Heartwood/Sapwood Characteristics of Populus euphratica Oliv. Trunks and Their Relationship with Soil Physicochemical Properties in the Lower Tarim River, Northwest China
Abstract
1. Introduction
2. Results
2.1. Physical Characteristics of Heartwood and Sapwood in Sample Tree Trunks
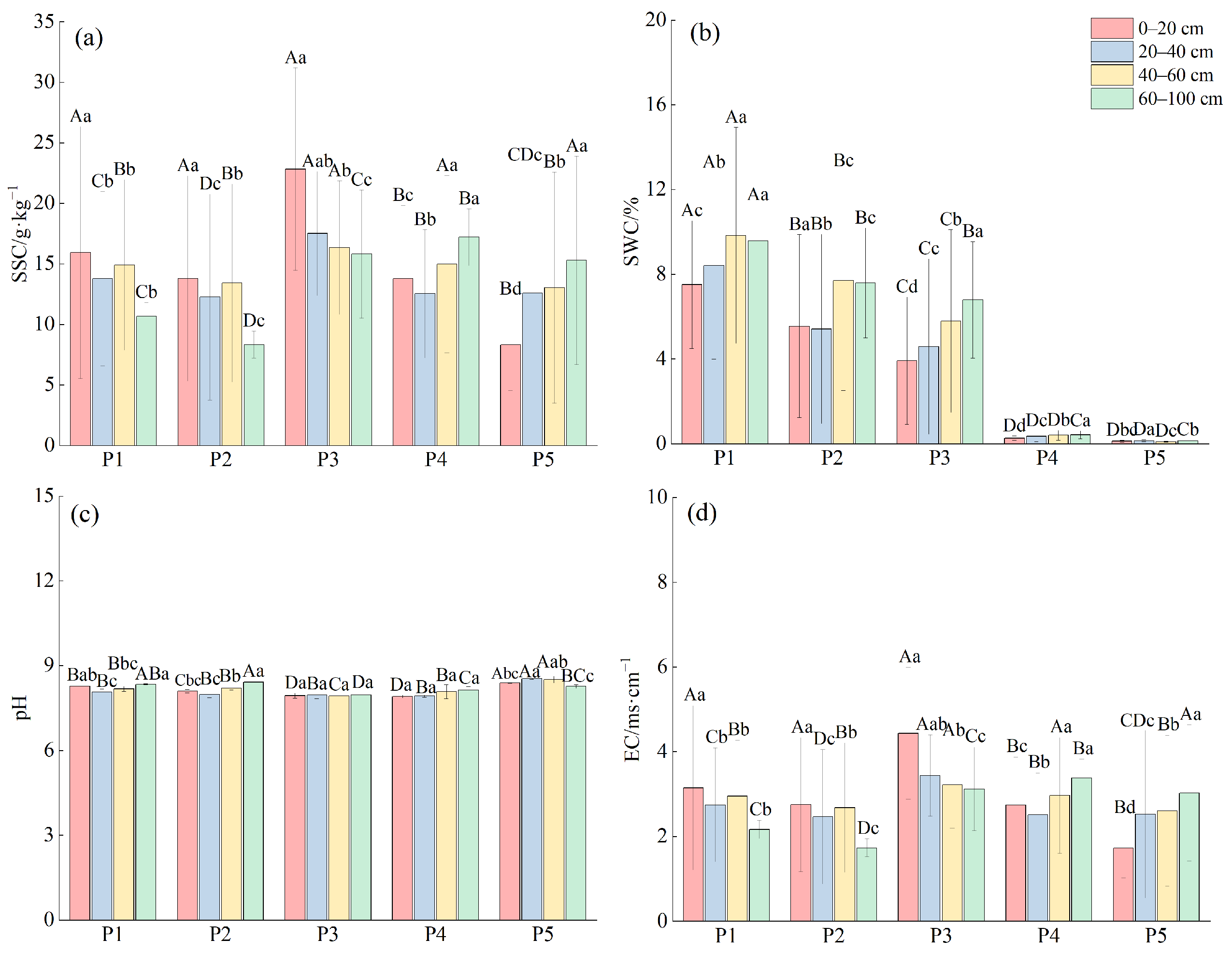
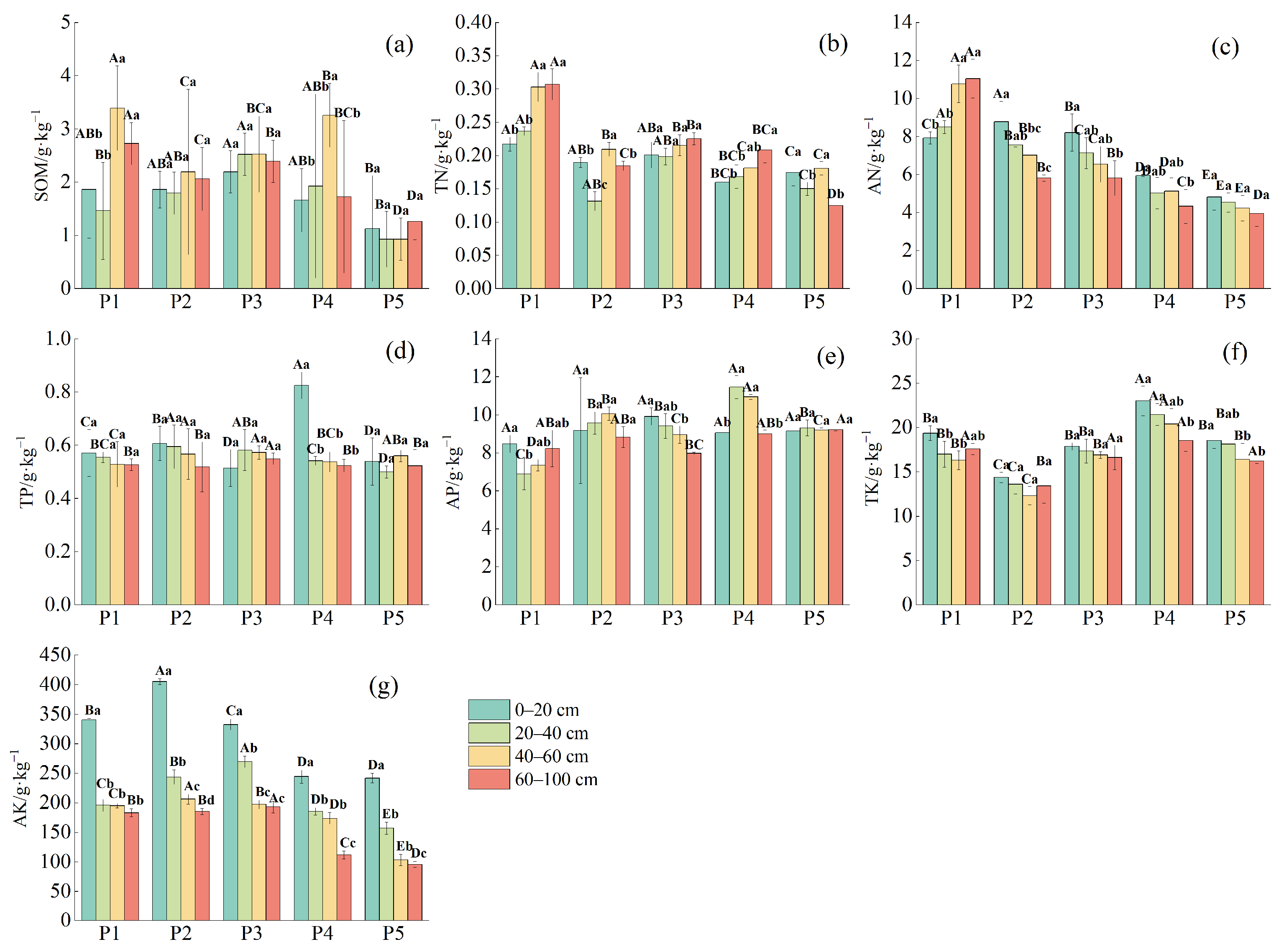
2.2. Analysis of Soil Physical and Chemical Properties
2.2.1. Characterization of Soil Water Salinity at Different Depths
2.2.2. Characterization of Soil Nutrients at Different Depths
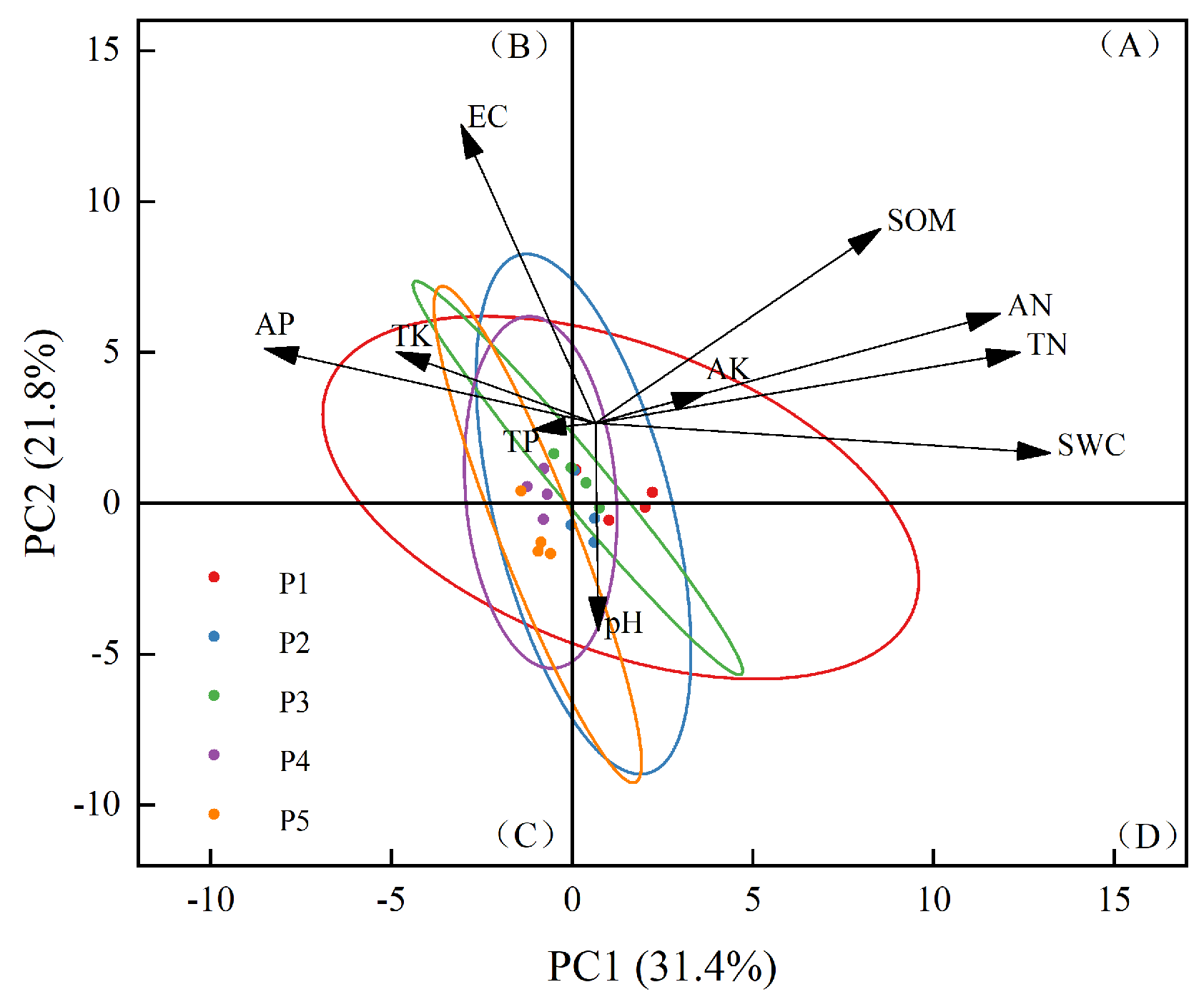
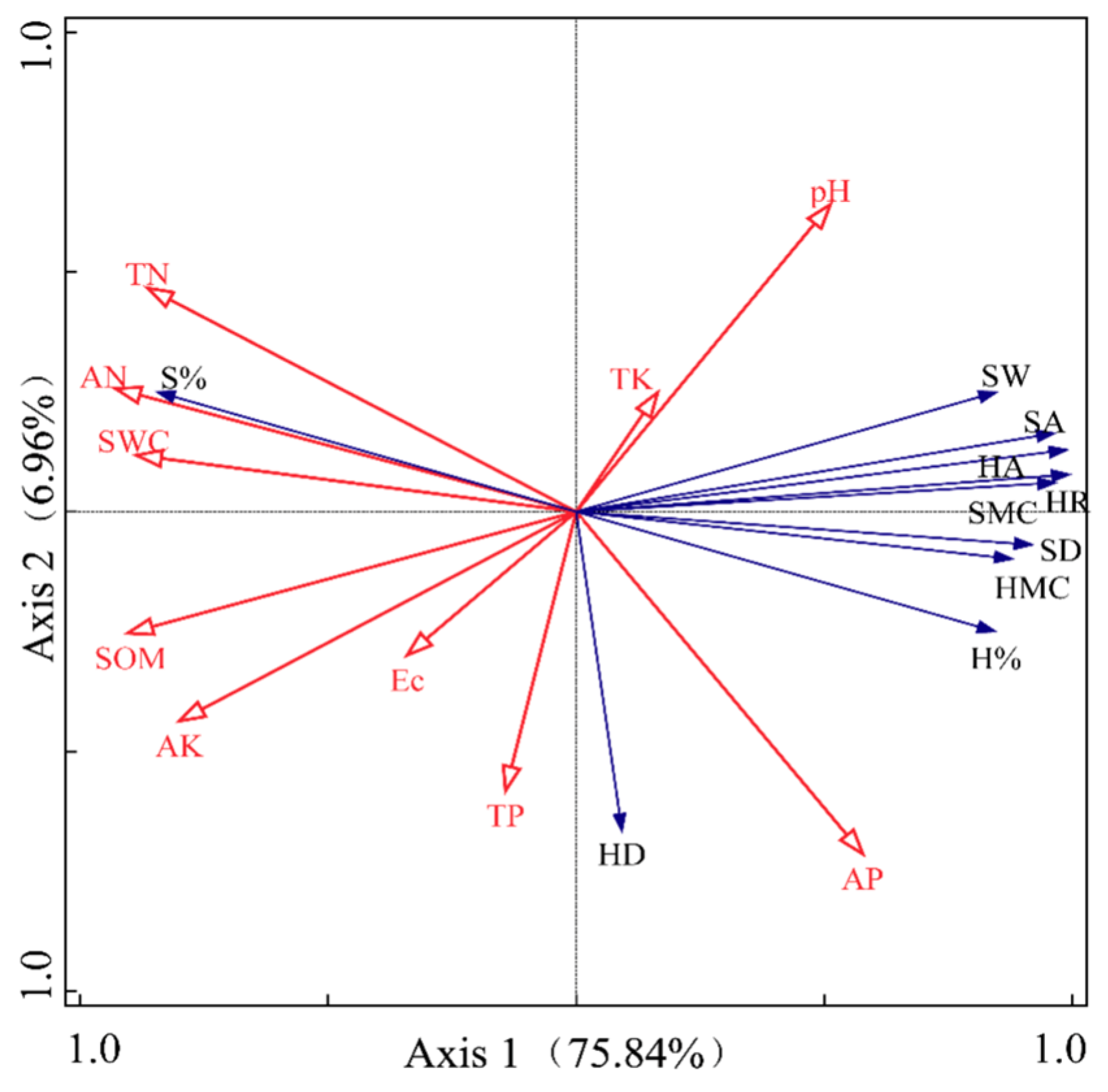
2.2.3. Principal Component Analysis of Soil Physical and Chemical Properties
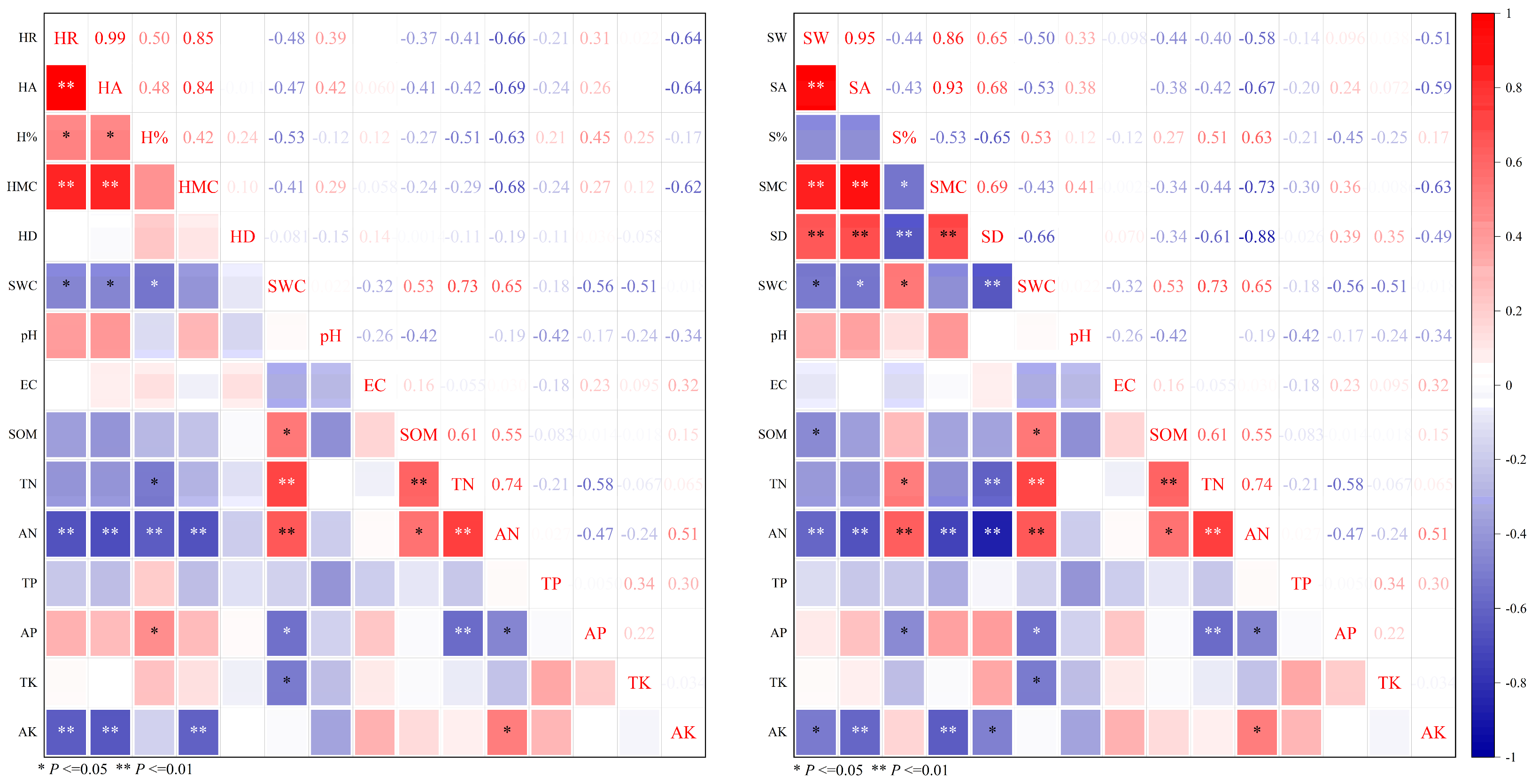
2.3. Relationship Between the Physical Characteristics of Heartwood and Sapwood in P. euphratica Trunks and the Physical and Chemical Properties of Soil
3. Discussion
3.1. Changing Patterns in the Physical Properties of Heartwood and Sapwood in P. euphratica Trunks
3.2. Influence of Soil Physicochemical Properties on the Physical Characteristics of Heartwood and Sapwood in P. euphratica Trunks
4. Materials and Methods
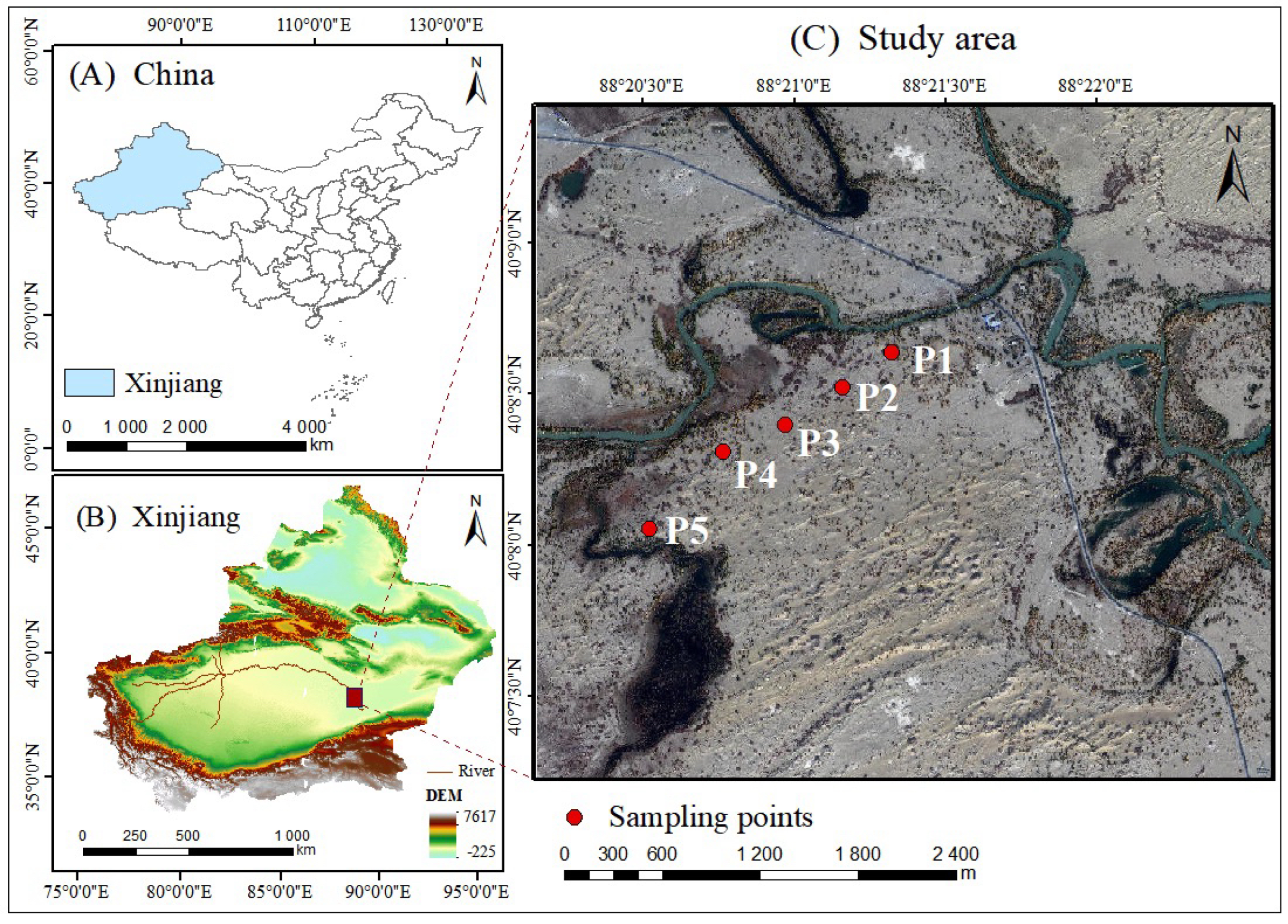
4.1. Overview of the Study Area
4.2. Sample Plot Setup and Data Acquisition
4.3. Soil Sample Collection and Processing
4.4. Data Processing and Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qin, L.; Shang, H.; Liu, W.; Fan, Y.T.; Liu, K.; Zhang, T.; Zhang, R. Differential response of radial growth and δ13C in Qinghai spruce (Picea crassifolia) to climate change on the southern and northern slopes of the Qilian Mountains in Northwest China. J. For. Res. 2024, 35, 58. [Google Scholar] [CrossRef]
- Yang, X.Q.; Li, L.P.; Yu, X.R.; Liu, Y.; Xie, S.D.; Zhu, G.L.; Xu, J.M.; Zhao, P. An untargeted metabolomics analysis of the components of heartwood and sapwood in 4 fast-growing Chinese fir (Cunninghamia lanceolata (Lamb.) Hook) clones. Trees 2024, 38, 339–356. [Google Scholar] [CrossRef]
