Chemotaxonomic Insights into Korean Daphne spp. and Wikstroemia spp. by Integrating Flavonoid Contents with Ecological Factors
Abstract
1. Introduction
2. Results
2.1. Sampling Sites and Environmental Conditions
2.2. Method Validation and Quantitative/Qualitative Analysis of Six Flavonoids
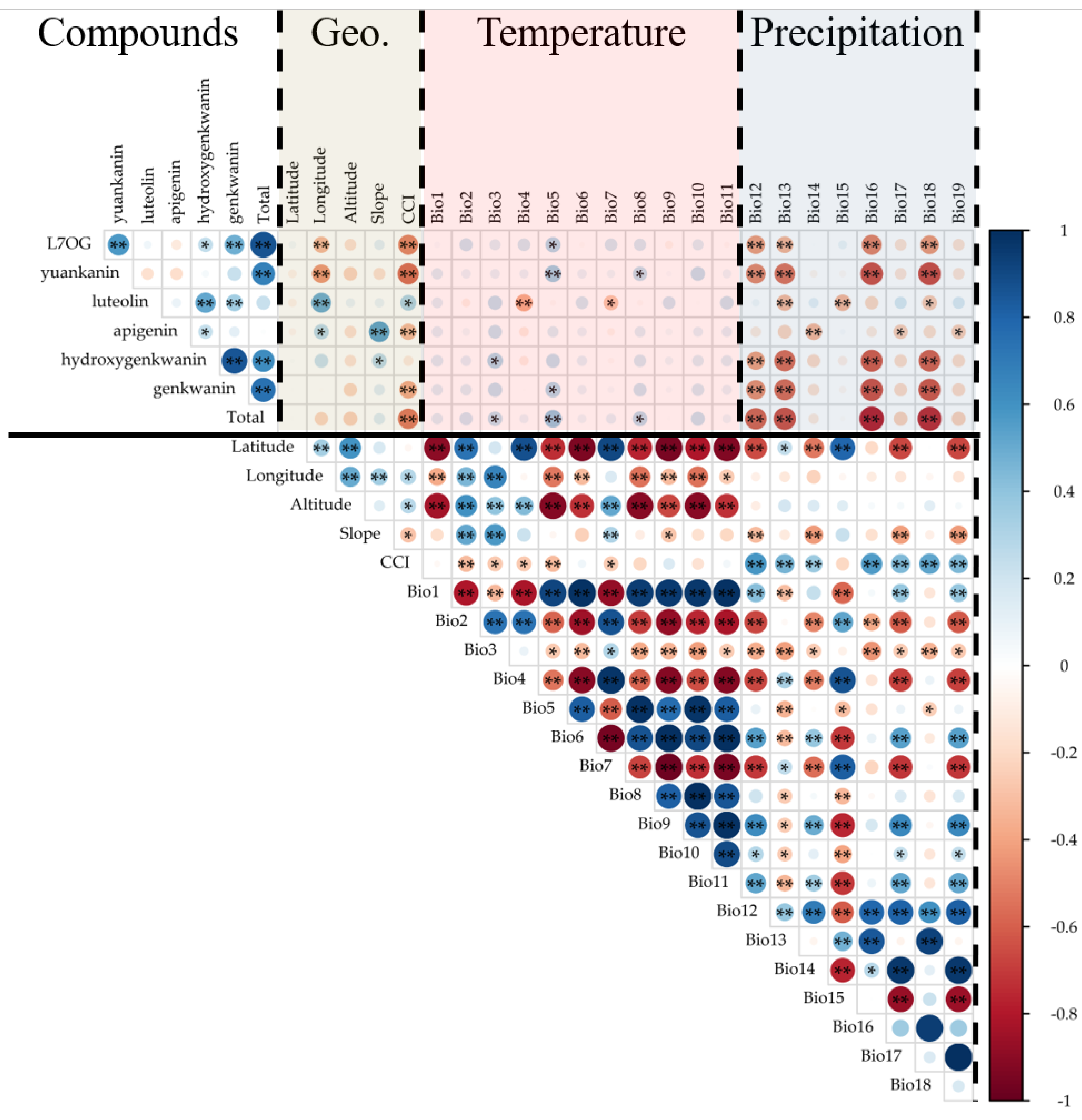
2.3. Correlation Between Environmental Variables and Flavonoid Contents
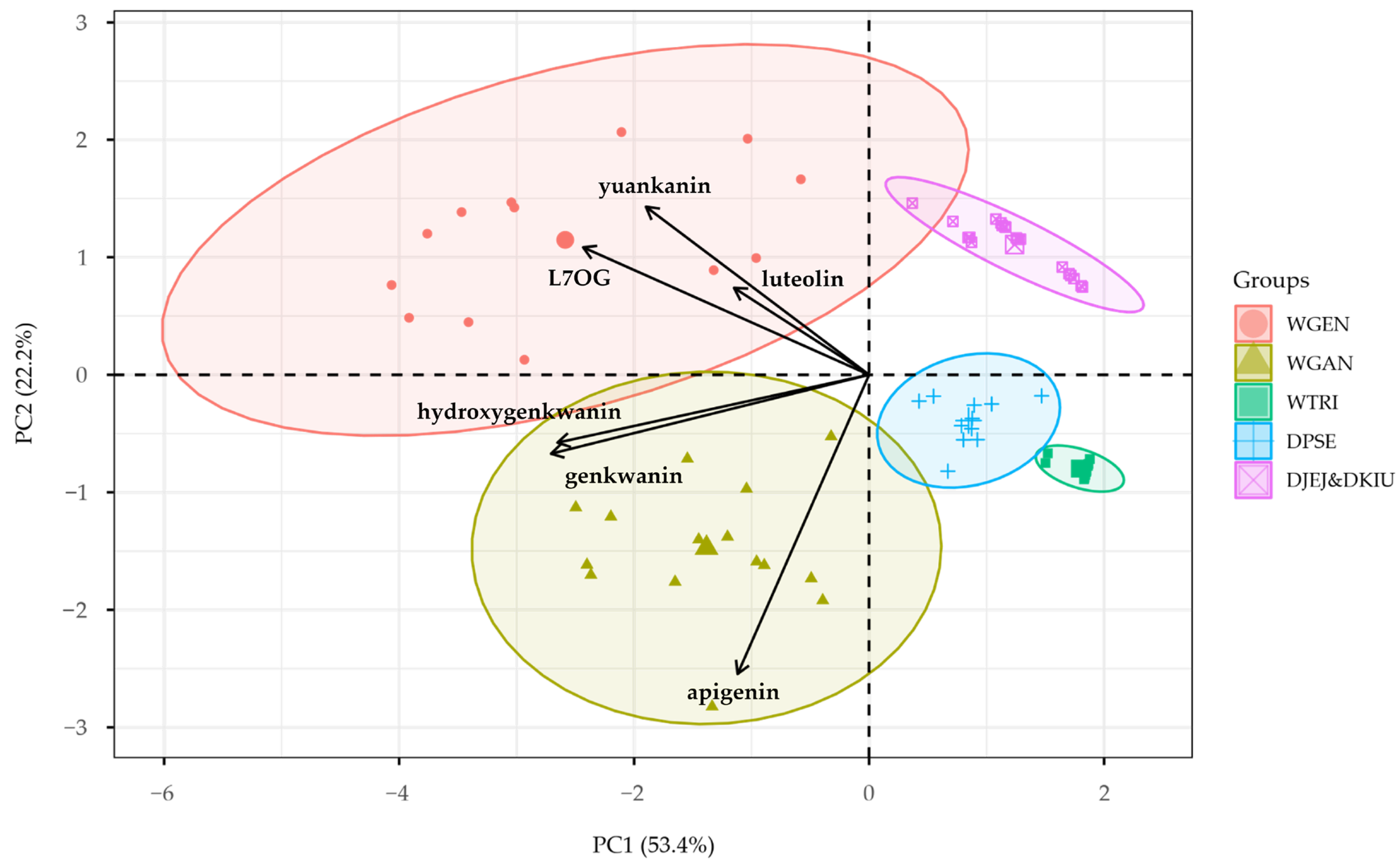
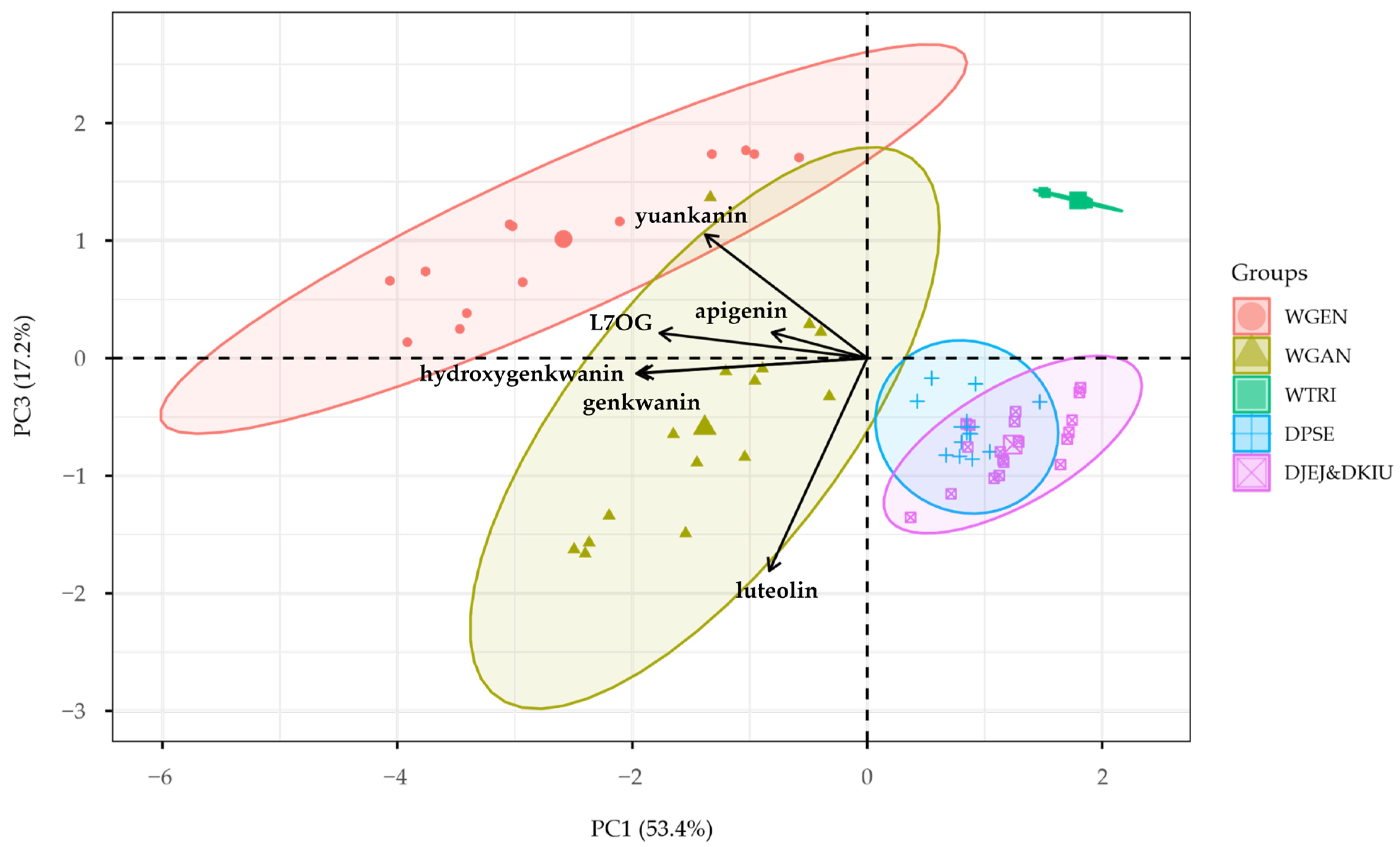
2.4. Multivariate Analysis of Flavonoid Profiles
3. Discussion
4. Materials and Methods
4.1. Plant Samples
4.2. Chemical Analysis
4.3. Environmental Variables
4.4. Statistical and Multivariate Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- de Jussieu, A.L. Genera Plantarum; Herissant et Barrois: Paris, France, 1789. [Google Scholar]
- Domke, W. Zur Kenntnis einiger Thymelaeaceen. Notizbl. Bot. Gart. Berlin-Dahlem 1932, 11, 348–363. [Google Scholar] [CrossRef]
- Herber, B.E. Thymelaeaceae. In Flowering Plants. Dicotyledons, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2003; pp. 373–396. [Google Scholar]
- Wang, Y.; Gilbert, M.G.; Mathew, B.; Brickell, C.D.; Nevling, L.I. Thymelaeaceae. In Flora of China; Science Press: Beijing, China; Missouri Botanical Garden Press: St. Louis, MO, USA, 2007; Volume 13, pp. 212–230. [Google Scholar]
- Lee, J.J.; Oh, S.H. A comparative morphological study of Thymelaeaceae in Korea. Korean J. Plant Taxon. 2017, 47, 207–221. [Google Scholar] [CrossRef]
- Jung, E.H.; Hong, S.P. The taxonomic consideration of leaf epidermal microstructure in Korean Thymelaeaceae. Korean J. Plant Taxon. 2003, 33, 421–433. [Google Scholar] [CrossRef]
- Jung, E.H.; Hong, S.P. Pollen morphology of Thymelaeaceae in Korea. Korean J. Plant Taxon. 2003, 33, 255–270. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, W.C.; Cao, W.; Zhang, Y.; Li, Z. Character of leaf epidermis and their systematic significance of Daphne and Wikstroemia (Thymelaeaceae). Plant Divers. Resour. 2015, 37, 493–512. [Google Scholar]
- Lee, S.Y.; Xu, K.W.; Huang, C.Y.; Lee, J.H.; Liao, W.B.; Zhang, Y.H.; Fan, Q. Molecular phylogenetic analyses based on the complete plastid genomes and nuclear sequences reveal Daphne (Thymelaeaceae) to be non-monophyletic as currently circumscribed. Plant Divers. 2022, 44, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, J.; Yan, F.; Wang, Z.; Zeng, X.; Zhang, J. Comparative analysis of the complete chloroplast genome of seven Wikstroemia taxa (Thymelaeaceae) provides insights into the genome structure and phylogenetic relationships. Planta 2025, 261, 40. [Google Scholar] [CrossRef] [PubMed]
