Natural Enemies Acquire More Prey Aphids from Hormone-Treated Insect-Attracting Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Exogenous Hormones
2.2. Insect Colony
2.3. Field Survey
2.3.1. Experimental Plot Design
2.3.2. C. monnieri Plot Cultivation
2.3.3. Arthropod Sampling in the C. monnieri Field
2.4. Life Table Study
2.5. Life Table Parameter Analysis
2.6. Statistical Analysis
3. Results
3.1. Field Investigation Results
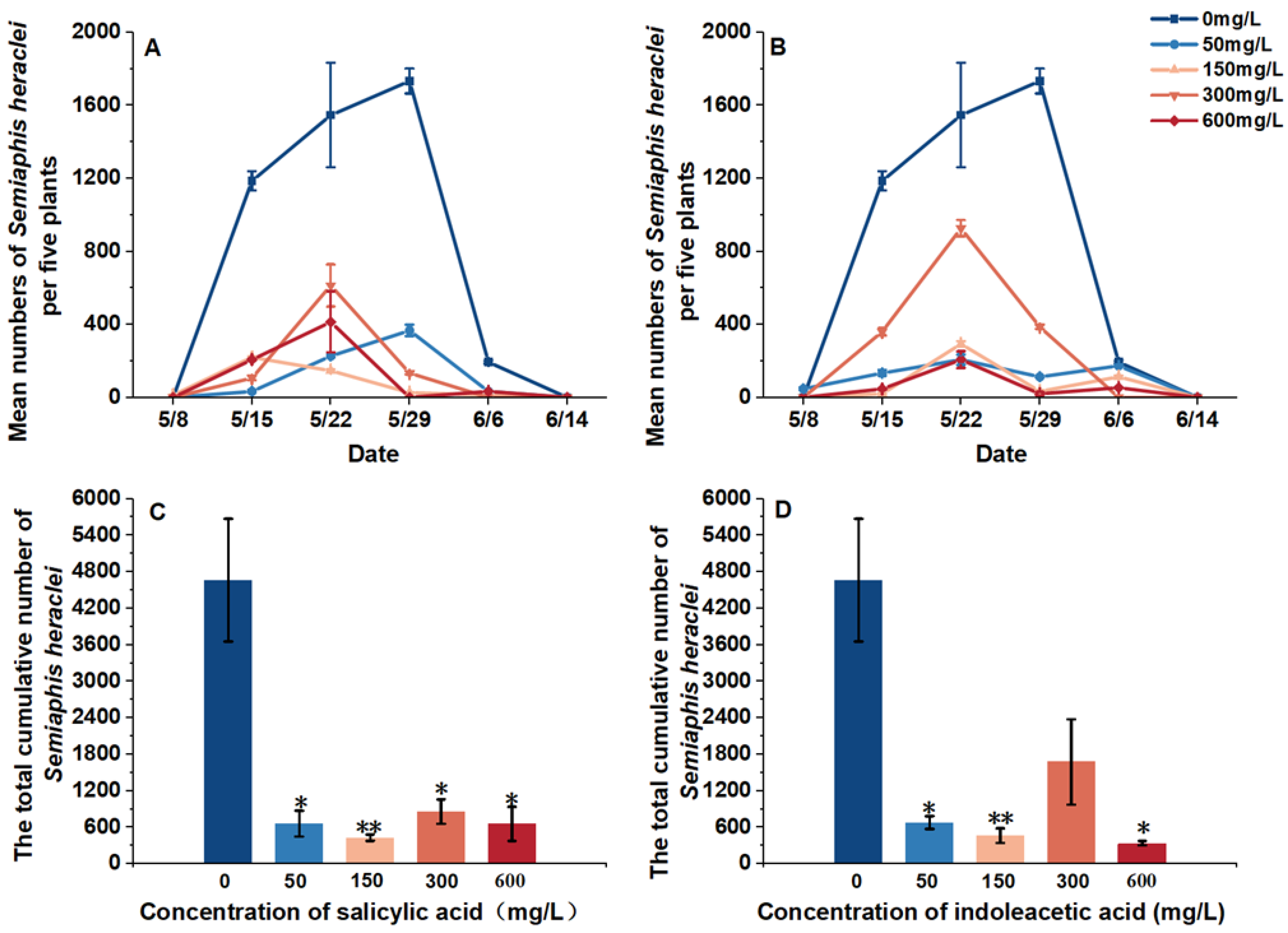
3.1.1. Variation of Field Aphids Abundance Under Different Hormone Treatment
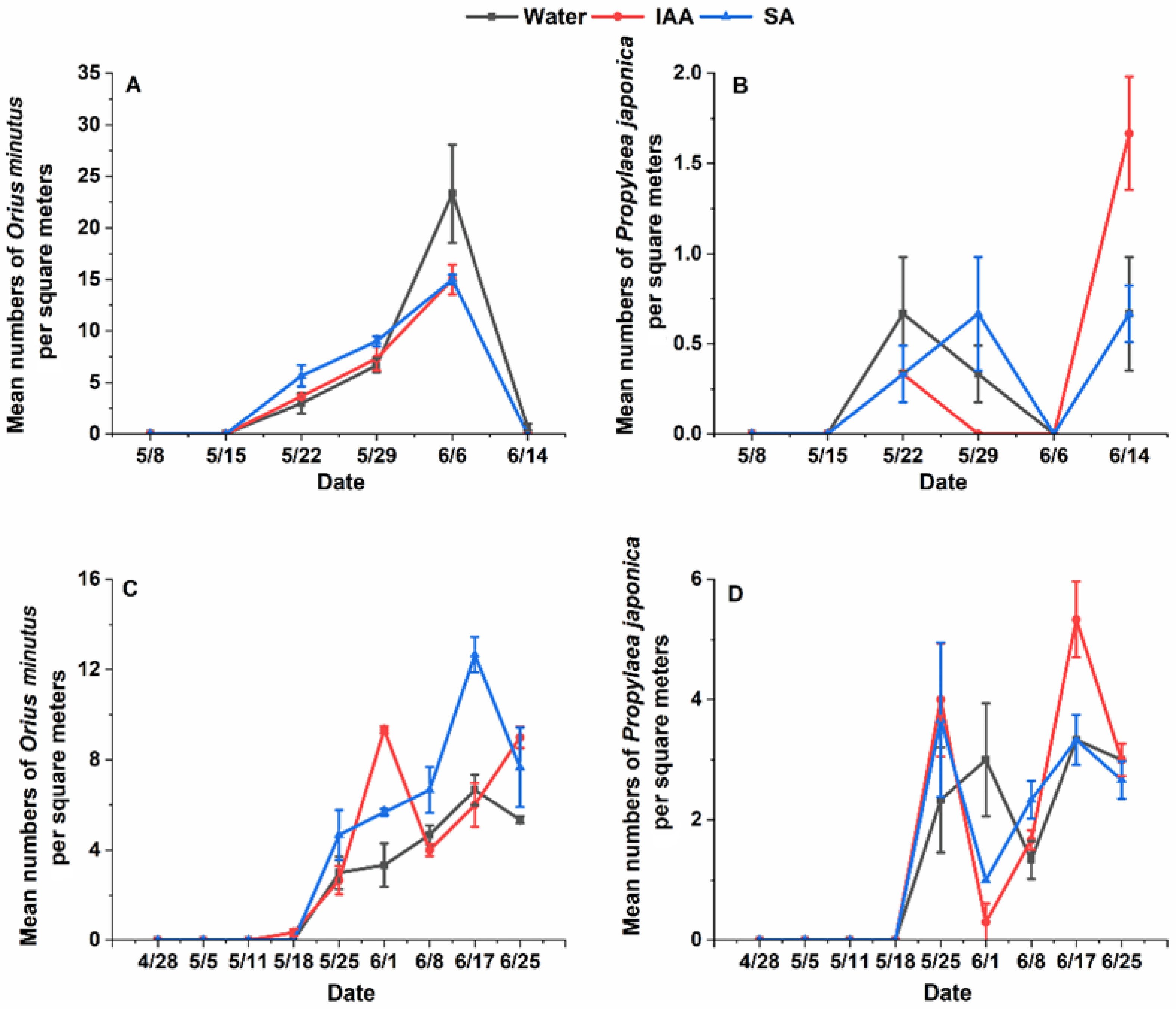
3.1.2. Variation in Field Natural Enemy Abundance Under Different Hormone Treatments
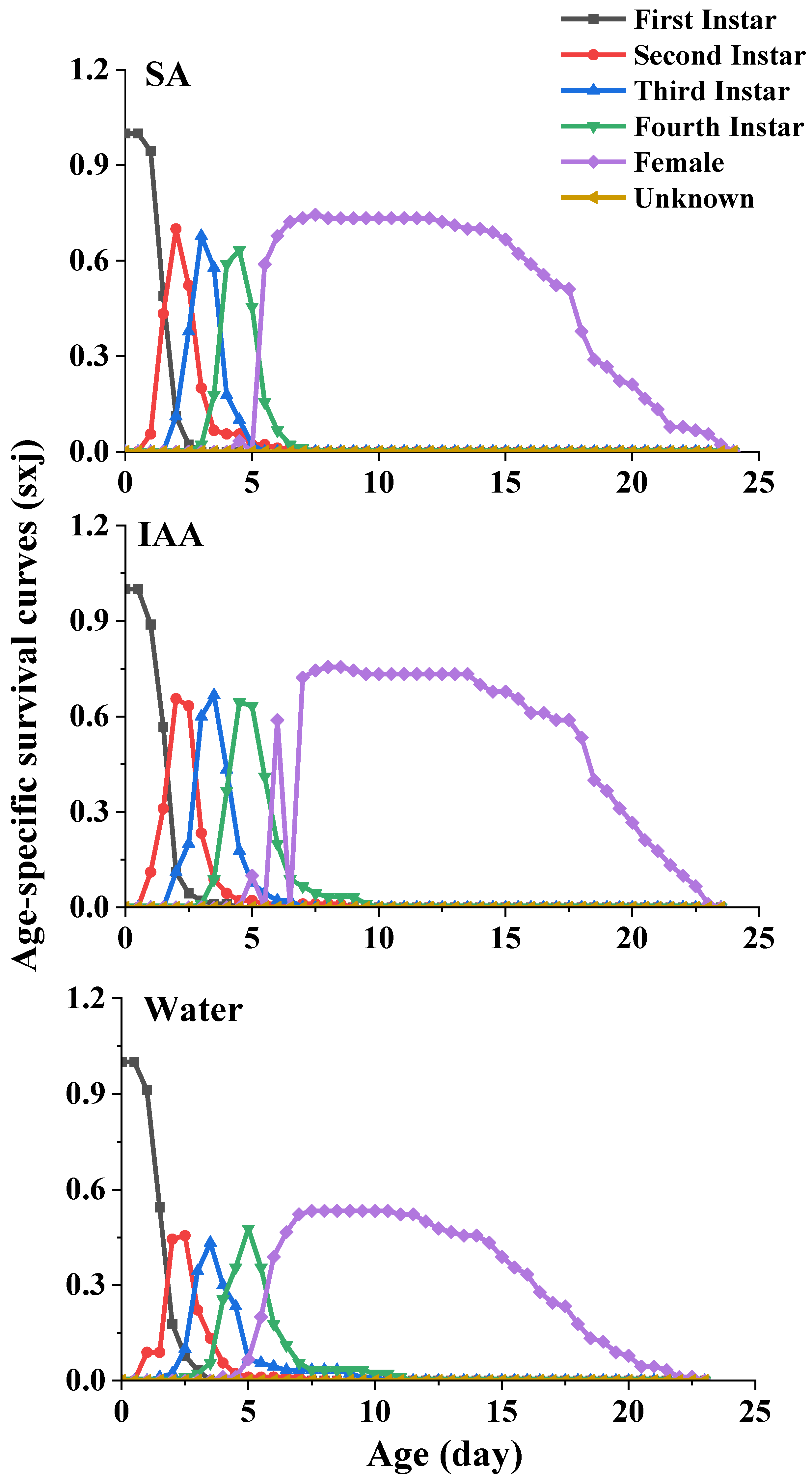
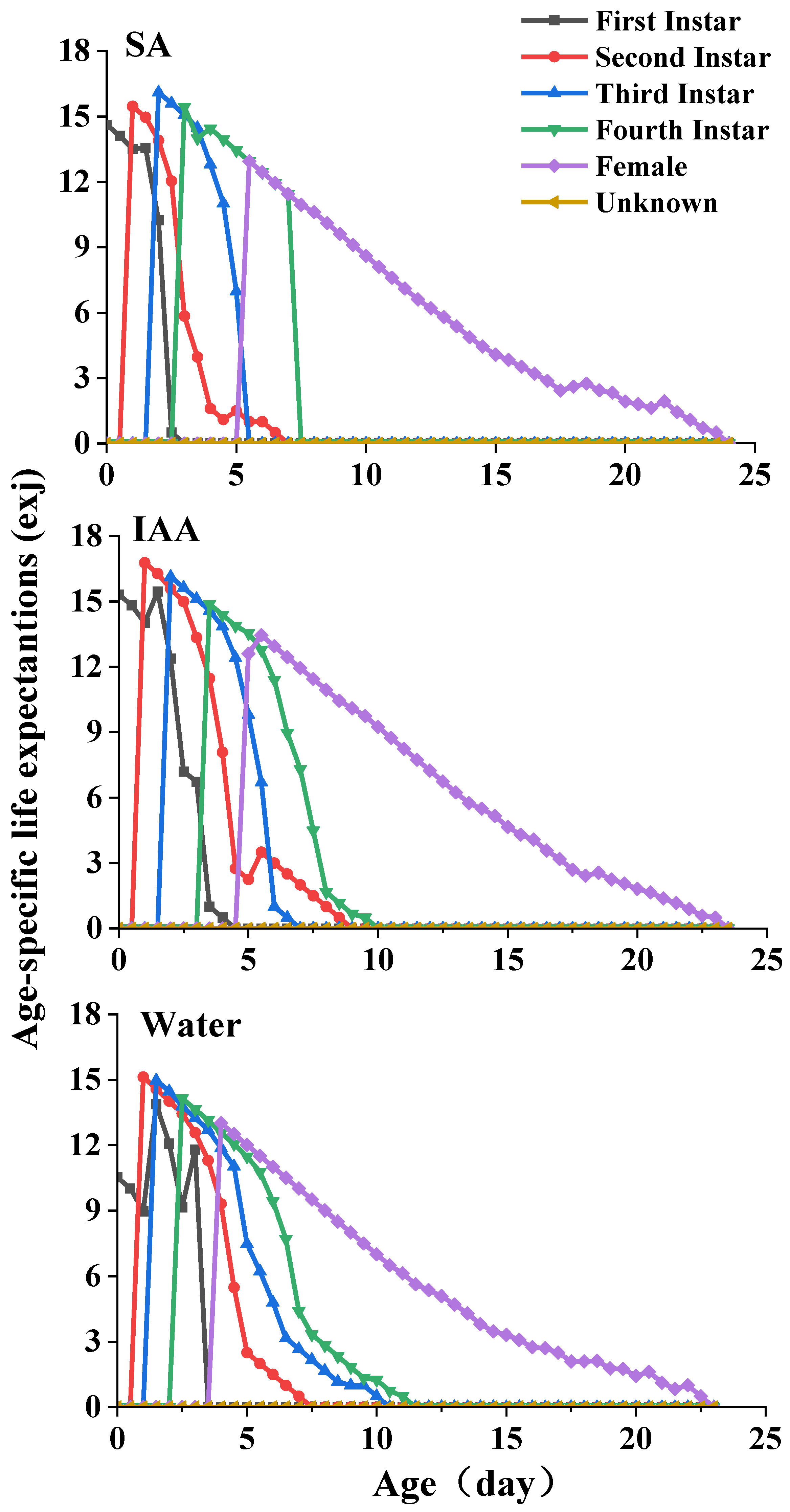
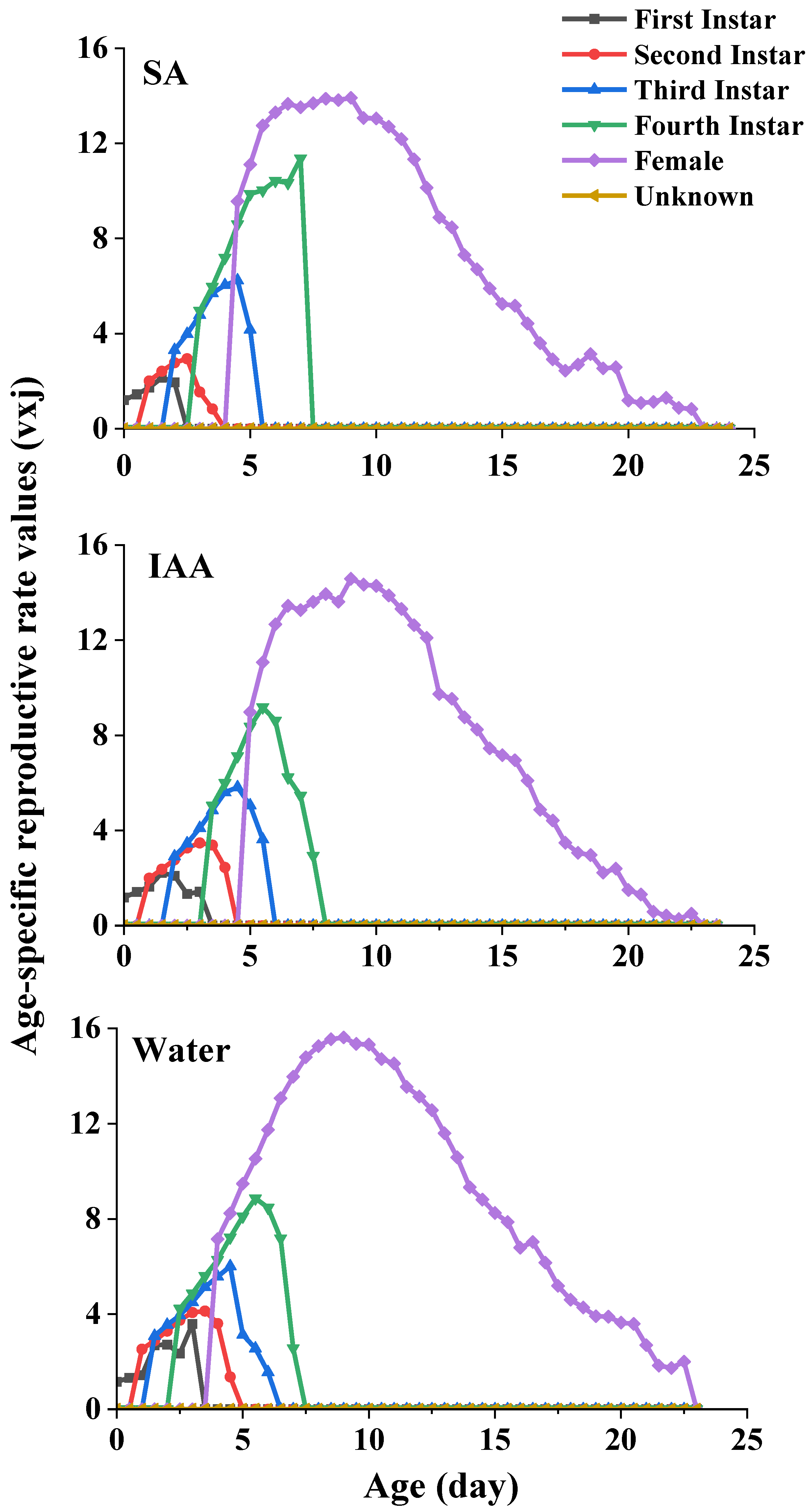
3.2. Life Table Experiment
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Santner, A.; Calderon-Villalobos, L.I.A.; Estelle, M. Plant hormones are versatile chemical regulators of plant growth. Nat. Chem. Biol. 2009, 5, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Milkereit, J.; Stoddard, C.S.; Dito, D.F.; Hodson, A.K. The influence of leaf traits and deficit irrigation on insect communities in mature green tomato production. Agric. Forest Entomol. 2020, 22, 119–128. [Google Scholar] [CrossRef]
- Miller, A.E.M.; Heyland, A. Endocrine interactions between plants and animals: Implications of exogenous hormone sources for the evolution of hormone signaling. Gen. Comp. Endocrinol. 2010, 166, 455–461. [Google Scholar] [CrossRef]
- Tejeda-Sartorius, O.; Soto-Hernández, R.M.; San Miguel-Chávez, R. Endogenous Hormone Profile and Sugars Display Differential Distribution in Leaves and Pseudobulbs of Laelia anceps Plants Induced and Non-Induced to Flowering by Exogenous Gibberellic Acid. Plants 2022, 11, 845. