Rhizosphere Engineering in Saline Soils: Role of PGPR and Organic Manures in Root–Soil Biochemical Interactions for Allium Crops
Abstract
1. Introduction
2. Results
2.1. Soil Fundamental Properties
2.2. Soil Microbial Parameters
2.3. Plant Parameters
2.4. Residual Effects
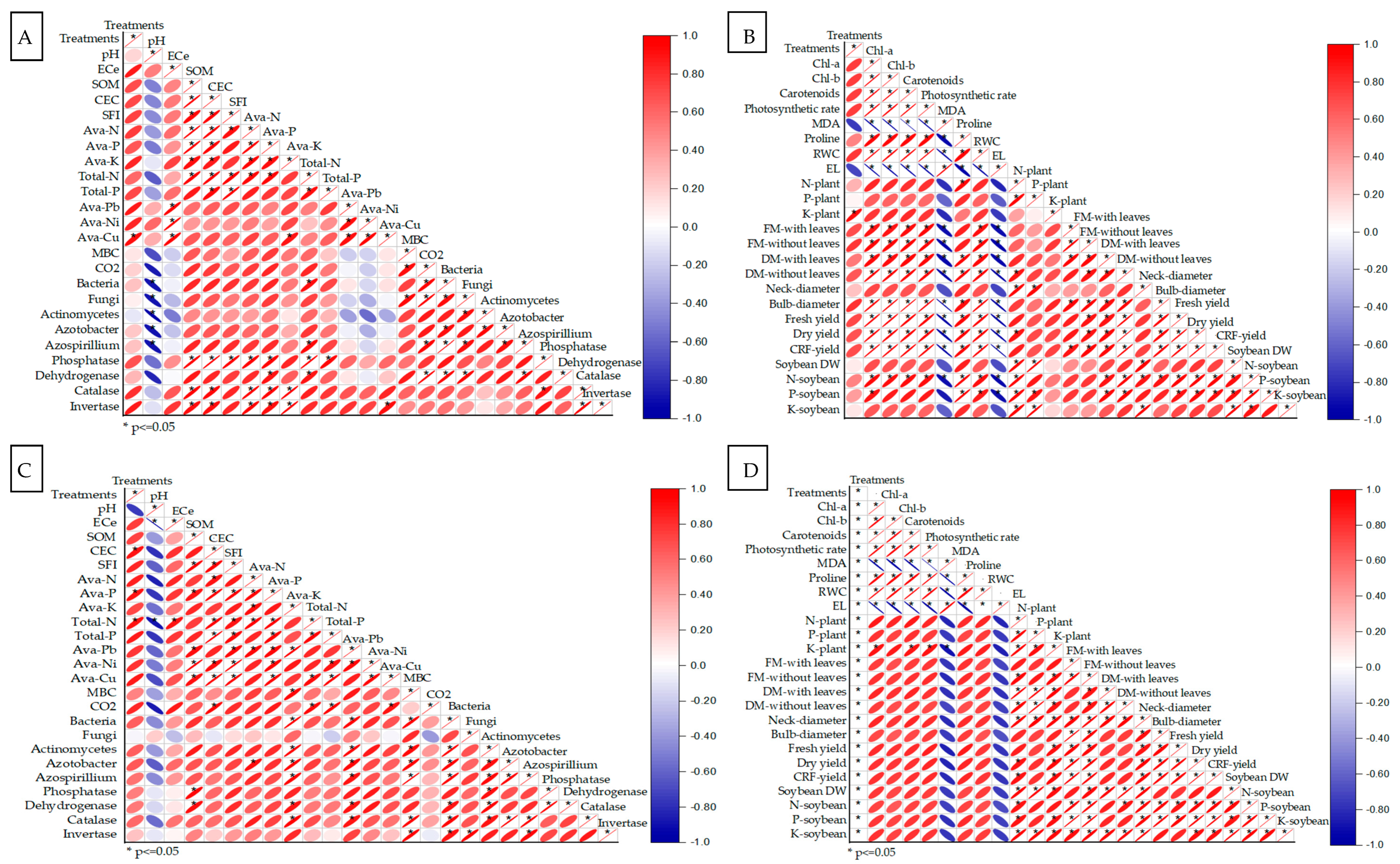
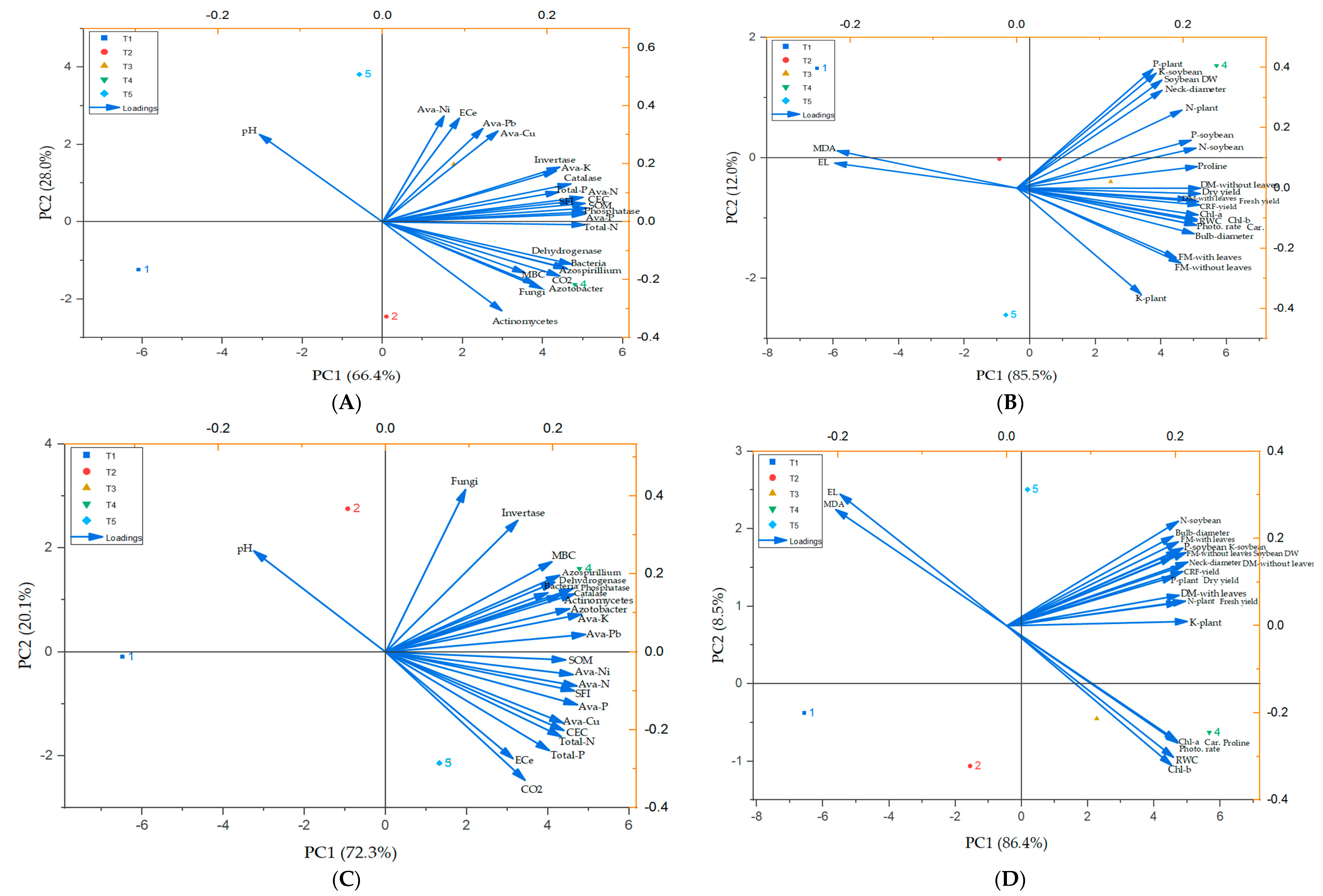
2.5. Soil–Plant Interactions (Pearson Correlation and PCA)
3. Discussion
3.1. Impact of Nitrogen Amendments on Soil Properties and Fertility
3.2. Microbial Responses to Nitrogen Amendments
3.3. Microbial Responses to Nitrogen Amendments
3.4. Enhancement of Photosynthetic Efficiency and Stress Tolerance
3.5. Nutrient Uptake and Yield Enhancement
3.6. Residual Impact of Nitrogen Amendments on Soybean Growth: Insights from the Bioassay Experiment
3.7. Implications for Sustainable Agriculture
4. Materials and Methods
4.1. Study Location and Experimental Setup
| Parameter | Soil | Sewage Sludge | Poultry Manure |
|---|---|---|---|
| pH * | 7.88 ± 0.01 | 5.54 ± 0.02 | 7.30 ± 0.01 |
| Electrical conductivity (ECe; soil paste extract at 25 °C; dS/m) | 3.47 ± 0.05 | 7.27 ± 0.08 | 9.24 ± 0.08 |
| Saturation percentage (%) | 77 ± 1 | 164 ± 2 | 125 ± 2 |
| Total organic carbon (TOC; %) | 1.29 ± 0.03 | 8.38 ± 0.05 | 9.35 ± 0.04 |
| Soil organic matter (SOM; g kg−1) | 22.2 ± 0.2 | 144.4 ± 1.4 | 161.2 ± 1.6 |
| C/N ratio | 13.16 ± 0.5 | 6.27 ± 0.3 | 7.14 ± 0.4 |
| Cation exchange capacity (CEC; cmolec/kg soil) | 33.9 ± 0.4 | 36.3 ± 0.5 | na |
| Available-N (mg/kg) | 42 ± 2.1 | 532 ± 22.2 | 490 ± 19.6 |
| Available-P (mg/kg) | 7 ± 0.05 | 54 ± 0.9 | 151 ± 2.4 |
| Available-K (mg/kg) | 103 ± 1.9 | 66 ± 0.9 | 515 ± 2.5 |
| Total-N (g/kg) | 0.98 ± 0.04 | 13.37 ± 0.81 | 13.09 ± 0.85 |
| Total-P (mg/kg) | 133 ± 21 | 444 ± 24 | 833 ± 28 |
| DTPA-Cu (mg/kg) | 0.92 ± 0.03 | 24.80 ± 0.91 | 4.90 ± 0.15 |
| DTPA-Ni (mg/kg) | 0.20 ± 0.01 | 2.80 ± 0.05 | 2.80 ± 0.03 |
| DTPA-Pb (mg/kg) | 0.70 ± 0.02 | 14.00 ± 0.08 | 3.60 ± 0.05 |
| Sand (%) | 18.20 ± 0.23 | na † | na |
| Silt (%) | 57.54 ± 1.21 | na | na |
| Clay (%) | 24.26 ± 0.98 | na | na |
| Texture class | Silty loam | na | na |
| Total bacterial count (CFU ⸸ 106/g soil) | 50.0 ± 1.21 | 133.0 ± 2.33 | 3.0 ± 0.02 |
| Total fungi count (CFU 106/g soil) | 1.6 ± 0.01 | 5.0 ± 0.02 | 0.9 ± 0.01 |
| Total actinomycetes count (CFU 106/g soil) | 3.3 ± 0.1 | 10.6 ± 0.3 | 1.8 ± 0.1 |
| Spore-forming bacteria count (CFU 106/g soil) | 0.5 ± 0.01 | 0.6 ± 0.01 | 0.2 ± 0.01 |
