Genotype-by-Environment Interaction Stability Analysis of New Quinoa (Chenopodium quinoa Willd.) Varieties in the Mediterranean Zone of Chile
Abstract
1. Introduction
2. Results
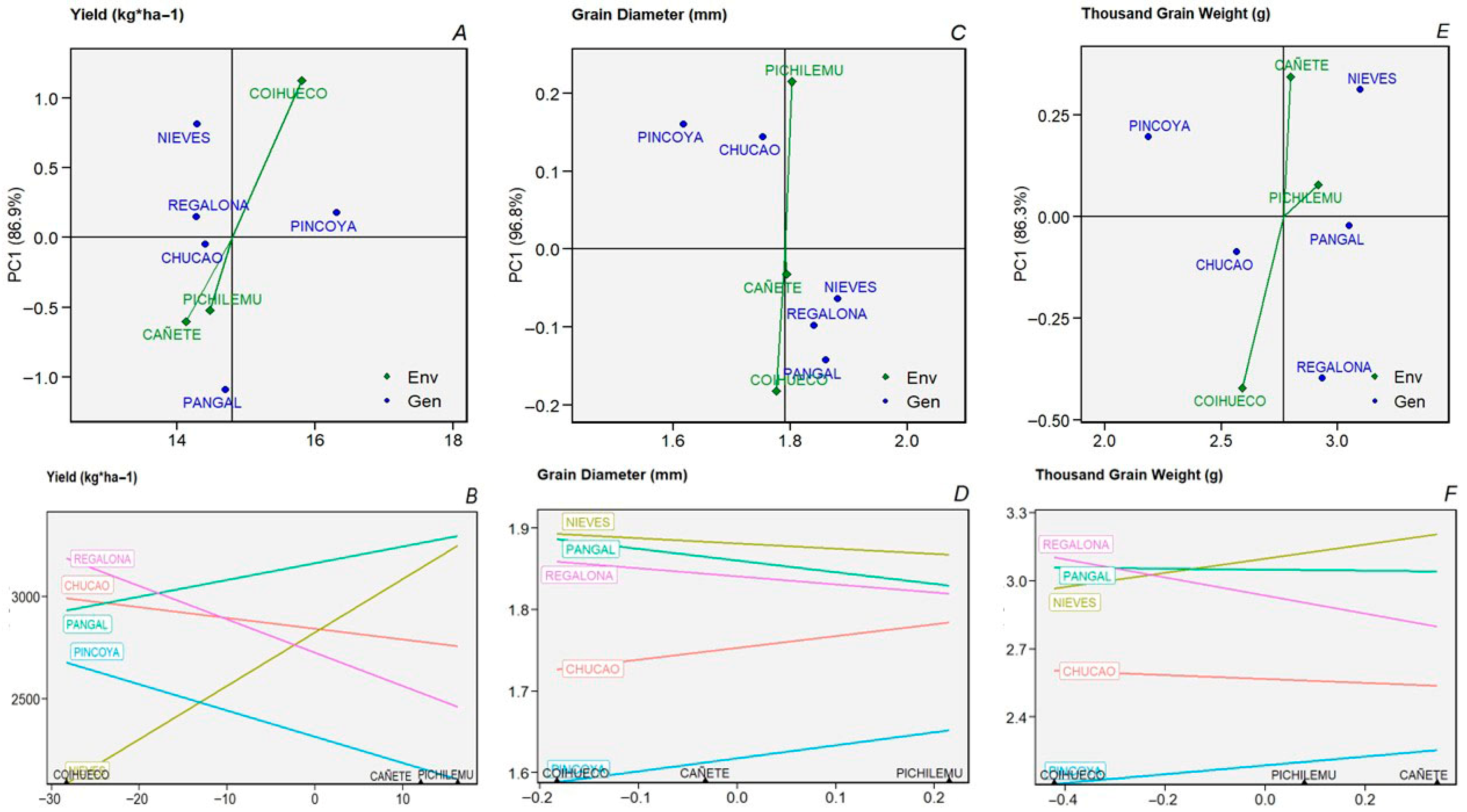
2.1. Environmental and Seasonal Variation in Productive Variables
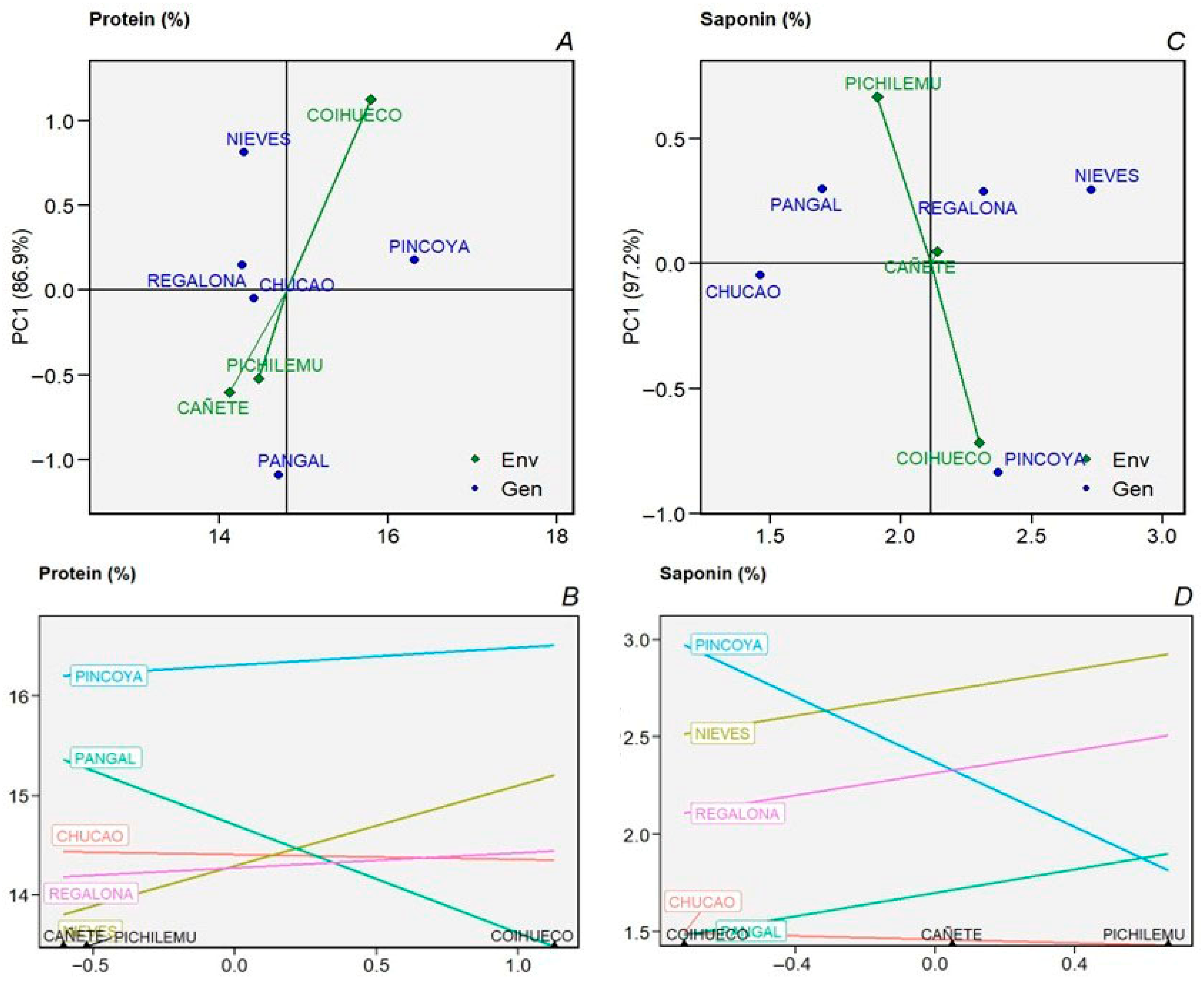
2.2. Environmental and Seasonal Variation in Grain Quality Variables
2.3. Cluster Analysis
2.4. AMMI—Biplot Model
3. Discussion
4. Materials and Methods
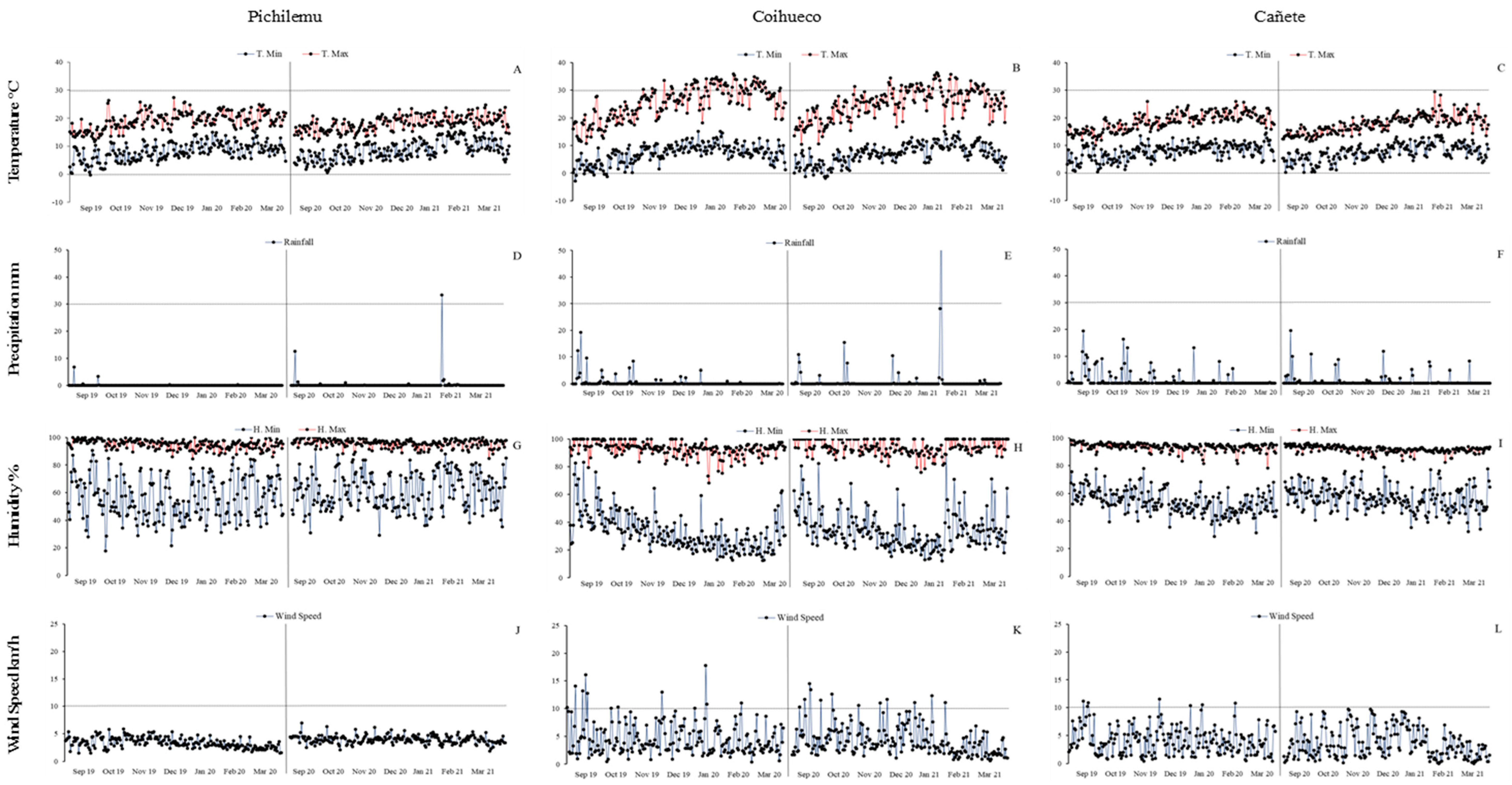
4.1. Study Areas and Experimental Design
4.2. Grain Quality Analysis
4.3. Statistical Analysis, AMMI Model
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Angeli, V.; Silva, P.M.; Massuela, D.C.; Khan, M.W.; Hamar, A.; Khajehei, F.; Graeff-Hönninger, S.; Piatti, C. Quinoa (Chenopodium quinoa Willd.): An overview of the potentials of the ‘golden grain’ and socio-economic and environmental aspects of its cultivation and marketization. Foods 2020, 9, 216. [Google Scholar] [CrossRef] [PubMed]
- Zevallos, V.F.; Herencia, L.I.; Chang, F.; Donnelly, S.; Ellis, H.J.; Ciclitira, P.J. Gastrointestinal effects of eating Quinoa (Chenopodium quinoa Willd.) in celiac patients. Am. J. Gastroenterol. 2014, 109, 270–278. [Google Scholar] [CrossRef]
- Hinojosa, L.; González, J.A.; Barrios-Masias, F.H.; Fuentes, F.; Murphy, K.M. Quinoa abiotic stress responses: A review. Plants 2018, 7, 106. [Google Scholar] [CrossRef] [PubMed]
- Tomaz, A.; Palma, J.F.; Ramos, T.; Costa, M.N.; Rosa, E.; Santos, M.; Boteta, L.; Dôres, J.; Patanita, M. Yield, technological quality and water footprints of wheat under Mediterranean climate conditions: A field experiment to evaluate the effects of irrigation and nitrogen fertilization strategies. Agric. Water Manag. 2021, 258, 107214. [Google Scholar] [CrossRef]
- del Pozo, A.; Ruf, K.; Alfaro, C.; Zurita, A.; Guerra, F.; Sagredo, B. Traits associated with higher productivity and resilience to drought-prone Mediterranean environments of coastal-lowland quinoa (Chenopodium quinoa Willd.). Field Crop. Res. 2023, 299, 108985. [Google Scholar] [CrossRef]
