Importance of OsRac1 in Signalling of Pigm-1 Mediated Resistance to Rice Blast Disease
Abstract
1. Introduction
2. Results
2.1. Amino Acid Sequence Analysis and Comparison Between Pigm-1 and Pit, Pia, Pid3, Pi9
2.2. Pigm-1 Interacts with OsRac1 by the NBS Domain
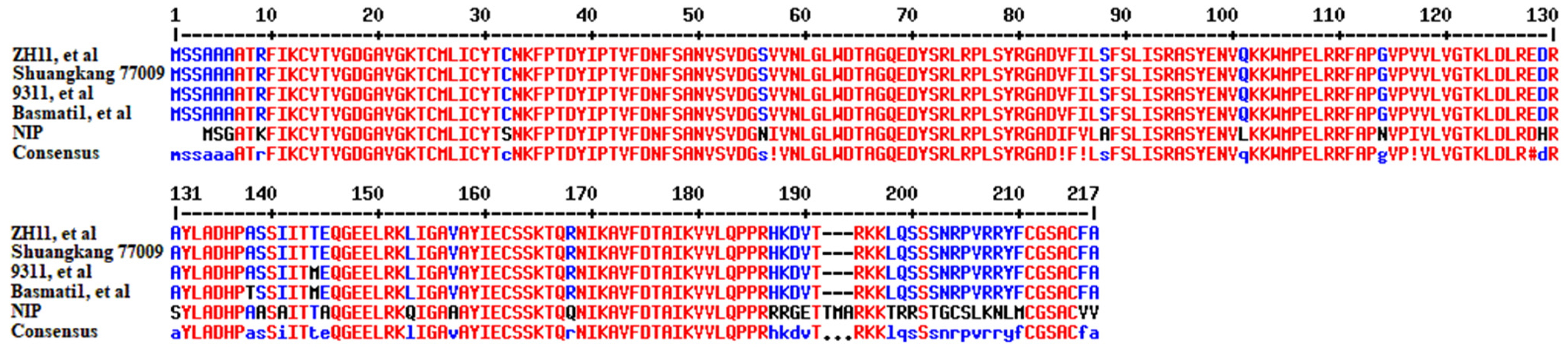
2.3. Amino Acid Sequence Analysis of OsRac1 in Different Rice Varieties
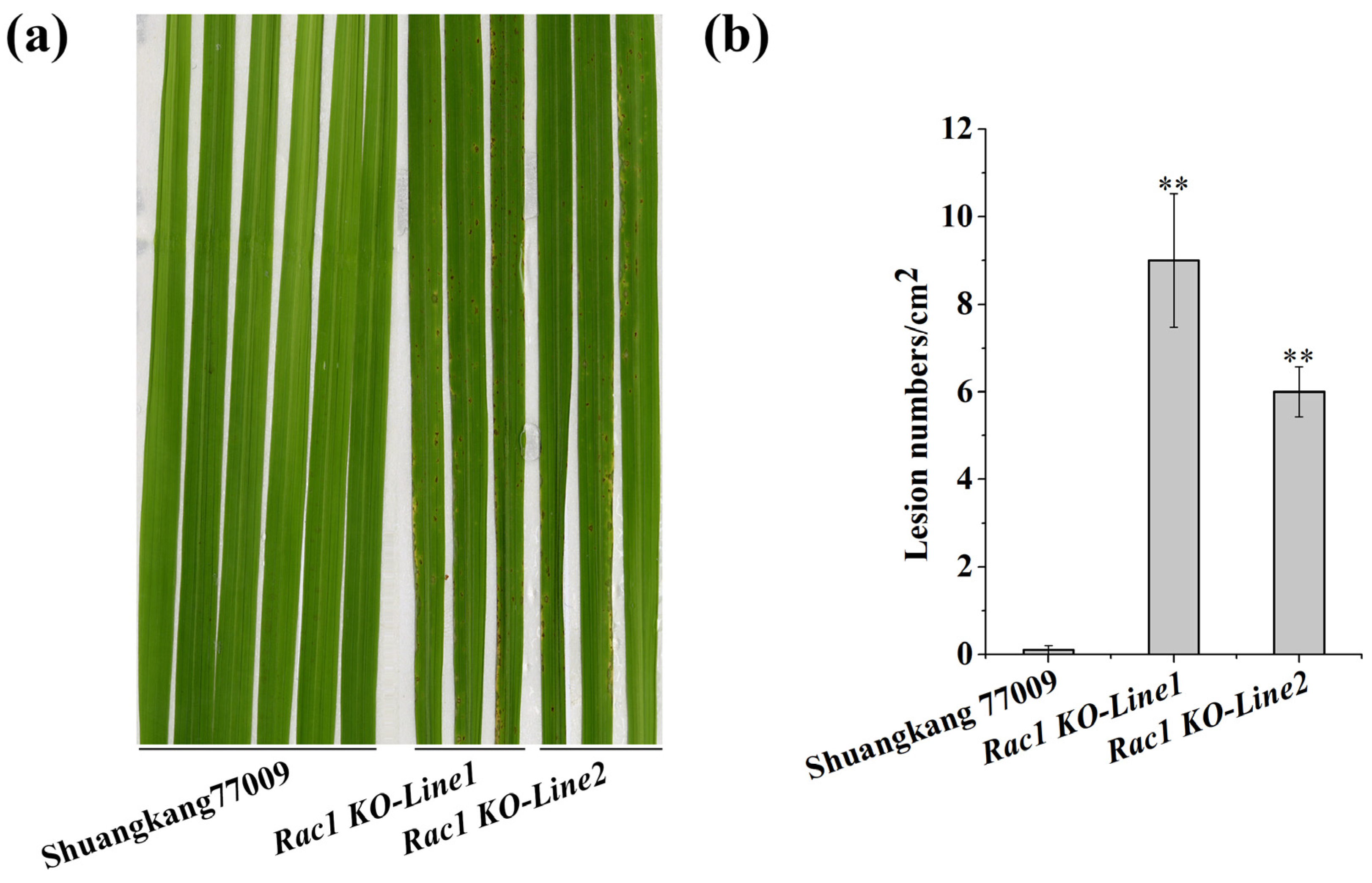
2.4. OsRac1 Is a Key Downstream Component of the Pigm-1 Protein
2.5. Pigm-1 Is Used to Improve Blast Resistance in 9311
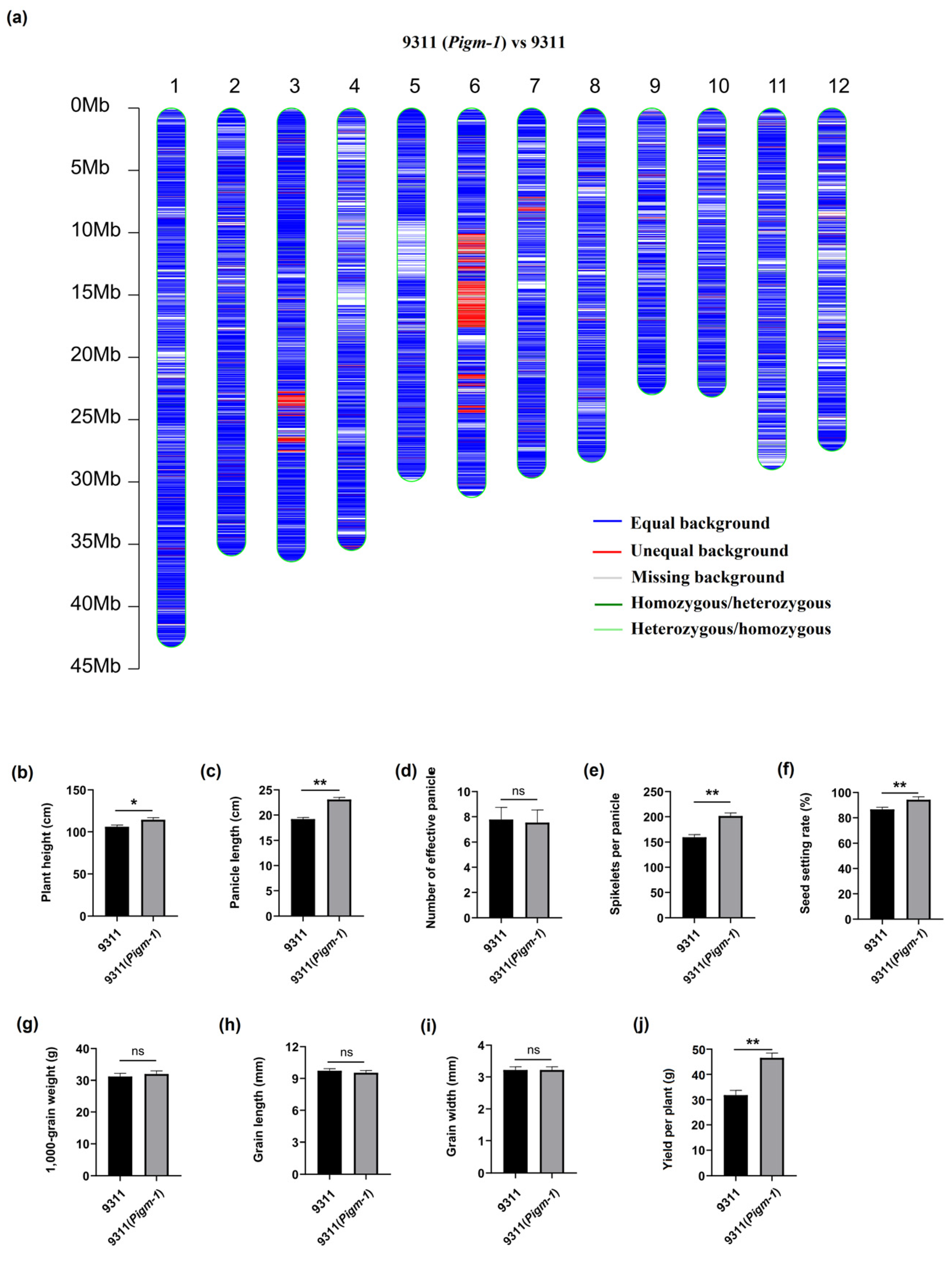
2.6. Genetic Background Analysis of Improved Line 9311(Pigm-1)
2.7. Analysis of the Main Agronomic Characteristics of the Improved Line 9311(Pigm-1)
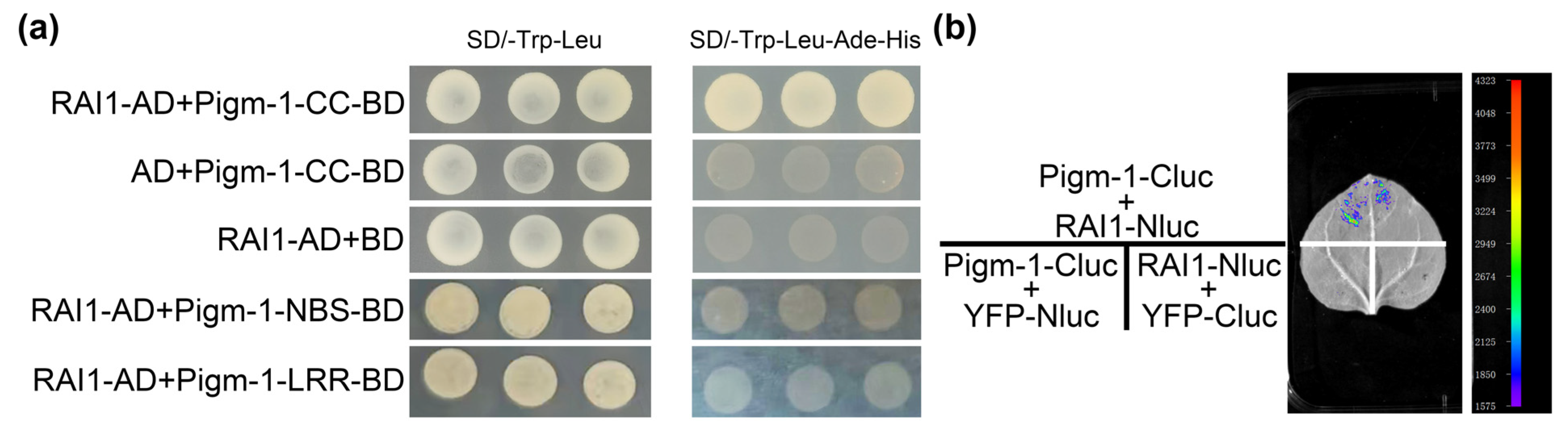
2.8. Pigm-1-CC Interacts with RAI1
3. Discussion
3.1. Application Prospect Analysis of the Pigm-1 Gene
3.2. OsRac1 Is Involved in Pigm-1-Mediated Blast Resistance
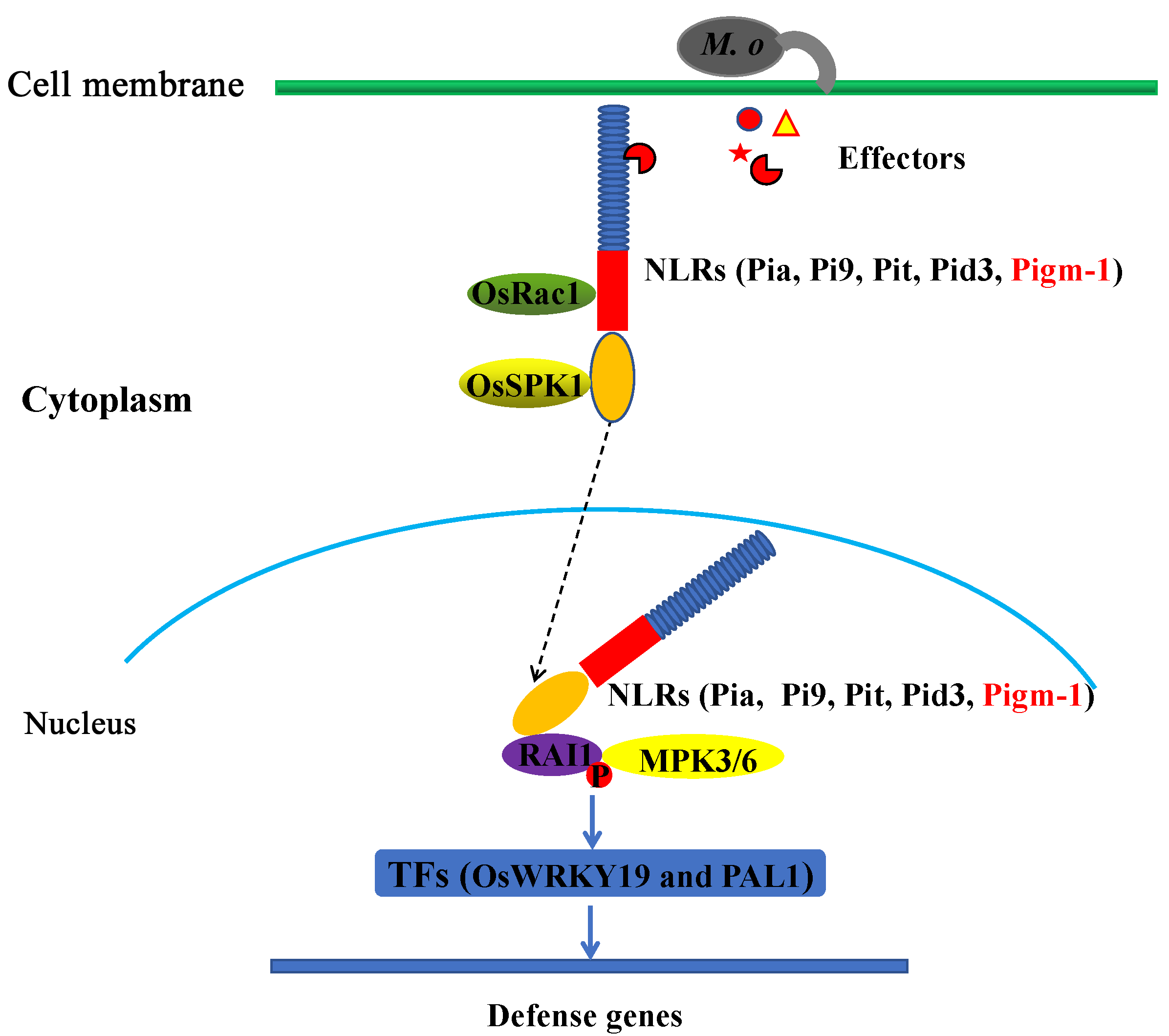
3.3. A Model for OsRac1 and RAI1-Mediated Disease Resistance
4. Materials and Methods
4.1. Plant Materials
4.2. Experiment on Incubation with Rice Blast Fungus
4.3. RNA and DNA Isolation
4.4. Targeted Mutagenesis of OsRac1 with CRISPR/Cas9
4.5. The LUC Assay
4.6. Y2H Assay