| Azotobacter count (CFU 104/g soil) | 8.8 ± 0.14 | nd ‡ | nd |
| Azospirillium count (CFU 104/g soil) | 2.1 ± 0.10 | nd | nd |
| Phosphatase activity (mg p-nitrophenol/g soil/h) | 35.5 ± 0.21 | nd | nd |
| Dehydrogenase activity (mg TPF/kg soil/day) | 6.91 ± 0.18 | nd | nd |
| Catalase activity (µmole H2O2/g soil/15 min) | 187 ± 13 | nd | nd |
| Invertase activity (µmole glucose/g soil/day) | 5.78 ± 0.11 | nd | nd |
4.2. Source of Used Materials and Their Preparation
4.3. Experimental Treatments
4.3.1. Onion Cultivation Treatments
4.3.2. Garlic Cultivation Treatments
4.4. Experimental Design and Crop Management
4.5. Soil Sampling and Analytical Procedures
4.5.1. Soil Physicochemical Analysis
4.5.2. Microbiological Analysis
4.6. Plant Sampling and Physiological Analysis
4.7. Bioassay Trial
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Munns, R.; Tester, M. Mechanisms of Salinity Tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- García-Caparrós, P.; Hasanuzzaman, M.; Lao, M.T. Oxidative Stress and Antioxidant Defense in Plants under Salinity. In Reactive Oxygen, Nitrogen and Sulfur Species in Plants; Hasanuzzaman, M., Fotopoulos, V., Nahar, K., Fujita, M., Eds.; Wiley: Hoboken, NJ, USA, 2019; pp. 291–309. ISBN 978-1-119-46869-1. [Google Scholar]
- Aghajanzadeh, T.A.; Reich, M.; Hawkesford, M.J.; Burow, M. Sulfur Metabolism in Allium Cepa Is Hardly Affected by Chloride and Sulfate Salinity. Arch. Agron. Soil Sci. 2019, 65, 945–956. [Google Scholar] [CrossRef]
- Semida, W.M.; Abdelkhalik, A.; Rady, M.O.A.; Marey, R.A.; Abd El-Mageed, T.A. Exogenously Applied Proline Enhances Growth and Productivity of Drought Stressed Onion by Improving Photosynthetic Efficiency, Water Use Efficiency and up-Regulating Osmoprotectants. Sci. Hortic. 2020, 272, 109580. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, Q.; Liu, M.; Zhou, H.; Ma, C.; Wang, P. Regulation of Plant Responses to Salt Stress. Int. J. Mol. Sci. 2021, 22, 4609. [Google Scholar] [CrossRef]
- Chang, P.-T.; Randle, W.M. Sodium Chloride Timing and Length of Exposure Affect Onion Growth and Flavor. J. Plant Nutr. 2005, 28, 1755–1766. [Google Scholar] [CrossRef]
- Ncayiyana, M.; Maboko, M.M.; Bertling, I. Yield and Nutritional Quality of Different Short-Day Onion Cultivars as Affected by Nitrogen Application. S. Afr. J. Plant Soil 2018, 35, 215–221. [Google Scholar] [CrossRef]
- Ricciardi, L.; Mazzeo, R.; Marcotrigiano, A.R.; Rainaldi, G.; Iovieno, P.; Zonno, V.; Pavan, S.; Lotti, C. Assessment of Genetic Diversity of the “Acquaviva Red Onion” (Allium Cepa L.) Apulian Landrace. Plants 2020, 9, 260. [Google Scholar] [CrossRef]