- Ma, R.K.; Luo, J.; Wang, W.; Fu, Y.L. Changes in the physiological activity of parenchyma cells in Dalbergia odorifera xylem and its relationship with heartwood formation. BMC Plant Biol. 2023, 23, 559. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.M.; Ding, C.J.; Wang, S.J.; Ma, C.M.; Zhang, C.; Li, Y.T.; Wang, J.M.; Yang, M.S.; Su, X.H. Effects of site conditions on growth and wood properties of Populus × euramericana cv. ‘74/76’. J. For. Res. 2023, 34, 401–414. [Google Scholar] [CrossRef]
- Pappas, C.; Belanger, N.; Bastien-Beaudet, G.; Couture, C.; D’Orangeville, L.; Duchesne, L.; Gennaretti, F.; Houle, D.; Hurley, A.G.; Kless, S.; et al. Xylem porosity, sapwood characteristics, and uncertainties in temperate and boreal forest water use. Agric. For. Meteorol. 2022, 323, 109092. [Google Scholar] [CrossRef]
- Song, J.; Betz, F.; Aishan, T.; Halik, U.; Abliz, A. Impact of water supply on the restoration of the severely damaged riparian plants along the Tarim River in Xinjiang, Northwest China. Ecol. Indic. 2024, 158, 111570. [Google Scholar] [CrossRef]
- Makoto, K.; Susloparova, E.; Tsuyama, I.; Shimase, T.; Nakaba, S.; Takahashi, N.; Yoshida, T. Influence of soil properties on the heartwood colour of Juglans mandshurica var. sachalinensis in a cool temperate forest. J. Wood Sci. 2021, 67, 1. [Google Scholar]
- Kint, V.; Vansteenkiste, D.; Aertsen, W.; De Vos, B.; Bequet, R.; Van Acker, J.; Muys, B. Forest structure and soil fertility determine internal stem morphology of Pedunculate oak: A modelling approach using boosted regression trees. Eur. J. For. Res. 2012, 131, 609–622. [Google Scholar] [CrossRef]
- Wind, C.; Arend, M.; Fromm, J. Potassium-Dependent Cambial Growth in Poplar. J. Plant Biol. 2004, 6, 30–37. [Google Scholar] [CrossRef]
- He, Q.; Ye, M.; Zhao, X.; Pan, X. Environmental Factors’ Effects on Stem Radial Variations of Populus euphratica in the Lower Reaches of the Tarim River in Northwestern China. Sustainability 2023, 15, 11556. [Google Scholar] [CrossRef]
- England, J.R.; Attiwill, P.M. Changes in sapwood permeability and anatomy with tree age and height in the broad-leaved evergreen species Eucalyptus regnans. Tree Physiol. 2007, 27, 1113–1124. [Google Scholar] [CrossRef]