- Emrani, N.; Maldonado-Taipe, N.; Hasler, M.; Patiranage, D.S.R.; Jung, C. Early Flowering and Maturity Promote the Successful Adaptation and High Yield of Quinoa (Chenopodium quinoa Willd.) in Temperate Regions. Plants 2024, 13, 2919. [Google Scholar] [CrossRef]
- De Bock, P.; Van Bockstaele, F.; Raes, K.; Vermeir, P.; Van der Meeren, P.; Eeckhout, M. Impact of tempering process on yield and composition of quinoa flour. LWT 2020, 140, 110808. [Google Scholar] [CrossRef]
- Fuentes, F. Genetic improvement of quinoa. Agric. Desierto 2014, 2008, 26. [Google Scholar]
- Dumschott, K.; Wuyts, N.; Alfaro, C.; Castillo, D.; Fiorani, F.; Zurita-Silva, A. Morphological and Physiological Traits Associated with Yield under Reduced Irrigation in Chilean Coastal Lowland Quinoa. Plants 2022, 11, 323. [Google Scholar] [CrossRef] [PubMed]
- Madrid, D.; Salgado, E.; Verdugo, G.; Olguín, P.; Bilalis, D.; Fuentes, F. Morphological traits defining breeding criteria for coastal quinoa in Chile. Not. Bot. Horti Agrobot. Cluj Napoca 2018, 46, 190–196. [Google Scholar] [CrossRef]
- Ruiz, K.B.; Biondi, S.; Oses, R.; Acuña-Rodríguez, I.S.; Antognoni, F.; Martinez-Mosqueira, E.A.; Coulibaly, A.; Canahua-Murillo, A.; Pinto, M.; Zurita-Silva, A.; et al. Quinoa biodiversity and sustainability for food security under climate change. A review. Agron. Sustain. Dev. 2014, 34, 349–359. [Google Scholar] [CrossRef]
- Bertero, H.D.; De La Vega, A.J.; Correa, G.; Jacobsen, S.E.; Mujica, A. Genotype and genotype-by-environment interaction effects for grain yield and grain size of quinoa (Chenopodium quinoa Willd.) as revealed by pattern analysis of international multi-environment trials. Field Crops Res. 2004, 89, 299–318. [Google Scholar] [CrossRef]