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.Y.; Du, W.F.; Wu, H.S.; Lyu, Y.; Ge, W.H.; Li, C.Y. Application of plant growth regulators in Chinese medicinal materials cultivation and reasearch progress of its impact on quality and yield of Chinese medicinal materials. China J. Tradit. Chin. Med. Pharm. 2022, 1587–1590. [Google Scholar]
- Blitzer, E.J.; Dormann, C.F.; Holzschuh, A.; Klein, A.M.; Rand, T.A.; Tscharntke, T. Spillover of functionally important organisms between managed and natural habitats. Agric. Ecosyst. Environ. 2012, 146, 34–43. [Google Scholar] [CrossRef]
- Ismail, M.A.; Amin, M.A.; Eid, A.M.; Hassan, S.E.; Mahgoub, H.A.M.; Lashin, I.; Abdelwahab, A.T.; Azab, E.; Gobouri, A.A.; Elkelish, A.; et al. Comparative study between exogenously applied plant growth hormones versus metabolites of microbial endophytes as plant growth-promoting for Phaseolus vulgaris L. Cells 2021, 10, 1059. [Google Scholar] [CrossRef]
- Huang, Y.-B.; Chi, H. Age-stage, two-sex life tables of Bactrocera cucurbitae (Coquillett) (Diptera: Tephritidae) with a discussion on the problem of applying female age-specifific life tables to insect populations. Insect Sci. 2012, 19, 263–273. [Google Scholar] [CrossRef]
- Ahn, J.J.; Choi, K.S. Population Parameters and Growth of Riptortus pedestris (Fabricius) (Hemiptera: Alydidae) under Fluctuating Temperature. Insects 2022, 13, 113. [Google Scholar] [CrossRef]
- Morandin, L.A.; Kremen, C. Hedgerow restoration promotes pollinator populations and exports native bees to adjacent fields. Ecol. Appl. 2013, 23, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Ouyang, F.; Chen, J.; Yang, Q.F.; Desneux, N.; Xiao, Y.L.; Zhang, J.P.; Ge, F. Biological control of Aphis spiraecola in apples using an insectary plant that attracts and sustains predators. Biol. Control 2021, 155, 104532. [Google Scholar] [CrossRef]
