The Optimization of the Debittering Process and the Exploration of Bitter Metabolites of Paeonia ostii ‘Fengdan’ Seeds
Abstract
1. Introduction
2. Results and Discussion
2.1. Acidic Solution Soaking Reduces the Total Polyphenol Content of Paeonia ostii ‘Fengdan’ Kernels
2.2. Effect of Each Factor/Level on Debittering
2.3. Effect of Debittering on Morphological Characteristics of Paeonia ostii ‘Fengdan’ Kernels
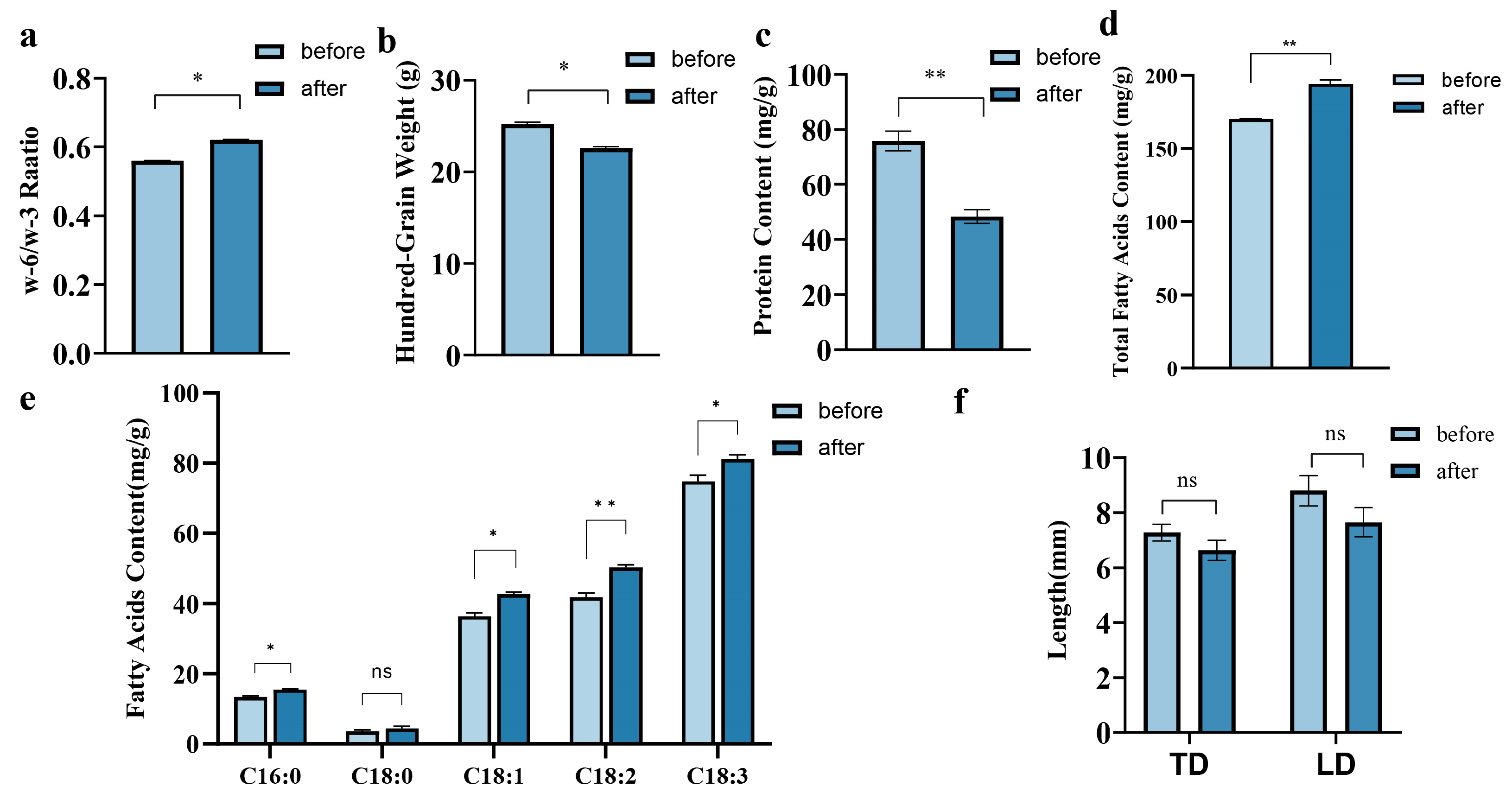
2.4. Effect of Debittering on Some Nutritional Qualities of Paeonia ostii ‘Fengdan’ Kernels
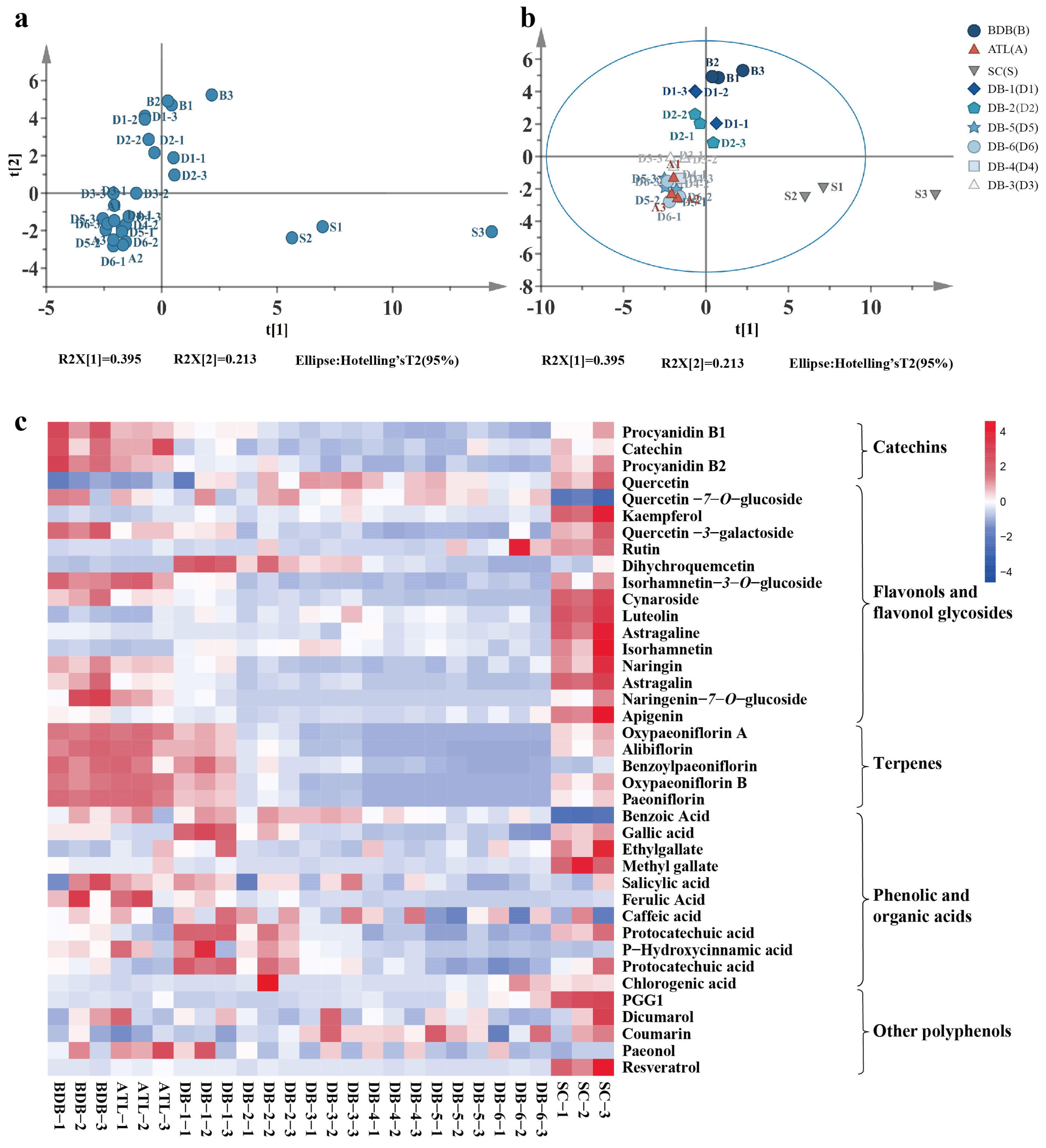
2.5. Analysis of Bitter Metabolites in Paeonia ostii ‘Fengdan’ Seeds
2.5.1. Bitter Metabolite Statistical Analysis
2.5.2. Differentially Accumulated Metabolites in Debittering
2.6. Specific Phenolic Metabolite Analysis
2.6.1. Monoterpene Glycosides
2.6.2. Flavonoids
Quercetins
Luteolin and Kaempferol
Catechins
2.6.3. Phenolic and Organic Acids
2.6.4. Resveratrol
3. Materials and Methods
3.1. Materials
3.1.1. Seed Materials
3.1.2. Chemicals and Reagents
3.2. Debittering Process of Paeonia ostii ‘Fengdan’ Seeds
3.2.1. Removal of Inner Seed Coat
3.2.2. Debittering by Acidic Solution
3.2.3. Determination of Total Polyphenol Content
3.2.4. Sensory Evaluation of Bitter Taste of ‘Fengdan’ Kernel
3.3. Nutritional Quality Evaluation Before and After Debittering
3.3.1. Determination of Fatty Acids’ Composition
3.3.2. Quantification of Soluble Protein Content
3.4. Identification of Polyphenol Compounds in Paeonia ostii ‘Fengdan’ Seeds During Debittering
3.4.1. Preparation of Seed Samples
3.4.2. LC-ESI-QQQ-MS Analysis of Methanol Extract
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, X.; Li, C.; Contreras, M.d.M.; Verardo, V.; Gómez-Caravaca, A.M.; Xing, C. Integrated Profiling of Fatty Acids, Sterols and Phenolic Compounds in Tree and Herbaceous Peony Seed Oils: Marker Screening for New Resources of Vegetable Oil. Foods 2020, 9, 770. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, T.; Yin, D.-X.; Song, P.; Hou, X.-G.; Qi, Q.; Qi, Z.-H. Major Fatty Acid Profiles and Bioactivity of Seed Oils from Ten Tree Peony Cultivars as a Potential Raw Material Source for the Cosmetics and Healthy Products. Chem. Biodivers. 2020, 17, e2000469. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhao, D.; Zhao, J. Study of the Cutting Mechanism of Oil Tree Peony Stem. Forests 2020, 11, 760. [Google Scholar] [CrossRef]