- Team, E. Onion Production Trade and Consumption A 360° Industry Report EssFeed; EssFeed: Hong Kong, China, 2025. [Google Scholar]
- Roa, S. Garlic Production by Country in 2025: Top 15 Countries. Available online: https://smallholdinghero.com/garlic/garlic-production-by-country (accessed on 20 September 2025).
- Geisseler, D.; Ortiz, R.S.; Diaz, J. Nitrogen Nutrition and Fertilization of Onions (Allium Cepa L.)—A Literature Review. Sci. Hortic. 2022, 291, 110591. [Google Scholar] [CrossRef]
- Venâncio, J.B.; Dias, N.D.S.; De Medeiros, J.F.; De Morais, P.L.D.; Do Nascimento, C.W.A.; Neto, O.N.D.S.; Sá, F.V.D.S. Yield and Morphophysiology of Onion Grown under Salinity and Fertilization with Silicon. Sci. Hortic. 2022, 301, 111095. [Google Scholar] [CrossRef]
- Chourasia, K.N.; More, S.J.; Kumar, A.; Kumar, D.; Singh, B.; Bhardwaj, V.; Kumar, A.; Das, S.K.; Singh, R.K.; Zinta, G.; et al. Salinity Responses and Tolerance Mechanisms in Underground Vegetable Crops: An Integrative Review. Planta 2022, 255, 68. [Google Scholar] [CrossRef] [PubMed]
- Forotaghe, Z.A.; Souri, M.K.; Jahromi, M.G.; Torkashvand, A.M. Physiological and Biochemical Responses of Onion Plants to Deficit Irrigation and Humic Acid Application. Open Agric. 2021, 6, 728–737. [Google Scholar] [CrossRef]
- Bhushan, C.; Yadav, A.K.; Gangwar, H.K.; Kumar, B.; Katiyar, S.K.; Vikram, N. Effect of Bio-Fertilizers on Growth and Yield of Garlic (Allium Sativum Linn.). Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 739–743. [Google Scholar] [CrossRef]
- Alshaal, T.; Alharbi, K.; Naif, E.; Rashwan, E.; Omara, A.E.-D.; Hafez, E.M. Strengthen Sunflowers Resilience to Cadmium in Saline-Alkali Soil by PGPR-Augmented Biochar. Ecotoxicol. Environ. Saf. 2024, 280, 116555. [Google Scholar] [CrossRef]
- Alshaal, T.; El-Ramady, H. Foliar Application: From Plant Nutrition to Biofortification. EBSS 2017, Vol.1, 71–83. [Google Scholar] [CrossRef]
- Tian, S.; Zhu, B.; Yin, R.; Wang, M.; Jiang, Y.; Zhang, C.; Li, D.; Chen, X.; Kardol, P.; Liu, M. Organic Fertilization Promotes Crop Productivity through Changes in Soil Aggregation. Soil Biol. Biochem. 2022, 165, 108533. [Google Scholar] [CrossRef]
- Shoaib, A.; Meraj, S.; Nafisa; Khan, K.A.; Javaid, M.A. Influence of Salinity and Fusarium Oxysporum as the Stress Factors on Morpho-Physiological and Yield Attributes in Onion. Physiol. Mol. Biol. Plants 2018, 24, 1093–1101. [Google Scholar] [CrossRef]
