). Saline stress (4.0 dS m
−1) decreased the stomatal conductance (
gs) of lettuce cultivars by 26.67%, 35.56%, and 32.56% for SVR 2005, Simpson and Grand Rapids, respectively, when compared to the control (0.53 dS m
−1) (
Table 1). In SVR 2005, exogenous application of ascorbic acid (ASC) and salicylic acid (SA) increased stomatal conductance by 22.72% and 31.82%, respectively, compared to salt stress. In Simpson, exogenous application of ASC and gibberellic acid (GA
3) increased stomatal conductance by 10.34% and 27.59%, respectively, relative to salt stress. For the cultivar Grand Rapids, all organic acids improved stomatal conductance in relation to salt stress, with increases of 17.24%, 10.34%, and 27.59%, respectively (
Table 1). Under saline stress condition, the lowest
gs value (0.22 mol (H
2O) m
−2 s
−1) was verified in cultivar SVR 2005.
Transpiration (
E) was decreased by 20.06%, 25.58%, and 26.25% in the treatment with saline stress (4.0 dS m
−1) for SVR 2005, Simpson and Grand Rapids, respectively, when compared to the control (0.53 dS m
−1) (
Table 1). In SVR 2005, exogenous application of ASC and SA increased
E by 18.92% and 27.03%, respectively, relative to saline stress. In Simpson, exogenous applications of ASC, GA
3, and SA increased transpiration by 11.34%, 9.62%, and 13.40% compared to saline stress treatment. For Grand Rapids, the organic acids that improved transpiration in relation to saline stress were ASC and SA, with increases of 26.69% and 28.83%, respectively (
Table 1). Under saline stress condition, the lowest
E value was verified in cultivar SVR 2005 and the highest
E value in cultivar Simpson (
Table 1).
Saline stress decreased the internal concentration of CO
2 (
Ci) in the cultivar Simpson, in relation to the control (0.53 dS m
−1), with decrease of 13.43% (
Table 1). Different behavior was observed in the cultivars SVR 2005 and Grand Rapids, not differing between the treatment under saline stress and control. For the Simpson cultivar, all organic acids (ASC, GA
3, and SA) increased
Ci by 15.95%, 6.47%, and 9.05% in relation to saline stress treatment, respectively (
Table 1). Under saline stress condition, the lowest
Ci value was verified in the Simpson cultivar and the highest in the SVR 2005 cultivar (
Table 1).
The assimilation rate of CO
2 (
AN) of the cultivars SVR 2005, Simpson, and Grand Rapids was decreased under saline stress (4.0 dS m
−1), 19.47%, 11,10%, and 18.37%, respectively, in relation to the control. In SVR 2005, exogenous application of ASC and SA increased
AN by 25.94% and 25.02%, respectively, compared to saline stress. In Simpson, the application of organic acids did not increase the assimilation rate of CO
2. For Grand Rapids, all organic acids improved the assimilation rate of CO
2 in relation to saline stress, with increases of 19.21% (ASC), 17.59% (GA
3), and 21.66% (SA) (
Table 1). Under saline stress conditions, the lowest
AN was found in the SVR 2005 cultivar and the highest in Simpson (
Table 1).
Saline stress increased the instantaneous water use efficiency (
WUE) of lettuce cultivars Simpson and Grand Rapids by 19.41% and 10.61%, respectively, when compared to the control (
Table 1). The application of GA
3 increased 14.14% in relation to saline stress in the cultivar Grands Rapids. Under saline stress condition, the lowest
WUE was verified in SVR 2005 cultivar and the highest in Simpson (
Table 1).
There was a decrease in instantaneous carboxylation efficiency (
AN/
Ci) in SVR 2005 and Grand Rapids cultivars of 20.63% and 14.10%, respectively, under saline stress condition (4.0 dS m
−1), compared to the control (0.53 dS m
−1). In lettuce cultivar SVR 2005, exogenous application of organic acids ASC and SA increased about 38.00% and 32.00%, respectively. For Grand Rapids, all organic acids improved instantaneous carboxylation efficiency compared to saline stress, with increases of 26.87% (ASC), 26.87% (GA
3), and 25.37% (SA) (
Table 1). Under saline stress conditions, the lowest
AN/
Ci was observed in the SVR 2005 cultivar and the highest in Simpson (
Table 1).
There was an isolated effect (
p ≤ 0.05) of lettuce cultivar factors and treatments for maximum PSII quantum efficiency (
Fv/
Fm) (
Table 2). There was an isolated effect of treatments for quantum efficiency of PSII (
Y) (
p ≤ 0.001), photochemical extinction coefficient (
qL) (
p ≤ 0.05), regulated photochemical extinction quantum yield (
YNPQ) (
p ≤ 0.001), and unregulated photochemical extinction quantum yield (
YNO) (
p ≤ 0.001) (
Table 2). Minimum fluorescence of illuminated plant tissue (
Fo’) was observed as an isolated effect of cultivar factor (
p ≤ 0.05) (
Table 2).