- Yu, S.; Du, S.; Yuan, J.; Hu, Y. Fatty Acid Profile in the Seeds and Seed Tissues of Paeonia L. Species as New Oil Plant Resources. Sci. Rep. 2016, 6, 26944. [Google Scholar] [CrossRef] [PubMed]
- Deng, R.; Gao, J.; Yi, J.; Liu, P. Could Peony Seeds Oil Become a High-Quality Edible Vegetable Oil? The Nutritional and Phytochemistry Profiles, Extraction, Health Benefits, Safety and Value-Added-Products. Food Res. Int. 2022, 156, 111200. [Google Scholar] [CrossRef]
- Cao, W.; Wang, Y.; Shehzad, Q.; Liu, Z.; Zeng, R. Effect of Different Solvents on the Extraction of Oil from Peony Seeds (Paeonia Suffruticosa Andr.): Oil Yield, Fatty Acids Composition, Minor Components, and Antioxidant Capacity. J. Oleo Sci. 2022, 71, 333–342. [Google Scholar] [CrossRef]
- Su, J.; Ma, C.; Liu, C.; Gao, C.; Nie, R.; Wang, H. Hypolipidemic Activity of Peony Seed Oil Rich in α-Linolenic, Is Mediated Through Inhibition of Lipogenesis and Upregulation of Fatty Acid β-Oxidation. J. Food Sci. 2016, 81, H1001–H1009. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, D.; Song, L.-M.; Xu, Q.; Li, H.; Xu, H. Chemical Profile and Antioxidant Activity of the Oil from Peony Seeds (Paeonia Suffruticosa Andr.). Oxid. Med. Cell. Longev. 2017, 2017, 9164905. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Shao, X.; Wei, Y.; Cheong, L.; Pan, L.; Tu, K. Rapid Detection of Adulterated Peony Seed Oil by Electronic Nose. J. Food Sci. Technol. 2018, 55, 2152–2159. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ma, X.; Li, Q.; Hu, Y.; Yang, J.; Song, Z. Effects of NSC in Different Organs and at Different Growth Stages on the Yield of Oil Peony Fengdan with Different Ages. Front. Plant Sci. 2023, 14, 1108668. [Google Scholar] [CrossRef]
- Zhang, Q.-Y.; Yu, R.; Xie, L.-H.; Rahman, M.M.; Kilaru, A.; Niu, L.-X.; Zhang, Y.-L. Fatty Acid and Associated Gene Expression Analyses of Three Tree Peony Species Reveal Key Genes for α-Linolenic Acid Synthesis in Seeds. Front. Plant Sci. 2018, 9, 106. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.K.K.; Gurumeenakshi, G.; Varadharaju, N.; Cheng, Y.-L.; Diosady, L.L. Debittering Moringa oleifera (Lam.) Leaves in Fortified South Indian Instant Soup. Chemosens. Percept. 2021, 14, 11–18. [Google Scholar] [CrossRef]
