Herbicidal and Antibacterial Secondary Metabolites Isolated from the Nicotiana tabacum-Derived Endophytic Fungus Aspergillus japonicus TE-739D
Abstract
1. Introduction
2. Results
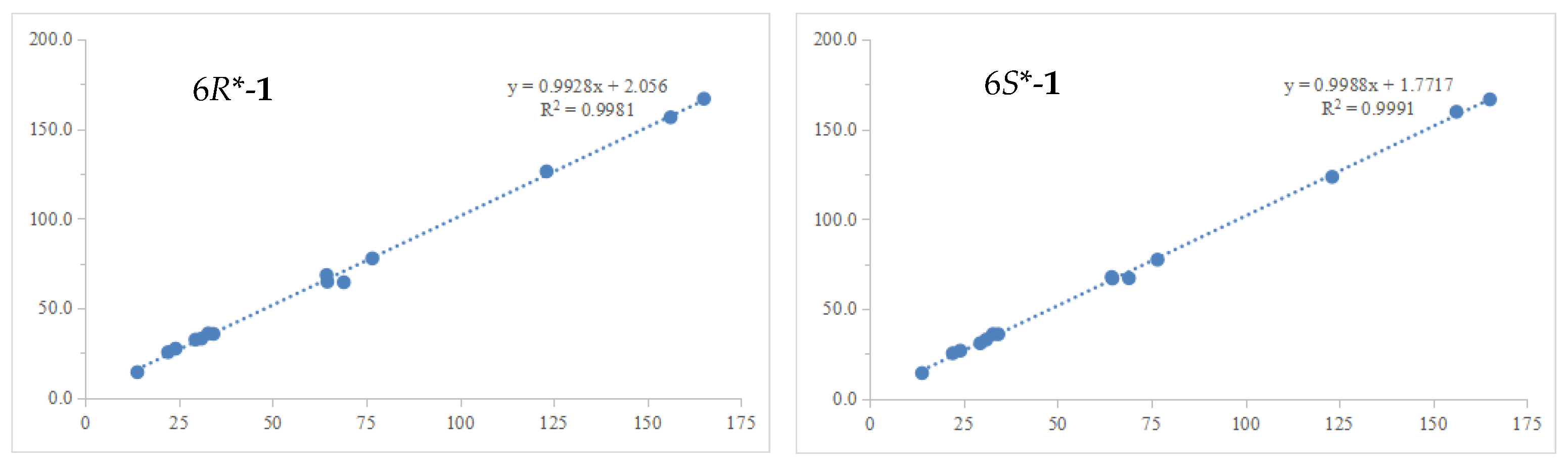
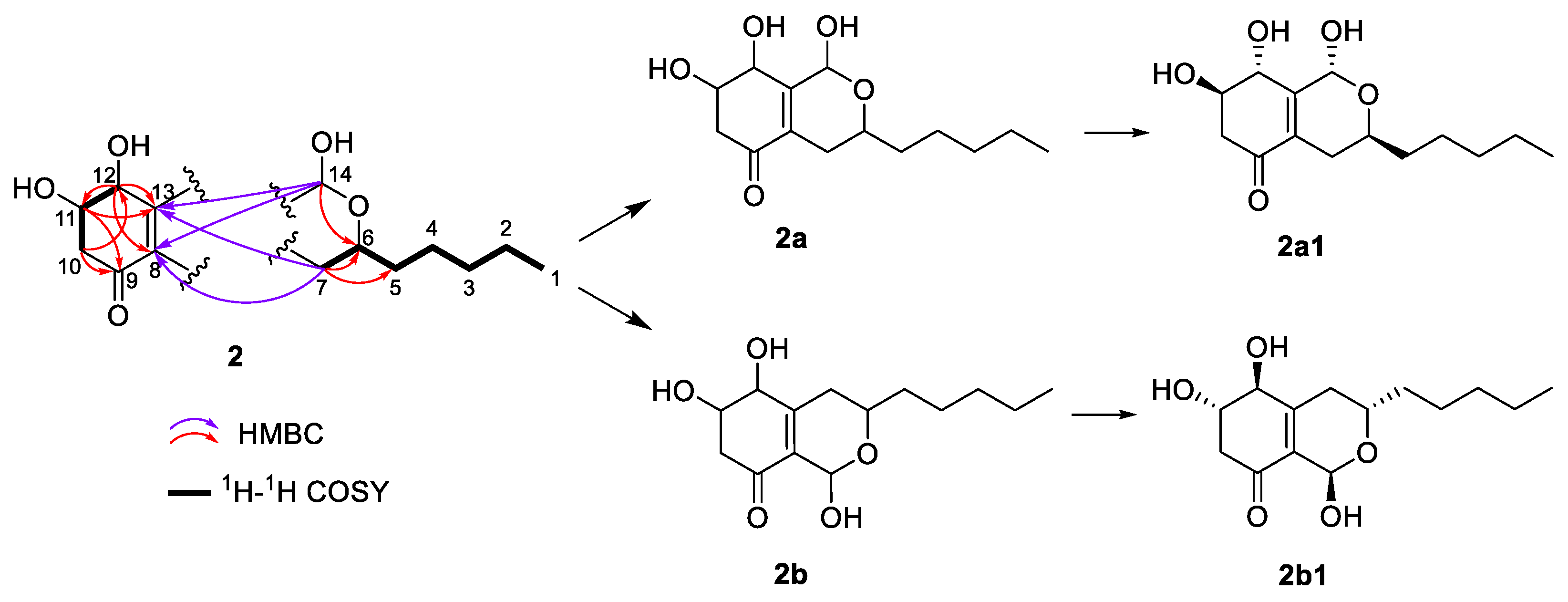
2.1. Structure Elucidation of Compounds
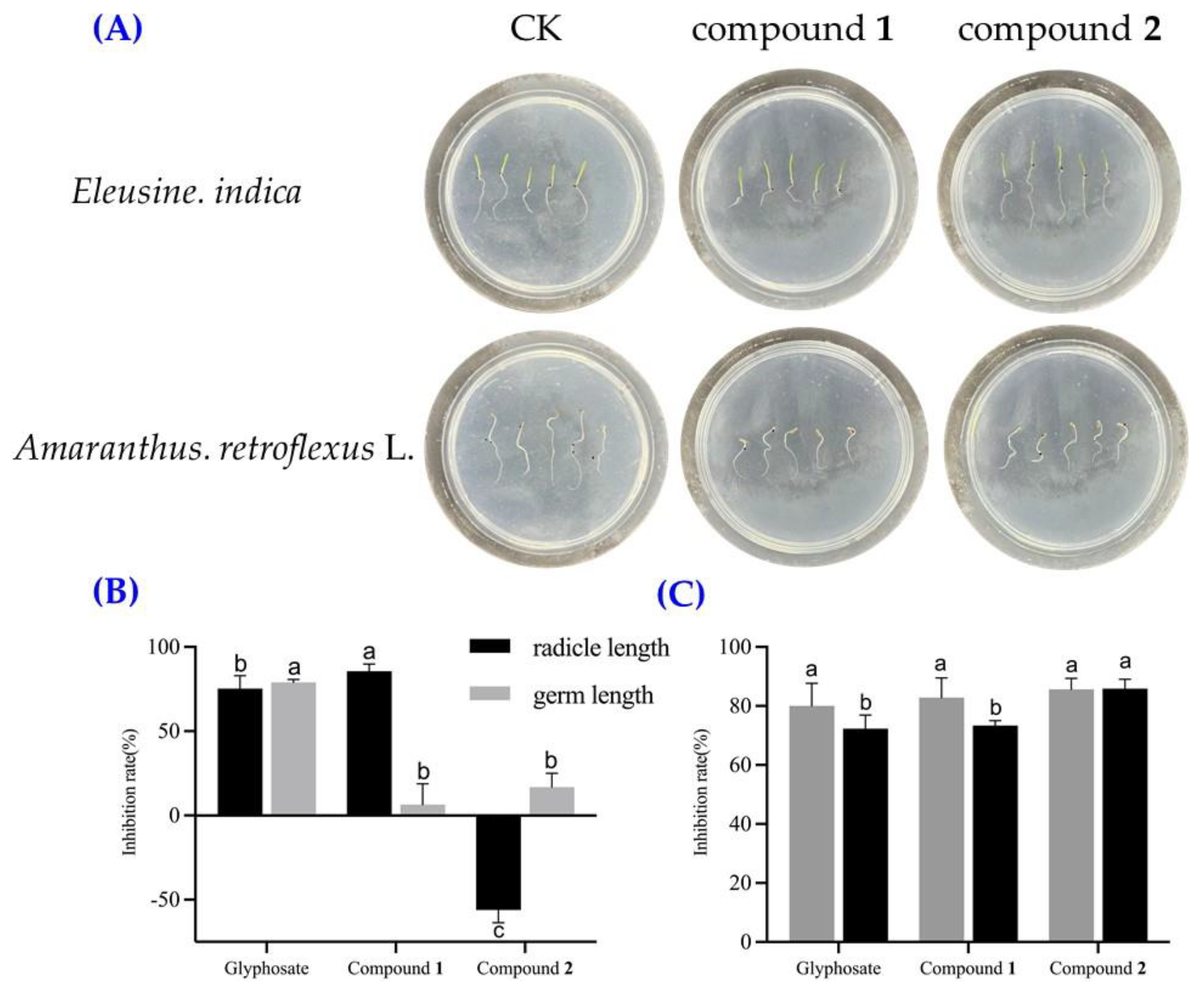
2.2. Herbicidal Activities of Compounds 1 and 2
2.3. Antibacterial Activities of Compounds 1–6
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Separation, Purification, and Identification of A. japonicus TE-739D
4.3. Fermentation and Extraction
4.4. Isolation
4.5. Spectral Data of the Isolated Compounds
4.5.1. Japonione A (1)
4.5.2. Japonione B (2)
4.6. Seed Germination Inhibition Assays
4.7. Antibacterial Assays
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gu, G.; Gong, X.; Xu, D.; Yang, Y.L.; Yin, R.Y.; Dai, J.G.; Zhu, K.; Lai, D.W.; Zhou, L.G. Diphenyl Ether Derivative Rhexocerins and Rhexocercosporins from the Endophytic Fungus Rhexocercosporidium sp. Dzf14 Active against Gram-Positive Bacteria with Multidrug-Resistance. J. Nat. Prod. 2023, 86, 1931–1938. [Google Scholar] [CrossRef] [PubMed]
- Gu, G.; Hou, X.W.; Xue, M.Y.; Jia, X.W.; Pan, X.Q.; Xu, D.; Dai, J.G.; Lai, D.W.; Zhou, L.G. Rosellichalasins A-H, cytotoxic cytochalasans from the endophytic fungus Rosellinia sp. Glinf021. Phytochemistry 2024, 222, 114103. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.L.; Wang, X.F.; Xu, K.; Li, W.; Chen, D.; Zhang, P. Characterization of a New Insecticidal Anthraquinone Derivative from an Endophyte of Acremonium vitellinum against Helicoverpa armigera. J. Agric. Food Chem. 2020, 68, 11480–11487. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Shan, T.; Mou, Y.; Zhou, L. Plant-Derived Bioactive Compounds Produced by Endophytic Fungi. Mini-Rev. Med. Chem. 2011, 11, 159–168. [Google Scholar] [CrossRef]