- Jiku, M.A.S.; Alimuzzaman, M.; Singha, A.; Rahaman, M.A.; Ganapati, R.K.; Alam, M.A.; Sinha, S.R. Response and Productivity of Garlic (Allium Sativum L.) by Different Levels of Potassium Fertilizer in Farm Soils. Bull. Natl. Res. Cent. 2020, 44, 9. [Google Scholar] [CrossRef]
- Chanchan, M.; Thapa, P.; Hore, J.K. Effect of Biofertilizers with Graded Levels of Nitrogen and Phosphorus on Growth and Yield of Garlic ( Allium Sativum L.). Rese. Crop. 2018, 19, 127. [Google Scholar] [CrossRef]
- Solouki, A.; Berna-Sicilia, J.Á.; Martinez-Alonso, A.; Ortiz-Delvasto, N.; Bárzana, G.; Carvajal, M. Onion Plants (Allium Cepa L.) React Differently to Salinity Levels According to the Regulation of Aquaporins. Heliyon 2023, 9, e13815. [Google Scholar] [CrossRef] [PubMed]
- Verma, N.V.; Jaiswal, R.K.; Ali, S.A.; Dhakad, R.K. Impact of Bio Fertilizers on Growth and Yield Attributes of Onion (Allium Cepa L.) Var. Aflr. Int. J. Curr. Microbiol. Appl. Sci. 2021, 10, 931–938. [Google Scholar] [CrossRef]
- Sharma, S.; Gupta, R.; Dugar, G.; Srivastava, A.K. Impact of Application of Biofertilizers on Soil Structure and Resident Microbial Community Structure and Function. In Bacteria in Agrobiology: Plant Probiotics; Maheshwari, D.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 65–77. ISBN 978-3-642-27514-2. [Google Scholar]
- Patil, M.; Jana, P.; Murumkar, C. Effect of Onion and Garlic Biowaste on Germination and Growth of Microgreens. Int. J. Sci. Rep. 2021, 7, 302. [Google Scholar] [CrossRef]
- Gebremichael, Y.; Woldetsadik, K.; Gedamu, F. Effect of Combined Application of Organic Manure and Inorganic Nitrogen on Marketable Yield, Shelf Life of Onion and Soil Fertility Status after Harvest. Asian Res. J. Agric. 2017, 6, 1–13. [Google Scholar] [CrossRef]
- Gupta, M.; Sharma, R.; Sharma, M.; Sharma, S.; Sharma, P. Influence of Inorganic Fertilizers with Biofertilizers on Biochemical Components, Growth and Yield of Garlic under Subtropical Irrigated Condition. J. Plant Nutr. 2024, 47, 837–849. [Google Scholar] [CrossRef]
- Chander, K.; Brookes, P.C. Is the Dehydrogenase Assay Invalid as a Method to Estimate Microbial Activity in Copper-Contaminated Soils? Soil Biol. Biochem. 1991, 23, 909–915. [Google Scholar] [CrossRef]
- Dick, W.A.; Cheng, L.; Wang, P. Soil Acid and Alkaline Phosphatase Activity as pH Adjustment Indicators. Soil Biol. Biochem. 2000, 32, 1915–1919. [Google Scholar] [CrossRef]