- Estivi, L.; Fusi, D.; Brandolini, A.; Hidalgo, A. Effect of Debittering with Different Solvents and Ultrasound on Carotenoids, Tocopherols, and Phenolics of Lupinus albus Seeds. Antioxidants 2022, 11, 2481. [Google Scholar] [CrossRef] [PubMed]
- Fontoin, H.; Saucier, C.; Teissedre, P.-L.; Glories, Y. Effect of pH, Ethanol and Acidity on Astringency and Bitterness of Grape Seed Tannin Oligomers in Model Wine Solution. Food Qual. Prefer. 2008, 19, 286–291. [Google Scholar] [CrossRef]
- Kore, V.T.; Chakraborty, I. Efficacy of Various Techniques on Biochemical Characteristics and Bitterness of Pummelo Juice. J. Food Sci. Technol. 2015, 52, 6073–6077. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Yan, B.; Chen, Z.; Wang, L.; Tang, W. Recent Technologies for the Extraction and Separation of Polyphenols in Different Plants: A Review. J. Renew. Mater. 2022, 10, 1471–1490. [Google Scholar] [CrossRef]
- Andrewes, P.; Busch, J.L.H.C.; de Joode, T.; Groenewegen, A.; Alexandre, H. Sensory Properties of Virgin Olive Oil Polyphenols: Identification of Deacetoxy-Ligstroside Aglycon as a Key Contributor to Pungency. J. Agric. Food Chem. 2003, 51, 1415–1420. [Google Scholar] [CrossRef] [PubMed]
- Ćurko, N.; Kovačević Ganić, K.; Gracin, L.; Đapić, M.; Jourdes, M.; Teissedre, P.L. Characterization of Seed and Skin Polyphenolic Extracts of Two Red Grape Cultivars Grown in Croatia and Their Sensory Perception in a Wine Model Medium. Food Chem. 2014, 145, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Fayeulle, N.; Preys, S.; Roger, J.-M.; Boulanger, R.; Hue, C.; Cheynier, V.; Sommerer, N. Multiblock Analysis to Relate Polyphenol Targeted Mass Spectrometry and Sensory Properties of Chocolates and Cocoa Beans. Metabolites 2020, 10, 311. [Google Scholar] [CrossRef]
- Suzuki, T.; Morishita, T.; Noda, T.; Ishiguro, K.; Otsuka, S.; Katsu, K. Breeding of Buckwheat to Reduce Bitterness and Rutin Hydrolysis. Plants 2021, 10, 791. [Google Scholar] [CrossRef]