- Yang, Q.F.; Ouyang, F.; Men, X.Y.; Ge, F. Discovery and utilization of a functional plant, rich in the natural enemies of insect pests, in northern China. Chin. J. Appl. Entomol. 2018, 55, 942–947. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Ouyang, F.; Su, J.Y.; Li, Z.; Yuan, Y.Y.; Sun, Y.C.; Sarkar, S.C.; Xiao, Y.L.; Ge, F. Intercropping flowering plants facilitate conservation, movement and biocontrol performance of predators in insecticide-free apple orchard. Agric. Ecosyst. Environ. 2022, 340, 108157. [Google Scholar] [CrossRef]
- Ouyang, F.; Men, X.Y.; Yang, B.; Su, J.W.; Zhang, Y.S.; Zhao, Z.H.; Ge, F. Maize benefits the predatory beetle, Propylea japonica (Thunberg), to provide potential to enhance biological control for aphids in cotton. PLoS ONE 2012, 7, e44379. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Ouyang, F.; Li, Z.; Yuan, Y.Y.; Yang, Q.F.; Ge, F. Cnidium monnieri (L.) Cusson Flower as a Supplementary Food Promoting the Development and Reproduction of Ladybeetles Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae). Plants 2023, 12, 1786. [Google Scholar] [CrossRef] [PubMed]
- Chi, H.; Liu, H. Two new methods for the study of insect population ecology. Bull. Insect. Zool. Acad. Sin. 1985, 24, 225–240. [Google Scholar]
- Akca, I.; Ayvaz, T.; Yazici, E.; Smith, C.L.; Chi, H. Demography and population projection of Aphis fabae (Hemiptera: Aphididae) with additional comments on life table research criteria. J. Econ. Entomol. 2015, 108, 1466–1478. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.M.; Chi, H.; Wang, R.C.; Wang, Y.P.; Xu, Y.Y.; Li, X.D.; Yin, P.; Zheng, F.Q. Demography and uncertainty of population growth of Conogethes punctiferalis (Lepidoptera: Crambidae) reared on five host plants with discussion on some life history statistics. J. Econ. Entomol. 2018, 111, 2143–2152. [Google Scholar] [CrossRef]