- Yue, H.; Gauch, H.G.; Wei, J.; Xie, J.; Chen, S.; Peng, H.; Bu, J.; Jiang, X. Genotype by Environment Interaction Analysis for Grain Yield and Yield Components of Summer Maize Hybrids across the Huanghuaihai Region in China. Agriculture 2022, 12, 602. [Google Scholar] [CrossRef]
- Hilmarsson, H.S.; Rio, S.; Sánchez, J.I.Y. Genotype by environment interaction analysis of agronomic spring barley traits in iceland using ammi, factorial regression model and linear mixed model. Agronomy 2021, 11, 499. [Google Scholar] [CrossRef]
- Manjarres-Hernández, E.H.; Morillo-Coronado, A.C.; Ojeda-Pérez, Z.Z.; Cárdenas-Chaparro, A.; Arias-Moreno, D.M. Characterization of the yield components and selection of materials for breeding programs of quinoa (Chenopodium quinoa Willd.). Euphytica 2021, 217, 101. [Google Scholar] [CrossRef]
- Zaid, I.U.; Zahra, N.; Habib, M.; Naeem, M.K.; Asghar, U.; Uzair, M.; Latif, A.; Rehman, A.; Ali, G.M.; Khan, M.R. Estimation of Genetic Variances and Stability Components of Yield-Related Traits of Green Super Rice at Multi-Environmental Conditions in Pakistan. Agronomy 2022, 12, 1157. [Google Scholar] [CrossRef]
- Sellami, M.H.; Pulvento, C.; Lavini, A. Agronomic practices and performances of quinoa under field conditions: A systematic review. Plants 2021, 10, 72. [Google Scholar] [CrossRef]
- Thiam, E.; Allaoui, A.; Benlhabib, O. Quinoa Productivity and Stability Evaluation through Varietal and Environmental Interaction. Plants 2021, 10, 714. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.L.; Luu, H.N.; Phan, T.H.N.; Chu, D.H.; Bertero, D.; Curti, N.; McKeown, P.C.; Spillane, C.; Bazile, D. Genotype by environment interaction across water regimes in relation to cropping season response of quinoa (Chenopodium quinoa). PLoS ONE 2024, 19, e0309777. [Google Scholar] [CrossRef]
- Laki, E.S.; Rabiei, B.; Marashi, H.; Jokarfard, V.; Börner, A. Association study of morpho-phenological traits in quinoa (Chenopodium quinoa Willd.) using SSR markers. Sci. Rep. 2024, 14, 5991. [Google Scholar] [CrossRef] [PubMed]
