Response of Plant Leaf Traits to Environmental Factors in Climax Communities at Varying Latitudes in Karst Regions
Abstract
1. Introduction
2. Study Area and Research Methodology
2.1. Overview of the Study Area
2.2. Sample Setup
2.3. Sample Collection and Processing
- (1)
- Plant leaf sampling and processing
- (2)
- Soil Collection
2.4. Determination of Functional Properties and Analysis of Samples
- (1)
- Elemental Determination
- (2)
- Determination of Functional Traits
2.5. Data Processing
3. Results and Analysis
3.1. Changes in Leaf Functional Traits of the Climax Community at Varying Latitudes
3.2. Correlations Between Leaf Traits in the Climax Community
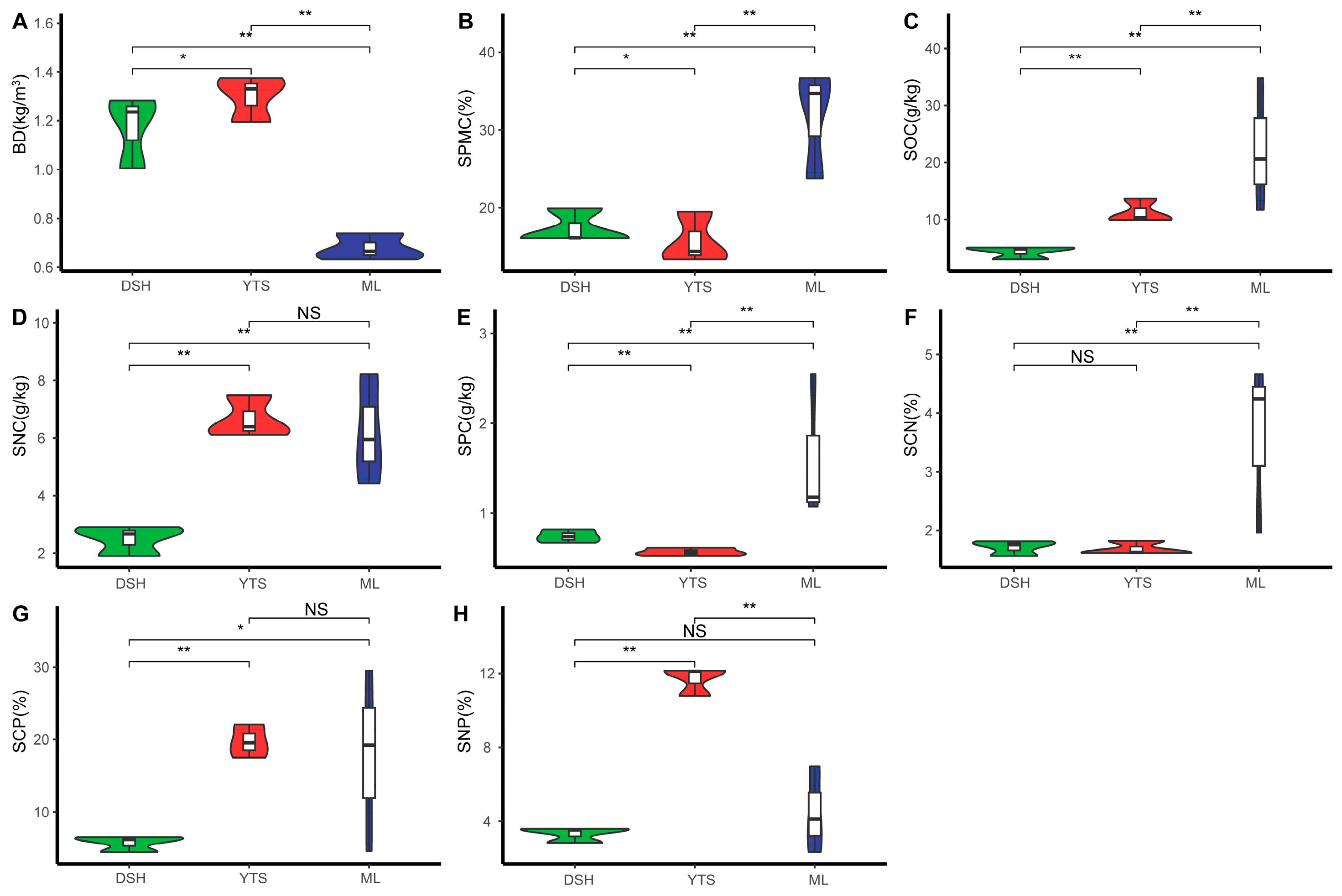
3.3. Changes in Environmental Factors of the Climax Community at Different Latitudes
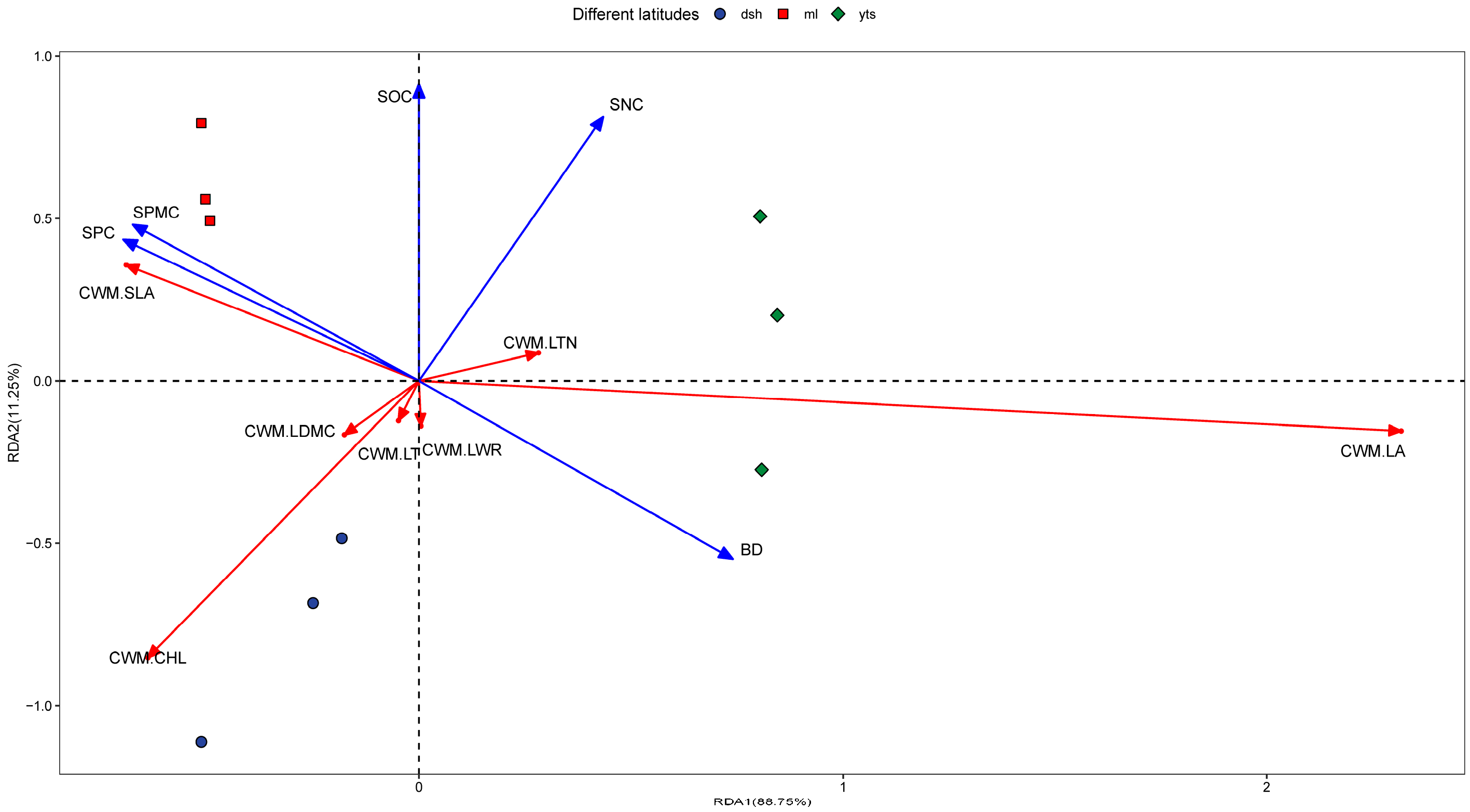
3.4. Relationships Between Environmental Factors and Leaf Traits in the Climax Community
4. Discussion
4.1. Changes in the Laws of Plant Functional Traits in Karst Vertex Communities Across Different Latitudes
4.2. Changes in Soil Factors in Karst Vertex Communities at Varying Latitudes
4.3. Response of Plant Functional Traits in the Climax Community to Environmental Factors at Different Latitudes
5. Conclusions
- (1)
- Karst plant communities display distinct functional trait combinations at varying latitudes. As latitude increases, the resource utilization traits shift to a combination characterized by high leaf thickness (LT) and low specific leaf area (SLA). In terms of overall changes, the functional traits of the apex communities exhibited some convergence effects, which may be attributed to the limited variation in environmental pressures with latitude in the karst region;
- (2)
- Bulk density (BD), soil microbial carbon (SPMC), soil nitrogen carbon (SNC), soil phosphorus content (SPC), and soil organic carbon (SOC) play a significant role in the changes in functional traits during the process of plant restoration. Plants are primarily limited by bulk density (BD) during growth, and most environmental factors exert a limiting effect on plant functional traits. Furthermore, many plant functional traits can only fulfill their roles under specific environmental conditions. Among the various responses of plant functional traits to environmental factors, community-weighted mean leaf area (CWM.LA) and community-weighted mean chlorophyll content (CWM.CHL) were found to be the most sensitive to soil factors.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, J.; Wang, Y.; He, N.; Ye, Z.; Chen, C.; Zang, R.; Feng, Y.; Lu, Q.; Li, J. Plant functional traits regulate soil bacterial diversity across temperate deserts. Sci. Total Environ. 2020, 715, 136976. [Google Scholar] [CrossRef] [PubMed]