4.7. Bioinformatics Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jones, J.D.G.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Dodds, N.; Rathjen, J.P. Plant immunity: Towards an integrated view of plant-pathogen interactions. Nat. Rev. Genet. 2010, 11, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Feng, B.M.; Zhou, J.M.; Tang, D.Z. Plant immune signaling: Advancing on two frontiers. J. Integr. Plant Biol. 2020, 62, 2–24. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, K.; Katagiri, F. Comparing signaling mechanisms engaged in pattern-triggered and effector-triggered immunity. Curr. Opin. Plant Biol. 2010, 13, 459–465. [Google Scholar] [CrossRef]
- Thomma, B.P.H.J.; Nurnberger, T.; Joosten, M.H.A.J. Of PAMPs and effectors: The blurred PTI-ETI dichotomy. Plant Cell 2011, 23, 4–15. [Google Scholar] [CrossRef]
- Ngou, B.P.H.; Ahn, H.K.; Ding, P.; Jones, J.D.G. Mutual potentiation of plant immunity by cell-surface and intracellular receptors. Nature 2021, 592, 110–115. [Google Scholar] [CrossRef]
- Yuan, M.H.; Jiang, Z.Y.; Bi, G.Z.; Nomura, K.; Liu, M.H.; Wang, Y.P.; Cai, B.Y.; Zhou, J.M.; He, S.Y.; Xin, X.F. Pattern-recognition receptors are required for NLR-mediated plant immunity. Nature 2021, 592, 105–109. [Google Scholar] [CrossRef]
- Tang, D.Z.; Wang, G.X.; Zhou, J.M. Receptor kinases in plant-pathogen interactions: More than pattern recognition. Plant Cell 2017, 29, 618–637. [Google Scholar] [CrossRef]
- Dangl, J.L.; Horvath, D.M.; Staskawicz, B.J. Pivoting the plant immune system from dissection to deployment. Science 2013, 341, 746–751. [Google Scholar] [CrossRef]