- Anchico-Jojoa, W.; Peixoto, R.; Spehar, C.R. Hybridization between progenies and agronomic characterization of the F2 generation in quinoa Hybridization between progenies and agronomic characterization of the F2 generation in quinoa. Crop Breed. Appl. Biotechnol. 2023, 23, 46292345. [Google Scholar] [CrossRef]
- Rincent, R.; Malosetti, M.; Ababaei, B.; Touzy, G.; Mini, A.; Bogard, M.; Martre, P.; Le Gouis, J.; van Eeuwijk, F. Using crop growth model stress covariates and AMMI decomposition to better predict genotype-by-environment interactions. Theor. Appl. Genet. 2019, 132, 3399–3411. [Google Scholar] [CrossRef]
- Bocianowski, J.; Warzecha, T.; Nowosad, K.; Bathelt, R. Genotype by environment interaction using AMMI model and estimation of additive and epistasis gene effects for 1000-kernel weight in spring barley (Hordeum vulgare L.). J. Appl. Genet. 2019, 60, 127–135. [Google Scholar] [CrossRef]
- Munda, S.; Sarma, N.; Lal, M. GxE interaction of 72 accessions with three year evaluation of Cymbopogon winterianus Jowitt. using regression coefficient and Additive Main effects and Multiplicative Interaction model (AMMI). Ind. Crop. Prod. 2020, 146, 112169. [Google Scholar] [CrossRef]
- FAO. Estado del Arte de la Quinua en el Mundo en 2013; FAO: Rome, Italy, 2014. [Google Scholar]
- Alandia, G.; Rodriguez, J.P.; Jacobsen, S.E.; Bazile, D.; Condori, B. Global expansion of quinoa and challenges for the Andean region. Glob. Food Secur. 2020, 26, 100429. [Google Scholar] [CrossRef]
- Pulvento, C.; Bazile, D. Worldwide Evaluations of Quinoa—Biodiversity and Food Security under Climate Change Pressures: Advances and Perspectives. Plants 2023, 12, 868. [Google Scholar] [CrossRef] [PubMed]
- Peterson, A.; Murphy, K. Tolerance of Lowland Quinoa Cultivars to Sodium Chloride and Sodium Sulfate Salinity. Crop Sci. Soc. Am. 2015, 55, 331–338. [Google Scholar] [CrossRef]
- Sankaran, S.; Espinoza, C.Z.; Hinojosa, L.; Ma, X.; Murphy, K. High-Throughput Field Phenotyping to Assess Irrigation Treatment Effects in Quinoa. Agrosystems Geosci. Environ. 2019, 2, 1–7. [Google Scholar] [CrossRef]