- He, N.; Li, Y.; Liu, C.; Xu, L.; Li, M.; Zhang, J.; He, J.; Tang, Z.; Han, X.; Ye, Q.; et al. Plant Trait Networks: Improved resolution of the dimensionality of adaptation. Trends Ecol. Evol. 2020, 35, 908–918. [Google Scholar] [CrossRef]
- Li, T.; Deng, Q.; Yuan, Z.Y.; Jiao, F. Latitudinal changes in plant stoichiometric and soil C,N,P stoichiometry in loess Plateau. Chin. J. Environ. Sci. 2015, 36, 2988–2996. [Google Scholar]
- Zhang, L.; Tan, X.; Dong, Z.; Zheng, J.; Yuan, Z.; Li, C. The relationship between plant functional traits and soil physicochemical properties in the riparian zones of downtown Chongqing. Acta Ecol. Sin. 2023, 43, 1892–1902, (In Chinese with English Abstract). [Google Scholar]
- Liu, M.; Li, L.; Che, Y.; Jiao, J. Functional traits of plant leaves at different succession stages in alpine meadow. Bull. Bot. Res. 2019, 39, 760–769, (In Chinese with English Abstract). [Google Scholar]
- Zhou, X.; Xin, J.; Huang, X.; Li, H.; Li, F.; Song, W. Linking leaf functional traits with soil and climate factors in forest ecosystems in China. Plants 2022, 11, 3545. [Google Scholar] [CrossRef]
- He, N.; Yan, P.; Liu, C.; Xu, L.; Li, M.; Van Meerbeek, K.; Zhou, G.; Zhou, G.; Liu, S.; Zhou, X.; et al. Predicting ecosystem productivity based on plant community traits. Trends Plant Sci. 2023, 28, 43–53. [Google Scholar] [CrossRef]
- Ackerly, D.; Cornwell, W. A trait-based approach to community assembly: Partitioning of species trait values into within- and among- community components. Ecol. Lett. 2007, 10, 135–145. [Google Scholar] [CrossRef]
- Corlett, R.; Westcott, D. Will plant movements keep up with climate change? Trends Ecol. Evol. 2013, 28, 482–488. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, H.; Wang, X.; Long, W.; Li, C.; Fang, Y.; Fu, M.; Zhu, K. Effects of functional diversity and phylogenetic diversity on the tropical cloud forest community assembly. Chin. J. Plant Ecol. 2019, 43, 217–226, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Kembel, S.; Hubbell, S. The phylogenetic structure of a neotropical forest tree community. Ecology 2006, 87, S86–S99. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liang, S.; Zhou, L.; Liang, S.; Ning, J.; Mo, J.; Li, D. Maintenance of species diversity in evergreen deciduous broad-leaved mixed forest of karst hills in central subtropical region based on functional traits. Acta Ecol. Sin. 2024, 44, 4400–4411, (In Chinese with English Abstract). [Google Scholar]