- Gu, G.; Hou, X.W.; Xue, M.Y.; Pan, X.Q.; Dong, J.; Yang, Y.L.; Amuzu, P.; Xu, D.; Lai, D.W.; Zhou, L.G. Diphenyl ethers from endophytic fungus Rhexocercosporidium sp. Dzf14 and their antibacterial activity by affecting homeostasis of cell membranes. Pest Manag. Sci. 2024, 80, 2658–2667. [Google Scholar] [CrossRef] [PubMed]
- Bang, S.; Kim, J.; Oh, J.; Kim, J.S.; Yu, S.R.; Deyrup, S.; Bahn, Y.S.; Shim, S.H. Rare beta-Resorcylic Acid Derivatives from a Halophyte-Associated Fungus Colletotrichum gloeosporioides JS0419 and Their Antifungal Activities. Mar. Drugs 2022, 20, 195. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.R.; Jiang, C.N.; Wang, H.S.; Gu, G.; Wang, M.; Sui, X.N.; Si, T.; Zhang, Z.F.; Zhang, P.; Zhao, D.L. Herbicidal Sorbicillinoid Analogs Cause Lignin Accumulation in Aspergillus aculeatus TE-65L. J. Agric. Food Chem. 2024, 72, 21102–21111. [Google Scholar] [CrossRef] [PubMed]
- Al-Fakih, A.A.; Almaqtri, W.Q.A. Overview on antibacterial metabolites from terrestrial Aspergillus spp. Mycology 2019, 10, 191–209. [Google Scholar] [CrossRef]
- Nisa, I.C.; Artasasta, M.A.; Chairunnisa, R.A.; Nurramadhani, A.; Giantana, T.G.; Ayunin, R. Review: Antibiotic-promising Aspergillus endophytic fungi. Malays. J. Microbiol. 2024, 20, 511–519. [Google Scholar]
- Li, H.; Fu, Y.; Song, F. Marine Aspergillus: A Treasure Trove of Antimicrobial Compounds. Mar. Drugs 2023, 21, 277. [Google Scholar] [CrossRef]
- Hussein, M.E.; Mohamed, O.G.; El-Fishawy, A.M.; El-Askary, H.I.; El-Senousy, A.S.; El-Beih, A.A.; Nossier, E.S.; Naglah, A.M.; Almehizia, A.A.; Tripathi, A.; et al. Identification of Antibacterial Metabolites from Endophytic Fungus Aspergillus fumigatus, isolated from Albizia lucidior Leaves (Fabaceae), Utilizing Metabolomic and Molecular Docking Techniques. Molecules 2022, 27, 1117. [Google Scholar] [CrossRef] [PubMed]
- Soltani, N.; Dille, J.A.; Burke, I.C.; Everman, W.; VanGessel, M.J.; Davis, V.M.; Sikkema, P.H. Potential Corn Yield Losses from Weeds in North America. Weed Technol. 2017, 30, 979–984. [Google Scholar] [CrossRef]