- Pinto, I.; Pereira, H.; Usenius, A. Heartwood and sapwood development within maritime pine (Pinus pinaster Ait.) stems. Trees 2004, 18, 284–294. [Google Scholar] [CrossRef]
- Curvo, K.R.; Silva, G.A.O.; Castro, V.R.; Gava, F.H.; Pereira, B.L.C.; Oliveira, A.C. Heartwood proportion and density of Tectona grandis L.f. wood from Brazilian fast-growing plantations at different ages. Eur. J. Wood Prod. 2024, 2, 357–369. [Google Scholar] [CrossRef]
- Aishan, T.; Jiang, W.; Cheng, Q.; Halik, Ü.; Betz, F.; Yusup, A. Quantitative diagnosis of internal wood damage in living trees and its relationship with soil physicochemical properties: The case of an endangered desert riparian forest in Xinjiang, NW China. For. Ecol. Manag. 2024, 561, 121880. [Google Scholar] [CrossRef]
- Aishan, T.; Mumin, R.; Halik, U.; Jiang, W.; Sun, Y.X.; Yusup, A.; Chen, T.Y. Patterns in Tree Cavities (Hollows) in Euphrates Poplar (Populus euphratica, Salicaceae) along the Tarim River in NW China. Forest 2024, 15, 421. [Google Scholar] [CrossRef]
- Wang, H.Z.; Han, L.; Xu, Y.L.; Niu, J.L. Model Analysis of the Stomatal Conductance Response to Light in Populus pruinosa at Different Temperatures in the Taklimakan Desert. Appl. Ecol. Environ. Sci. 2015, 24, 741–748. [Google Scholar]
- Aye, T.N.; Brännström, A.; Carlsson, L. Prediction of tree sapwood and heartwood profiles using pipe model and branch thinning theory. Tree Physiol. 2022, 42, 2174–2185. [Google Scholar] [CrossRef]
- Luo, B.; He, R.; Yang, Y. A review of physiological function of sapwood and formation mechanism of heartwood. J. Beijing For. Univ. 2018, 40, 120–129. [Google Scholar]
- Luo, J.; Ma, R.K.; Wei, P.L.; Fu, Y.L. Variation on radial and axial of heartwood and sapwood in Eucalyptus cloeziana. J. Beijing For. Univ. 2021, 43, 32–140. [Google Scholar]
- Castagneri, D.; Regev, L.; Boaretto, E.; Carrer, M. Xylem anatomical traits reveal different strategies of two Mediterranean oaks to cope with drought and warming. Environ. Exp. Bot. 2017, 133, 128–138. [Google Scholar] [CrossRef]
