Integrated Transcriptome–Metabolome Analysis Reveals the Flavonoids Metabolism Mechanism of Maize Radicle in Response to Low Temperature
Abstract
1. Introduction
2. Results
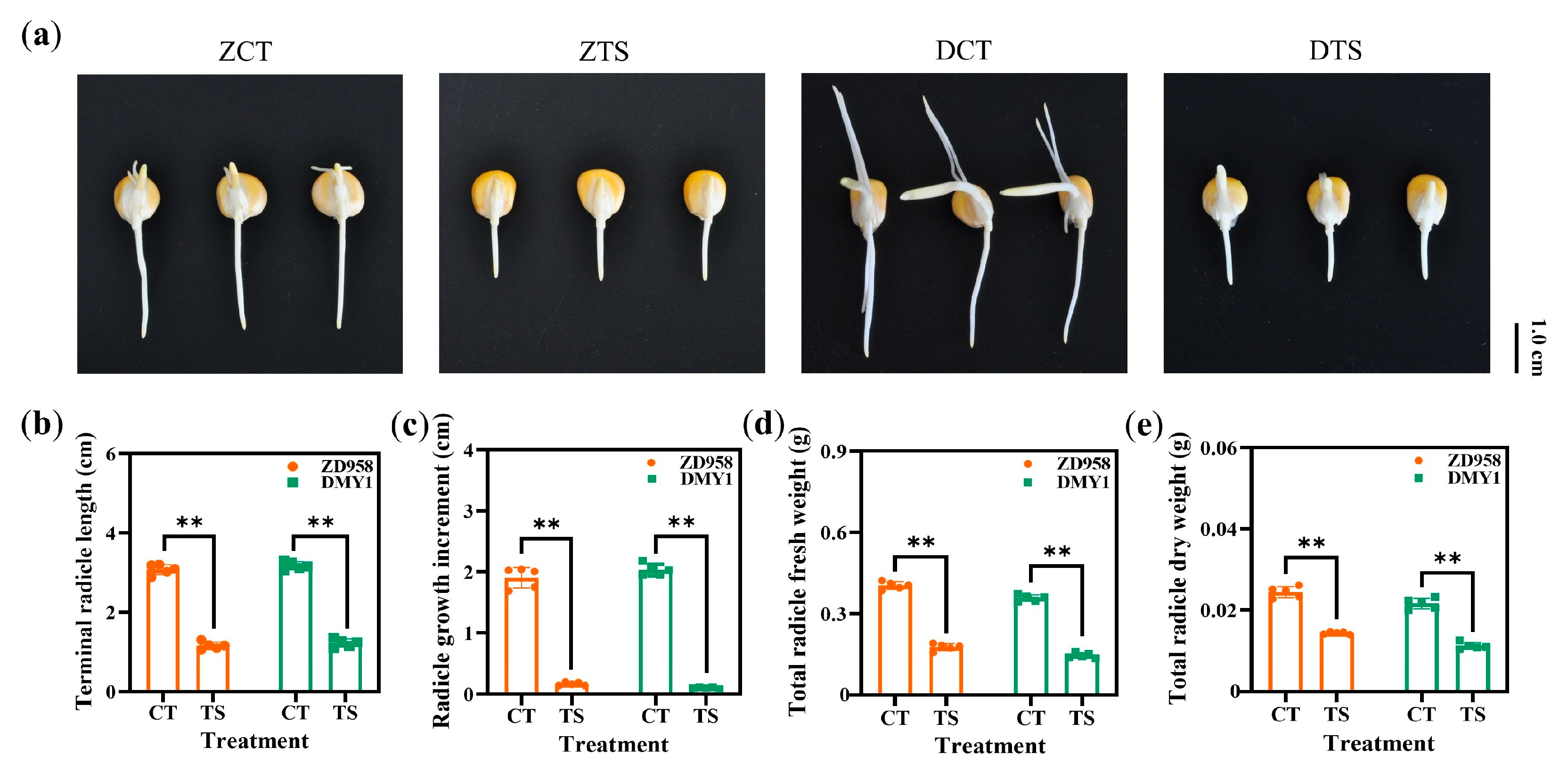
2.1. Effect of TS on the Phenotypic Characteristics of Maize Radicles