- Chi, H.; Su, H.Y. Age-stage, two-sex life tables of Aphidius gifuensis (Ashmead) (Hymenoptera: Braconidae) and its host Myzus persicae (Sulzer) (Homoptera: Aphididae) with mathematical proof of the relationship between female fecundity and the net reproductive rate. Environ. Entomol. 2006, 35, 10–21. [Google Scholar] [CrossRef]
- Hu, M.X.; Zhang, L.X. Effects of exogenous plant hormones on insect resistance and natural enemies of plants. Eng. J. Heilongjiang Univ. 2019, 10, 91–96. [Google Scholar] [CrossRef]
- Shi, M.Z.; Li, J.Y.; Jian, B.D.; Fu, J.W.; Zheng, L.Z.; Chi, H. Indirect effect of elevated CO2 on population parameters and growth of Agasicles hygrophila (Coleoptera: Chrysomelidae), a biocontrol agent of Alligatorweed (Amaranthaceae). J. Econ. Entomol. 2019, 112, 1120–1129. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.L.; Ma, K.S.; Chi, H.; Hou, Y.M.; Gao, X.W. Transgenerational hormetic effects of sublethal dose of flupyradifurone on the green peach aphid, Myzus persicae (Sulzer) (Hemiptera: Aphididae). PLoS ONE 2019, 14, e0208058. [Google Scholar] [CrossRef] [PubMed]
- Chi, H. Life-table analysis incorporating both sexes and variable development rates among individuals. Environ. Entomol. 1988, 17, 26–34. [Google Scholar] [CrossRef]
- Tuan, S.J.; Lee, C.C.; Chi, H. Population and damage projection of Spodoptera litura (F.) on peanuts (Arachis hypogaea L.) under different conditions using the age-stage, two-sex life table. Pest Manag. Sci. 2014, 70, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, K.; Zhu, K.Y.; Tian, Y.F.; Yu, Q.; Zhang, W.Y.; Wang, Z.Q. Effect of exogenous plant hormones on agronomic and physiological performance of a leaf early-senescent rice mutant osled. Plant Growth Regul. 2020, 92, 517–533. [Google Scholar] [CrossRef]
- Goodman, D. Optimal life histories, optimal notation, and the value of reproductive value. Am. Nat. 1982, 119, 803–823. [Google Scholar] [CrossRef]