- Dorji, Y.; Isasa, E.; Pierick, K. Insights into the relationship between hydraulic safety, hydraulic efficiency and tree structural complexity from terrestrial laser scanning and fractal analysis. Trees 2024, 38, 221–239. [Google Scholar] [CrossRef]
- Kijowska-Oberc, J.; Staszak, A.M.; Kamiński, J.; Ratajczak, E. Adaptation of Forest Trees to Rapidly Changing Climate. Forests 2020, 11, 123. [Google Scholar] [CrossRef]
- Hillabrand, R.M.; Dyck, M.; Landhäusser, S.M. Increases in sap flow and storage can compensate for successively greater losses of conducting area in large trees. Agric. For. Meteorol. 2023, 333, 109395. [Google Scholar] [CrossRef]
- Kühnhammer, K.; Haren, J.V.; Kübert, A.; Bailey, K.; Dubbert, M.; Hu, J.; Ladd, S.N.; Meredith, L.K.; Werner, C.; Beyer, M. Deep roots mitigate drought impacts on tropical trees despite limited quantitative contribution to transpiration. Sci. Total Environ. 2023, 893, 164763. [Google Scholar] [CrossRef]
- Chen, Z.C.; Li, S.; Wan, X.C.; Liu, S.R. Strategies of tree species to adapt to drought from leaf stomatal regulation and stem embolism resistance to root properties. Front. Plant Sci. 2022, 13, 926535. [Google Scholar] [CrossRef]
- Ndayambaza, B.; Si, J.; Deng, Y.; Jia, B.; He, X.; Zhou, D.; Wang, C.; Zhu, X.; Liu, Z.; Qin, J. The Euphrates Poplar Responses to Abiotic Stress and Its Unique Traits in Dry Regions of China (Xinjiang and Inner Mongolia): What Should We Know? Genes 2023, 14, 2213. [Google Scholar] [CrossRef]
- Gerchow, M.; Marshall, J.D.; Kühnhammer, K. Thermal imaging of increment cores: A new method to estimate sapwood depth in trees. Trees 2023, 37, 349–359. [Google Scholar] [CrossRef]
- Traoré, M.; Kaal, J.; Cortizas, A.M. Variation of wood color and chemical composition in the stem cross-section of oak (Quercus spp.) trees, with special attention to the sapwood-heartwood transition zone. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 285, 121893. [Google Scholar] [CrossRef]
- Kafuti, C.; Lehnebach, R.; Bourland, N.; Beeckman, H.; Van Acker, J.; Luambua, N.K.; Van den Bulcke, J. Earlier onset and slower heartwood investment in faster-growing trees of African tropical species. Ann. Bot. 2024, 133, 905–916. [Google Scholar] [CrossRef]