- Moshiashvili, G.; Tabatadze, N.; Mshvildadze, V. The genus Daphne: A review of its traditional uses, phytochemistry and pharmacology. Fitoterapia 2020, 143, 104540. [Google Scholar] [CrossRef]
- Huan, D.Q.; Hop, N.Q.; Son, N.T. Wikstroemia: A review on its phytochemistry and pharmacology. Curr. Pharm. Biotechnol. 2024, 25, 563–598. [Google Scholar] [CrossRef]
- GBIF Occurrence Download. Available online: https://doi.org/10.15468/39omei (accessed on 29 August 2025).
- World Flora Online (WFO). Daphne genkwa Siebold & Zucc. Published on the Internet. 2025. Available online: http://www.worldfloraonline.org/taxon/wfo-0000637613 (accessed on 29 August 2025).
- Krishnaswamy, N.R. Flavonoids as chemotaxonomic markers: A review. Rheedea 1993, 3, 1–11. [Google Scholar]
- Amin, A.; Park, C.-H. Chemotaxonomy, an efficient tool for medicinal plant identification: Current trends and limitations. Plants 2025, 14, 123. [Google Scholar] [CrossRef]
- Harborne, J.B. Phytochemical Methods: A Guide to Modern Techniques of Plant Analysis; Springer: Dordrecht, The Netherlands, 1998. [Google Scholar]
- Wink, M. Evolution of secondary metabolites from an ecological and molecular phylogenetic perspective. Phytochemistry 2003, 64, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Mabberley, D.J. Mabberley’s Plant-Book: A Portable Dictionary of Plants; Cambridge University Press: Cambridge, UK, 2017. [Google Scholar]
- Singh, R. Chemotaxonomy: Principles and practices. J. Pharmacogn. Phytochem. 2016, 5, 325–331. [Google Scholar]
- Umoh, O.T. Chemotaxonomy of flowering plants. Asian Plant Res. J. 2020, 5, 43–52. [Google Scholar] [CrossRef]
- Kubitzki, K. The Families and Genera of Vascular Plants; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Fraga, B.M.; Hernández, M.G.; Fernández, C.; Santana, J.M.H. A chemotaxonomic study of nine Canarian Sideritis species. Phytochemistry 2009, 70, 1038–1048. [Google Scholar] [CrossRef]
- Koshovyi, O.; Raal, A.; Myha, M.M.M.; Ilina, T.; Borodina, N.; Komissarenko, A. The phytochemical and chemotaxonomic study of Salvia spp. growing in Ukraine. J. Appl. Biol. Biotechnol. 2020, 8, 29–36. [Google Scholar] [CrossRef]