- Brooks, M.E.; Kristensen, K.; van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Mächler, M.; Bolker, B.M. glmmTMB balances speed and flexibility among packages for Zero inflated Generalized Linear Mixed Modeling. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef]
- Mollah, M.M.I.; Choi, H.W.; Yeam, I.; Lee, J.M.; Kim, Y. Salicylic acid, a plant hormone, suppresses phytophagous insect immune response by interrupting HMG-Like DSP1. Front. Physiol. 2021, 12, 744272. [Google Scholar] [CrossRef]
- Chi, H.; Güncan, A.; Kavousi, A.; Gharakhani, G.; Atlihan, R.; Özgökçe, M.S.; Shirazi, J.; Amir-Maafi, M.; Maroufpoor, M.; Roya, T. TWOSEX-MSChart: The key tool for life table research and education. Entomol. Gen. 2022, 42, 845–849. [Google Scholar] [CrossRef]
- Di, N.; Wang, S.; Ridsdill-Smith, J.; Chen, Y.F.; Harwood, J.D.; Zhang, K.; Liu, T.X. Nitrogen and plant growth regulator affect plant detoxification metabolism and tritrophic interactions among Triticum aestivum, Sitobion avenae and Aphelinus asychis. Entomol. Gen. 2021, 41, 369–384. [Google Scholar] [CrossRef]
- Lin, Y.B.; Lin, X.H.; Ding, C.; Ding, C.H.; Xia, M.; Xue, R.R.; Sun, Z.X.; Chen, D.Q.; Zhu-Salzman, K.; Zeng, R.S. Priming of rice defense against a sap-sucking insect pest brown planthopper by silicon. J. Pest Sci. 2022, 95, 1371–1385. [Google Scholar] [CrossRef]
- Bartlett, L.; Connor, E.F. Exogenous phytohormones and the induction of plant galls by insects. Arthropod-Plant Interact. 2014, 8, 339–348. [Google Scholar] [CrossRef]
- Cipollini, D.; Enright, S.; Traw, M.B.; Bergelson, J. Salicylic acid inhibits jasmonic acid-induced resistance of Arabidopsis thaliana to Spodoptera exigua. Mol. Ecol. 2004, 13, 1643–1653. [Google Scholar] [CrossRef]