The maximum PSII quantum efficiency (
Fv/
Fm) showed a significant difference by the mean test only for the treatments factor, with a difference only between the application of gibberellic and salicylic acids under saline stress, with SA being only 2.09% higher than AG
3 (
Table 2). There was a decrease in PSII quantum efficiency (
Y) in the treatments with gibberellic acids of 24.25%, compared to the control treatment (0.53 dS m
−1) (
Table 2). In relation to saline stress (4.0 dS m
−1), there was only a decrease in
Y (16.64%) when gibberellic acid was used. Regarding minimum fluorescence of illuminated plant tissue (
Fo’), there was only a difference between the control treatment and the treatment under saline stress, with an increase in
Fo’ of 24.17% under saline stress (
Table 2).
The photochemical extinction coefficient (
qL) showed a difference between the treatment under saline stress and the treatment with the application of gibberellic acid. There was a decrease in
qL of about 40.91% in the treatment under GA
3 application compared to saline stress (
Table 2). The regulated photochemical extinction quantum yield (
YNPQ) showed an increase of 26.69% in the treatment under saline stress compared to the control. Among the treatments under saline stress, the highest
YNPQ was found in the treatment with AG
3, with an increase of 25.79% compared to saline stress (
Table 2). The unregulated photochemical extinction quantum yield (
YNO) was highest in the treatment under AG
3 application, with an increase of 45.83% and 55.55% compared to the control and to the treatment under saline stress, respectively (
Table 2).
The interaction between treatments and lettuce cultivars was significant for electron transport rate (
ETR) and leaf temperature (
Tl) (
p ≤ 0.001) (
Table 3).
There was an increase in electron transport rate (
ETR) of 184.91%, 148.35%, and 161.27% for lettuce cultivars SVR 2005, Simpson, and Grand Rapids, respectively, in the treatment with saline stress, when compared to the control (
Table 3). The application of salicylic acid caused a higher
ETR in the Simpson cultivar (63.42), followed by SVR 2005 (39.60) and Grand Rapids (25.56) (
Table 3).
Saline stress decreased leaf temperature (
Tl) by 2.48%, 3.04%, and 1.65% in SVR 2005, Simpson, and Grand Rapids lettuce cultivars, respectively, compared to the control (
Table 3). In SVR 2005, exogenous applications of ASC, GA
3, and SA increased leaf temperature by 3.09%, 3.33%, and 3.51% relative to saline stress. In Simpson, exogenous applications of ASC, GA
3, and SA increased leaf temperature by 0.72%, 2.29%, and 4.41% compared to saline stress. Grand Rapids followed the same trend: the ASC, GA
3, and SA increased
Tl relative to saline stress, with increases of 2.93%, 0.78%, and 1.50%, respectively (
Table 3).
2.2. Production, Biomass, Tissue Water Content and Leaf Concentration of K+ and Na+
The interaction between treatments and lettuce cultivars was significant for production (P) and number of leaves (
NL) (