- Miao, L.; Liu, M.; Xiao, Y.; Yang, C.; Wang, Q.; Wang, M. Studies on phylogeny and functional traits structure of plant communities at different latitudes. Acta Ecol. Sin. 2024, 44, 317–329. [Google Scholar]
- Fang, J.; Wang, X.; Shen, Z.; Tang, Z.; He, J.; Yu, D.; Jiang, Y.; Wang, Z.; Zheng, C.; Zhu, J.; et al. Methods and protocols for plant community inventory. Biodivers. Sci. 2009, 17, 533–548, (In Chinese with English Abstract). [Google Scholar]
- Bao, S.D. Soil Agrochemical Analysis, 3rd ed.; Agricultural Press: Beijing, China, 2005; pp. 45–52. [Google Scholar]
- NY/T2017-2011; Determination of Nitrogen, Phosphorus and Potassium in Plants. Ministry of Agriculture: Beijing, China, 2011.
- LY/T1228-2015; Determination of Nitrogen in Forest Soil. Ministry of Agriculture: Beijing, China, 2015.
- LY/T1232-2015; Phosphorus Determination Methods of Forest Soils. Ministry of Agriculture: Beijing, China, 2015.
- Pérezharguindeguy, N.; Díaz, S.; Garnier, E. New handbook for standardised measurement of plant functional traits worldwide. Aust. J. Bot. 2013, 61, 167–234. [Google Scholar] [CrossRef]
- Lavorel, S.; Grigulis, K.; McIntyre, S.; Williams, N.; Garden, D.; Dorrough, J.; Berman, S.; Quétier, F.; Thébault, A.; Bonis, A. Assessing functional diversity in the field-methodology matters. Fun. Ecol. 2008, 22, 134–147. [Google Scholar] [CrossRef]
- The SPSSAU Project. SPSSAU, Version 25.0. Online Application Software. 2022. Available online: https://www.spssau.com (accessed on 12 February 2024).
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. 2020. Available online: https://www.R-project.org/ (accessed on 12 February 2024).
- Reich, P. The world-wide “fast-slow” plant economics spectrum: A traits manifesto. J. Ecol. 2014, 102, 275–301. [Google Scholar] [CrossRef]
- Wright, I.; Reich, P.; Westoby, M.; Ackerly, D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef]
- Liu, R.H.; Bai, J.L.; Bao, H.; Zhao, J.J.; Jiang, Y.; Liang, S.C.; Li, Y.J. Variation and correlation in functional traits of main woody plants in the cyclobalanopsis glauca community in the karst hills of Guilin, southwest China. Chin. J. Plant Ecol. 2020, 44, 828–841, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Liu, M.X.; Nan, X.N.; Zhang, G.J.; Li, B.W.; Xu, L.; Mu, R.L.; Li, L.; Yu, R.X. Relationship between species diversity and functional diversity of plant communities on different slopes in alpine meadow. Acta Ecol. Sin. 2021, 41, 5398–5407, (In Chinese with English Abstract). [Google Scholar]
