Different Genotypes of the Rare and Threatened Moss Physcomitrium eurystomum (Funariaceae) Exhibit Different Resilience to Zinc and Copper Stress
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Plant Material and In Vitro Cultivation
4.2. Experimental Design
4.3. Morphogenetic and Physiological Parameters
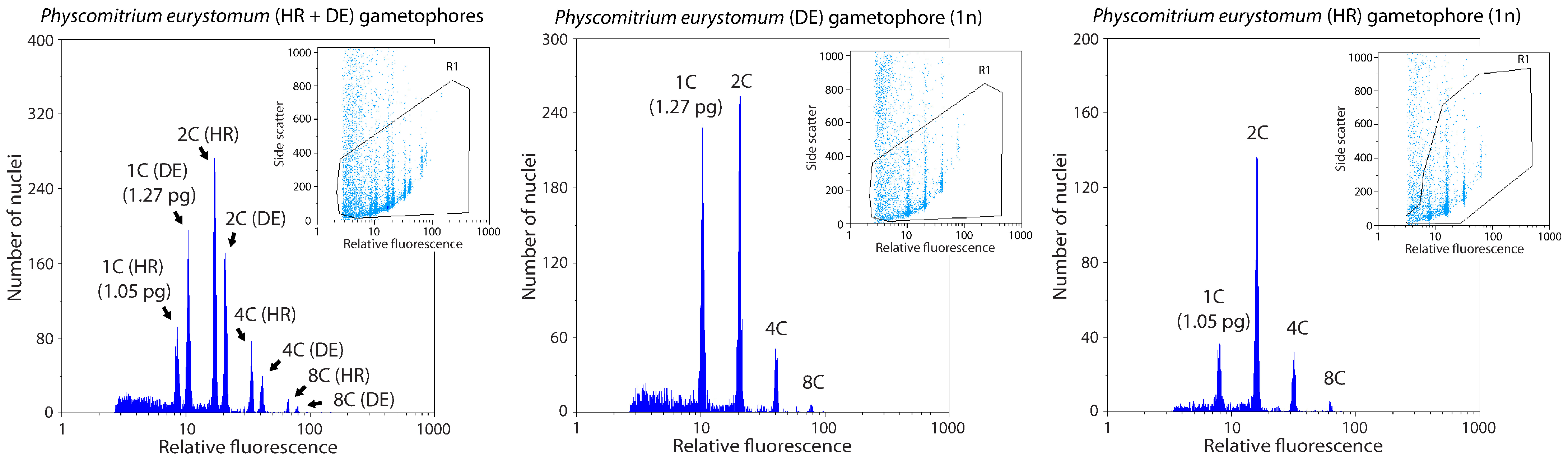
4.4. Flow Cytometry Analysis
[(the sample peak mean)/(the standard peak mean)].
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sabovljević, M.; Weidinger, M.; Sabovljević, A.; Stanković, J.; Adlassnig, W.; Lang, I. Metal accumulation in the acrocarp moss Atrichum undulatum under controlled conditions. Environ. Pollut. 2020, 256, 113397. [Google Scholar] [CrossRef] [PubMed]
- Bansal, P.; Srivastava, A. Sporophyte characterization and sporogenesis in Physcomitrium eurystomum Sendtn. (Bryophyta: Funariaceae). Caryologia 2017, 70, 120–127. [Google Scholar] [CrossRef]
- Chetia, T.; Roy, H. Physcomitrium eurystomum Sendtn. (Funariaceae): A rare species recorded for Assam, India. J. Threatened Taxa 2024, 16, 25474–25477. [Google Scholar] [CrossRef]
- Hodgetts, N.; Calix, M.; Englefield, E.; Fettes, N.; Garcia Criado, M.; Patin, L.; Nieto, A.; Bergamini, A.; Bisang, I.; Baisheva, E.; et al. A Miniature World in Decline: European Red List of Mosses, Liverworts and Hornworts; IUCN: Brussels, Belgium, 2019; ISBN 9782831719931/9782831719948. [Google Scholar]
- Hodgetts, N.G.; Lockhart, N. Checklist and country status of European bryophytes—Update 2020. In Irish Wildlife Manuals 123; National Parks and Wildlife Service, Department of Culture, Heritage and the Gaeltacht: Dublin, Ireland, 2020. [Google Scholar]
- Sabovljević, M.S.; Pantović, J.P.; Širka, P.; Vujičić, M.M.; Sabovljević, A.D.; Papp, B. Red-list of moss species of Serbia: 2024 assessment. Bot. Serb. 2024, 48, 207–222. [Google Scholar] [CrossRef]
- Martinčič, A. New checklist and the red list of the mosses (Bryophyta) of Slovenia. Hacquetia 2024, 23, 69–118. [Google Scholar] [CrossRef]
- Stešević, D.; Andjić, B.; Stanišić-Vujačić, M. Physcomitrium eurystomum Sendt. a new moss species in the bryophyte flora of Montenegro. Acta Bot. Croat. 2020, 79, 95–97. [Google Scholar] [CrossRef]
- Li, J.; Yu, H.; Luan, Y. Meta-analysis of the copper, zinc, and cadmium absorption capacities of aquatic plants in heavy metal-polluted water. Int. J. Environ. Res. Pub. Health 2015, 12, 14958–14973. [Google Scholar] [CrossRef]
- Fu, Z.; Wu, F.; Chen, L.; Xu, B.; Feng, C.; Bai, Y.; Liao, H.; Sun, S.; Giesy, J.P.; Guo, W. Copper and zinc, but not other priority toxic metals, pose risks to native aquatic species in a large urban lake in Eastern China. Environ. Pollut. 2016, 219, 1069–1076. [Google Scholar] [CrossRef]
- Kaamoush, M.; El-Agawany, N.; Salhin, H.E.; Zeiny, A. Monitoring the effect of nickel, copper, and zinc on growth and photosynthetic pigments of Spirulina platensis with suitability investigation in Idku Lake. Environ. Sci. Pollut. Res. 2022, 29, 78942–78959. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.; Singh, P.; Mankotia, S.; Swain, J.; Satbhai, S. Iron homeostasis in plants and its crosstalk with copper, zinc, and manganese. Plant Stress 2021, 1, 100008. [Google Scholar] [CrossRef]