- Deng, Y.W.; Zhai, K.R.; Xie, Z.; Yang, D.Y.; Zhu, X.D.; Liu, J.Z.; Wang, X.; Qin, P.; Yang, Y.Z.; Zhang, G.M.; et al. Epigenetic regulation of antagonistic receptors confers rice blast resistance with yield balance. Science 2017, 355, 962–965. [Google Scholar] [CrossRef]
- Berken, A. ROPs in the spotlight of plant signal transduction. Cell. Mol. Life Sci. 2006, 63, 2446–2459. [Google Scholar] [CrossRef] [PubMed]
- Nibau, C.; Wu, H.M.; Cheung, A.Y. RAC/ROP GTPases: ‘hubs’ for signal integration and diversification in plants. Trends Plant Sci. 2006, 11, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Kawano, Y.; Akamatsu, A.; Hayashi, K.; Housen, Y.; Okuda, J.; Yao, A.; Nakashima, A.; Takahashi, H.; Yoshida, H.; Wong, H.L.; et al. Activation of a Rac GTPase by the NLR family disease resistance protein Pit plays a critical role in rice innate immunity. Cell Host Microbe 2010, 7, 362–375. [Google Scholar] [CrossRef] [PubMed]
- Kawano, Y.; Shimamoto, K. Early signaling network in rice PRR and R-mediated immunity. Curr. Opin. Plant Biol. 2013, 16, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Kawano, Y.; Kaneko-Kawano, T.; Shimamoto, K. Rho family GTPase-dependent immunity in plants and animals. Front. Plant Sci. 2014, 5, 522. [Google Scholar] [CrossRef] [PubMed]