- Kim, H.-J.; Son, D.C.; Kim, H.-J.; Choi, K.; Oh, S.-H.; Kang, S.-H. The chemotaxonomic classification of Korean Campanulaceae based on triterpene, sterol, and polyacetylene contents. Biochem. Syst. Ecol. 2017, 74, 11–18. [Google Scholar] [CrossRef]
- Treutter, D. Significance of flavonoids in plant resistance: A review. Environ. Chem. Lett. 2005, 3, 147–157. [Google Scholar] [CrossRef]
- Winkel-Shirley, B. Flavonoid biosynthesis: A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol. 2002, 126, 485–493. [Google Scholar] [CrossRef]
- Bazargani, M.M.; Falahati-Anbaran, M.; Rohloff, J. Comparative analyses of phytochemical variation within and between congeneric species of willow herb, Epilobium hirsutum and E. parviflorum: Contribution of environmental factors. Front. Plant Sci. 2021, 11, 595190. [Google Scholar] [CrossRef]
- Adzet, T.; Martinez, F. Flavonoids in the leaves of thymus: A Chemotaxonomic survey. Biochem. Syst. Ecol. 1981, 9, 293–295. [Google Scholar] [CrossRef]
- Mierziak, J.; Kostyn, K.; Kulma, A. Flavonoids as important molecules of plant interactions with the environment. Molecules 2014, 19, 16240–16265. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Lv, S.; Zhao, L.; Gao, T.; Yu, C.; Hu, J.; Ma, F. Advances in the study of the function and mechanism of the action of flavonoids in plants under environmental stresses. Planta 2023, 257, 108. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.D.; Yu, Y.G.; Yang, D.F.; Qi, Z.C.; Liu, R.Z.; Deng, F.T.; Cai, Z.X.; Li, Y.; Sun, Y.F.; Liang, Z.S. Chemotaxonomic variation in secondary metabolite contents and their correlation between environmental factors in Salvia miltiorrhiza Bunge from natural habitat of China. Ind. Crops Prod. 2018, 113, 335–347. [Google Scholar] [CrossRef]
- Lee, S.; Oh, D.G.; Lee, S.; Kim, G.R.; Lee, J.S.; Son, Y.K.; Bae, C.-H.; Yeo, J.; Lee, C.H. Chemotaxonomic metabolite profiling of 62 indigenous plant species and its correlation with bioactivities. Molecules 2015, 20, 19719–19734. [Google Scholar] [CrossRef]
- Uranishi, R.; Aedla, R.; Alsaadi, D.H.M.; Wang, D.; Kusakari, K.; Osaki, H.; Sugimura, K.; Watanabe, T. Evaluation of environmental factor effects on the polyphenol and flavonoid content in the leaves of Chrysanthemum indicum L. and its habitat suitability prediction mapping. Molecules 2024, 29, 927. [Google Scholar] [CrossRef]