- Dawood, S.; Shabbir, G.; Malik, S.I.; Raja, N.I.; Shah, Z.H.; Rauf, M.; Al Zahrani, Y.; Alghabari, F.; Alsamadany, H.; Shahzad, K.; et al. Delineation of Physiological, Agronomic and Genetic Responses of Different Wheat Genotypes under Drought Condition. Agronomy 2022, 12, 1056. [Google Scholar] [CrossRef]
- Miranda, M.; Vega-Gálvez, A.; Martinez, E.; López, J.; Rodríguez, M.J.; Henríquez, K.; Fuentes, F. Diversidade genética e comparação das características físico-químicas e nutricionais de seis genótipos de quinoa (Chenopodium quinoa willd.) cultivados no chile. Cienc. Tecnol. Aliment. 2012, 32, 835–843. [Google Scholar] [CrossRef]
- Fuentes, F.; Martinez, E.; Hinrichsen, P.; Jellen, E.; Maughan, P. Assessment of genetic diversity patterns in Chilean quinoa (Chenopodium quinoa Willd.) germplasm using multiplex fluorescent microsatellite markers. Conserv. Genet. 2009, 10, 369–377. [Google Scholar] [CrossRef]
- Bazile, D.; Jacobsen, S.-E.; Verniau, A. The Global Expansion of Quinoa: Trends and Limits. Front. Plant Sci. 2016, 7, 622. [Google Scholar] [CrossRef]
- Rehman, H.U.; Alharby, H.F.; Al-Zahrani, H.S.; Bamagoos, A.A.; Alsulami, N.B.; Alabdallah, N.M.; Iqbal, T.; Wakeel, A. Enriching Urea with Nitrogen Inhibitors Improves Growth, N Uptake and Seed Yield in Quinoa (Chenopodium quinoa Willd) Affecting Photochemical Efficiency and Nitrate Reductase Activity. Plants 2022, 11, 371. [Google Scholar] [CrossRef]
- Jarvis, D.E.; Ho, Y.S.; Lightfoot, D.J.; Schmöckel, S.M.; Li, B.; Borm, T.J.A.; Ohyanagi, H.; Mineta, K.; Michell, C.T.; Saber, N.; et al. The genome of Chenopodium quinoa. Nature 2017, 542, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Morillo, A.C.; Manjarres, E.H.; Mora, M.S. Afrosymetric method for quantifying saponins in Chenopodium quinoa Willd. from Colombia. Braz. J. Biol. 2022, 82, e262716. [Google Scholar] [CrossRef] [PubMed]