- Akamatsu, A.; Wong, H.L.; Fujiwara, M.; Okuda, J.; Nishide, K.; Uno, K.; Imai, K.; Umemura, K.; Kawasaki, T.; Kawano, Y.; et al. An OsCEBiP/OsCERK1-OsRacGEF1-OsRac1 module is an essential component of chitin-induced rice immunity. Cell Host Microbe 2013, 13, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.T.; Hamada, S.; Fujiwara, M.; Zhu, T.H.; Thao, N.P.; Wong, H.L.; Krishna, P.; Ueda, T.; Kaku, H.; Shibuya, N.; et al. The Hop/Sti1-Hsp90 chaperone complex facilitates the maturation and transport of a PAMP receptor in rice innate immunity. Cell Host Microbe 2010, 7, 185–196. [Google Scholar] [CrossRef]
- Ono, E.; Wong, H.L.; Kawasaki, T.; Hasegawa, M.; Kodama, O.; Shimamoto, K. Essential role of the small GTPase Rac in disease resistance of rice. Proc. Natl. Acad. Sci. USA 2001, 98, 759–764. [Google Scholar] [CrossRef]
- Wang, Q.; Li, Y.Y.; Ishikawa, K.; Kosami, K.I.; Uno, K.; Nagawa, S.; Tan, L.; Du, J.M.; Shimamoto, K.; Kawano, Y. Resistance protein Pit interacts with the GEF OsSPK1 to activate OsRac1 and trigger rice immunity. Proc. Natl. Acad. Sci. USA 2018, 115, 11551–11560. [Google Scholar] [CrossRef]
- Yu, M.X.; Zhou, Z.Z.; Liu, X.; Yin, D.D.; Li, D.Y.; Zhao, X.F.; Li, X.B.; Li, S.P.; Chen, R.J.; Lu, L.; et al. The OsSPK1–OsRac1–RAI1 defense signaling pathway is shared by two distantly related NLR proteins in rice blast resistance. Plant Physiol. 2021, 187, 2852–2864. [Google Scholar] [CrossRef]
- Yang, D.W.; Li, S.P.; Lu, L.; Fang, J.; Tan, D.Z. Identification and application of the Pigm-1 gene in rice disease-resistance breeding. Plant Biol. 2020, 22, 1022–1029. [Google Scholar] [CrossRef] [PubMed]