- Colonges, K.; Seguine, E.; Saltos, A.; Davrieux, F.; Minier, J.; Jimenez, J.-C.; Lahon, M.-C.; Calderon, D.; Subia, C.; Sotomayor, I.; et al. Diversity and Determinants of Bitterness, Astringency, and Fat Content in Cultivated Nacional and Native Amazonian Cocoa Accessions from Ecuador. Plant Genome 2022, 15, e20218. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, Z.; Chen, X.; Guo, Z.; Wen, L.; Kan, J. Evaluation of Bitter Compounds in Zanthoxylum Schinifolium Sieb. et Zucc. by Instrumental and Sensory Analyses. Food Chem. 2022, 390, 133180. [Google Scholar] [CrossRef]
- Gao, C.; Tello, E.; Peterson, D.G. Identification of Compounds That Enhance Bitterness of Coffee Brew. Food Chem. 2023, 415, 135674. [Google Scholar] [CrossRef] [PubMed]
- Pascual, O.; González-Royo, E.; Gil, M.; Gómez-Alonso, S.; García-Romero, E.; Canals, J.M.; Hermosín-Gutíerrez, I.; Zamora, F. Influence of Grape Seeds and Stems on Wine Composition and Astringency. J. Agric. Food Chem. 2016, 64, 6555–6566. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.-Z.; Tang, J.-M.; Ni, J.; Zheng, T.-T.; Zhou, Y.; Sun, D.-Y.; Li, G.-N.; Liu, P.; Niu, L.-X.; Zhang, Y.-L. Comprehensive Metabolite Profile of Multi-Bioactive Extract from Tree Peony (Paeonia Ostii and Paeonia Rockii) Fruits Based on MS/MS Molecular Networking. Food Res. Int. 2021, 148, 110609. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Xiao, F.; Gong, J.; Yu, T. Applied Orthogonal Experiment Design for the Optimum Microwave-Assisted Extraction Conditions of Polysaccharides from Rhodiolae Radix. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 179–185. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cui, L.; Hou, X.; Li, W.; Leng, Y.; Zhang, Y.; Li, X.; Hou, Y.; Liu, Z.; Kang, W. Dynamic Changes of Secondary Metabolites and Tyrosinase Activity of Malus Pumila Flowers. BMC Chem. 2019, 13, 81. [Google Scholar] [CrossRef]
- Qu, J.-H.; Du, B.; Peng, F.; Wang, T.-K.; Yang, Y.-D. Optimisation of Triterpenoids Extraction from Anli Pears (Pyrus ussuriensis Maxim) by Pressurised Liquid Extraction. Qual. Assur. Saf. Crops Foods 2016, 8, 105–110. [Google Scholar] [CrossRef]
- Yang, D.M.; Zhu, X.Y.; Wang, Q.S.; Zhang, Q.; Wang, H.L.; Ying, T.J. Optimization of Extraction Conditions of Total Phenol from Lotus (Gaertn) Rhizome. J. Sci. Ind. Res. 2012, 71, 200–204. [Google Scholar]
- Ertaş, N.; Bilgiçli, N. Effect of Different Debittering Processes on Mineral and Phytic Acid Content of Lupin (Lupinus albus L.) Seeds. J. Food Sci. Technol. 2014, 51, 3348–3354. [Google Scholar] [CrossRef]