- Stanković, J.; Janković, S.; Lang, I.; Vujičić, M.; Sabovljević, M.; Sabovljević, A. The toxic metal stress in two mosses of different growth forms under axenic and controlled conditions. Bot. Serb. 2021, 45, 31–47. [Google Scholar] [CrossRef]
- Sassmann, S.; Weidinger, M.; Adlassnig, W.; Hofhansl, F.; Bock, B.; Lang, I. Zinc and copper uptake in Physcomitrella patens: Limitations and effects on growth and morphology. Environ. Experim. Bot. 2015, 118, 12–20. [Google Scholar] [CrossRef]
- Fu, X.; Yang, H.; Pangestu, F.; Nikolau, B.J. Failure to maintain acetate homeostasis by acetate-activating enzymes impacts plant development. Plant Physiol. 2020, 182, 1256–1271. [Google Scholar] [CrossRef] [PubMed]
- Tossi, V.E.; Martínez Tosar, L.J.; Laino, L.E.; Iannicelli, J.; Regalado, J.J.; Escandón, A.S.; Baroli, I.; Causin, H.F.; Pitta-Álvarez, S.I. Impact of polyploidy on plant tolerance to abiotic and biotic stresses. Front. Plant Sci. 2022, 13, 869423. [Google Scholar] [CrossRef]
- Paape, T.; Akiyama, R.; Cereghetti, T.; Onda, Y.; Hirao, A.S.; Kenta, T.; Shimizu, K.K. Experimental and field data support range expansion in an allopolyploid Arabidopsis owing to parental legacy of heavy metal hyperaccumulation. Front. Genetics 2020, 11, 565854. [Google Scholar] [CrossRef]
- Ćosić, M.; Vujičić, M.M.; Sabovljević, M.S.; Sabovljević, A.D. Effects of salt on selected bryophyte species tested under controlled conditions. Bot. Serb. 2020, 44, 27–35. [Google Scholar] [CrossRef]
- Printarakul, N.; Adulkittichai, K.; Meeinkuirt, W. Effects of copper accumulation on growth and development of Scopelophila cataractae grown in vitro. Ecotox. Environ. Safety 2022, 245, 114127. [Google Scholar] [CrossRef]
- Rajčić, M.V.; Šircelj, H.; Matić, N.A.; Pavkov, S.D.; Poponessi, S.; Tosti, T.B.; Sabovljević, A.D.; Sabovljević, M.S.; Vujičić, M.M. Effects of the salt stress duration and intensity on developmental and physiological features of the moss Polytrichum formosum. Plants 2024, 13, 1438. [Google Scholar] [CrossRef] [PubMed]
- Sabovljević, A.; Vujičić, M.; Stanković, J.; Sabovljević, M. Effects of zinc and copper on development and survival of the moss Atrichum undulatum in controlled conditions. Bot. Serb. 2018, 42, 181–184. [Google Scholar] [CrossRef]
- Schillaci, L.; Djaković, N.; Lang, I. Is a combination of metals more toxic to mosses than a single metal? Plants 2023, 12, 3960. [Google Scholar] [CrossRef]
- Xu, E.; Liu, Y.; Gu, D.; Zhan, X.; Li, J.; Zhou, K.; Zhang, P.; Zou, Y. Molecular mechanisms of plant responses to copper: From deficiency to excess. Int. J. Mol. Sci. 2024, 25, 6993. [Google Scholar] [CrossRef] [PubMed]
- Shakya, K.; Chettri, M.K.; Sawidis, T. Impact of heavy metals (copper, zinc, and lead) on the chlorophyll content of some mosses. Arch. Environ. Con. Tox. 2008, 54, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Bano, S.; Rao, A.; Agnihotri, N. Effect of zinc and cadmium on chlorophyll content of mosses Brachythecium rutabulum and Mnium cuspidatum. Int. J. Life Sci. Res. 2018, 6, 267–278. [Google Scholar]