- Hu, G.Z.; Liu, H.Y.; Shangguan, H.L.; Wu, X.C.; Xu, X.T.; Williams, M. The role of heartwood water storage for sem-arid trees under drought. Agric. For. Meteorol. 2018, 256, 534–541. [Google Scholar] [CrossRef]
- Wen, L.; Chen, S.; Wei, P.; Fu, Y. Morphological, Physiological, Biochemical and Metabolite Analyses of Parenchyma Cells Reveal Heartwood Formation Mechanism of Schima superba. Forests 2024, 15, 984. [Google Scholar] [CrossRef]
- Li, J.; Hu, S.J.; Sheng, Y.; He, X.R. Whole-Plant Water Use and Hydraulics of Populus euphratica and Tamarix ramosissima Seedlings in Adaption to Groundwater Variation. Water 2022, 14, 1869. [Google Scholar] [CrossRef]
- Elizabeth, R.; Rogers, R.S.; Zalesny, J.; Lin, C.H.; Vinhal, R.A. Intrinsic and extrinsic factors influencing Populus water use: A literature review. J. Environ. Manag. 2023, 348, 119180. [Google Scholar]
- Zhou, H.H.; Li, W.H. The effects of oasis ecosystem hydrological processes on soil salinization in the lower reaches of the Tarim River, China. Ecohydrology 2013, 6, 1009–1020. [Google Scholar] [CrossRef]
- Fu, A.H.; Li, W.H.; Chen, Y.N. The threshold of soil moisture and salinity influencing the growth of Populus euphratica and Tamarix ramosissima in the extremely arid region. Environ. Earth Sci. 2012, 66, 2519–2529. [Google Scholar] [CrossRef]
- Zou, S.Y.; Li, D.D.; Di, N.; Liu, J.Q.; Li, L.Y.; Liu, Y.; Xi, B.Y.; Coleman, M. Stand development modifies effects of soil water availability on poplar fine-root traits: Evidence from a six-year experiment. Plant Soil 2022, 480, 165–184. [Google Scholar] [CrossRef]
- Tang, W.Z.; Liu, X.; Liang, X.Y.; Liu, H.; Yu, K.L.; He, P.C.; McAdam, S.; Zhao, H.; Ye, Q. Hydraulic vulnerability difference between branches and roots increases with environmental aridity. Oecologia 2024, 205, 177–190. [Google Scholar] [CrossRef]
- Zhao, Z.G.; Shen, W.; Wang, C.S.; Jia, H.Y.; Zeng, J. Heartwood variations in mid-aged plantations of Erythrophleum fordii. J. For. Res. 2021, 32, 2375–2383. [Google Scholar] [CrossRef]