- He, N.Q.; Yang, D.W.; Zheng, X.H.; Huang, F.H.; Cheng, C.P.; Ye, N. Improving blast resistance of R20 by molecular marker-assisted selection of the Pigm-1 gene. J. Nucl. Agric. Sci. 2022, 36, 0245–0250. [Google Scholar]
- Zhou, Z.Z.; Pang, Z.Q.; Zhao, S.L.; Zhang, L.L.; Lv, Q.M.; Yin, D.D.; Li, D.Y.; Liu, X.; Zhao, X.F.; Li, X.B.; et al. Importance of OsRac1 and RAI1 in signalling of nucleotide-binding site leucine-rich repeat protein-mediated resistance to rice blast disease. New Phytol. 2019, 223, 828–838. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.H.; Saijo, Y.; Mauch, S.; Biskup, C.; Bieri, S.; Keller, B.; Seki, H.; Ulker, B.; Somssich, I.E.; Schulze-Lefert, P. Nuclear activity of MLA immune receptors links isolate-specific and basal disease-resistance responses. Science 2007, 315, 1098–1103. [Google Scholar] [CrossRef]
- Chang, C.; Yu, D.S.; Jiao, J.; Jing, S.J.; Schulze-Lefert, P.; Shen, Q.H. Barley MLA immune receptors directly interfere with antagonistically acting transcription factors to initiate disease resistance signaling. Plant Cell 2013, 25, 1158–1173. [Google Scholar] [CrossRef]
- Inoue, H.; Hayashi, N.; Matsushita, A.; Liu, X.Q.; Nakayama, A.; Sugano, S.; Jiang, C.J.; Takatsuji, H. Blast resistance of CC-NB-LRR protein Pb1 is mediated by WRKY45 through protein-protein interaction. Proc. Natl. Acad. Sci. USA 2013, 110, 9577–9582. [Google Scholar] [CrossRef]