- Li, Q.Q.; Xu, F.; Chen, Z.; Teng, Z.F.; Sun, K.; Li, X.C.; Yu, J.Y.; Zhang, G.X.; Liang, Y.; Huang, X.H.; et al. Synergistic interplay of ABA and BR signal in regulating plant growth and adaptation. Nat. Plants 2021, 7, 1108–1118. [Google Scholar] [CrossRef] [PubMed]
- Poitou, L.; Robinet, C.; Suppo, C.; Rousselet, J.; Laparie, M.; Pincebourde, S. When insect pests build their own thermal niche: The hot nest of the pine processionary moth. J. Therm. Biol. 2021, 98, 102947. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.F.; Men, X.Y.; Zhao, W.L.; Li, C.; Zhang, Q.Q.; Cai, Z.P.; Ge, F.; Ouyang, F. Flower strips as a bridge habitat facilitate the movement of predatory beetles from wheat to maize crops. Pest Manag. Sci. 2021, 77, 1839–1850. [Google Scholar] [CrossRef] [PubMed]
- Dudt, J.F.; Shure, D.J. The influence of light and nutrients on foliar phenolics and insect herbivory. Ecology 1994, 75, 86–98. [Google Scholar] [CrossRef]
- Gayer, C.; Berger, J.; Dieterich, M.; Gallé, R.; Reidl, K.; Witty, R.; Ben, A.; Woodcock Batáry, P. Flowering fields, organic farming and edge habitats promote diversity of plants and arthropods on arable land. J. Appl. Ecol. 2021, 58, 1155–1166. [Google Scholar] [CrossRef]
- Schubert, L.F.; Hellwig, N.; Kirmer, A.; Schmid-Egger, C.; Schmidt, A.; Dieker, P.; Tischew, S. Habitat quality and surrounding landscape structures influence wild bee occurrence in perennial wildflower strips. Basic Appl. Ecol. 2022, 60, 76–86. [Google Scholar] [CrossRef]
- Chi, H.; You, M.; Atlihan, R.; Smith, C.L.; Kavousi, A.; Özgökçe, M.S.; Güncan, A.; Tuan, S.J.; Fu, J.W.; Xu, Y.Y.; et al. Age-stage, two-sex life table: An introduction to theory, data analysis, and application. Entomol. Gen. 2020, 40, 103–124. [Google Scholar] [CrossRef]
- Heinen, R.; Steinauer, K.; De Long, J.R.; Jongen, R.; Biere, A.; Harvey, J.A.; Bezemer, M. Exogenous application of plant hormones in the field alters aboveground plant–insect responses and belowground nutrient availability, but does not lead to differences in plant–soil feedbacks. Arthropod-Plant Interact. 2020, 14, 559–570. [Google Scholar] [CrossRef]