- Betas, I.; Alma, M.H.; Goker, Y.; Yuksel, A.; Gundogan, R. Influence of site on sapwood and heartwood ratios of Turkish Calabrain pine. For. Prod. J. 2003, 53, 48–50. [Google Scholar]
- Wright, S.J.; Yavitt, J.B.; Wurzburger, N.; Turner, B.L.; Tanner, E.V.J.; Sayer, E.J.; Santiago, L.S.; Kaspari, M.; Hedin, L.O.; Harms, K.E.; et al. Potassium, phosphorus, or nitrogen limit root allocation, tree growth, or litter production in a lowland tropical forest. Ecology 2011, 92, 1616–1625. [Google Scholar] [CrossRef]
- Aishan, T.; Halik, Ü.; Betz, F.; Tiyip, T.; Ding, J.L.; Nuermaimaiti, Y. Modeling height–diameter relationship for Populus euphratica in the Tarim riparian forest ecosystem, Northwest China. J. For. Re. 2016, 27, 889–900. [Google Scholar] [CrossRef]
- Xu, Q.; Ye, M.; Xu, H.L.; Wang, X.Y.; Zhao, X.F. Effects of ecological water conveyance on the composition, diversity and stability of plant community in the lower reaches of Tarim River. Chin. J. Ecol. 2018, 37, 2603–2610. [Google Scholar]
- Wang, S.J.; Chen, B.H.; Li, H.Q. Euphrates Poplar Forest, 1st ed.; China Environmental Science Press: Beijing, China, 1996; pp. 1–199. [Google Scholar]
- Han, J.X.; Song, X.Y.; Fu, H.Y.; Liu, C.G.; Yang, F.S. Effects of the decomposition agent application on the physicochemical properties and microbial community structure of wheat straw-returning soil. Environ. Sci. Technol. 2024, 35, 103668. [Google Scholar] [CrossRef]
| Index | DBH | 15–25 cm | 25–35 cm | 35–45 cm | >45 cm | Average | |
|---|---|---|---|---|---|---|---|
| Height | |||||||
| HR | 0.3 m | 8.64 ± 1.62 Da | 11.09 ± 1.58 Ca | 15.63 ± 1.82 Ba | 18.91 ± 0.87 Aa | 12.80 ± 4.01 | |
| 0.8 m | 8.33 ± 1.94 Da | 10.95 ± 0.75 Ca | 14.96 ± 1.96 Ba | 18.90 ± 1.17 Aa | 12.48 ± 3.99 | ||
| 1.3 m | 7.93 ± 1.36 Da | 11.15 ± 0.90 Ca | 15.08 ± 1.55 Ba | 18.34 ± 1.00 Aa | 12.37 ± 3.87 | ||
| SW | 0.3 m | 1.95 ± 0.44 Ca | 2.53 ± 0.56 Ba | 2.73 ± 0.40 ABa | 3.00 ± 0.16 Aa | 2.49 ± 0.57 | |
| 0.8 m | 1.91 ± 0.33 Ca | 2.27 ± 0.42 Cab | 2.69 ± 0.63 Ba | 3.17 ± 0.08 Aab | 2.42 ± 0.61 | ||
| 1.3 m | 1.82 ± 0.39 Ca | 2.11 ± 0.25 Cb | 2.48 ± 0.54 Ba | 3.30 ± 0.21 Ab | 2.30 ± 0.61 | ||