- Zhang, Z.K.; Zheng, X.X.; Lin, H.Z.; Lin, X.; Hang, L.J. Summary of changes in plant functional traits and environmental factors in different successional stages of island plants. Acta Ecol. Sin. 2019, 39, 3749–3758, (In Chinese with English Abstract). [Google Scholar]
- Li, Y.J.; Zheng, J.M.; Wang, G.Z.; Zhou, J.X.; Liu, Y.G.; Ha, W.X. A study of functional traits of natural secondary forests and their influencing factors in different succession stages in Karst areas: A case study of Dahei mountain, Yunnan province. Acta Geosci. Sin. 2021, 42, 397–406, (In Chinese with English Abstract). [Google Scholar]
- Wu, X. Effects of plants functional traits and diversity on forest biomass and productivity along latitudinal gradient and during successional stages. Chin. J. Plant Ecol. 2018, 42, 562–572, (In Chinese with English Abstract). [Google Scholar]
- Zhang, J.Y. Characteristics of plant communities across the natural tropical coniferous forest -broadleaved forest ecotones in Hainan Island, China. Trends Ecol. Evol. 2020, 35, 908–918, (In Chinese with English Abstract). [Google Scholar]
- Lu, M.M.; Huang, X.C.; Ci, X.Q.; Yang, G.P.; Li, J. Phylogenetic community structure of subtropical forests along elevational gradients in Ailao Mountains of southwest China. Chin. Biodivers. 2014, 22, 438–448, (In Chinese with English Abstract). [Google Scholar]
- Wang, Y.; Zhang, L.; Chen, J.; Feng, L.; Li, F.; Yu, L. Functional diversity of plant communities in relationship to leaf and soil stoichiometry in karst areas of southwest China. Forests 2022, 13, 864. [Google Scholar] [CrossRef]
- Keddy, E. Assembly and response rules: Two goals for predictive community ecology. J. Veg. Sci. 1992, 3, 157–164. [Google Scholar] [CrossRef]
- Mayfield, M.; Levine, J. Opposing effects of competitive exclusion on the phylogenetic structure of communities. Ecol. Lett. 2010, 13, 1085–1093. [Google Scholar] [CrossRef]
- Liu, M.X. Studies on physiological and leaf morphological traits for photosynthesis on different slopes in a subalpine meadow. Acta Ecol. Sin. 2017, 37, 8526–8536, (In Chinese with English Abstract). [Google Scholar]
- Kang, L.L. Effects of Karst Habitat Heterogeneity on Plant Leaf Functional Traits and Soil Nutrients. Master’s Thesis, Guizhou University, Guiyang, China, 2021. (In Chinese with English Abstract). [Google Scholar]