- Anwar, M.P.; Islam, A.K.M.M.; Yeasmin, S.; Rashid, M.H.; Juraimi, A.S.; Ahmed, S.; Shrestha, A. Weeds and Their Responses to Management Efforts in A Changing Climate. Agronomy 2021, 11, 1921. [Google Scholar] [CrossRef]
- Heap, I. Global perspective of herbicide-resistant weeds. Pest Manag. Sci. 2014, 70, 1306–1315. [Google Scholar] [CrossRef]
- Zhao, D.L.; Han, X.B.; Wang, M.; Zeng, Y.T.; Li, Y.Q.; Ma, G.Y.; Liu, J.; Zheng, C.J.; Wen, M.X.; Zhang, Z.F.; et al. Herbicidal and Antifungal Xanthone Derivatives from the Alga-Derived Fungus Aspergillus versicolor D5. J. Agric. Food Chem. 2020, 68, 11207–11214. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xu, K.W.; Wang, M.; Wu, P.; Zhang, Z.R.; Gao, X.; Li, Y.Q.; Wu, G.X.; Zhang, C.S.; Zhao, D.L. Phytotoxic and Antimicrobial Terrein Derivatives and Butenolides Isolated from the Endophytic Fungus Aspergillus terreus HT5. J. Agric. Food Chem. 2024, 71, 20713–20723. [Google Scholar] [CrossRef]
- Lodewyk, M.W.; Siebert, M.R.; Tantillo, D.J. Computational prediction of 1H and 13C chemical shifts: A useful tool for natural product, mechanistic, and synthetic organic chemistry. Chem. Rev. 2012, 112, 1839–1862. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Fang, Y.; Zhang, M.; Lin, A.; Zhu, T.; Gu, Q.; Zhu, W. Six new ergosterols from the marine-derived fungus Rhizopus sp. Steroids 2008, 73, 19–26. [Google Scholar] [CrossRef]
- Esumi, T.; Makado, G.; Zhai, H.F.; Shimizu, Y.; Mitsumoto, Y.; Fukuyama, Y. Efficient synthesis and structure–activity relationship of honokiol, a neurotrophic biphenyl-type neolignan. Bioorg. Med. Chem. Lett. 2004, 14, 2621–2625. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Jensen, P.R.; Fenical, W. A cyclic carbonate and related polyketides from a marine-derived fungus of the genus Phoma. Phytochemistry 2003, 64, 571–574. [Google Scholar] [CrossRef]