| HA | 0.3 m | 242.15 ± 84.49 Da | 393.77 ± 112.92 Ca | 776.52 ± 180.59 Ba | 1125.06 ± 104.18 Aa | 526.57 ± 316.31 | |
| 0.8 m | 228.93 ± 100.30 Da | 378.15 ± 52.08 Ca | 714.31 ± 178.49 Ba | 1125.96 ± 141.67 Aa | 538.39 ± 330.98 | ||
| 1.3 m | 203.14 ± 66.10 Da | 392.58 ± 62.95 Ca | 721.64 ± 144.68 Ba | 1058.74 ± 116.72 Aa | 526.57 ± 316.31 | ||
| SA | 0.3 m | 120.30 ± 40.87 Da | 198.78 ± 60.30 Ca | 293.35 ± 65.40 Ba | 383.97 ± 20.87 Aa | 229.83 ± 105.24 | |
| 0.8 m | 111.83 ± 29.05 Da | 172.49 ± 32.57 Cab | 278.49 ± 85.76 Ba | 408.02 ± 26.27 Aa | 219.09 ± 113.26 | ||
| 1.3 m | 100.67 ± 29.18 Da | 162.04 ± 24.33 Cb | 255.97 ± 62.73 Ba | 409.98 ± 42.36 Aa | 209.18 ± 112.98 | ||
| HD | 0.3 m | 0.35 ± 0.04 Aa | 0.39 ± 0.06 Aa | 0.38 ± 0.04 Aa | 0.37 ± 0.01 Aa | 0.37 ± 0.02 | |
| 0.8 m | 0.37 ± 0.04 Ab | 0.41 ± 0.05 Aa | 0.39 ± 0.03 Aab | 0.37 ± 0.01 Ab | 0.38 ± 0.02 | ||
| 1.3 m | 0.38 ± 0.02 Cc | 0.42 ± 0.03 Aa | 0.39 ± 0.02 BCbc | 0.4 ± 0.03 ABab | 0.4 ± 0.02 | ||
| SD | 0.3 m | 0.37 ± 0.05 Cb | 0.38 ± 0.04 BCb | 0.44 ± 0.04 Aa | 0.43 ± 0.01 ABa | 0.4 ± 0.04 | |
| 0.8 m | 0.36 ± 0.04 Bc | 0.39 ± 0.05 ABbc | 0.42 ± 0.03 Aab | 0.44 ± 0.01 Aa | 0.4 ± 0.04 | ||
| 1.3 m | 0.36 ± 0.02 Cc | 0.4 ± 0.03 Bb | 0.43 ± 0.01 Aa | 0.44 ± 0.01 Aa | 0.41 ± 0.03 | ||
| HMC | 0.3 m | 27.53 ± 2.93 Dd | 31.97 ± 3.13 Cc | 39.64 ± 6.3 Bb | 44.46 ± 1.23 Aa | 35.9 ± 7.59 | |
| 0.8 m | 26.93 ± 3.76 Cc | 30.13 ± 4.1 Cc | 36.87 ± 4.89 Bb | 42.31 ± 3.21 Aa | 34.06 ± 6.88 | ||
| 1.3 m | 28.63 ± 1.44 Bb | 28.14 ± 2.12 Bb | 34.87 ± 5.73 Aa | 37.51 ± 3.49 Aa | 32.29 ± 4.64 | ||
| SMC | 0.3 m | 13.65 ± 1.72 Cc | 18.64 ± 6.17 Bb | 25.58 ± 3.03 Aa | 28.31 ± 0.99 Aa | 21.54 ± 6.65 | |
| 0.8 m | 13.8 ± 0.99 Cc | 17.45 ± 3.47 Bb | 25.61 ± 3.25 Aa | 27.97 ± 0.26 Aa | 21.23 ± 6.65 | ||
| 1.3 m | 14.11 ± 0.83 Cd | 17.81 ± 3.58 Cc | 24.84 ± 0.77 Bb | 30.58 ± 2.15 Aa | 21.84 ± 7.34 | ||
| H% | 0.3 m | 0.66 ± 0.05 Ba | 0.66 ± 0.06 Ba | 0.72 ± 0.03 Aa | 0.74 ± 0.02 Aa | 0.69 ± 0.06 | |
| 0.8 m | 0.65 ± 0.07 Ba | 0.69 ± 0.05 ABab | 0.72 ± 0.05 Aa | 0.73 ± 0.01 Aab | 0.69 ± 0.06 | ||
| 1.3 m | 0.66 ± 0.06 Ba | 0.71 ± 0.03 ABb | 0.74 ± 0.06 Aa | 0.72 ± 0.02 Ab | 0.70 ± 0.06 | ||
