(aS)-Glucosciadopitysin, a New Biflavonoid Glycoside from the Leaves of Ginkgo biloba and Osteogenic Activity of Bioflavonoids
Abstract
1. Introduction
2. Results and Discussion
2.1. Isolation of Compounds from the Leaves of G. biloba
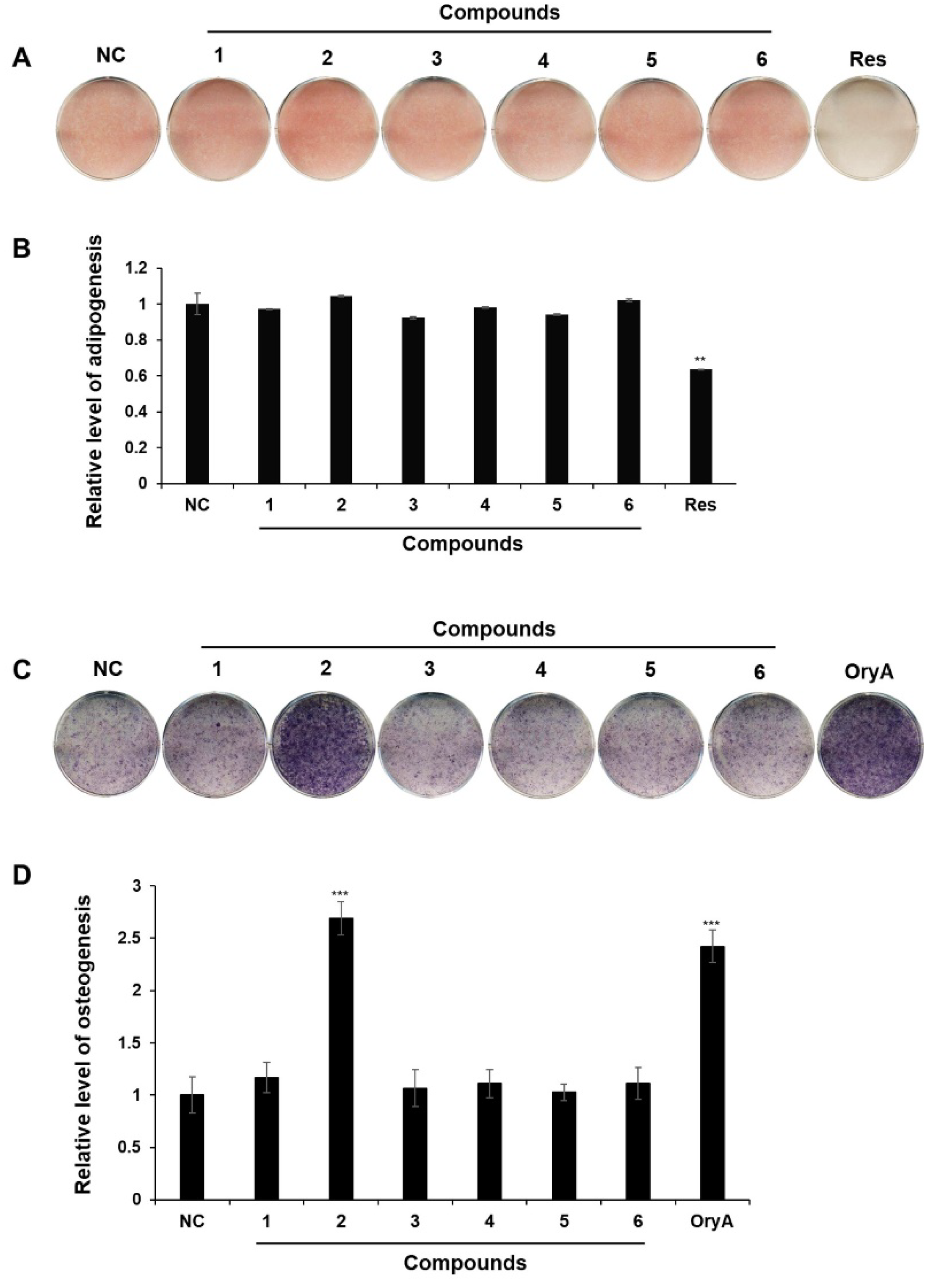
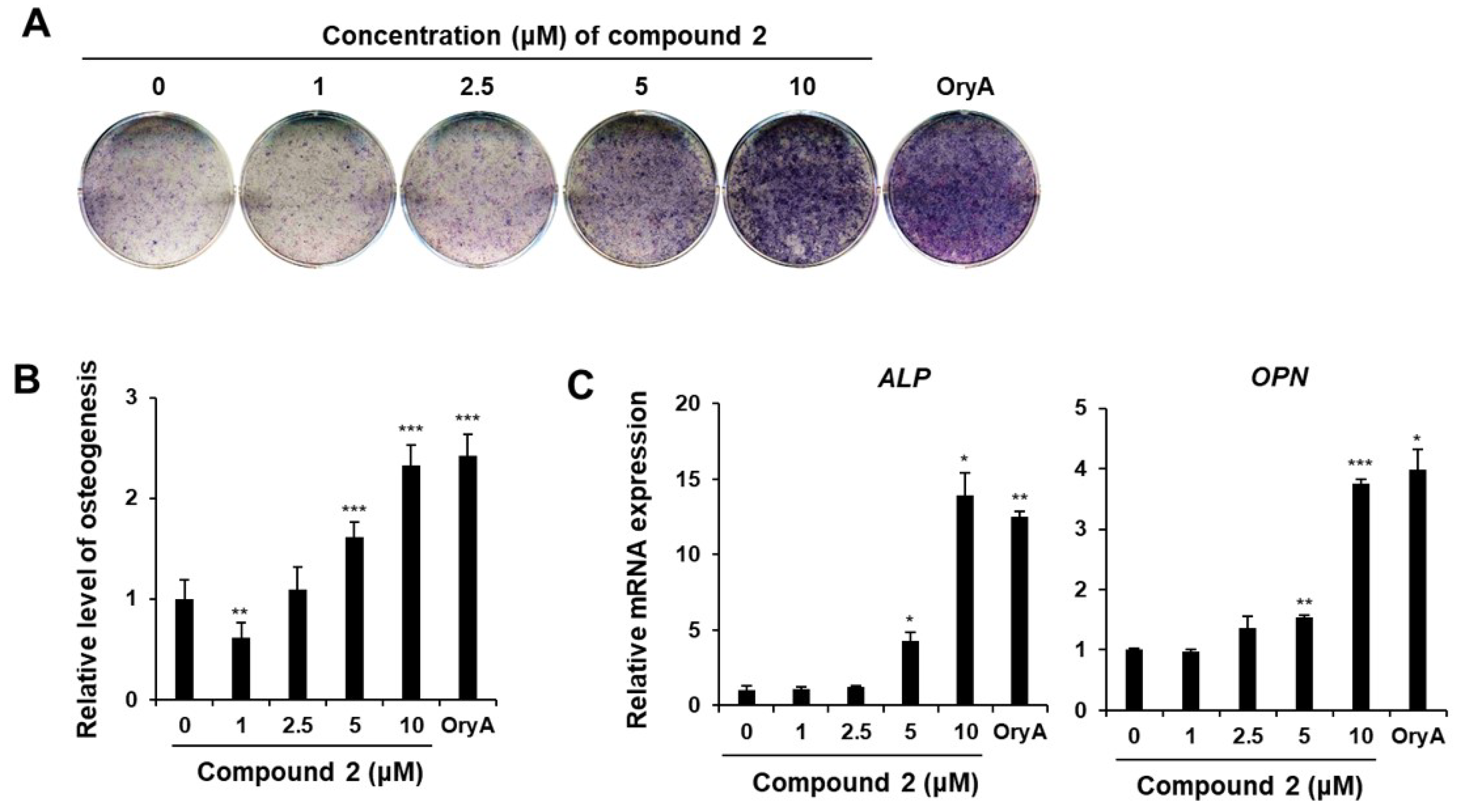
2.2. The Regulatory Effects of Compounds 1–6 on MSC Differentiation into Adipocytes and Osteoblasts
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
(aS)-Glucosciadopitysin (1)
3.4. Acid Hydrolysis and Absolute Configuration Determination of the Sugar Moiety of 1
3.5. Electronic Circular Dichroism (ECD) Calculations
3.6. Cell Culture and Differentiation
3.7. Oil Red O Staining
3.8. Alkaline Phosphatase (ALP) Staining
3.9. ALP Activity
3.10. mRNA Isolation and Real-Time Polymerase Chain Reaction (PCR)
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Makino, S.; Fukuda, K.; Miyoshi, S.; Konishi, F.; Kodama, H.; Pan, J.; Sano, M.; Takahashi, T.; Hori, S.; Abe, H.; et al. Cardiomyocytes can be generated from marrow stromal cells in vitro. J. Clin. Investig. 1999, 103, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Yoon, D.S.; Kim, H.O.; Lee, J.W. Characterization of different subpopulations from bone marrow-derived mesenchymal stromal cells by alkaline phosphatase expression. Stem Cells. Dev. 2012, 21, 2958–2968. [Google Scholar] [CrossRef] [PubMed]
- Kruger, T.E.; Miller, A.H.; Godwin, A.K.; Wang, J. Bone sialoprotein and osteopontin in bone metastasis of osteotropic cancers. Crit. Rev. Oncol. Hematol. 2014, 89, 330–341. [Google Scholar] [CrossRef] [PubMed]
- Whyte, M.P. Hypophosphatasia and the role of alkaline phosphatase in skeletal mineralization. Endocr. Rev. 1994, 15, 439–461. [Google Scholar]