p ≤ 0.001), and for shoot dry mass (
SDM) (
p ≤ 0.05) (
Table 4).
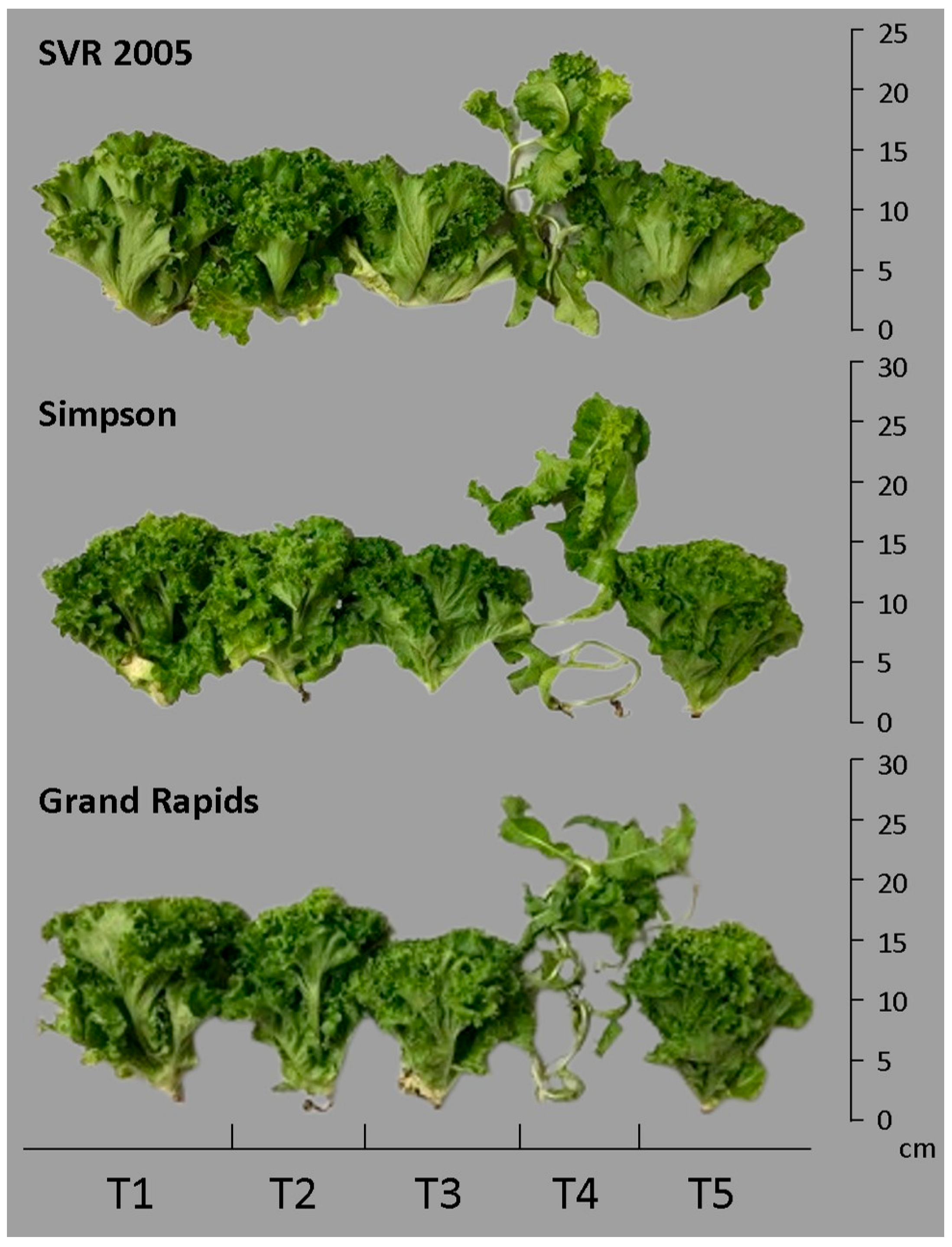
Lettuce cultivars that received applications of ascorbic and salicylic acids showed normal growth, but gibberellic acid application caused disturbed growth of lettuce plants (
Figure 1). Production (
PROD) decreased by 29.53% and 34.93% for cultivars SVR 2005 and Simpson, respectively, under saline stress of 4.0 dS m
−1 and compared to the control (
Table 4). In the Simpson cultivar, the application of SA increased production by 24.85%, compared to saline stress, whereas in the cultivars SVR 2005 and Grand Rapids there was no increase by the application of organic acids (
Table 4 and
Figure 1). Under saline stress conditions, the lowest production was observed in the SVR 2005 and Simpson cultivars and the highest in the Grand Rapids cultivar (
Table 4).
Saline stress decreased the number of leaves (
NL) in the Simpson cultivar by 39.23% compared to the control. In SVR 2005 and Grand Rapids, there were no differences between control and saline stress. In Simpson, ASC and SA increased
NL by 27.85% and 37.97%, respectively, compared to saline stress. In Grand Rapids, ASC caused a decrease in
NL of 16.50% compared to the saline stress treatment (
Figure 1). The treatment with ASC showed the highest
NL value (20.20) in the Simpson cultivar, followed by Grand Rapids (17.20) and SVR 2005 (14.60) (
Table 4).
There was no statistical difference in shoot dry mass (
SDM) when comparing saline stress and control for the three cultivars investigated. The application of AG
3 to lettuce cultivars caused a decrease in
SDM in all cultivars compared to saline stress, with a decrease of 50.11%, 61.52%, and 37.86% for SVR 2005, Simpson, and Grand Rapids, respectively (
Table 4). The lowest
SDM in the treatment of saline stress was found in cultivar SVR 2005, with Simpson and Grand Rapids higher, but not statistically different.
There was an isolated effect for the factors treatments and cultivars for tissue water content (TWC) (
p ≤ 0.001) (
Table 5). There was no significant effect for the potassium K
+ content (
p > 0.05), there was an isolated effect of treatments (
p ≤ 0.001) and cultivars (
p ≤ 0.05) on leaf Na
+ concentration, and, for the sodium/potassium ratio (Na
+/K
+), there was an isolated effect of treatments (
p ≤ 0.001) (
Table 5).
The application of ascorbic and gibberellic acid caused a decrease of 1.55% and 1.27%, respectively, in TWC, when compared to the control (
Table 5). The cultivar SVR 2005 showed higher TWC compared to the cultivars Simpson and Grand Rapids.
Saline stress increased Na
+ by 121.86% in relation to the control, and in the treatments with the application of organic acids there was no significant difference in relation to saline stress. Among the cultivars, the cultivar Simpson concentrated more sodium than the cultivar Grand Rapids, but neither differed from SVR 2005. In the Na
+/K
+ ratio, there was an increase in saline stress of 107.69% compared to the control (
Table 5).