- Pathan, S.; Siddiqui, R.A. Nutritional Composition and Bioactive Components in Quinoa (Chenopodium quinoa Willd.) Greens: A Review. Nutrients 2022, 14, 558. [Google Scholar] [CrossRef]
- Escribano, J.; Cabanes, J.; Jiménez-Atiénzar, M.; Ibañez-Tremolada, M.; Gómez-Pando, L.R.; García-Carmona, F.; Gandía-Herrero, F. Characterization of betalains, saponins and antioxidant power in differently colored quinoa (Chenopodium quinoa) varieties. Food Chem. 2017, 234, 285–294. [Google Scholar] [CrossRef]
- Marques, L.; Gonçalves, M.C.; Davide, L.M.C.; Santos, A.D.; Candido, L.S. Adaptability and Stability of Maize Genotypes in Growing Regions of Central Brazil. Rev. Ceres 2021, 68, 201–211. [Google Scholar] [CrossRef]
- Kakabouki, I.P.; Roussis, I.; Hela, D.; Papastylianou, P.; Folina, A.; Bilalis, D. Root growth dynamics and productivity of quinoa (Chenopodium quinoa Willd.) in response to fertilization and soil tillage. Folia Hortic. 2019, 31, 277–291. [Google Scholar] [CrossRef]
- Fuentes, F.; Bhargava, A. Morphological Analysis of Quinoa Germplasm Grown Under Lowland Desert Conditions. Agron. Crop. Sci. 2011, 197, 124–134. [Google Scholar] [CrossRef]
- Yasui, Y.; Hirakawa, H.; Oikawa, T.; Toyoshima, M.; Matsuzaki, C.; Ueno, M.; Mizuno, N.; Nagatoshi, Y.; Imamura, T.; Miyago, M.; et al. Draft genome sequence of an inbred line of Chenopodium quinoa, an allotetraploid crop with great environmental adaptability and outstanding nutritional properties. DNA Res. 2016, 23, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, H.; Carbajal, Y.; Gonzales, M.; Vásquez, V.; López, A. Determinación de la actividad insecticida de la saponina de la quinua (Chenopodium quinua) en larvas de Drosophila melanogaster. Sci. Agropecu. 2019, 10, 39–45. [Google Scholar] [CrossRef]
- Di Rienzo, J.; Casanoves, F.; Balzarini, M.; Gonzalez, L. InfoStat Manual del Usuario. 2015, Volume 2011, pp. 167–188. Available online: https://www.researchgate.net/publication/283491340_Infostat_manual_del_usuario (accessed on 12 May 2025).