- Townsend, P.D.; Dixon, C.H.; Slootweg, E.J.; Sukarta, O.C.A.; Yang, A.W.H.; Hughes, T.R.; Sharples, G.J.; Palsson, L.O.; Takken, F.L.W.; Goverse, A.; et al. The intracellular immune receptor Rx1 regulates the DNA-binding activity of a Golden2-like transcription factor. J. Biol. Chem. 2018, 293, 3218–3233. [Google Scholar] [CrossRef]
- Zhai, K.R.; Deng, Y.W.; Liang, D.; Tang, J.; Yan, B.X.; Yin, X.; Lin, H.; Chen, F.; Yang, D.Y.; Xie, Z.; et al. RRM transcription factors interact with NLRs and regulate broad-spectrum blast resistance in rice. Mol. Cell 2019, 74, 996–1009. [Google Scholar] [CrossRef]
- Lieberherr, D.; Thao, N.P.; Nakashima, A.; Umemura, K.; Kawasaki, T.; Shimamoto, K. A sphingolipid elicitor-inducible mitogen-activated protein kinase is regulated by the small GTPase OsRac1 and heterotrimeric G-protein in rice. Plant Physiol. 2005, 138, 1644–1652. [Google Scholar] [CrossRef]
- Thao, N.P.; Chen, L.; Nakashima, A.; Hara, S.; Umemura, K.; Takahashi, A.; Shirasu, K.; Kawasaki, T.; Shimamoto, K. RAR1 and HSP90 form a complex with Rac/Rop GTPase and function in innate-immune responses in rice. Plant Cell 2007, 19, 4035–4045. [Google Scholar] [CrossRef]
- Nakashima, A.; Chen, L.; Thao, N.P.; Fujiwara, M.; Wong, H.L.; Kuwano, M.; Wong, H.L.; Kuwano, M.; Umemura, K.; Shirasu, K.; et al. RACK1 functions in rice innate immunity by interacting with the Rac1 immune complex. Plant Cell 2008, 20, 2265–2279. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Oikawa, T.; Kyozuka, J.; Wong, H.L.; Umemura, K.; Kishi-Kaboshi, M.; Takahashi, A.; Kawano, Y.; Kawasaki, T.; Shimamoto, K. The bHLH Rac immunity1 (RAI1) is activated by OsRac1 via OsMAPK3 and OsMAPK6 in rice immunity. Plant Cell Physiol. 2012, 53, 740–754. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.W.; Li, S.P.; Xiao, Y.P.; Lu, L.; Zheng, Z.C.; Tang, D.Z.; Cui, H.T. Transcriptome analysis of rice response to blast fungus identified core genes involved in immunity. Plant Cell Environ. 2021, 44, 3103–3121. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.C.; Thompson, W.F. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980, 8, 4321–4325. [Google Scholar] [CrossRef]
- He, N.Q.; Zhan, G.P.; Huang, F.H.; Abou-Elwafa, S.F.; Yang, D.W. Fine mapping and cloning of a major QTL qph12, which simultaneously affects the plant height, panicle length, spikelet number and yield in rice (Oryza sativa L.). Front. Plant Sci. 2022, 13, 878558. [Google Scholar] [CrossRef]
- Xie, K.B.; Zhang, J.W.; Yang, Y.N. Genome-wide prediction of highly specific guide RNA spacers for CRISPR-Cas9-mediated genome editing in model plants and major crops. Mol. Plant 2014, 7, 923–926. [Google Scholar] [CrossRef]
- Ma, X.L.; Chen, L.T.; Zhu, Q.L.; Chen, Y.L.; Liu, Y.G. Rapid decoding of sequence-specific nuclease-induced heterozygous and biallelic mutations by direct sequencing of PCR products. Mol. Plant 2015, 8, 1285–1287. [Google Scholar] [CrossRef]