- Zhao, L.; Liang, N.; Lang, D.; Zhou, D.; Dong, X.; Peng, J.; Liu, L.; Pan, B.; Xing, B. Heating Methods Generate Different Amounts of Persistent Free Radicals from Unsaturated Fatty Acids. Sci. Total Environ. 2019, 672, 16–22. [Google Scholar] [CrossRef]
- Alshatwi, A.A.; Subash-Babu, P. Effects of Increasing Ratios of Dietary Omega-6/Omega-3 Fatty Acids on Human Monocyte Immunomodulation Linked with Atherosclerosis. J. Funct. Foods 2018, 41, 258–267. [Google Scholar] [CrossRef]
- Jiménez-Martínez, C.; Campos-Mendiola, R.; Sánchez-Espíndola, M.E.; Jiménez-Aparicio, A.; Gutiérrez-López, G.; Dávila-Ortiz, G. Microstructural Changes in Lupinus Campestris Seed in Response to Three Thermal Debittering Treatments. J. Sci. Food Agric. 2009, 89, 2399–2404. [Google Scholar] [CrossRef]
- Zhang, L.; Wei, W. Anti-Inflammatory and Immunoregulatory Effects of Paeoniflorin and Total Glucosides of Paeony. Pharmacol. Ther. 2020, 207, 107452. [Google Scholar] [CrossRef]
- Xin, Q.; Yuan, R.; Shi, W.; Zhu, Z.; Wang, Y.; Cong, W. A Review for the Anti-Inflammatory Effects of Paeoniflorin in Inflammatory Disorders. Life Sci. 2019, 237, 116925. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-L.; Wang, Y.-T.; Guo, Z.-Y.; Zhang, N.-N.; Wang, Y.-Y.; Hu, D.; Wang, Z.-Z.; Zhang, Y. Efficacy of Paeoniflorin on Models of Depression: A Systematic Review and Meta-Analysis of Rodent Studies. J. Ethnopharmacol. 2022, 290, 115067. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Chen, Y.; Tumilty, S.; Liu, L.; Luo, L.; Yin, H.; Xie, Y. Paeoniflorin Is a Promising Natural Monomer for Neurodegenerative Diseases via Modulation of Ca2+ and ROS Homeostasis. Curr. Opin. Pharmacol. 2022, 62, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Magar, R.T.; Sohng, J.K. A Review on Structure, Modifications and Structure-Activity Relation of Quercetin and Its Derivatives. J. Microbiol. Biotechnol. 2020, 30, 11–20. [Google Scholar] [CrossRef]
- Rolnik, A.; Olas, B. The Plants of the Asteraceae Family as Agents in the Protection of Human Health. Int. J. Mol. Sci. 2021, 22, 3009. [Google Scholar] [CrossRef] [PubMed]