- Williams, R.H.; Hayes, J.D. The Breeding Implications of Studies on Yield and Its Components in Contrasting Genotypes of Spring Barley. 1977. Available online: https://www.jstor.org/stable/23777721 (accessed on 12 May 2025).
- Mwiinga, B.; Sibiya, J.; Kondwakwenda, A.; Musvosvi, C.; Chigeza, G. Genotype x environment interaction analysis of soybean (Glycine max (L.) Merrill) grain yield across production environments in Southern Africa. Field Crop. Res. 2020, 256, 107922. [Google Scholar] [CrossRef]

| Parameter | Yield (kg·ha−1) | Diameter Grains (mm) | Thousand-Grain Weight (g) | Protein (%) | Saponin (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | F | p | |
| Year | 71.07 * | p < 0.001 | 65.89 * | p < 0.001 | 3.13 ns | 0.082 | 62.81 * | p < 0.001 | 0.01 * | p < 0.001 |
| Genotype | 18.27 * | p < 0.001 | 63.56 * | p < 0.001 | 38.64 * | p < 0.001 | 64.28 * | p < 0.001 | 32.09 * | p < 0.001 |
| Environment | 25.70 * | p < 0.001 | 1.60 ns | 0.210 | 11.74 * | p < 0.001 | 112.45 * | p < 0.001 | 2.76 * | p < 0.001 |
| Year × environment | 2.12 * | p < 0.001 | 21.01 * | p < 0.001 | 20.43 * | p < 0.001 | 154.15 * | p < 0.001 | 2.73 * | p < 0.001 |
| Year × genotype | 1.14 * | p < 0.001 | 0.92 ns | 0.459 | 1.52 ns | 0.208 | 12.29 * | p < 0.001 | 2.65 * | p < 0.001 |
| Genotype × environment | 12.95 * | p < 0.001 | 1.51 ns | 0.173 | 1.15 ns | 0.342 | 15.01 * | p < 0.001 | 1.62 * | p < 0.001 |
| Year × environment × genotype | 1.81 * | p < 0.001 | 1.10 ns | 0.379 | 1.72 ns | 0.111 | 10.56 * | p < 0.001 | 2.36 * | p < 0.001 |
| Yield (kg·ha−1) | Diameter Grain (mm) | Thousand-Grain Weight (g) | Protein (%) | Saponin (%) | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Varieties | Mean | S.D. | Min | Max | C.V. | Mean | S.D. | Min | Max | C.V. | Mean | S.D. | Min | Max | C.V. | Mean | S.D. | Min | Max | C.V. | Mean | S.D. | Min | Max | C.V. |
| Nieves | 2824 ab | 888.52 | 1300 | 4133 | 31.46 | 1.88 a | 0.09 | 1.67 | 2.01 | 4.91 | 3.10 a | 0.41 | 2.19 | 3.73 | 13.14 | 14.29 b | 1.83 | 11.88 | 18.00 | 12.82 | 2.73 a | 0.53 | 1.60 | 4.10 | 19.47 |
| Pangal | 3162 a | 610.32 | 2100 | 4344 | 19.30 | 1.86 a | 0.10 | 1.68 | 2.09 | 5.46 | 3.05 a | 0.43 | 2.51 | 4.28 | 14.10 | 14.70 b | 1.54 | 12.31 | 16.63 | 10.50 | 1.70 bc | 0.33 | 0.90 | 2.30 | 19.46 |
| Pincoya | 2314 c | 310.10 | 1833 | 2889 | 13.70 | 1.62 c | 0.10 | 1.53 | 1.93 | 6.11 | 2.19 c | 0.42 | 1.59 | 3.41 | 19.29 | 16.31 a | 1.53 | 13.75 | 19.44 | 9.39 | 1.95 a | 0.68 | 1.00 | 3.20 | 28.76 |
| Chucao | 2842 ab | 478.76 | 2167 | 3950 | 16.84 | 1.75 b | 0.08 | 1.67 | 1.97 | 4.35 | 2.57 b | 0.25 | 2.30 | 3.28 | 9.90 | 14.41 b | 1.33 | 12.19 | 16.19 | 9.25 | 1.46 c | 0.37 | 0.90 | 2.30 | 25.30 |