- Wang, S.Q.; Yu, G.R. Ecological stoichiometry characteristics of ecosystem carbon, nitrogen and phosphorus elements. Acta Ecol. Sin. 2008, 28, 3937–3947, (In Chinese with English Abstract). [Google Scholar]
- Cleveland, C.C.; Liptzin, D. C: N: P Stoichiometry in soil: Is there a “redfield ratio” for the microbial biomass? Biogeochemistry 2007, 85, 235–246. [Google Scholar] [CrossRef]
- Tian, H.; Chen, G.; Zhang, C.; Jerry, M.; Chars, A. Pattern and variation of C: N: P ratios in China’s soils: A synthesis of observational data. Biogeochemistry 2010, 98, 139–151. [Google Scholar] [CrossRef]
- Liu, N.; Yu, L.F.; Zhao, Q.; Wu, Y.N.; Yan, L.B. C: N: P stoichiometry of leaf-litter-soil continuum in secondary forests of the rocky desertification regions of the karst plateau. Chin. J. Appl. Environ. Biol. 2020, 26, 681–688, (In Chinese with English Abstract). [Google Scholar]
- Liu, Y. Effects of Soil Depth on Plant Community Structure and Biomass in Karst Region. Ph.D. Thesis, Southwest University, Chongqing, China, 2020. (In Chinese with English Abstract). [Google Scholar]
- Wang, Q.; Rong, L.; Wang, M.J.; Ye, T.M.; Li, T.T.; Yang, W.S.; Li, X. Response of leaf functional traits of woody plants to degraded karst soil in central Guizhou. Earth Environ. 2022, 50, 639–647, (In Chinese with English Abstract). [Google Scholar]
- Liu, X.J.; Ma, K.P. Plant functional traits-concepts, applications and future directions. Sci. Sin. Vitae 2015, 45, 325–339, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Chen, J.; Feng, L.; Li, F.; Yu, L. Study on the relationship between functional characteristics and environmental factors in karst plant communities. Ecol. Evol. 2022, 12, e9335. [Google Scholar] [CrossRef]
- Zhou, T.; Cui, Y.C.; Ye, Y.Y.; Zhao, W.J.; Hou, Y.J.; Wu, P.; Ding, F.J. Leaf functional traits of typical karst forest plants under different niches. J. Cent. South Univ. For. Technol. 2022, 42, 129–140, (In Chinese with English Abstract). [Google Scholar]
- Shui, W.; Guo, P.P.; Zhu, S.F.; Feng, J.; Sun, X.; Li, H. Variation of plant functional traits and adaptive strategy of woody species in degraded karst tiankeng of Yunnan province. Sci. Geogr. Sin. 2022, 42, 1295–1306, (In Chinese with English Abstract). [Google Scholar]
- Huang, L.J.; Yu, Y.M.; An, X.F.; Yu, L.L.; Xue, Y.G. Leaf functional traits, species diversity and functional diversity of plant community in Tiankeng forests. Acta Ecol. Sin. 2022, 42, 10264–10275, (In Chinese with English Abstract). [Google Scholar]