- Yan, H.J.; Zhao, Y.F.; Shi, H.; Li, J.; Wang, Y.C.; Tang, D.Z. BRASSINOSTEROID-SIGNALING KINASE1 phosphorylates MAPKKK5 to regulate immunity in Arabidopsis. Plant Physiol. 2018, 176, 2991–3002. [Google Scholar] [CrossRef]
- Zhao, Y.F.; Wu, G.H.; Shi, H.; Tang, D.Z. RECEPTOR-LIKE KINASE 902 associates with and phosphorylates BRASSINOSTEROID-SIGNALING KINASE1 to regulate plant immunity. Mol. Plant 2019, 12, 59–70. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, D.; He, N.; Huang, F.; Chen, J.; Yu, M.; Jin, Y.; Lin, S.; Li, S. Importance of OsRac1 in Signalling of Pigm-1 Mediated Resistance to Rice Blast Disease. Plants 2025, 14, 217. https://doi.org/10.3390/plants14020217
Yang D, He N, Huang F, Chen J, Yu M, Jin Y, Lin S, Li S. Importance of OsRac1 in Signalling of Pigm-1 Mediated Resistance to Rice Blast Disease. Plants. 2025; 14(2):217. https://doi.org/10.3390/plants14020217
Chicago/Turabian StyleYang, Dewei, Niqing He, Fenghuang Huang, Jialin Chen, Minxiang Yu, Yidan Jin, Shaojun Lin, and Shengping Li. 2025. "Importance of OsRac1 in Signalling of Pigm-1 Mediated Resistance to Rice Blast Disease" Plants 14, no. 2: 217. https://doi.org/10.3390/plants14020217
APA StyleYang, D., He, N., Huang, F., Chen, J., Yu, M., Jin, Y., Lin, S., & Li, S. (2025). Importance of OsRac1 in Signalling of Pigm-1 Mediated Resistance to Rice Blast Disease. Plants, 14(2), 217. https://doi.org/10.3390/plants14020217