- Park, B.Y.; Min, B.S.; Ahn, K.S.; Kwon, O.K.; Joung, H.; Bae, K.H.; Lee, H.K.; Oh, S.R. Daphnane diterpene esters isolated from flower buds of Daphne genkwa induce apoptosis in human myelocytic HL-60 cells and suppress tumor growth. J. Ethnopharmacol. 2007, 111, 496–503. [Google Scholar] [CrossRef]
- Kim, M.A.; Kang, K.; Lee, H.J.; Kim, M.; Kim, C.Y.; Nho, C.W. Apigenin isolated from Daphne genkwa Siebold et Zucc. inhibits 3T3-L1 preadipocyte differentiation through a modulation of mitotic clonal expansion. Life Sci. 2014, 101, 64–72. [Google Scholar] [CrossRef]
- Jiang, C.P.; He, X.; Yang, X.L.; Zhang, S.L.; Li, H.; Song, Z.J.; Zhang, C.F.; Yang, Z.L.; Li, P.; Wang, C.Z.; et al. Anti-rheumatoid arthritis activity of flavonoids from Daphne genkwa. Phytomedicine 2014, 21, 830–837. [Google Scholar] [CrossRef]
- Zhou, D.C.; Zheng, G.; Jia, L.Y.; He, X.; Zhang, C.F.; Wang, C.Z.; Yuan, C.S. Comprehensive evaluation on anti-inflammatory and anti-angiogenic activities in vitro of fourteen flavonoids from Daphne genkwa based on the combination of efficacy coefficient method and principal component analysis. J. Ethnopharmacol. 2021, 268, 113683. [Google Scholar] [CrossRef]
- Hu, Y.; Pan, R.; Wang, Y.; Ma, M.; Peng, Y.; Fan, W.; Zhang, R.; Nian, H.; Zhu, J. Daphne genkwa: Ethnopharmacology, phytochemistry and pharmacology of an important traditional Chinese medicine. Fitoterapia 2024, 177, 106089. [Google Scholar] [CrossRef]
- Tang, W.; Eisenbrand, G. Chinese Drugs of Plant Origin, Chemistry, Pharmacology, and Use in Traditional and Modern Medicine; Springer-Verlag: Berlin, Germany, 1992; pp. 429–436. [Google Scholar]
- Sun, Y.W.; Bao, Y.; Yu, H.; Chen, Q.; Lu, F.; Zhai, S.; Zhang, C.F.; Li, F.; Wang, C.Z.; Yuan, C.S. Anti-rheumatoid arthritis effects of flavonoids from Daphne genkwa. Int. Immunopharmacol. 2020, 83, 106384. [Google Scholar] [CrossRef]
- Xie, H.; Liang, Y.; Ito, Y.; Wang, X.; Chen, R.; He, J.; Li, H.; Zhang, T. Preparative isolation and purification of four flavonoids from Daphne genkwa Sieb. et Zucc. by high-speed countercurrent chromatography. J. Liq. Chromatogr. Relat. Technol. 2011, 34, 2360–2372. [Google Scholar] [CrossRef]