- Fabjan, N.; Rode, J.; Kosir, I.J.; Wang, Z.; Zhang, Z.; Kreft, I. Tartary Buckwheat (Fagopyrum tataricum Gaertn.) as a Source of Dietary Rutin and Quercitrin. J. Agric. Food Chem. 2003, 51, 6452–6455. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, S.D.; de Sousa, L.R.F.; Burger, M.C.M.; Lima, M.I.S.; da Silva, M.F.D.G.; Fernandes, J.B.; Vieira, P.C. Evaluation of Flavonols and Derivatives as Human Cathepsin B Inhibitor. Nat. Prod. Res. 2015, 29, 2212–2214. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Kroeker, A.; He, S.; Kozak, R.; Audet, J.; Mbikay, M.; Chrétien, M. Prophylactic Efficacy of Quercetin 3-β-O-d-Glucoside against Ebola Virus Infection. Antimicrob. Agents Chemother. 2016, 60, 5182–5188. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Shin, S.Y.; Lee, Y.; Park, Y.; Kim, B.G.; Ahn, J.-H.; Chong, Y.; Lee, Y.H.; Lim, Y. Rhamnetin Production Based on the Rational Design of the Poplar O-Methyltransferase Enzyme and Its Biological Activities. Bioorg. Med. Chem. Lett. 2011, 21, 3866–3870. [Google Scholar] [CrossRef] [PubMed]
- Gendrisch, F.; Esser, P.R.; Schempp, C.M.; Wölfle, U. Luteolin as a Modulator of Skin Aging and Inflammation. BioFactors Oxf. Engl. 2021, 47, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Park, C.M.; Song, Y.-S. Luteolin and Luteolin-7-O-Glucoside Inhibit Lipopolysaccharide-Induced Inflammatory Responses through Modulation of NF-Kappa B/AP-1/PI3K-Akt Signaling Cascades in RAW 264.7 Cells. Nutr. Res. Pract. 2013, 7, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Harris, G.K.; Qian, Y.; Leonard, S.S.; Sbarra, D.C.; Shi, X. Luteolin and Chrysin Differentially Inhibit Cyclooxygenase-2 Expression and Scavenge Reactive Oxygen Species but Similarly Inhibit Prostaglandin-E2 Formation in RAW 264.7 Cells1. J. Nutr. 2006, 136, 1517–1521. [Google Scholar] [CrossRef] [PubMed]
- Sabzichi, M.; Hamishehkar, H.; Ramezani, F.; Sharifi, S.; Tabasinezhad, M.; Pirouzpanah, M.; Ghanbari, P.; Samadi, N. Luteolin-Loaded Phytosomes Sensitize Human Breast Carcinoma MDA-MB 231 Cells to Doxorubicin by Suppressing Nrf2 Mediated Signalling. Asian Pac. J. Cancer Prev. 2014, 15, 5311–5316. [Google Scholar] [CrossRef]
- Poinsaut, P.; Gerland, C. Les Tanins: Synergies Entre Tanins Des Raisins et Tanins Oenologiques. Mag. Trimest. D’information Prof. 1999, 26, 11–12. [Google Scholar]
- Musial, C.; Kuban-Jankowska, A.; Gorska-Ponikowska, M. Beneficial Properties of Green Tea Catechins. Int. J. Mol. Sci. 2020, 21, 1744. [Google Scholar] [CrossRef] [PubMed]