- Ardanuy, A.; Lee, M.S.; Albajes, R. Landscape context influences leafhopper and predatory Orius spp. abundances in maize fields. Agric. For. Entomol. 2018, 20, 81–92. [Google Scholar] [CrossRef]
- Coulson, R.N.; Franklin, R.T. Microenvironmental Measurements for the Dioryctria amatella-zimmermani Complex in Shortleaf Pine. 1. Insect Introduction, Temperature, Humidity, and Vapor Pressure Deficit. J. Econ. Entomol. 1970, 63, 558–564. [Google Scholar] [CrossRef]
- Read, R.G.; Adames, A.J.; Galindo, P. A model of microenvironment and man-biting tropical insects. Environ. Entomol. 1978, 7, 547–552. [Google Scholar] [CrossRef]
- Torrico-Bazoberry, D.; Pinto, C.F.; Davyt-Colo, J. Response to selected ecological parameters by Leptus hringuri Haitlinger, 2000 larvae (Trombidiformes: Erythraeidae) parasitizing treehoppers (Hemiptera: Membracidae) from Bolivia on two host-plant species. Int. J. Acarol. 2020, 46, 174–179. [Google Scholar] [CrossRef]
- Vaz, P.G.; Bugalho, M.N.; Fedriani, J.M. Unravelling associations between tree-seedling performance, herbivory, competition, and facilitation in high nature value farmlands. J. Environ. Manag. 2019, 232, 1066–1074. [Google Scholar] [CrossRef] [PubMed]
- Wallis, R.L.; Turner, J.E. Insects overwintering in the warm microenvironment of drainage ditches in central Washington. Environ. Entomol. 1972, 1, 107–109. [Google Scholar] [CrossRef]

| Parameters | Control | Treatments | ||||
|---|---|---|---|---|---|---|
| n | Clean Water | n | SA | n | IAA | |
| First stage | 56 | 2.04 ± 0.08 a | 81 | 1.78 ± 0.042 b | 77 | 1.82 ± 0.054 ab |
| Second stage | 55 | 1.21 ± 0.05 a | 69 | 0.96 ± 0.047 b | 74 | 1.2 ± 0.048 a |
| Third stage | 52 | 1.28 ± 0.06 a | 67 | 1.28 ± 0.052 a | 72 | 1.36 ± 0.036 a |