- Du, W.J.; Ji, J.; Wang, L.; Lan, X.Y.; Li, J.; Lei, J.Q.; He, X.; Zhang, C.F.; Huang, W.Z.; Wang, Z.Z.; et al. Relationship between the UPLC-Q-TOF-MS fingerprinted constituents from Daphne genkwa and their anti-inflammatory, anti-oxidant activities. Biomed. Chromatogr. 2017, 31, e4012. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Biological Resources. Red Data Book of the Republic of Korea, Volume 5: Vascular Plants; National Institute of Biological Resources: Incheon, Republic of Korea, 2020; 510p. [Google Scholar]
- Korea National Arboretum. The National Red List of Vascular Plants in Korea; Korea National Arboretum: Pocheon, Republic of Korea, 2021; 399p. [Google Scholar]
- Laoué, J.; Deguilloux, M.F.; Fichot, R.; Brignolas, F.; Badeau, V.; Villar, M. Plant flavonoids in Mediterranean species: A focus on flavonols as protective metabolites under climate stress. Front. Plant Sci. 2022, 13, 869938. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.; Yadav, A.; Jadhav, P.; Kunjir, S.; Khandelwal, A.; Jangid, K. Flavonoids in plant–environment interactions and stress responses. Environ. Exp. Bot. 2024, 215, 105653. [Google Scholar] [CrossRef]
- Zheng, J.; Tian, W.; Yang, C.; Shi, W.; Cao, P.; Long, J.; Xiao, L.; Wu, Y.; Liang, J.; Li, X.; et al. Identification of flavonoids in Plumula nelumbinis and evaluation of their antioxidant properties from different habitats. Ind. Crops Prod. 2019, 127, 36–45. [Google Scholar] [CrossRef]
- Ohtsuki, T.; Murai, Y.; Iwashina, T.; Setoguchi, H. Geographical differentiation inferred from flavonoid content between coastal and freshwater populations of the coastal plant Lathyrus japonicus (Fabaceae). Biochem. Syst. Ecol. 2013, 51, 243–250. [Google Scholar] [CrossRef]
- Qaderi, M.M.; Martel, A.B.; Strugnell, C.A. Environmental factors regulate plant secondary metabolites. Plants 2023, 12, 447. [Google Scholar] [CrossRef]
- Sampaio, B.L.; Edrada-Ebel, R.; Da Costa, F.B. Effect of the environment on the secondary metabolic profile of Tithonia diversifolia: A model for environmental metabolomics of plants. Sci. Rep. 2016, 6, 29265. [Google Scholar] [CrossRef]
- Chen, W.; Fu, X.; Xu, Y.; Zhang, H.; Yu, J.; Zhou, Y.; Zhang, Y. A review of classification, biosynthesis, biological activities and potential applications of flavonoids. Molecules 2023, 28, 4982. [Google Scholar] [CrossRef]