- Jebara, S.; Jebara, M.; Limam, F.; Aouani, M.E. Changes in Ascorbate Peroxidase, Catalase, Guaiacol Peroxidase and Superoxide Dismutase Activities in Common Bean (Phaseolus Vulgaris) Nodules under Salt Stress. J. Plant Physiol. 2005, 162, 929–936. [Google Scholar] [CrossRef]
- Vitolo, M.; Borzani, W. Measurement of Invertase Activity of Cells of Saccharomyces Cerevisiae. Anal. Biochem. 1983, 130, 469–470. [Google Scholar] [CrossRef]
- Ncayiyana, M.; Maboko, M.M.; Bertling, I. Alterations in Yield, Physicochemical Components and Mineral Composition of Onion Following Organic Manure and Inorganic Nitrogen Application. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2018, 68, 213–219. [Google Scholar] [CrossRef]
- Backes, C.; Villas Bôas, R.L.; Godoy, L.J.G.D.; Vargas, P.F.; Santos, A.J.M. Determination of Growth and Nutrient Accumulation in Bella Vista Onion. Rev. Caatinga 2018, 31, 246–254. [Google Scholar] [CrossRef]
- Sparks, D.L.; Soil Science Society of America; American Society of Agronomy (Eds.) Methods of Soil Analysis. Part 3: Chemical Methods; Soil Science Society of America Book Series; Soil Science Society of America: American Society of Agronomy: Madison, WI, USA, 1996; ISBN 978-0-89118-825-4. [Google Scholar]
- Cottenie, A. Soil and Plant Testing as a Basis of Fertilizer Recommendations; FAO Soils Bulletin; Food and Agriculture Organization of the United Nations: Rome, Italy, 1980; ISBN 978-92-5-100956-7. [Google Scholar]
- Jackson, M.L. Soil Chemical Analysis; Prentice Hall of India Pvt. Ltd.: New Delhia, India, 1973. [Google Scholar]
- Abd-el-Malek, Y.; Hosny, I.; Shawky, B.T. Studies on Azotobacters Prevailing in Egyptian Soils. Zentralblatt für Bakteriologie, Parasitenkunde, Infektionskrankheiten und Hygiene. Zweite Naturwissenschaftliche Abt. Mikrobiol. Landwirtsch. Technol. Und Des Umweltschutzes 1979, 134, 498–506. [Google Scholar] [CrossRef]
- Dobereiner, J.; Marriel, I.E.; Nery, M. Ecological Distribution of Spirillum Lipoferum Beijerinck. Can. J. Microbiol. 1976, 22, 1464–1473. [Google Scholar] [CrossRef]
- Qi, Y.; Darilek, J.L.; Huang, B.; Zhao, Y.; Sun, W.; Gu, Z. Evaluating Soil Quality Indices in an Agricultural Region of Jiangsu Province, China. Geoderma 2009, 149, 325–334. [Google Scholar] [CrossRef]
- Olsen, S.R.; Cole, C.V.; Watanabe, F.S. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; USDA Circular No. 939; US Government Printing Office: Washington, DC, USA, 1954. [Google Scholar]