- Narukawa, M.; Kimata, H.; Noga, C.; Watanabe, T. Taste Characterisation of Green Tea Catechins. Int. J. Food Sci. Technol. 2010, 45, 1579–1585. [Google Scholar] [CrossRef]
- Del Olmo, A.; Calzada, J.; Nuñez, M. Benzoic Acid and Its Derivatives as Naturally Occurring Compounds in Foods and as Additives: Uses, Exposure, and Controversy. Crit. Rev. Food Sci. Nutr. 2017, 57, 3084–3103. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Rui, Y.; Guo, S.; Luan, F.; Liu, R.; Zeng, N. Ferulic Acid: A Review of Its Pharmacology, Pharmacokinetics and Derivatives. Life Sci. 2021, 284, 119921. [Google Scholar] [CrossRef]
- Tian, B.; Liu, J. Resveratrol: A Review of Plant Sources, Synthesis, Stability, Modification and Food Application. J. Sci. Food Agric. 2020, 100, 1392–1404. [Google Scholar] [CrossRef] [PubMed]
- Pastor, R.F.; Restani, P.; Di Lorenzo, C.; Orgiu, F.; Teissedre, P.-L.; Stockley, C.; Ruf, J.C.; Quini, C.I.; Garcìa Tejedor, N.; Gargantini, R.; et al. Resveratrol, Human Health and Winemaking Perspectives. Crit. Rev. Food Sci. Nutr. 2019, 59, 1237–1255. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, F.; Gao, X.-J.; Yong, J.-J.; Zhang, W.-H.; Zhao, J.-J.; Wang, H.-Q. Effects of different drying methods on content of bioactive component and antioxidant activity in Lycium ruthenicum. Zhongguo Zhong Yao Za Zhi Zhongguo Zhongyao Zazhi China J. Chin. Mater. Medica 2017, 42, 3926–3931. [Google Scholar] [CrossRef]
- Zhang, Q.; Yu, R.; Sun, D.; Rahman, M.M.; Xie, L.; Hu, J.; He, L.; Kilaru, A.; Niu, L.; Zhang, Y. Comparative Transcriptome Analysis Reveals an Efficient Mechanism for α-Linolenic Acid Synthesis in Tree Peony Seeds. Int. J. Mol. Sci. 2019, 20, 65. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.-Z.; Ni, J.; Tang, J.-M.; Sun, D.-Y.; Yan, Z.-G.; Zhang, J.; Niu, L.-X.; Zhang, Y.-L. Bioactive Components, Antioxidant and Antimicrobial Activities of Paeonia rockii Fruit during Development. Food Chem. 2021, 343, 128444. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Tang, W.; Wang, H.; Sheng, Y.; Tan, X.; Shi, Y.; Fan, W.; Ge, S. Phosphoric Acid Pretreatment of Poplar to Optimize Fermentable Sugars Production Based on Orthogonal Experimental Design. Front. Chem. 2023, 11, 1119215. [Google Scholar] [CrossRef] [PubMed]