- Liu, W.; Zhang, L.; Xuan, K.; Hu, C.; Liu, S.; Liao, L.; Li, B.; Jin, F.; Shi, S.; Jin, Y. Alpl prevents bone ageing sensitivity by specifically regulating senescence and differentiation in mesenchymal stem cells. Bone Res. 2018, 6, 27. [Google Scholar] [CrossRef]
- Sodek, J.; Ganss, B.; McKee, M.D. Osteopontin. Crit. Rev. Oral Biol. Med. 2000, 11, 279–303. [Google Scholar] [CrossRef]
- Singh, B.; Kaur, P.; Gopichand; Singh, R.D.; Ahuja, P.S. Biology and chemistry of Ginkgo biloba. Fitoterapia 2008, 79, 401–418. [Google Scholar] [CrossRef]
- Liu, Y.; Xin, H.; Zhang, Y.; Che, F.; Shen, N.; Cui, Y. Leaves, seeds and exocarp of Ginkgo biloba L. (Ginkgoaceae): A Comprehensive Review of Traditional Uses, phytochemistry, pharmacology, resource utilization and toxicity. J. Ethnopharmacol. 2022, 298, 115645. [Google Scholar] [CrossRef]
- van Beek, T.A. Chemical analysis of Ginkgo biloba leaves and extracts. J. Chromatogr. A 2002, 967, 21–55. [Google Scholar] [CrossRef]
- Jeong, S.Y.; Lee, K.H.; Kim, J.K.; Ahn, D.; Kim, H.; Chung, S.J.; Yoon, S.Y.; Kim, K.H. Ginkgolic Acid Derivatives from Ginkgo biloba Show Inhibitory Activity against Protein Tyrosine Phosphatases Associated with Insulin Resistance. Appl. Sci. 2023, 13, 13220. [Google Scholar] [CrossRef]
- Menezes, J.; Diederich, M.F. Bioactivity of natural biflavonoids in metabolism-related disease and cancer therapies. Pharmacol. Res. 2021, 167, 105525. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Chen, C.; Zhang, Z.; Wu, Z.; Fan, X.; Zhang, Z.; Di, W.; Shi, L. Ginkgo biloba extract promotes osteogenic differentiation of human bone marrow mesenchymal stem cells in a pathway involving Wnt/beta-catenin signaling. Pharmacol. Res. 2015, 97, 70–78. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, J.; Gu, X.; Zhang, X.; Shi, S.; Liu, C. Effects of the extract of Ginkgo biloba on the differentiation of bone marrow mesenchymal stem cells in vitro. Am. J. Transl. Res. 2016, 8, 3032–3040. [Google Scholar]
- Lucinda, L.M.; Vieira, B.J.; Oliveira, T.T.; Sa, R.C.; Peters, V.M.; Reis, J.E.; Guerra, M.O. Evidences of osteoporosis improvement in Wistar rats treated with Ginkgo biloba extract: A histomorphometric study of mandible and femur. Fitoterapia 2010, 81, 982–987. [Google Scholar] [CrossRef]
- Zhu, B.; Xue, F.; Zhang, C.Q.; Li, G.Y. Ginkgolide B promotes osteoblast differentiation via activation of canonical Wnt signalling and alleviates osteoporosis through a bone anabolic way. Cell Mol. Med. 2019, 23, 5782–5793. [Google Scholar] [CrossRef]
- Lee, B.S.; So, H.M.; Kim, S.; Kim, J.K.; Kim, J.C.; Kang, D.M.; Ahn, M.J.; Ko, Y.J.; Kim, K.H. Comparative evaluation of bioactive phytochemicals in Spinacia oleracea cultivated under greenhouse and open field conditions. Arch. Pharm. Res. 2022, 45, 795–805. [Google Scholar] [CrossRef]
- Cho, H.; Kim, K.H.; Han, S.H.; Kim, H.-J.; Cho, I.-H.; Lee, S. Structure determination of heishuixiecaoline A from Valeriana fauriei and its content from different cultivated regions by HPLC/PDA Analysis. Nat. Prod. Sci. 2022, 28, 181–186. [Google Scholar] [CrossRef]
- Yu, J.S.; Jeong, S.Y.; Li, C.S.; Oh, T.; Kwon, M.; Ahn, J.S.; Ko, S.K.; Ko, Y.J.; Cao, S.G.; Kim, K.H. New phenalenone derivatives from the Hawaiian volcanic soil-associated fungus FT729 and their inhibitory effects on indoleamine 2,3-dioxygenase 1 (IDO1). Arch. Pharm. Res. 2022, 45, 105–113. [Google Scholar] [CrossRef]