- Song, L.; Pan, M.; Zhao, R.; Deng, J.; Wu, Y. Recent advances, challenges and perspectives in enantioselective release. J. Control. Release 2020, 324, 156–171. [Google Scholar] [CrossRef]
- Kim, Y.S.; Lee, J.Y.; Park, J.; Hwang, W.; Lee, J.; Park, D. Synthesis and microbiological evaluation of honokiol derivatives as new antimicrobial agents. Arch. Pharm. Res. 2010, 33, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, P.; Reboredo-Durán, J.; Muñoz, L.; Freitas, H.; González, L. Herbicidal properties of the commercial formulation of methyl cinnamate, a natural compound in the invasive silver wattle (Acacia dealbata). Weed Sci. 2019, 68, 69–78. [Google Scholar] [CrossRef]
- Masi, M.; Freda, F.; Sangermano, F.; Calabrò, V.; Cimmino, A.; Cristofaro, M.; Meyer, S.; Evidente, A. Radicinin, a fungal phytotoxin as a target-specific bioherbicide for invasive buffelgrass (Cenchrus ciliaris) Control. Molecules 2019, 24, 1086. [Google Scholar] [CrossRef]
- Anteyi, W.O.; Klaiber, I.; Rasche, F. Diacetoxyscirpenol, a Fusarium exometabolite, prevents efficiently the incidence of the parasitic weed Striga hermonthica. BMC Plant Biol. 2022, 22, 84. [Google Scholar] [CrossRef]
- Berestetskiy, A. Modern Approaches for the Development of New Herbicides Based on Natural Compounds. Plants 2023, 12, 234. [Google Scholar] [CrossRef] [PubMed]
- Raiyemo, D.A.; Montgomery, J.S.; Cutti, L.; Abdollahi, F.; Llaca, V.; Fengler, K.; Lopez, A.J.; Morran, S.; Saski, C.A.; Nelson, D.R.; et al. Chromosome-level assemblies of Amaranthus palmeri, Amaranthus retroflexus, and Amaranthus hybridus allow for genomic comparisons and identification of a sex-determining region. Genomics, 2024, in press.
- Gu, G.; Jia, X.; Wang, W.; Li, P.; Zhao, S.; Zhou, Z.; Yin, R.; Lai, D.; Song, S.; Zhou, L. Culturable Endophytic Fungi from Glycyrrhiza inflata Distributed in Xinjiang, China with Antifungal Activity. Microbiol. Res. 2021, 12, 829–839. [Google Scholar] [CrossRef]