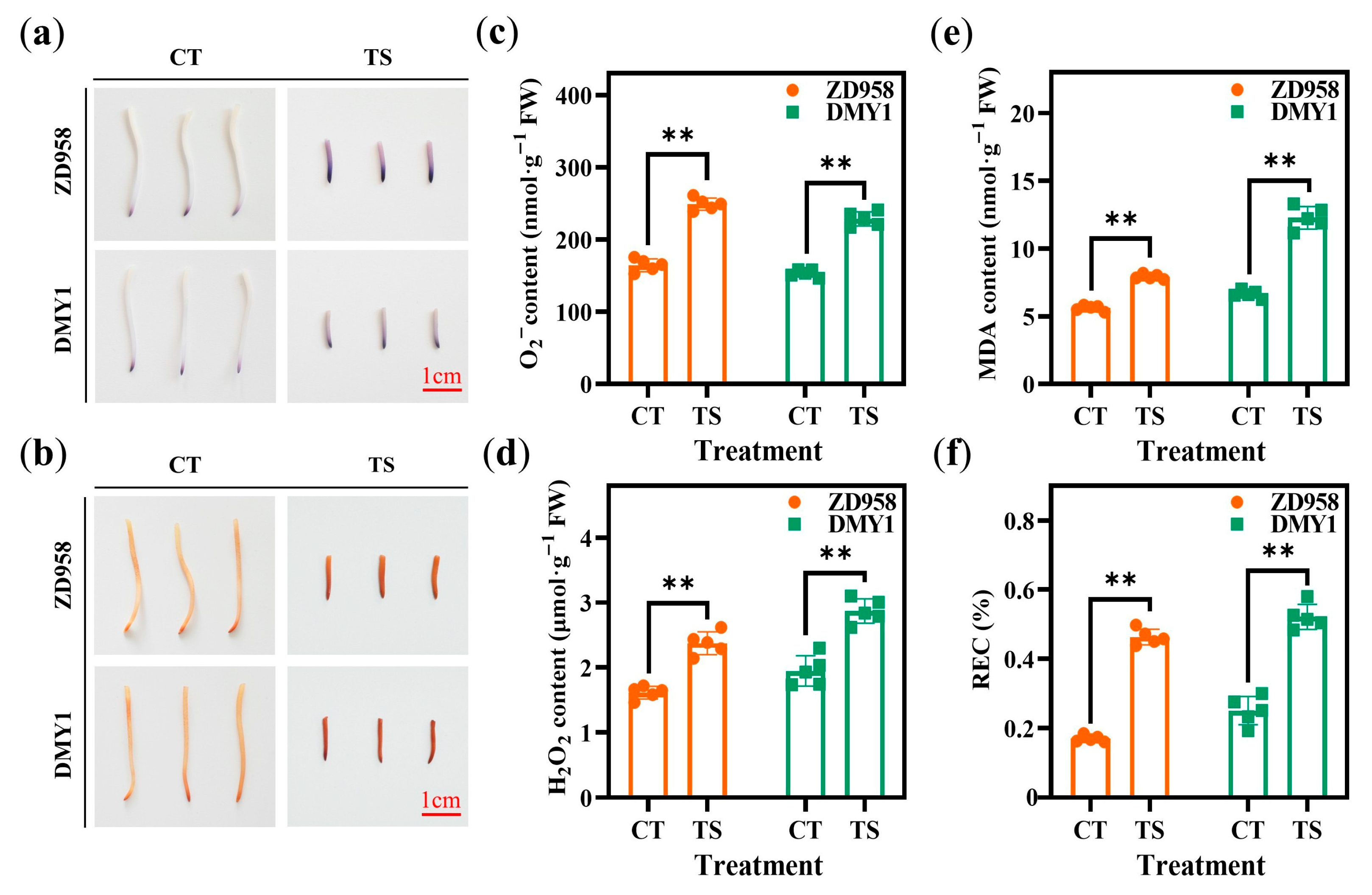
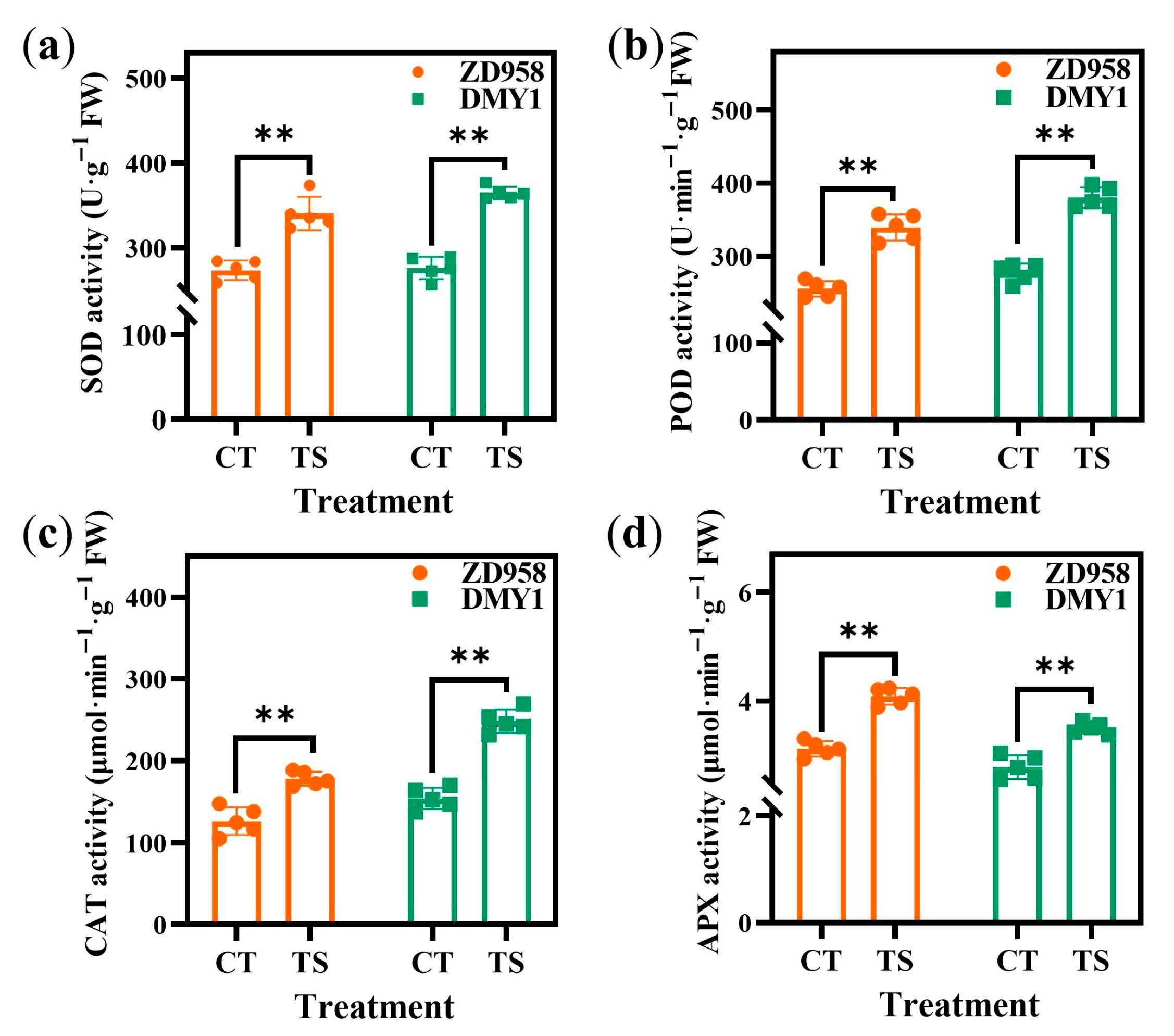
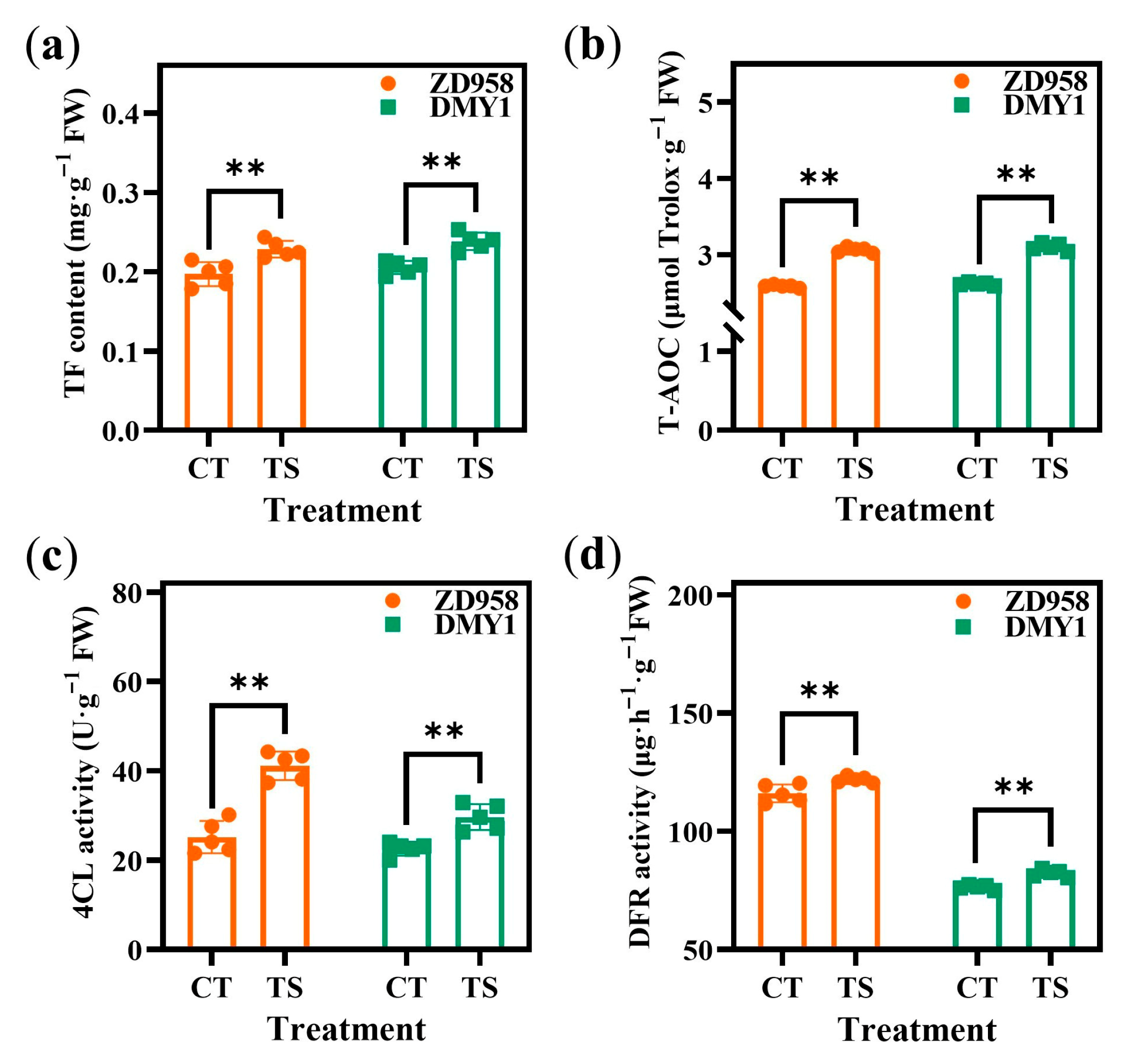