- Lee, S.; Jang, M.; Ryoo, R.; Roh, J.; Ko, S.K.; Kim, K.H. New autophagy-modulating lanostane-type triterpenoids from a hallucinogenic poisonous mushroom Gymnopilus orientispectabilis. Arch. Pharm. Res. 2024, 47, 272–287. [Google Scholar] [CrossRef]
- Lee, D.E.; Park, K.H.; Hong, J.H.; Kim, S.H.; Park, K.M.; Kim, K.H. Anti-osteoporosis effects of triterpenoids from the fruit of sea buckthorn (Hippophae rhamnoides) through the promotion of osteoblast differentiation in mesenchymal stem cells, C3H10T1/2. Arch. Pharm. Res. 2023, 46, 771–781. [Google Scholar] [CrossRef]
- Chen, K.; Hu, Z.M.; Song, W.; Wang, Z.L.; He, J.B.; Shi, X.M.; Cui, Q.H.; Qiao, X.; Ye, M. Diversity of O-Glycosyltransferases Contributes to the Biosynthesis of Flavonoid and Triterpenoid Glycosides in Glycyrrhiza uralensis. ACS Synth. Biol. 2019, 8, 1858–1866. [Google Scholar] [CrossRef]
- Li, S.-H.; Zhang, H.-J.; Niu, X.-M.; Yao, P.; Sun, H.-D.; Fong, H.H. Chemical Constituents from Amentotaxus yunnanensis and Torreya yunnanensis. J. Nat. Prod. 2003, 66, 1002–1005. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Nakashima, T.; Ueda, T.; Tomii, K.; Kouno, I. Facile discrimination of aldose enantiomers by reversed-phase HPLC. Chem. Pharm. Bull. 2007, 55, 899–901. [Google Scholar] [CrossRef]
- Bringmann, G.; Price Mortimer, A.J.; Keller, P.A.; Gresser, M.J.; Garner, J.; Breuning, M. Atroposelective synthesis of axially chiral biaryl compounds. Angew. Chem. Int. Ed. Engl. 2005, 44, 5384–5427. [Google Scholar] [CrossRef]