- Tremper, A.H.; Burton, A.S.; Higgs, D.E. Field and laboratory exposures of two moss species to low metal pollution. J. Atm. Chem. 2004, 49, 111–120. [Google Scholar] [CrossRef]
- Brown, D.H.; Wells, J.M. Physiological effects of heavy metals on the moss Rhytidiadelphus squarrosus. Ann. Bot. 1990, 66, 641–647. [Google Scholar] [CrossRef]
- Boquete, M.T.; Bermúdez-Crespo, J.; Aboal, J.R.; Callbaeira, A.; Fernandez, J.A. Assessing the effects of heavy metal contamination on the proteome of the moss Pseudoscleropodium purum cross-transplanted between different areas. Environ. Sci. Pollut. Res. 2014, 21, 2191–2200. [Google Scholar] [CrossRef]
- Quartacci, M.F.; Cosi, E.; Navari-Izzo, F. Lipids and NADPH-dependent superoxide production in plasma membrane vesicles from roots of wheat grown under copper deficiency or excess. J. Exp. Bot. 2001, 52, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, Z.; Sardar, A.; Shabbir, A.; Abbas, G.; Shamshad, S.; Khalid, S.; Murtaza, G.; Dumat, C.; Shahid, M. Copper uptake, essentiality, toxicity, detoxification and risk assessment in soil-plant environment. Chemosphere 2020, 259, 127436. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Fang, X.; Chen, M.; Wang, L.; Xia, J.; Wang, Z.; Fang, J.; Tran, L.-S.P.; Shangguan, L. Copper stress in grapevine: Consequences, responses, and a novel mitigation strategy using 5-aminolevulinic acid. Environ. Pollut. 2022, 307, 119561. [Google Scholar] [CrossRef] [PubMed]
- Adrees, M.; Ali, S.; Rizwan, M.; Ibrahim, M.; Abbas, F.; Farid, M.; Zia-ur-Rehman, M.; Irshad, M.K.; Bharwana, S.A. The effect of excess copper on growth and physiology of important food crops: A review. Environ. Sci. Pollut. Res. 2015, 22, 8148–8162. [Google Scholar] [CrossRef]
- Feil, S.B.; Pii, Y.; Valentinuzzi, F.; Tiziani, R.; Mimmo, T.; Cesco, S. Copper toxicity affects phosphorus uptake mechanisms at molecular and physiological levels in Cucumis sativus plants. Plant Physiol. Biochem. 2020, 157, 138–147. [Google Scholar] [CrossRef]
- Stanković, J.; Sabovljević, A.; Sabovljević, M. Bryophytes and heavy metals: A review. Acta Bot. Croat. 2018, 77, 109–118. [Google Scholar] [CrossRef]
- Sutter, K.; Jung, K.; Krauss, G.J. Effects of heavy metals on the nitrogen metabolism of the aquatic moss Fontinalis antipyretica L. ex Hedw. Environ. Sci. Pollut. Res. 2002, 9, 417–421. [Google Scholar] [CrossRef]
- Panda, S.K.; Choudhury, S. Changes in nitrate reductase activity and oxidative stress response in the moss Polytrichum commune subjected to chromium, copper and zinc phytotoxicity. Braz. J. Plant Physiol. 2005, 17, 191–197. [Google Scholar] [CrossRef]
- Nomura, T.; Hasezawa, S. Regulation of gemma formation in the copper moss Scopelophila cataractae by environmental copper concentrations. J. Plant Res. 2011, 124, 631–638. [Google Scholar] [CrossRef]
- McDaniel, S.F.; von Stackelberg, M.; Richardt, S.; Quatrano, R.S.; Reski, R.; Rensing, S.A. The speciation history of the Physcomitrium-Physcomitrella species complex. Evolution 2010, 64, 217–231. [Google Scholar] [CrossRef]