| Fourth stage | 48 | 1.49 ± 0.06 a | 67 | 1.43 ± 0.041 a | 69 | 1.51 ± 0.043 a |
| Adult | 48 | 11.07 ± 0.39 b | 67 | 12.99 ± 0.362 a | 69 | 12.89 ± 0.41 ab |
| Pre-adult | 48 | 5.94 ± 0.10 a | 67 | 5.46 ± 0.068 b | 69 | 5.86 ± 0.076 a |
| Total | 90 | 10.52 ± 0.80 b | 90 | 14.62 ± 0.748 a | 90 | 15.32 ± 0.747 a |
| Fecundity (F) | 72 | 44.35 ± 0.87 a | 80 | 50.60 ± 1.057 a | 78 | 49.17 ± 1.203 a |
| APOP | 48 | 1.41 ± 0.080 a | 67 | 0.35 ± 0.046 b | 69 | 0.54 ± 0.058 c |
| TPOP | 48 | 7.34 ± 0.16 a | 67 | 5.81 ± 8.082 b | 69 | 6.4 ± 0.086 c |
| Parameters | SA | IAA | Clean Water |
|---|---|---|---|
| Intrinsic rate of increase, r (d−1) | 0.37 ± 0.003 a | 0.35 ± 0.001 b | 0.29 ± 0.003 c |
| Finite rate of increase, λ (d−1) | 1.45 ± 0.004 a | 1.41 ± 0.004 b | 1.33 ± 0.003 c |
| Net reproductive rate, R0 | 37.67 ± 0.574 a | 37.70 ± 1.045 a | 23.66 ± 0.629 b |
| Mean generation rate T, (d) | 9.78 ± 0.054 c | 10.50 ± 0.026 b | 11.22 ± 0.021 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, X.; Zhang, X.; Han, G.; Sarkar, S.C.; Ge, F. Natural Enemies Acquire More Prey Aphids from Hormone-Treated Insect-Attracting Plants. Plants 2025, 14, 147. https://doi.org/10.3390/plants14020147
Jiang X, Zhang X, Han G, Sarkar SC, Ge F. Natural Enemies Acquire More Prey Aphids from Hormone-Treated Insect-Attracting Plants. Plants. 2025; 14(2):147. https://doi.org/10.3390/plants14020147
Chicago/Turabian StyleJiang, Xiaosheng, Xingrui Zhang, Guodong Han, Shovon Chandra Sarkar, and Feng Ge. 2025. "Natural Enemies Acquire More Prey Aphids from Hormone-Treated Insect-Attracting Plants" Plants 14, no. 2: 147. https://doi.org/10.3390/plants14020147
APA StyleJiang, X., Zhang, X., Han, G., Sarkar, S. C., & Ge, F. (2025). Natural Enemies Acquire More Prey Aphids from Hormone-Treated Insect-Attracting Plants. Plants, 14(2), 147. https://doi.org/10.3390/plants14020147