- Righini, S.; Rodriguez, E.J.; Berosich, C.; Grotewold, E.; Casati, P. Apigenin produced by maize flavone synthase I and II protects plants against UV-B-induced damage. Plant Cell Environ. 2018, 42, 495–508. [Google Scholar] [CrossRef] [PubMed]
- Tattini, M.; Galardi, C.; Pinelli, P.; Massai, R.; Remorini, D.; Agati, G. Flavonoids accumulate in leaves and glandular trichomes of Phillyrea latifolia exposed to excess solar radiation. New Phytol. 2000, 148, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Hodaei, M.; Rahimmalek, M.; Arzani, A.; Talebi, M. The effect of water stress on phytochemical accumulation, bioactive compounds and expression of key genes involved in flavonoid biosynthesis in Chrysanthemum morifolium L. Ind. Crops Prod. 2018, 120, 295–304. [Google Scholar] [CrossRef]
- Ma, D.; Sun, D.; Wang, C.; Li, Y.; Guo, T. Expression of flavonoid biosynthesis genes and accumulation of flavonoid in wheat leaves in response to drought stress. Plant Physiol. Biochem. 2014, 80, 60–66. [Google Scholar] [CrossRef]
- Cho, W.B.; Han, E.K.; Choi, G.; Lee, J.H. The complete chloroplast genome of Daphne kiusiana, an evergreen broad-leaved shrub on Jeju Island. Conserv. Genet. Resour. 2018, 10, 103–106. [Google Scholar] [CrossRef]
- Yoo, S.C.; Park, J.; Oh, S.H. Complete chloroplast genome of Daphne pseudomezereum var. koreana (Thymelaeaceae). Mitochondrial DNA Part B Resour. 2023, 8, 305–309. [Google Scholar] [CrossRef]
- Han, E.K.; Hong, J.C.; Park, J.; Park, J.S.; Oh, S.H.; Lee, J.H. Genetic and demographic signatures accompanying the evolution of the selfing syndrome in Daphne kiusiana, an evergreen shrub. Plant Commun. 2023, 4, 100557. [Google Scholar]
- Keem, M.J.; Kim, T.Y.; Park, N.J.; Choi, S.; Paik, J.H.; Jo, B.-G.; Kwon, T.H.; Kim, S.N.; Lee, S.R.; Yang, M.H. Isolation, characterization, and anti-allergic evaluation of phytochemicals from Wikstroemia trichotoma. Nutrients 2025, 17, 1552. [Google Scholar] [CrossRef]
- Bazargani, M.M.; Bloor, S.J.; Fomsgaard, I.S.; Nielsen, J.K.; Ørgaard, M.; Ravn, H.W. Comparative analyses of phytochemical variation within and between congeneric species of willow herb, Epilobium hirsutum and E. parviflorum: Contribution to chemotaxonomy. Biochem. Syst. Ecol. 2021, 96, 104267. [Google Scholar]
- Kuljarusnont, S.; Iwakami, S.; Iwashina, T.; Tungmunnithum, D. Flavonoids and other phenolic compounds for physiological roles, plant species delimitation, and medical benefits: A promising view. Molecules 2024, 29, 5351. [Google Scholar] [CrossRef]