- Wang, S.Q.; Zhu, X.F.; Wang, X.N.; Shen, T.; Xiang, F.; Lou, H.X. Flavonoids from and their cardioprotective effects against doxorubicin-induced toxicity in H9c2 cells. Phytochemistry 2013, 87, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.F.; Xie, C.H.; Jian, R.J.; Kang, J.; Li, Y.; Zhuang, C.L.; Yang, F.; Zhang, L.L.; Lai, L.; Wu, T.; et al. Biflavonoids from Caper (Capparis spinosa L.) Fruits and Their Effects in Inhibiting NF-kappa B Activation. J. Agric. Food Chem. 2011, 59, 3060–3065. [Google Scholar] [CrossRef] [PubMed]
- Agnolet, S.; Jaroszewski, J.W.; Verpoorte, R.; Staerk, D. 1H NMR-based metabolomics combined with HPLC-PDA-MS-SPE-NMR for investigation of standardized Ginkgo biloba preparations. Metabolomics 2010, 6, 292–302. [Google Scholar] [CrossRef]
- Bedir, E.; Tatli, I.I.; Khan, R.A.; Zhao, J.; Takamatsu, S.; Walker, L.A.; Goldman, P.; Khan, I.A. Biologically active secondary metabolites from Ginkgo biloba. J. Agric. Food. Chem. 2002, 50, 3150–3155. [Google Scholar] [CrossRef]
- Fajriah, S.; Megawati, M.; Darmawan, A. Apigenin, an anticancer active compound isolated from Macaranga gigantifolia leaves. J. Trop. Life Sci. 2016, 6, 7–9. [Google Scholar]
- Coulerie, P.; Nour, M.; Maciuk, A.; Eydoux, C.; Guillemot, J.C.; Lebouvier, N.; Hnawia, E.; Leblanc, K.; Lewin, G.; Canard, B.; et al. Structure-activity relationship study of biflavonoids on the Dengue virus polymerase DENV-NS5 RdRp. Planta Med. 2013, 79, 1313–1318. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, M.-X.; Wang, X.-Y.; Yang, Y.-L.; Gong, X.; Wang, C.-C.; Xu, J.-F.; Li, M.-H. Assessment of components of Gingko biloba leaves collected from different regions of China that contribute to its antioxidant effects for improved quality monitoring. Food Sci. Technol. 2020, 41, 676–683. [Google Scholar] [CrossRef]