- Božović, D.P.; Rimac, A.; Vujičić, M.M.; Singh, P.; Goga, M.; Li, M.; Varotto, C.; Sabovljević, A.D.; Sabovljević, M.S. The developmental and physiological traits of rare and threatened moss Physcomitrium eurystomum Sendtn. (Funariaceae) valuable for its conservation. Phyton Int. J. Exp. Bot. 2024, 93, 2949–2961. [Google Scholar] [CrossRef]
- Ostendorf, A.K.; van Gessel, N.; Malkowsky, Y.; Sabovljević, M.S.; Rensing, S.A.; Roth-Nebelsick, A. Polyploidization within the Funariaceae—A key principle behind speciation, sporophyte reduction, and the high variance of spore diameters? Bryophyte Diver. Evol. 2021, 43, 164–179. [Google Scholar] [CrossRef]
- Reski, R.; Abel, W.O. Induction of budding on chloronemata and caulonemata of the moss Physcomitrella patens, using isopentenyladenine. Planta 1985, 165, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Ćosić, M.V.; Sabovljević, M.S.; Papp, B.; Giba, Z.S.; Šinžar-Sekulić, J.B.; Sabovljević, A.D.; Vujičić, M.M. Micropropagation of rare bryo-halophyte Hennediella heimii. Bot. Serb. 2022, 46, 187–195. [Google Scholar] [CrossRef]
- Jadranin, B.Z.; Ćosić, M.V.; Božović, D.P.; Vujičić, M.M.; Ignatov, M.S.; Ignatova, E.A.; Sabovljević, A.D.; Sabovljević, M.S. An insight into the biology of the rare and peculiar moss Pterygoneurum sibiricum (Pottiaceae): A conservation physiology approach. Plants 2023, 12, 1359. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Goga, M.; Ručová, D.; Kolarčik, V.; Sabovljević, M.; Bačkor, M.; Lang, I. Usnic acid, as a biotic factor, changes the ploidy level in mosses. Ecol. Evol. 2018, 8, 2781–2787. [Google Scholar] [CrossRef] [PubMed]
- Schönswetter, P.; Suda, J.; Popp, M.; Weiss-Schneeweiss, H.; Brochmann, C. Circumpolar phylogeography of Juncus biglumis (Juncaceae) inferred from AFLP fingerprints, cpDNA sequences, nuclear DNA content and chromosome numbers. Mol. Phylogen. Evol. 2007, 42, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Šmarda, P.; Knápek, O.; Březinová, A.; Horová, L.; Grulich, V.; Danihelka, J.; Veselý, P.; Šmerda, J.; Rotreklová, O.; Bureš, P. Genome sizes and genomic guanine+cytosine (GC) contents of the Czech vascular flora with new estimates for 1700 species. Preslia 2019, 91, 117–142. [Google Scholar] [CrossRef]
- Shang, L.; He, W.; Wang, T.; Yang, Y.; Xu, Q.; Zhao, X.; Yang, L.; Zhang, H.; Li, X.; Lv, Y.; et al. A complete assembly of the rice Nipponbare reference genome. Mol. Plant 2023, 16, 1232–1236. [Google Scholar] [CrossRef]
- Loureiro, J.; Rodriguez, E.; Doležel, J.; Santos, C. Two new nuclear isolation buffers for plant DNA flow cytometry: A test with 37 species. Ann. Bot. 2007, 100, 875–888. [Google Scholar] [CrossRef]
- Paľová, M.; Ručová, D.; Goga, M.; Kolarčik, V. Spatial and temporal patterns of endopolyploidy in mosses. Genes 2021, 12, 27. [Google Scholar] [CrossRef]
- Doležel, J.; Bartoš, J. Plant DNA flow cytometry and estimation of nuclear genome size. Ann. Bot. 2005, 95, 99–110. [Google Scholar] [CrossRef] [PubMed]
- R Core Team, R. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 25 December 2022).