- Wang, X.R.; Xing, Y.J. Research progress on the effects of environmental factors and community dynamics on plant species diversity. Int. J. Ecol. 2021, 10, 608–617, (In Chinese with English Abstract). [Google Scholar] [CrossRef]




| Sample Site | Dominant Species | Cover (%) | Elevation (m) | Longitude (°) | Latitude (°) |
|---|---|---|---|---|---|
| DSH | Fagus longipetiolata, Cunninghamia lanceolata, Woonyoungia septentrionalis, Synedrella nodiflora | 85 | 645.58 | 107.576389 E | 29.102778 N |
| YTS | Liquidambar formosana, Rhus chinensis, Styrax confusus, Pteridium aquilinum | 80 | 667.83 | 108.116208 E | 27.110187 N |
| ML | Cornus wilsoniana, Lindera communis, Nandina domestica, Selaginella tamariscina | 90 | 744.35 | 108.022222 E | 25.258333 N |
| Functional Feature Type | Data Type | Attribute |
|---|---|---|
| Leaf thickness (LT) | numerical value | Leaf blade thickness (mm) |
| Leaf area (LA) | numerical value | Mean leaf blade area of species (cm2) |
| Specific leaf area (SLA) | numerical value | Fresh leaf area/leaf dry mass (mm2/mg) |
| Chlorophyll (CHL) | numerical value | Leaf blade chlorophyll content (%) |
| Leaf aspect ratio (LWR) | numerical value | Leaf blade length to width ratio (%) |
| Leaf tissue density (LTN) | numerical value | Leaf blade dry weight/leaf volume (g/cm3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, G.; Wang, Y.; Chen, Z.; Ji, Y.; Lu, Y.; Liang, Y.; Zhou, R.; Tao, J. Response of Plant Leaf Traits to Environmental Factors in Climax Communities at Varying Latitudes in Karst Regions. Plants 2025, 14, 183. https://doi.org/10.3390/plants14020183
Xie G, Wang Y, Chen Z, Ji Y, Lu Y, Liang Y, Zhou R, Tao J. Response of Plant Leaf Traits to Environmental Factors in Climax Communities at Varying Latitudes in Karst Regions. Plants. 2025; 14(2):183. https://doi.org/10.3390/plants14020183
Chicago/Turabian StyleXie, Gang, Yang Wang, Zhifeng Chen, Yangyang Ji, Yao Lu, Yi Liang, Ruiyu Zhou, and Juan Tao. 2025. "Response of Plant Leaf Traits to Environmental Factors in Climax Communities at Varying Latitudes in Karst Regions" Plants 14, no. 2: 183. https://doi.org/10.3390/plants14020183
APA StyleXie, G., Wang, Y., Chen, Z., Ji, Y., Lu, Y., Liang, Y., Zhou, R., & Tao, J. (2025). Response of Plant Leaf Traits to Environmental Factors in Climax Communities at Varying Latitudes in Karst Regions. Plants, 14(2), 183. https://doi.org/10.3390/plants14020183