- Jeong, S.Y.; Alishir, A.; Zhang, S.; Zhang, Y.; Choi, S.; Pang, C.; Bae, H.Y.; Jung, W.H.; Kim, K.H. Identification of Obscurolide-Type Metabolites and Antifungal Metabolites from the Termite-Associated Streptomyces neopeptinius BYF101. J. Nat. Prod. 2023, 86, 1891–1900. [Google Scholar] [CrossRef] [PubMed]

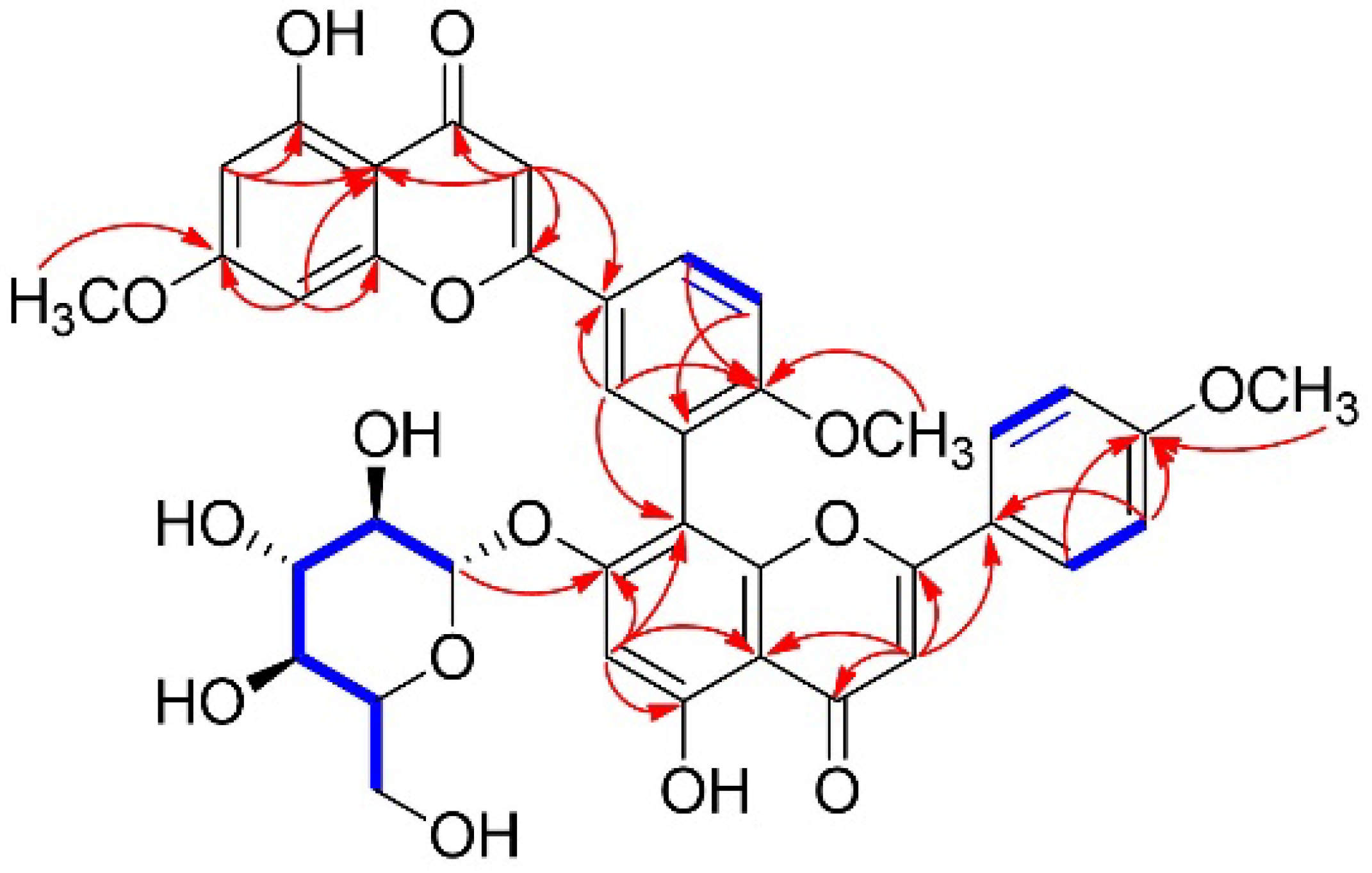

| Position | 1 | Sciadopitysin [23] | ||
|---|---|---|---|---|
| δC a, Type | δH, (J in Hz) | δC, Type | δH, (J in Hz) | |
| 1 | ||||
| 2 | 164.5, C | 164.3, C | ||
| 3 | 103.4, CH | 6.79, s | 104.1, CH | 6.97, s |
| 4 | 182.5, C | 182.8, C | ||
| 5 | 161.5, C | 161.5, C | ||
| 6 | 97.9, CH | 6.35, d (2.0) | 98.6, CH | 6.58, d (2.4) |
| 7 | 165.9, C | 165.8, C | ||
| 8 | 91.9, CH | 6.66, d (2.0) | 92.8, CH | 6.70, d (2.0) |
| 9 | 157.9, C | 155.5, C | ||
| 10 | 104.8, C | 104.9, C | ||
| 1′ | 121.5, C | 123.4, C | ||
| 2′ | 131.2, CH | 8.08, d (2.0) | 131.9, CH | 8.51, d (2.0) |
| 3′ | 122.9, C | 123.1, C | ||
| 4′ | 160.1, C | 162.6, C | ||
| 5′ | 111.1, CH | 7.35, d (8.5) | 111.8, CH | 7.36, d (8.8) |
| 6′ | 128.1, CH | 8.16, dd (8.5, 2.0) | 128.4, CH | 8.16, dd (8.8, 2.4) |
| 1′′ | ||||
| 2′′ | 164.4, C | 164.3, C | ||
| 3′′ | 102.6, CH | 6.75, s | 104.7, CH | 7.11, s |
| 4′′ | 181.0, C | 182.9, C | ||
| 5′′ | 161.1, C | 162.4, C | ||
| 6′′ | 98.5, CH | 6.82, s | 99.7, CH | 6.91, s |
| 7′′ | 160.4, C | 163.3, C | ||
| 8′′ | 106.2, C | 104.7, C | ||
| 9′′ | ND b | 158.0, C | ||
| 10′′ | 105.3, C | 105.8, C | ||
| 1′′′ | 122.9, C | 123.8, C | ||
| 2′′′ | 127.6, CH | 7.59, d (9.0) | 128.1, CH | 7.75, d (8.8) |
| 3′′′ | 114.1, CH | 6.92, d (9.0) | 114.8, CH | 7.00, d (8.8) |
| 4′′′ | 163.2, C | 162.8, C | ||