- Travaini, M.L.; Sosa, G.M.; Ceccarelli, E.A.; Walter, H.; Cantrell, C.L.; Carrillo, N.J.; Dayan, F.E.; Meepagala, K.M.; Duke, S.O. Khellin and Visnagin, Furanochromones from Ammi visnaga (L.) Lam., as Potential Bioherbicides. J. Agric. Food Chem. 2016, 64, 9475–9487. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Liu, Y.; Li, T.; Liu, X.; Hao, Z.; Ding, S.; Panichayupakaranant, P.; Zhu, K.; Shen, J. Plant Natural Flavonoids Against Multidrug Resistant Pathogens. Adv. Sci. 2021, 8, 2100749. [Google Scholar] [CrossRef]





| Position | 1 a | 2 b | ||
|---|---|---|---|---|
| δH Mult. (J in Hz) | δC Type | δH Mult. (J in Hz) | δC Type | |
| 1 | 0.87 t (6.8) | 13.9 CH3 | 0.91 t (6.9) | 14.4 CH3 |
| 2 | 1.29 m | 22.1 CH2 | 1.36 m | 23.7 CH2 |
| 3 | 1.28 m | 31.0 CH2 | 1.34 m | 32.9 CH2 |
| 4 | 1.38 m 1.32 m | 24.1 CH2 | 1.51 m 1.43 m | 26.1 CH2 |
| 5 | 1.67 ddt (13.7, 8.3, 5.2) 1.58 ddt (13.7, 10.9, 5.6) | 34.2 CH2 | 1.55 m | 36.4 CH2 |
| 6 | 4.33 ddt (9.0, 7.5, 5.6) | 76.6 CH | 3.89 ddt (11.1, 7.9, 4.1) | 67.3 CH |
| 7 | 2.40 m | 29.4 CH2 | 2.34 ddd (17.6, 3.8, 2.2) 1.79 dd (17.6, 11.1) | 28.4 CH2 |
| 8 | 156.2 C | 133.0 C | ||
| 9 | 4.22 t (8.3) | 64.4 CH | 198.9 C | |
| 10 | 1.90 m 1.80 ddd (12.6, 10.1, 2.0) | 32.8 CH2 | 2.83 dd (16.3, 4.2) 2.45 dd (16.3, 8.4) | 43.8 CH2 |
| 11 | 3.75 d (2.0) | 69.0 CH | 3.98 ddd (8.4, 6.1, 4.2) | 72.4 CH |
| 12 | 4.01 br. s | 64.6 CH | 4.30 dt (6.1, 2.2) | 71.0 CH |
| 13 | 123.1 C | 152.3 C | ||
| 14 | 165.1 C | 5.67 s | 89.2 CH | |
| 9-OH | 4.94 br. s | |||
| 11-OH | 4.80 br. s | |||
| 12-OH | 5.09 br. s | |||
| Position | Exp. δC | Cal. δC a (6R*-1) | Δδ (Cal. − Exp.) | Cal. δC a (6S*-1) | Δδ (Cal. − Exp.) |
|---|---|---|---|---|---|
| 1 | 13.9 | 14.2 | 0.3 | 14.2 | 0.3 |
| 2 | 22.1 | 25.4 | 3.3 | 25.3 | 3.2 |
| 3 | 31 | 33.0 | 2.0 | 32.8 | 1.8 |
| 4 | 24.1 | 27.4 | 3.3 | 26.7 | 2.6 |