- Brookes, P.C.; Landman, A.; Pruden, G.; Jenkinson, D.S. Chloroform Fumigation and the Release of Soil Nitrogen: A Rapid Direct Extraction Method to Measure Microbial Biomass Nitrogen in Soil. Soil Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]
- Ölinger, R.; Beck, T.; Heilmann, B.; Beese, F. Soil Respiration. In Methods in Soil Biology; Schinner, F., Öhlinger, R., Kandeler, E., Margesin, R., Eds.; Springer: Berlin/Heidelberg, Germany, 1996; pp. 93–110. ISBN 978-3-642-64633-1. [Google Scholar]
- Louw, H.A.; Webley, D.M. The Bacteriology of the Root Region of the Oat Plant Grown under Controlled Pot Culture Conditions. J. Appl. Bacteriol. 1959, 22, 216–226. [Google Scholar] [CrossRef]
- Johnson, J.L.; Temple, K.L. Some Variables Affecting the Measurement of “Catalase Activity” in Soil. Soil Sci. Soc. Am. J. 1964, 28, 207–209. [Google Scholar] [CrossRef]
- Barrs, H.; Weatherley, P. A Re-Examination of the Relative Turgidity Technique for Estimating Water Deficits in Leaves. Aust. Jnl. Biol. Sci. 1962, 15, 413. [Google Scholar] [CrossRef]
- Bajji, M.; Kinet, J.-M.; Lutts, S. The Use of the Electrolyte Leakage Method for Assessing Cell Membrane Stability as a Water Stress Tolerance Test in Durum Wheat. Plant Growth Regul. 2002, 36, 61–70. [Google Scholar] [CrossRef]
- Du, Z.; Bramlage, W.J. Modified Thiobarbituric Acid Assay for Measuring Lipid Oxidation in Sugar-Rich Plant Tissue Extracts. J. Agric. Food Chem. 1992, 40, 1566–1570. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid Determination of Free Proline for Water-Stress Studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. [34] Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1987; Volume 148, pp. 350–382. ISBN 978-0-12-182048-0. [Google Scholar]
- Ou, H.; Zhou, L.; Huang, J.; Zeng, Y.; Zhu, X.; Xie, R.; Tan, H.; Huang, B. Effects of long-term different fertilization on sugarcane yield stability, fertilizer contribution rate and nutrition loss. Sci. Agric. Sin. 2018, 51, 1931–1939. [Google Scholar] [CrossRef]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods; Wiley Blackwell: New Delhi, India, 2014; ISBN 978-81-265-5138-5. [Google Scholar]
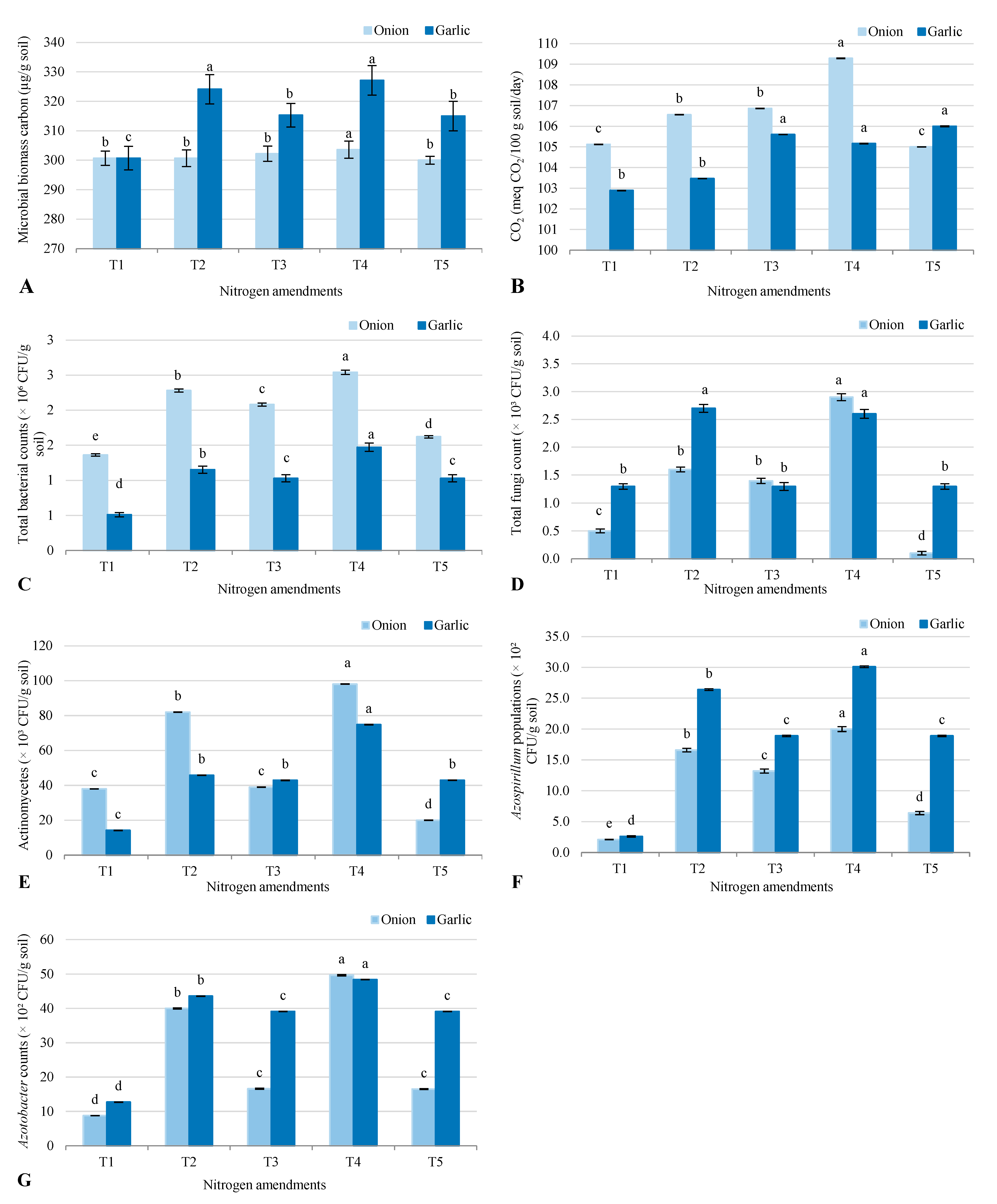
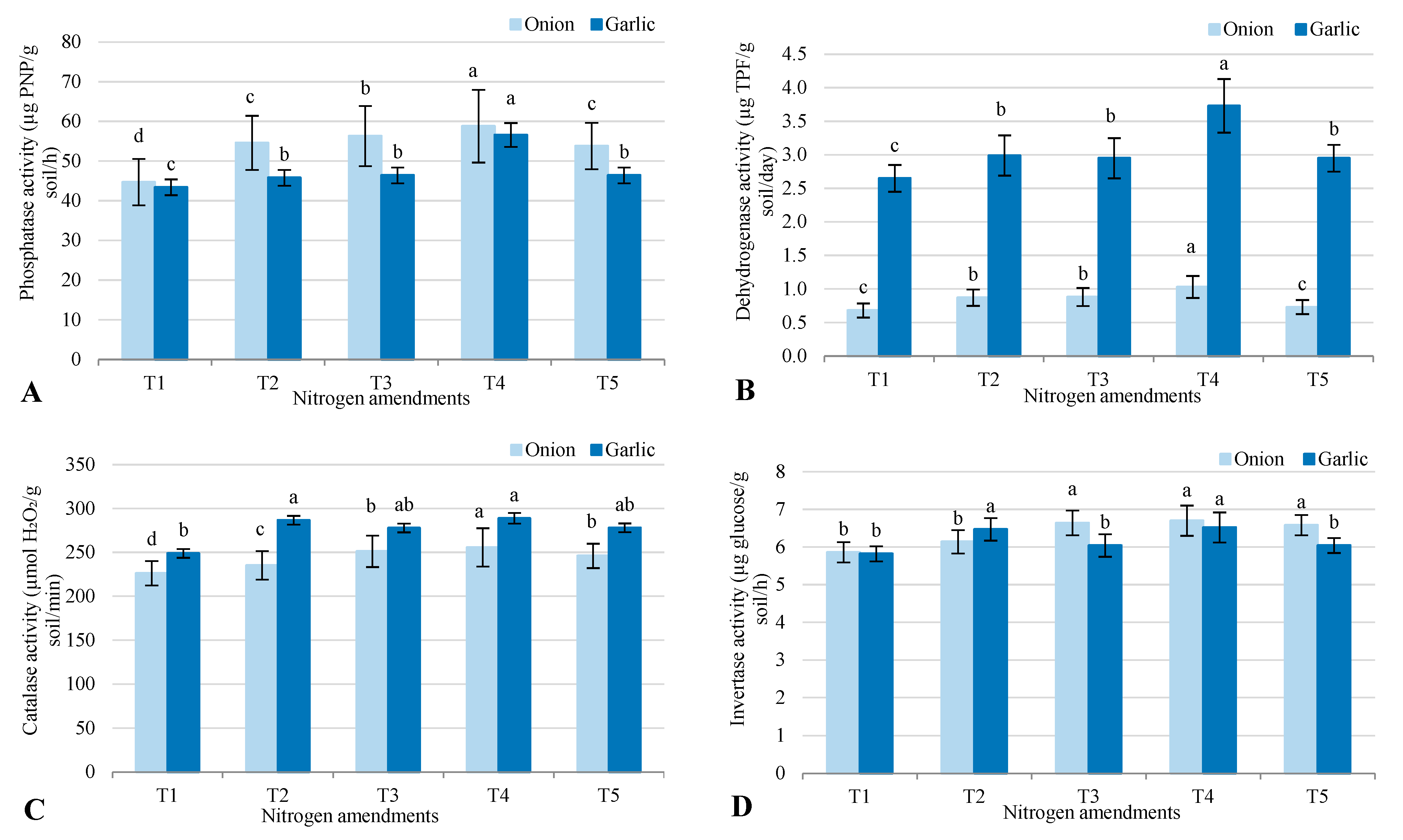

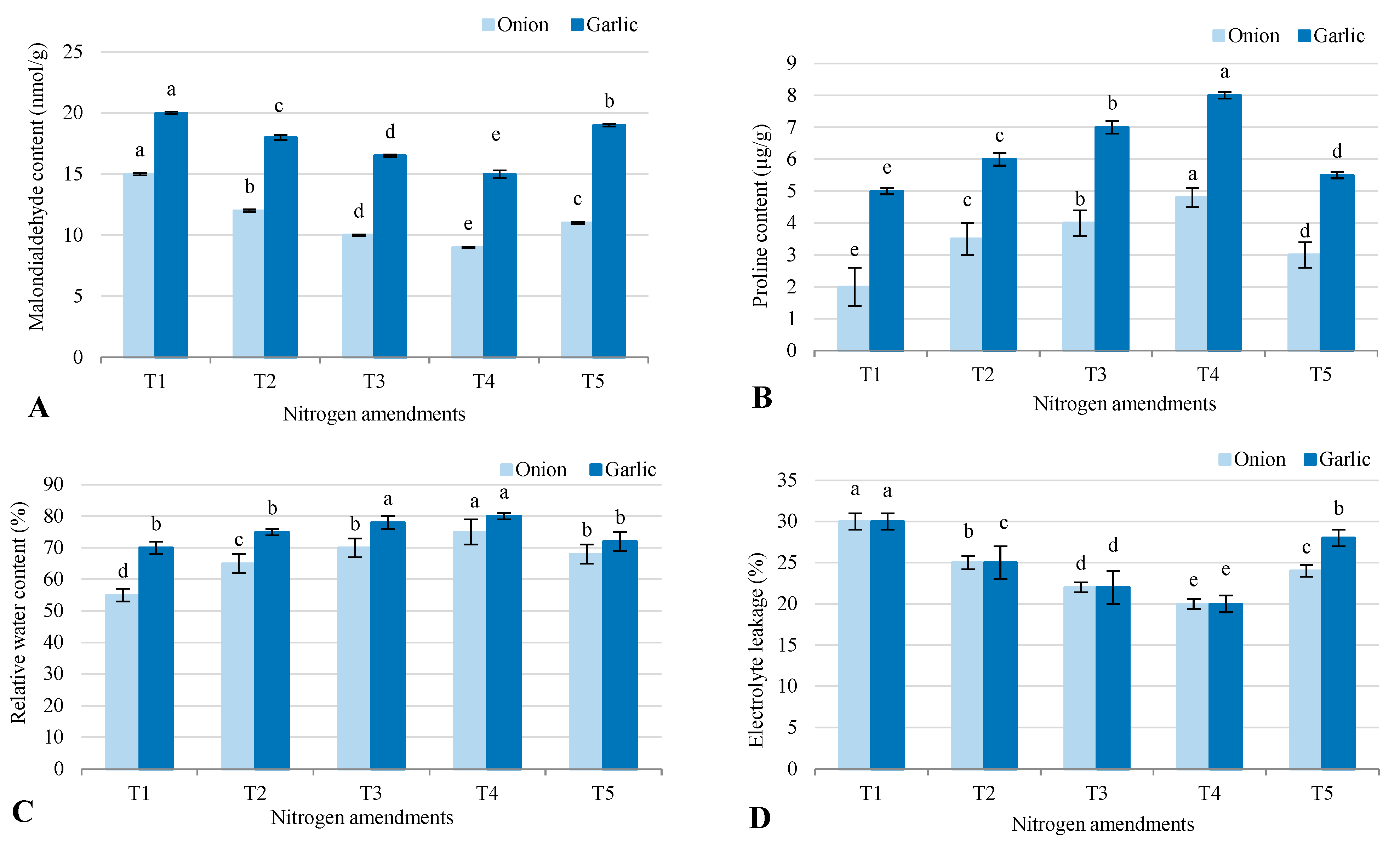
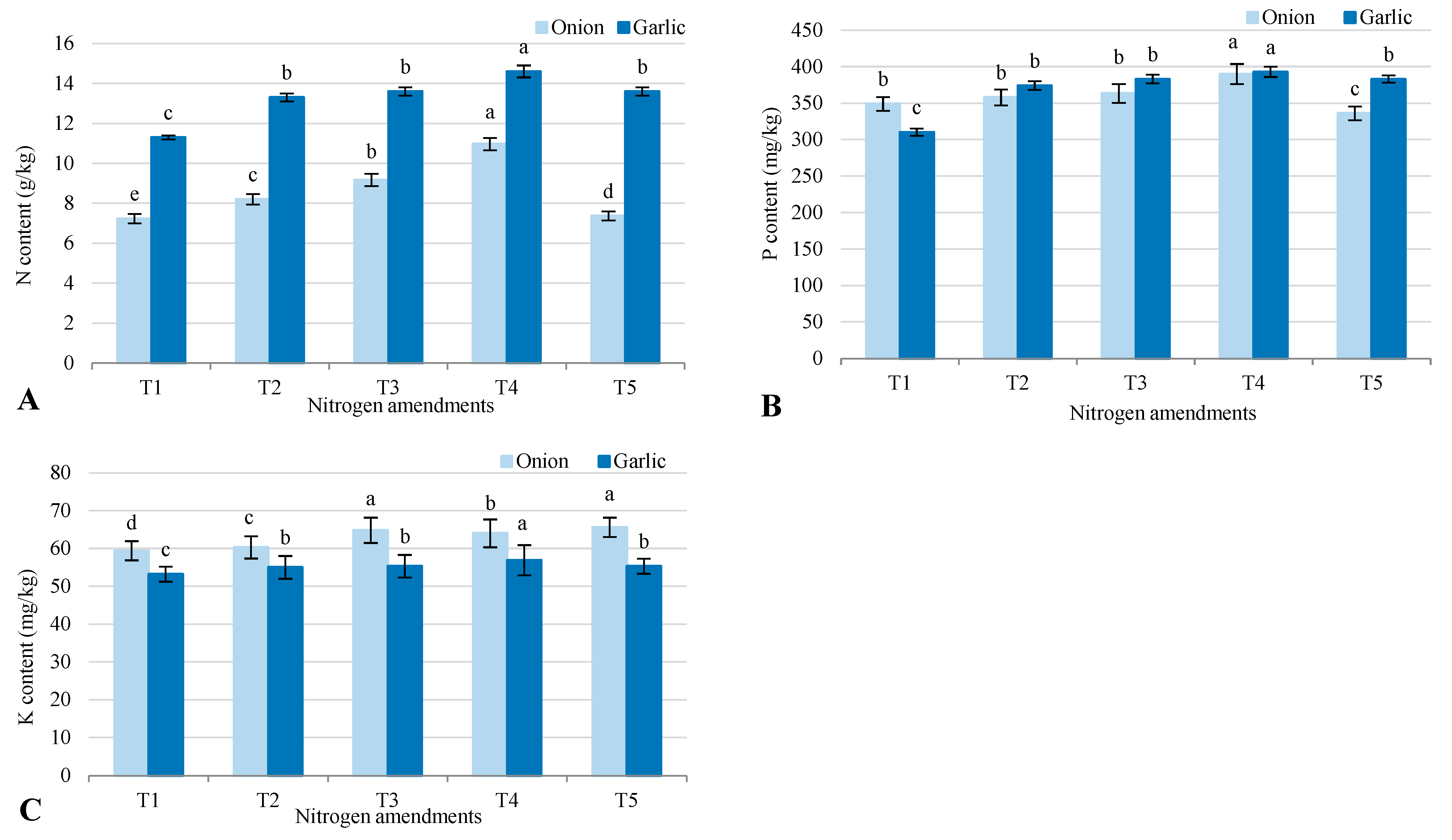

| T1 | T2 | T3 | T4 | T5 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Onion | Garlic | Onion | Garlic | Onion | Garlic | Onion | Garlic | Onion | Garlic | |