- Elkin, L.; Kay, M.; Higgins, J.; Wobbrock, J. An Aligned Rank Transform Procedure for Multifactor Contrast Tests. In Proceedings of the 34th Annual ACM Symposium on User Interface Software and Technology, Association for Computing Machinery, New York, NY, USA, 10–14 October 2021. [Google Scholar]
- Wobbrock, J.; Findlater, L.; Gergle, D.; Higgins, J. The Aligned Rank Transform for Nonparametric Factorial Analyses Using Only ANOVA Procedures. In Proceedings of the ACM Conference on Human Factors in Computing Systems (CHI 2011), Vancouver, BC, Canada, 7–12 May 2011; ACM Press: New York, NY, USA, 2011; pp. 143–146. [Google Scholar]
- Kay, M.; Elkin, L.; Higgins, J.; Wobbrock, J. mjskay/ARTool: ARTool 0.11.0 (v0.11.0). Zenodo. 2021. Available online: https://zenodo.org/records/4721941 (accessed on 20 December 2022).



| Parameter | Time | G | C | G × C |
|---|---|---|---|---|
| Index of multiplication | 2 h | 0.1329 | 5.2346 ** | 14.4338 *** |
| 24 h | 9.83698 ** | 0.77777 | 12.33015 *** | |
| Diameter of secondary protonema patch | 2 h | 690.2289 *** | 7.6139 *** | 2.9338 |
| 24 h | 556.978 *** | 9.338 *** | 18.761 *** | |
| Total chlorophyll content | 2 h | 1.43918 | 0.26049 | 0.70407 |
| 24 h | 0.305874 | 0.332833 | 0.088626 |
| Parameter | Time | G | C | G × C |
|---|---|---|---|---|
| Index of multiplication | 2 h | 206.703 *** | 39.088 *** | 33.927 *** |
| 24 h | 19.6348 *** | 165.9156 *** | 3.8402 * | |
| Diameter of secondary protonema patch | 2 h | 102.78 *** | 177.32 *** | 906.67 *** |
| 24 h | 72.168 *** | 137.309 *** | 677.689 *** | |
| Total chlorophyll content | 2 h | 0.0032258 | 5.554 * | 1.0518 |
| 24 h | 8.7461 ** | 49.7198 *** | 3.2631 |
| Treatment | Time | DE Genotype | HR Genotype |
|---|---|---|---|
| Control | 2 h | 100% | 100% |
| 24 h | 100% | 100% | |
| Zinc acetate 200 µM | 2 h | 100% | 100% |
| 24 h | 100% | 100% | |
| Zinc acetate 700 µM | 2 h | 100% | 100% |
| 24 h | 100% | 100% | |
| Copper acetate 200 µM | 2 h | 100% | 100% |
| 24 h | 100% | 97.2% | |
| Copper acetate 700 µM | 2 h | 88.9% | 27.8% |
| 24 h | 66.7% | 13.9% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Božović, D.P.; Ćosić, M.V.; Kolarčik, V.; Goga, M.; Varotto, C.; Li, M.; Sabovljević, A.D.; Sabovljević, M.S. Different Genotypes of the Rare and Threatened Moss Physcomitrium eurystomum (Funariaceae) Exhibit Different Resilience to Zinc and Copper Stress. Plants 2025, 14, 224. https://doi.org/10.3390/plants14020224
Božović DP, Ćosić MV, Kolarčik V, Goga M, Varotto C, Li M, Sabovljević AD, Sabovljević MS. Different Genotypes of the Rare and Threatened Moss Physcomitrium eurystomum (Funariaceae) Exhibit Different Resilience to Zinc and Copper Stress. Plants. 2025; 14(2):224. https://doi.org/10.3390/plants14020224
Chicago/Turabian StyleBožović, Djordje P., Marija V. Ćosić, Vladislav Kolarčik, Michal Goga, Claudio Varotto, Mingai Li, Aneta D. Sabovljević, and Marko S. Sabovljević. 2025. "Different Genotypes of the Rare and Threatened Moss Physcomitrium eurystomum (Funariaceae) Exhibit Different Resilience to Zinc and Copper Stress" Plants 14, no. 2: 224. https://doi.org/10.3390/plants14020224
APA StyleBožović, D. P., Ćosić, M. V., Kolarčik, V., Goga, M., Varotto, C., Li, M., Sabovljević, A. D., & Sabovljević, M. S. (2025). Different Genotypes of the Rare and Threatened Moss Physcomitrium eurystomum (Funariaceae) Exhibit Different Resilience to Zinc and Copper Stress. Plants, 14(2), 224. https://doi.org/10.3390/plants14020224