| 5 | 34.2 | 35.6 | 1.4 | 35.9 | 1.7 |
| 6 | 76.6 | 77.7 | 1.1 | 77.3 | 0.7 |
| 7 | 29.4 | 32.4 | 3.0 | 30.8 | 1.4 |
| 8 | 156.2 | 156.5 | 0.3 | 159.7 | 3.5 |
| 9 | 64.4 | 68.5 | 4.1 | 67.7 | 3.3 |
| 10 | 32.8 | 35.9 | 3.1 | 35.9 | 3.1 |
| 11 | 69.0 | 64.4 | −4.6 | 67.0 | -2.0 |
| 12 | 64.6 | 64.7 | 0.1 | 66.9 | 2.3 |
| 13 | 123.1 | 126.3 | 3.2 | 123.4 | 0.3 |
| 14 | 165.1 | 166.8 | 1.7 | 166.6 | 1.5 |
| RMSD | 2.1 | RMSD | 1.8 | ||
| MAE | 1.1 | MAE | 0.8 |
| Compound | Ralstonia solanacearum | Xanthomonas oryzae | Bacillus cereus | Bacillus subtilis |
|---|---|---|---|---|
| 1 | >128 | >128 | >128 | >128 |
| 2 | >128 | >128 | >128 | >128 |
| 3 | >128 | >128 | >128 | >128 |
| 4 | >128 | >128 | 32 | 16 |
| 5 | >128 | >128 | 16 | 16 |
| 6 | >128 | >128 | >128 | >128 |
| Chloramphenicol a | 16 | 4 | 16 | 16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Yuan, X.; Huang, X.; Zhang, P.; Gu, G. Herbicidal and Antibacterial Secondary Metabolites Isolated from the Nicotiana tabacum-Derived Endophytic Fungus Aspergillus japonicus TE-739D. Plants 2025, 14, 173. https://doi.org/10.3390/plants14020173
Wang H, Yuan X, Huang X, Zhang P, Gu G. Herbicidal and Antibacterial Secondary Metabolites Isolated from the Nicotiana tabacum-Derived Endophytic Fungus Aspergillus japonicus TE-739D. Plants. 2025; 14(2):173. https://doi.org/10.3390/plants14020173
Chicago/Turabian StyleWang, Haisu, Xiaolong Yuan, Xinrong Huang, Peng Zhang, and Gan Gu. 2025. "Herbicidal and Antibacterial Secondary Metabolites Isolated from the Nicotiana tabacum-Derived Endophytic Fungus Aspergillus japonicus TE-739D" Plants 14, no. 2: 173. https://doi.org/10.3390/plants14020173
APA StyleWang, H., Yuan, X., Huang, X., Zhang, P., & Gu, G. (2025). Herbicidal and Antibacterial Secondary Metabolites Isolated from the Nicotiana tabacum-Derived Endophytic Fungus Aspergillus japonicus TE-739D. Plants, 14(2), 173. https://doi.org/10.3390/plants14020173