| S% | 0.3 m | 0.34 ± 0.05 Aa | 0.34 ± 0.06 Aa | 0.28 ± 0.03 Ba | 0.26 ± 0.02 Bb | 0.31 ± 0.06 | |
| 0.8 m | 0.35 ± 0.07 Aa | 0.31 ± 0.05 ABab | 0.28 ± 0.05 Ba | 0.27 ± 0.01 Bab | 0.31 ± 0.06 | ||
| 1.3 m | 0.34 ± 0.06 Aa | 0.29 ± 0.03 ABb | 0.26 ± 0.06 Ba | 0.28 ± 0.02 Ba | 0.30 ± 0.06 | ||
| Plot | Distance from River/m | Longitude and Latitude | Elevation Level/m | Types of Stands | Average DBH/cm | Average TH/m |
|---|---|---|---|---|---|---|
| P1 | 50 | 40°08′10″, 88°22′48″ | 820 | middle-aged forest | 22.11 ± 7.82 | 6.42 ± 2.29 |
| P2 | 300 | 40°08′11″, 88°22′34″ | 818 | middle-aged forest | 26.18 ± 0.09 | 6.67 ± 2.16 |
| P3 | 500 | 40°08′08″, 88°22′04″ | 818 | middle-aged forest | 21.25 ± 8.91 | 7.73 ± 4.39 |
| P4 | 750 | 40°08′03″, 88°21′56″ | 820 | mature forest | 31.52 ± 7.68 | 9.8 ± 5.2 |
| P5 | 1050 | 40°07′56″, 88°21′47″ | 821 | overmature forest | 47.58 ± 4.12 | 5.42 ± 1.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.; Aishan, T.; Wang, N.; Halik, Ü.; Yao, S. Heartwood/Sapwood Characteristics of Populus euphratica Oliv. Trunks and Their Relationship with Soil Physicochemical Properties in the Lower Tarim River, Northwest China. Plants 2025, 14, 154. https://doi.org/10.3390/plants14020154
Chen T, Aishan T, Wang N, Halik Ü, Yao S. Heartwood/Sapwood Characteristics of Populus euphratica Oliv. Trunks and Their Relationship with Soil Physicochemical Properties in the Lower Tarim River, Northwest China. Plants. 2025; 14(2):154. https://doi.org/10.3390/plants14020154
Chicago/Turabian StyleChen, Tongyu, Tayierjiang Aishan, Na Wang, Ümüt Halik, and Shiyu Yao. 2025. "Heartwood/Sapwood Characteristics of Populus euphratica Oliv. Trunks and Their Relationship with Soil Physicochemical Properties in the Lower Tarim River, Northwest China" Plants 14, no. 2: 154. https://doi.org/10.3390/plants14020154
APA StyleChen, T., Aishan, T., Wang, N., Halik, Ü., & Yao, S. (2025). Heartwood/Sapwood Characteristics of Populus euphratica Oliv. Trunks and Their Relationship with Soil Physicochemical Properties in the Lower Tarim River, Northwest China. Plants, 14(2), 154. https://doi.org/10.3390/plants14020154