- Mason, C.M.; Bowsher, A.W.; Crowell, B.L.; Celoy, R.M.; Tsai, C.J.; Donovan, L.A. Macroevolution of leaf defenses and secondary metabolites across the genus Helianthus. New Phytol. 2016, 209, 1720–1733. [Google Scholar] [CrossRef]
- Frazer, G.W.; Canham, C.D.; Lertzman, K.P. Gap Light Analyzer (GLA), Version 2.0; Simon Fraser University: Burnaby, BC, Canada; Institute of Ecosystem Studies: Millbrook, NY, USA, 1999. [Google Scholar]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1 km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
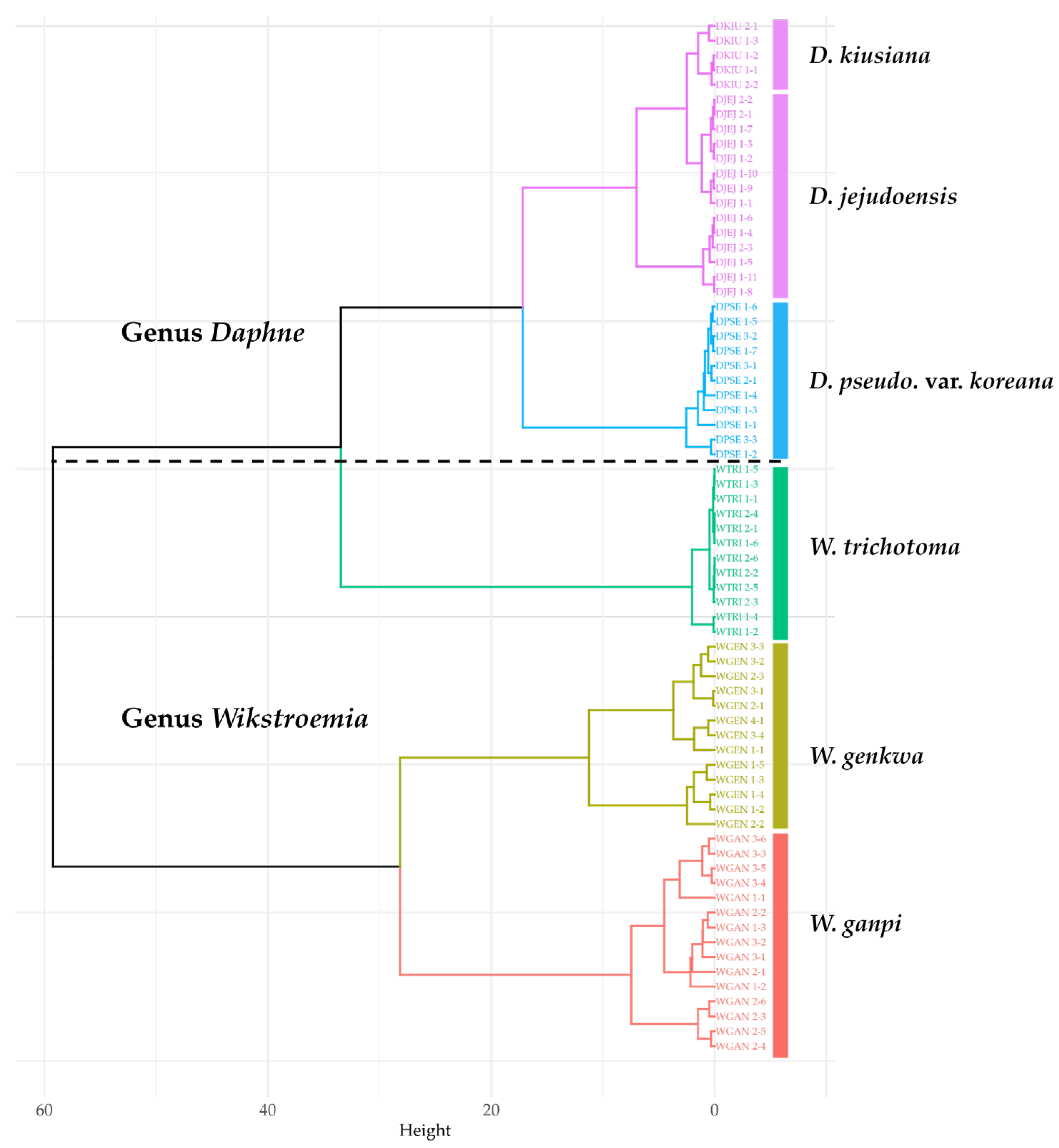
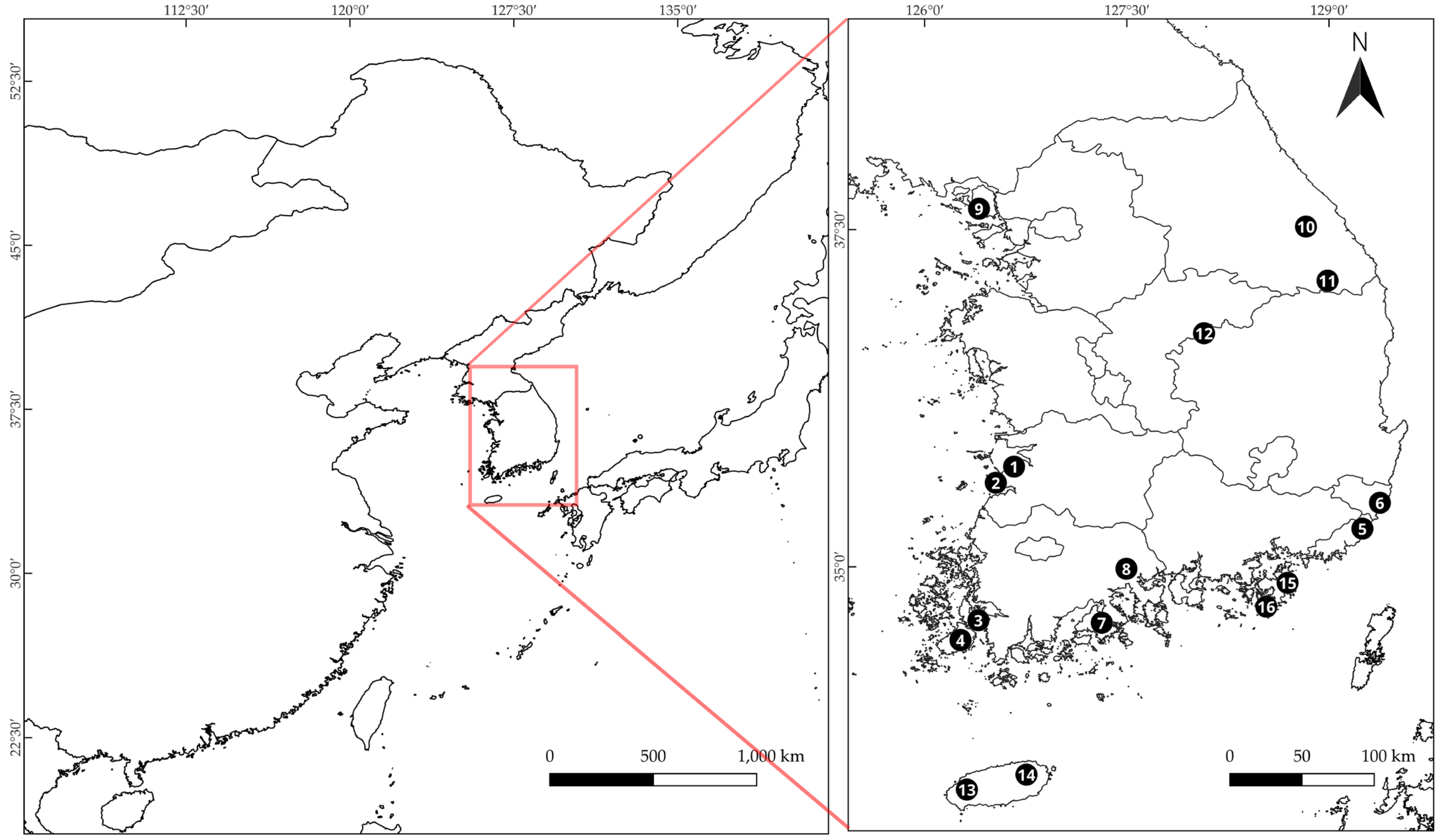
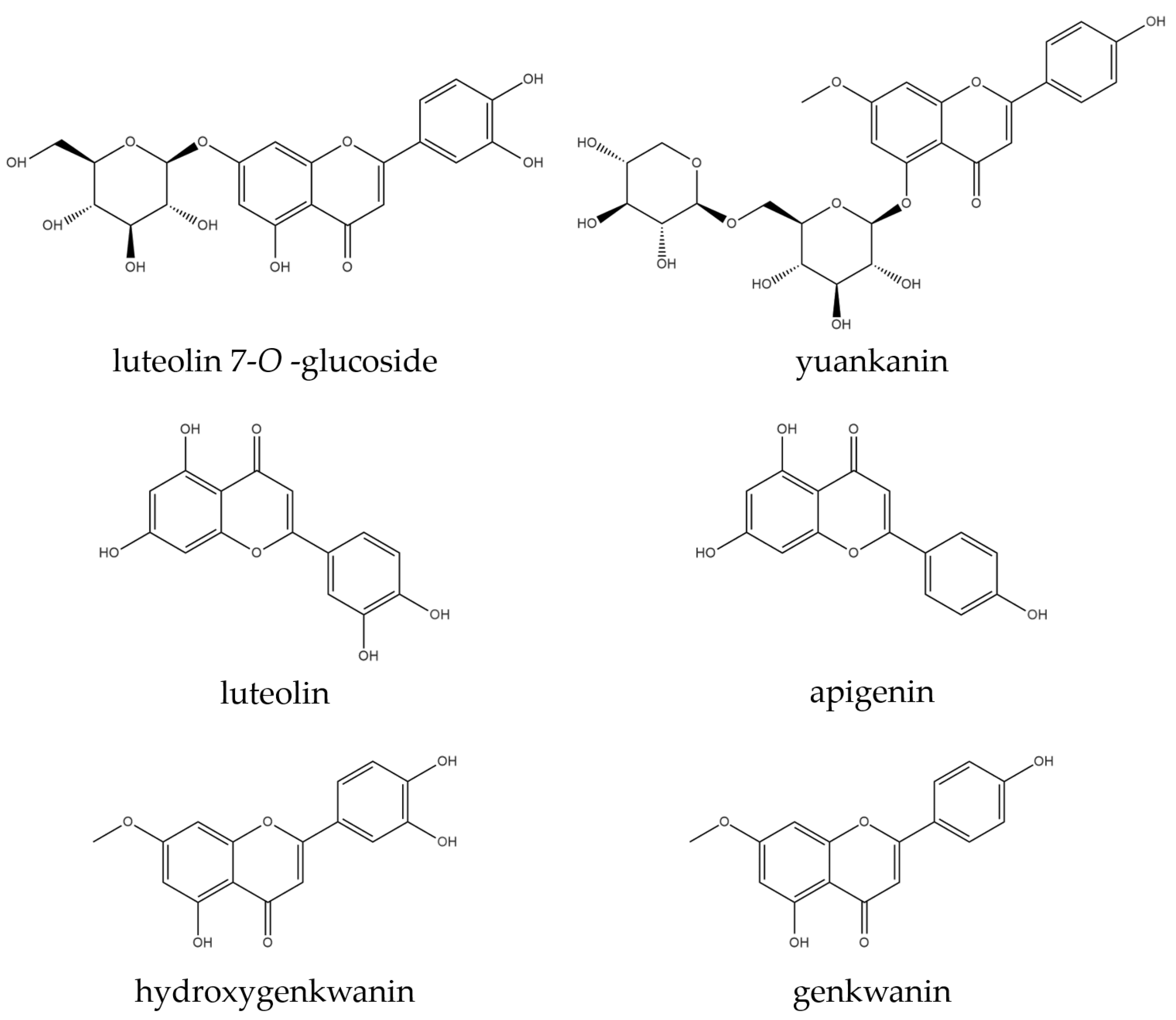

| Taxa | No | Abbr. | Sample No. | Lat. | Long. | Alt. (m) | Slope (%) | CCI (%) |
|---|---|---|---|---|---|---|---|---|
| Genus Wikstroemia | ||||||||
| W. genkwa (Siebold & Zucc.) Domke | 1 | WGEN | 1-1 to 5 | 35.60 | 126.49 | 174.60 | 35.00 | 0.02 |
| 2 | WGEN | 2-1 to 3 | 35.59 | 126.50 | 33.20 | 25.00 | 0.00 | |
| 3 | WGEN | 3-1 to 4 | 34.57 | 126.32 | 48.80 | 5.00 | 0.08 | |
| 4 | WGEN | 4-1 | 34.56 | 126.30 | 43.30 | 3.00 | 0.04 | |
| W. ganpi (Siebold & Zucc.) Maxim | 5 | WGAN | 1-1 to 3 | 35.23 | 129.24 | 42.50 | 15.00 | 0.00 |
| 6 | WGAN | 2-1 to 6 | 35.54 | 129.41 | 268.00 | 45.00 | 0.81 | |
| 7 | WGAN | 3-1 to 6 | 34.60 | 127.42 | 73.00 | 60.00 | 0.08 | |
| W. trichotoma (Thunb.) Makino | 8 | WTRI | 1-1 to 6 | 34.98 | 127.47 | 78.00 | 40.00 | 0.33 |
| 9 | WTRI | 2-1 to 6 | 37.72 | 126.38 | 123.60 | 14.00 | 0.59 | |
| Genus Daphne | ||||||||
| D. pseudomezereum var. koreana (Nakai) Hamaya | 10 | DPSE | 1-1 to 7 | 37.59 | 128.89 | 956.00 | 30.00 | 0.54 |
| 11 | DPSE | 2-1 | 36.73 | 128.02 | 1192.00 | 43.00 | 0.66 | |
| 12 | DPSE | 3-1 to 3 | 37.15 | 128.89 | 756.00 | 25.00 | 0.59 | |
| D. jejudoensis M.Kim | 13 | DJEJ | 1-1 to 11 | 33.54 | 126.72 | 190.80 | 10.00 | 0.76 |
| 14 | DJEJ | 2-1 to 3 | 33.32 | 126.29 | 87.60 | 15.00 | 0.69 | |
| D. kiusiana Miq. | 15 | DKIU | 1-1 to 3 | 34.73 | 128.61 | 115.00 | 8.00 | 0.85 |
| 16 | DKIU | 2-1 to 2 | 34.73 | 128.68 | 107.00 | 20.00 | 0.88 | |
| Compound | Regression Equation | Correlation Coefficient | Linear Range (μg/mL) | LOD (μg/mL) | LOQ (μg/mL) |
|---|---|---|---|---|---|
| L7OG | y a = 5115.6x b + 3158.9 | 0.9999 | 1.0–400.0 | 0.05 | 0.15 |