| Regalona | 2724 b | 422.94 | 1933 | 3478 | 15.52 | 1.84 a | 0.06 | 1.72 | 1.99 | 3.38 | 2.94 a | 0.20 | 2.71 | 3.41 | 6.95 | 14.27 b | 1.16 | 12.13 | 16.06 | 8.13 | 2.32 ab | 0.32 | 1.80 | 2.70 | 13.67 |
| Mean | 2773 | 1866 | 3758 | 1.79 | 1.65 | 1.99 | 2.77 | 2.26 | 3.62 | 14.79 | 12.55 | 17.26 | 2.03 | 1.24 | 2.92 | ||||||||||
| Yield (kg·ha−1) | Diameter Grain (mm) | Thousand-Grain Weight (g) | Protein (%) | Saponin (%) | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Environment | Mean | S.D. | Min | Max | C.V. | Mean | S.D. | Min | Max | C.V. | Mean | S.D. | Min | Max | C.V. | Mean | S.D. | Min | Max | C.V. | Mean | S.D. | Min | Max | C.V. |
| Pichilemu | 2975 a | 718.30 | 1833 | 4344 | 24.14 | 1.80 a | 0.15 | 1.53 | 2.09 | 8.22 | 2.92 a | 0.57 | 1.96 | 4.28 | 19.42 | 14.47 b | 1.36 | 12.50 | 16.94 | 19.42 | 1.91 a | 0.67 | 0.90 | 3.30 | 35.09 |
| Coihueco | 2453 b | 518.91 | 1300 | 3233 | 21.15 | 1.78 a | 0.12 | 1.54 | 1.96 | 6.90 | 2.59 b | 0.43 | 1.59 | 3.17 | 16.59 | 15.80 a | 1.50 | 12.31 | 19.44 | 16.59 | 2.05 a | 1.73 | 1.30 | 4.10 | 28.66 |
| Cañete | 2892 a | 512.48 | 2000 | 3833 | 17.72 | 1.79 a | 0.12 | 1.53 | 2.01 | 6.66 | 2.80 ab | 0.42 | 1.84 | 3.73 | 15.12 | 14.12 b | 1.64 | 11.88 | 17.25 | 15.12 | 2.14 a | 0.60 | 1.00 | 3.20 | 28.01 |
| Mean | 2773 | 1711 | 3803 | 1.79 | 1.53 | 2.02 | 2.77 | 1.79 | 3.72 | 14.79 | 12.23 | 17.87 | 2.03 | 1.06 | 3.53 | ||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olguín, P.; Contreras, S.; Rojas, C.; Fuentes, F. Genotype-by-Environment Interaction Stability Analysis of New Quinoa (Chenopodium quinoa Willd.) Varieties in the Mediterranean Zone of Chile. Plants 2025, 14, 3007. https://doi.org/10.3390/plants14193007
Olguín P, Contreras S, Rojas C, Fuentes F. Genotype-by-Environment Interaction Stability Analysis of New Quinoa (Chenopodium quinoa Willd.) Varieties in the Mediterranean Zone of Chile. Plants. 2025; 14(19):3007. https://doi.org/10.3390/plants14193007
Chicago/Turabian StyleOlguín, Pablo, Samuel Contreras, Claudia Rojas, and Francisco Fuentes. 2025. "Genotype-by-Environment Interaction Stability Analysis of New Quinoa (Chenopodium quinoa Willd.) Varieties in the Mediterranean Zone of Chile" Plants 14, no. 19: 3007. https://doi.org/10.3390/plants14193007
APA StyleOlguín, P., Contreras, S., Rojas, C., & Fuentes, F. (2025). Genotype-by-Environment Interaction Stability Analysis of New Quinoa (Chenopodium quinoa Willd.) Varieties in the Mediterranean Zone of Chile. Plants, 14(19), 3007. https://doi.org/10.3390/plants14193007