| pH | 8.20 ± 0.03 b | 8.20 ± 0.02 a | 7.90 ± 0.03 d | 8.10 ± 0.03 b | 8.10 ± 0.03 c | 8.00 ± 0.03 c | 7.90 ± 0.03 d | 8.10 ± 0.03 b | 8.30 ± 0.01 a | 8.00 ± 0.02 c |
| ECe ‡ | 4.00 ± 0.15 d | 3.84 ± 0.05 c | 4.06 ± 0.12 d | 4.38 ± 0.10 b | 4.49 ± 0.18 b | 4.99 ± 0.10 a | 4.27 ± 0.17 c | 4.38 ± 0.07 b | 4.71 ± 0.18 a | 4.99 ± 0.10 a |
| SOM ¥ (%) | 22.8 ± 0.40 d | 22.2 ± 0.20 c | 23.8 ± 0.41 c | 22.4 ± 0.30 c | 24.3 ± 0.40 b | 23.4 ± 0.30 b | 24.7 ± 0.41 a | 24.8 ± 0.30 a | 23.9 ± 0.20 c | 23.4 ± 0.50 b |
| CEC ⸸ | 34.4 ± 0.50 d | 34.1 ± 0.40 c | 41.2 ± 0.53 c | 36.1 ± 0.50 b | 43.5 ± 0.59 b | 46.2 ± 0.50 a | 45.3 ± 0.65 a | 46.7 ± 0.50 a | 41.7 ± 0.28 c | 46.2 ± 0.50 a |
| SFI * | 0.94 ± 0.03 b | 0.94 ± 0.02 b | 0.99 ± 0.04 a | 0.95 ± 0.02 b | 0.99 ± 0.04 a | 0.98 ± 0.02 a | 1.00 ± 0.05 a | 1.00 ± 0.03 a | 0.99 ± 0.03 a | 0.98 ± 0.02 a |
| Ava-N (mg/kg) | 27.3 ± 1.32 e | 28.0 ± 2.00 c | 33.0 ± 1.34 d | 33.0 ± 3.00 b | 37.4 ± 1.56 b | 35.4 ± 2.50 a | 39.7 ± 1.56 a | 35.7 ± 2.50 a | 35.1 ± 0.72 c | 35.4 ± 2.00 a |
| Ava-P (mg/kg) | 8.5 ± 0.19 d | 9.5 ± 0.30 d | 12.3 ± 0.24 c | 10.9 ± 0.40 c | 15.4 ± 0.34 b | 13.0 ± 0.30 b | 17.9 ± 0.14 a | 13.6 ± 0.50 a | 12.9 ± 0.46 c | 13.0 ± 0.30 b |
| Ava-K (mg/kg) | 91 ± 1.02 c | 95 ± 2.00 c | 94 ± 1.24 c | 103 ± 2.50 b | 105 ± 1.05 b | 103 ± 2.50 b | 107 ± 1.36 a | 108 ± 3.00 a | 103 ± 2.82 b | 103 ± 3.00 b |
| Total-N (g/kg) | 1.23 ± 0.05 d | 1.22 ± 0.02 b | 1.44 ± 0.05 b | 1.26 ± 0.03 b | 1.45 ± 0.05 b | 1.32 ± 0.03 a | 1.51 ± 0.06 a | 1.31 ± 0.04 a | 1.39 ± 0.05 c | 1.32 ± 0.02 a |
| Total-P (g/kg) | 0.22 ± 0.01 b | 0.25 ± 0.00 a | 0.25 ± 0.01 a | 0.25 ± 0.00 a | 0.25 ± 0.00 a | 0.26 ± 0.01 a | 0.25 ± 0.00 a | 0.26 ± 0.01 a | 0.25 ± 0.00 a | 0.26 ± 0.01 a |
| Ava-Pb (mg/kg) | 0.61 ± 0.03 c | 0.61 ± 0.02 c | 0.65 ± 0.04 b | 0.65 ± 0.02 b | 0.69 ± 0.04 b | 0.66 ± 0.02 b | 0.66 ± 0.05 b | 0.69 ± 0.03 a | 0.72 ± 0.03 a | 0.66 ± 0.02 a |
| Ava-Ni (mg/kg) | 0.39 ± 0.02 d | 0.38 ± 0.01 c | 0.38 ± 0.03 d | 0.39 ± 0.01 c | 0.45 ± 0.03 b | 0.42 ± 0.01 b | 0.42 ± 0.04 c | 0.45 ± 0.02 a | 0.48 ± 0.02 a | 0.42 ± 0.01 b |
| Ava-Cu (mg/kg) | 1.33 ± 0.11 c | 1.33 ± 0.03 c | 1.37 ± 0.12 c | 1.37 ± 0.04 c | 1.97 ± 0.13 a | 1.48 ± 0.04 b | 1.84 ± 0.15 b | 1.97 ± 0.05 a | 2.10 ± 0.11 a | 1.84 ± 0.05 b |
| Parameter | T1 | T2 | T3 | T4 | T5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Onion | Garlic | Onion | Garlic | Onion | Garlic | Onion | Garlic | Onion | Garlic | |
| Fresh mass with leaves (g/plant) | 51.4 ± 0.12 d | 68.5 ± 23 c | 99.5 ± 0.15 b | 70.6 ± 32 c | 101.6 ± 0.16 b | 84.4 ± 33 b | 105.3 ± 0.16 a | 92.1 ± 35 a | 97.9 ± 0.09 c | 84.4 ± 25 b |