| Processing Combinations | pH | Temperature (°C) | Processing Time (h) | Polyphenol Content (mgGAE/g) |
|---|---|---|---|---|
| 1 | 3 | 35 | 24 | 29.9253 ± 1.1078 |
| 2 | 3 | 45 | 36 | 43.5259 ± 2.0622 |
| 3 | 3 | 55 | 48 | 9.3822 ± 1.0635 |
| 4 | 5 | 35 | 36 | 44.6006 ± 2.1256 |
| 5 | 5 | 45 | 48 | 40.4397 ± 1.1195 |
| 6 | 5 | 55 | 24 | 53.3822 ± 1.1474 |
| 7 | 7 | 35 | 48 | 34.4310 ± 2.0603 |
| 8 | 7 | 45 | 24 | 45.0201 ± 1.2101 |
| 9 | 7 | 55 | 36 | 41.8218 ± 2.098 |
| K1 | 27.6111 | 36.3190 | 42.7759 | |
| K2 | 46.1408 | 42.9952 | 43.3161 | |
| K3 | 40.4243 | 34.8621 | 28.0843 | |
| R | 18.5297 | 8.1331 | 15.2318 |
| Factors | Degrees of Freedom (df) | Sum of Squares (s) | Variance | F Value | p Value | Significance |
|---|---|---|---|---|---|---|
| pH | 2 | 1979.092 | 989.546 | 27.474 | 0.000 | ** |
| Temperature (°C) | 2 | 207.096 | 103.548 | 2.875 | 0.080 | ns |
| Processing Time (h) | 2 | 1153.083 | 576.542 | 16.007 | 0.000 | ** |
| Levels | Factors | ||
|---|---|---|---|
| A-pH | B-Temperature/°C | C-Processing Time/h | |
| 1 | 3 | 35 | 24 |
| 2 | 5 | 45 | 36 |
| 3 | 7 | 55 | 48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Xu, Y.; Liu, X.; Su, Q.; Zhang, J.; Zhang, X.; Guo, X.; Zhang, Y.; Zhang, Q. The Optimization of the Debittering Process and the Exploration of Bitter Metabolites of Paeonia ostii ‘Fengdan’ Seeds. Plants 2025, 14, 198. https://doi.org/10.3390/plants14020198
Li S, Xu Y, Liu X, Su Q, Zhang J, Zhang X, Guo X, Zhang Y, Zhang Q. The Optimization of the Debittering Process and the Exploration of Bitter Metabolites of Paeonia ostii ‘Fengdan’ Seeds. Plants. 2025; 14(2):198. https://doi.org/10.3390/plants14020198
Chicago/Turabian StyleLi, Shuting, Yanfeng Xu, Xinyue Liu, Qizhen Su, Junyu Zhang, Xinran Zhang, Xinmiao Guo, Yanlong Zhang, and Qingyu Zhang. 2025. "The Optimization of the Debittering Process and the Exploration of Bitter Metabolites of Paeonia ostii ‘Fengdan’ Seeds" Plants 14, no. 2: 198. https://doi.org/10.3390/plants14020198
APA StyleLi, S., Xu, Y., Liu, X., Su, Q., Zhang, J., Zhang, X., Guo, X., Zhang, Y., & Zhang, Q. (2025). The Optimization of the Debittering Process and the Exploration of Bitter Metabolites of Paeonia ostii ‘Fengdan’ Seeds. Plants, 14(2), 198. https://doi.org/10.3390/plants14020198