| 5′′′ | 114.1, CH | 6.92, d (9.0) | 114.8, CH | 7.00, d (8.8) |
| 6′′′ | 127.6, CH | 7.59, d (9.0) | 128.1, CH | 7.75, d (8.8) |
| 1⁗ | 100.1, CH | 5.19, d (7.5) | ||
| 2⁗ | 73.1, CH | 3.25, dd (9.0, 7.5) | ||
| 3⁗ | 76.6, CH | 3.45, dd (9.5, 9.0) | ||
| 4⁗ | 69.7, CH | 3.37, dd (9.5, 9.0) | ||
| 5⁗ | 76.9, CH | 3.50, ddd (9.0, 5.5, 2.5) | ||
| 6⁗a | 60.7, CH2 | 3.76, dd (12.0, 5.5) | ||
| 6⁗b | 3.92, dd (12.0, 2.5) | |||
| 7-OCH3 | 55.0, CH3 | 3.87, s | 56.0, CH3 | 3.90, s |
| 4′-OCH3 | 54.9, CH3 | 3.81, s | 55.8, CH3 | 3.75, s |
| 4′′′-OCH3 | 54.5, CH3 | 3.80, s | 55.3, CH3 | 3.63, s |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, S.Y.; Lee, K.H.; Kim, S.H.; Yang, M.H.; Lee, G.; Kim, K.H. (aS)-Glucosciadopitysin, a New Biflavonoid Glycoside from the Leaves of Ginkgo biloba and Osteogenic Activity of Bioflavonoids. Plants 2025, 14, 261. https://doi.org/10.3390/plants14020261
Jeong SY, Lee KH, Kim SH, Yang MH, Lee G, Kim KH. (aS)-Glucosciadopitysin, a New Biflavonoid Glycoside from the Leaves of Ginkgo biloba and Osteogenic Activity of Bioflavonoids. Plants. 2025; 14(2):261. https://doi.org/10.3390/plants14020261
Chicago/Turabian StyleJeong, Se Yun, Kwang Ho Lee, Seon Hee Kim, Min Hye Yang, Gakyung Lee, and Ki Hyun Kim. 2025. "(aS)-Glucosciadopitysin, a New Biflavonoid Glycoside from the Leaves of Ginkgo biloba and Osteogenic Activity of Bioflavonoids" Plants 14, no. 2: 261. https://doi.org/10.3390/plants14020261
APA StyleJeong, S. Y., Lee, K. H., Kim, S. H., Yang, M. H., Lee, G., & Kim, K. H. (2025). (aS)-Glucosciadopitysin, a New Biflavonoid Glycoside from the Leaves of Ginkgo biloba and Osteogenic Activity of Bioflavonoids. Plants, 14(2), 261. https://doi.org/10.3390/plants14020261