| Fresh mass without leaves (g/plant) | 43.6 ± 0.12 d | 46.5 ± 23d | 68.8 ± 0.15 c | 51.1 ± 32 c | 74.5 ± 0.16 b | 58.1 ± 33 b | 76.3 ± 0.16 a | 62.8 ± 35 a | 72.7 ± 0.09 b | 58.1 ± 25 b |
| Dry mass with leaves (g/plant) | 7.9 ± 0.71 d | 16.3 ± 20 c | 10.5 ± 0.77 b | 21.2 ± 30 b | 10.5 ± 0.95 b | 21.6 ± 32 b | 11.3 ± 1.1 a | 23.1 ± 29 a | 9.7 ± 0.44 c | 21.6 ± 27 b |
| Dry mass without leaves (g/plant) | 5.3 ± 2.54 c | 11.4 ± 2.1 c | 6.1 ± 2.62 b | 14.4 ± 1.9 b | 6.7 ± 2.67 a | 15.2 ± 1.8 a | 7.2 ± 2.70 a | 15.4 ± 1.5 a | 6.2 ± 1.32 b | 15.2 ± 1.1 a |
| Neck diameter (cm) | 1.86 ± 0.25 c | 0.77 ± 0.2 c | 1.85 ± 0.15 c | 0.84 ± 0.3 b | 1.91 ± 0.16 b | 0.89 ± 0.2 b | 1.98 ± 0.17 a | 0.93 ± 0.2 a | 1.84 ± 0.08 c | 0.89 ± 0.4 b |
| Bulb diameter (cm) | 3.8 ± 0.78 d | 4.4 ± 0.2 b | 4.5 ± 0.85 c | 4.4 ± 0.4 b | 5.0 ± 0.90 a | 4.8 ± 0.5 a | 5.2 ± 1.0 a | 5.0 ± 0.4 a | 4.8 ± 0.44 b | 4.8 ± 0.3 a |
| Fresh yield (ton/ha) | 9.8 ± 1.63 d | 10.7 ± 0.02 c | 10.6 ± 1.68 c | 10.8 ± 0.03 c | 11.3 ± 1.70 b | 11.3 ± 0.04 b | 11.8 ± 1.74 a | 12.2 ± 0.04 a | 10.9 ± 0.84 c | 11.3 ± 0.05 b |
| Dry yield (ton/ha) | 8.8 ± 96 d | 9.0 ± 0.03 d | 9.8 ± 107 c | 9.2 ± 0.01 c | 10.5 ± 342 b | 9.7 ± 0.02 b | 11.1 ± 541 a | 10.2 ± 0.02 a | 9.9 ± 611 c | 9.7 ± 0.01 b |
| CRF † | nc ‡ | - | 9.7 ± 0.12 d | 1.8 ± 1.8 c | 15.9 ± 0.23 b | 6.9 ± 2.2 b | 20.1 ± 0.25 a | 11.5 ± 2.3 a | 11.2 ± 0.22 c | 6.9 ± 2.1 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshaal, T.; Elhawat, N.; Veres, S. Rhizosphere Engineering in Saline Soils: Role of PGPR and Organic Manures in Root–Soil Biochemical Interactions for Allium Crops. Plants 2025, 14, 3075. https://doi.org/10.3390/plants14193075
Alshaal T, Elhawat N, Veres S. Rhizosphere Engineering in Saline Soils: Role of PGPR and Organic Manures in Root–Soil Biochemical Interactions for Allium Crops. Plants. 2025; 14(19):3075. https://doi.org/10.3390/plants14193075
Chicago/Turabian StyleAlshaal, Tarek, Nevien Elhawat, and Szilvia Veres. 2025. "Rhizosphere Engineering in Saline Soils: Role of PGPR and Organic Manures in Root–Soil Biochemical Interactions for Allium Crops" Plants 14, no. 19: 3075. https://doi.org/10.3390/plants14193075
APA StyleAlshaal, T., Elhawat, N., & Veres, S. (2025). Rhizosphere Engineering in Saline Soils: Role of PGPR and Organic Manures in Root–Soil Biochemical Interactions for Allium Crops. Plants, 14(19), 3075. https://doi.org/10.3390/plants14193075