| yuankanin | y = 1950.1x + 2021.7 | 0.9999 | 25.0–400.0 | 0.06 | 0.19 |
| luteolin | y = 70,466x + 119.01 | 1.0000 | 0.5–16.0 | 0.01 | 0.02 |
| apigenin | y = 10,596x − 2562.8 | 0.9996 | 1.0–32.0 | 0.04 | 0.11 |
| hydroxygenkwanin | y = 15,859x − 8070.6 | 1.0000 | 5.0–400.0 | 0.06 | 0.17 |
| genkwanin | y = 14,910x + 7178.8 | 0.9998 | 5.0–160.0 | 0.05 | 0.15 |
| Compound | Concentration (µg/mL) | Intra-Day a | Inter-Day b | ||
|---|---|---|---|---|---|
| Concentration Found (µg/mL) | RSD (%) | Concentration Found (µg/mL) | RSD (%) | ||
| luteolin 7-O-glucoside | 10 | 9.40 | 0.07 | 9.42 | 0.21 |
| 20 | 19.91 | 0.05 | 19.96 | 0.27 | |
| 40 | 41.55 | 0.55 | 41.75 | 0.08 | |
| yuankanin | 25 | 24.44 | 0.44 | 24.46 | 0.09 |
| 50 | 49.01 | 0.24 | 49.15 | 0.31 | |
| 100 | 100.16 | 0.06 | 100.23 | 0.06 | |
| luteolin | 1 | 1.00 | 0.56 | 0.99 | 0.23 |
| 4 | 4.11 | 0.03 | 4.10 | 0.22 | |
| 16 | 16.18 | 0.04 | 16.18 | 0.01 | |
| apigenin | 4 | 4.06 | 0.09 | 4.05 | 0.39 |
| 8 | 8.20 | 0.05 | 8.19 | 0.12 | |
| 16 | 16.09 | 0.22 | 16.15 | 0.29 | |
| hydroxygenkwanin | 5 | 5.16 | 0.55 | 5.16 | 0.36 |
| 10 | 10.04 | 0.08 | 10.03 | 0.09 | |
| 40 | 40.04 | 0.02 | 40.06 | 0.04 | |
| genkwanin | 5 | 4.94 | 0.20 | 4.93 | 0.30 |
| 10 | 10.04 | 0.01 | 10.03 | 0.10 | |
| 40 | 40.81 | 0.04 | 40.84 | 0.14 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Son, Y.; Kim, J.A.; Son, H.J.; Kim, H.-J.; Park, W.-G. Chemotaxonomic Insights into Korean Daphne spp. and Wikstroemia spp. by Integrating Flavonoid Contents with Ecological Factors. Plants 2025, 14, 3059. https://doi.org/10.3390/plants14193059
Son Y, Kim JA, Son HJ, Kim H-J, Park W-G. Chemotaxonomic Insights into Korean Daphne spp. and Wikstroemia spp. by Integrating Flavonoid Contents with Ecological Factors. Plants. 2025; 14(19):3059. https://doi.org/10.3390/plants14193059
Chicago/Turabian StyleSon, Yonghwan, Ji Ah Kim, Ho Jun Son, Hyun-Jun Kim, and Wan-Geun Park. 2025. "Chemotaxonomic Insights into Korean Daphne spp. and Wikstroemia spp. by Integrating Flavonoid Contents with Ecological Factors" Plants 14, no. 19: 3059. https://doi.org/10.3390/plants14193059
APA StyleSon, Y., Kim, J. A., Son, H. J., Kim, H.-J., & Park, W.-G. (2025). Chemotaxonomic Insights into Korean Daphne spp. and Wikstroemia spp. by Integrating Flavonoid Contents with Ecological Factors. Plants, 14(19), 3059. https://doi.org/10.3390/plants14193059