2.2. Effect of TS on Physiological and Biochemical Indicators of Maize Radicles
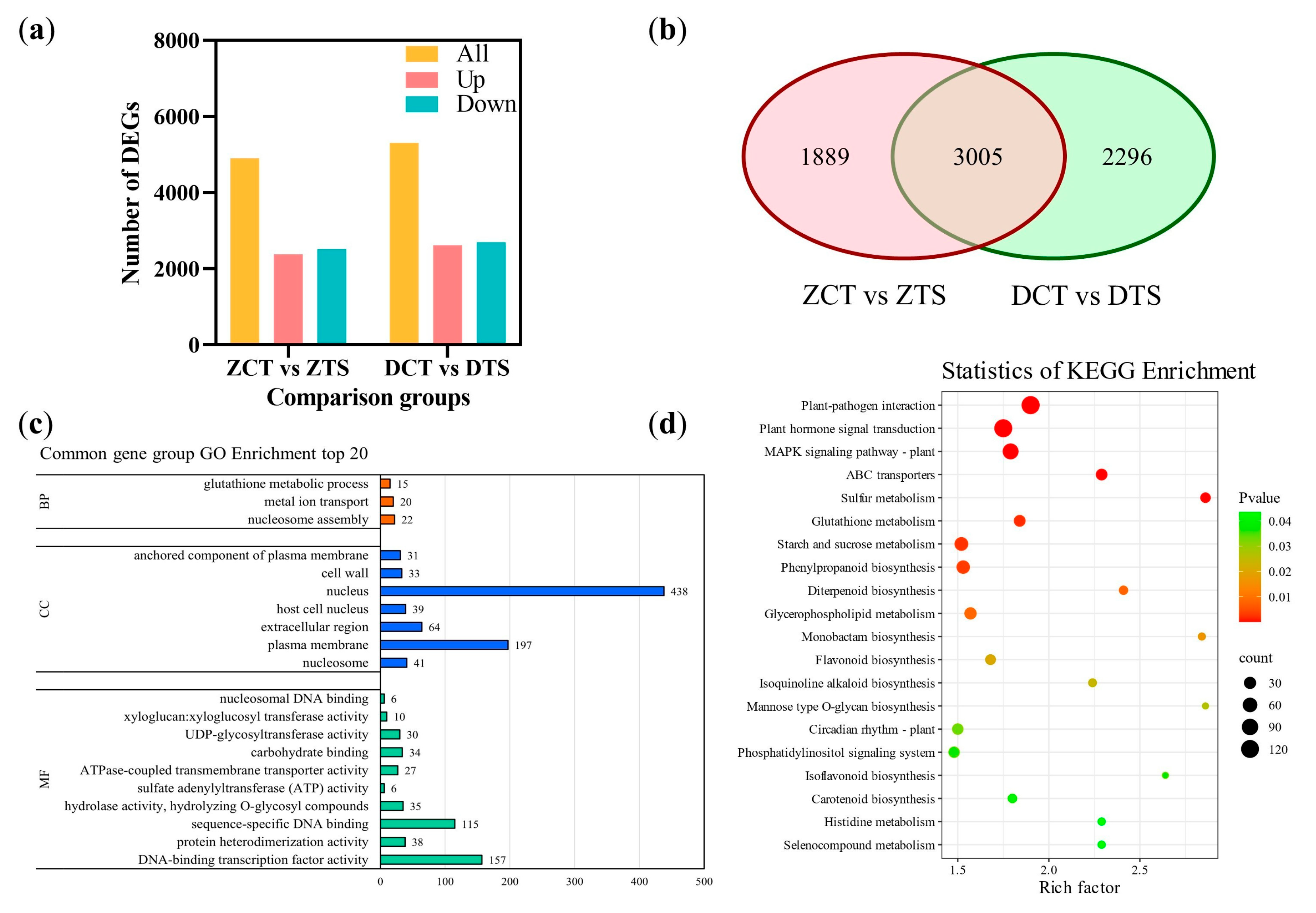
2.3. RNA-Seq and Differentially Expressed Gene Analysis of Maize Radicles Under TS
2.4. Co-Expression Trend Analysis of DEGs in Maize Radicles Under TS
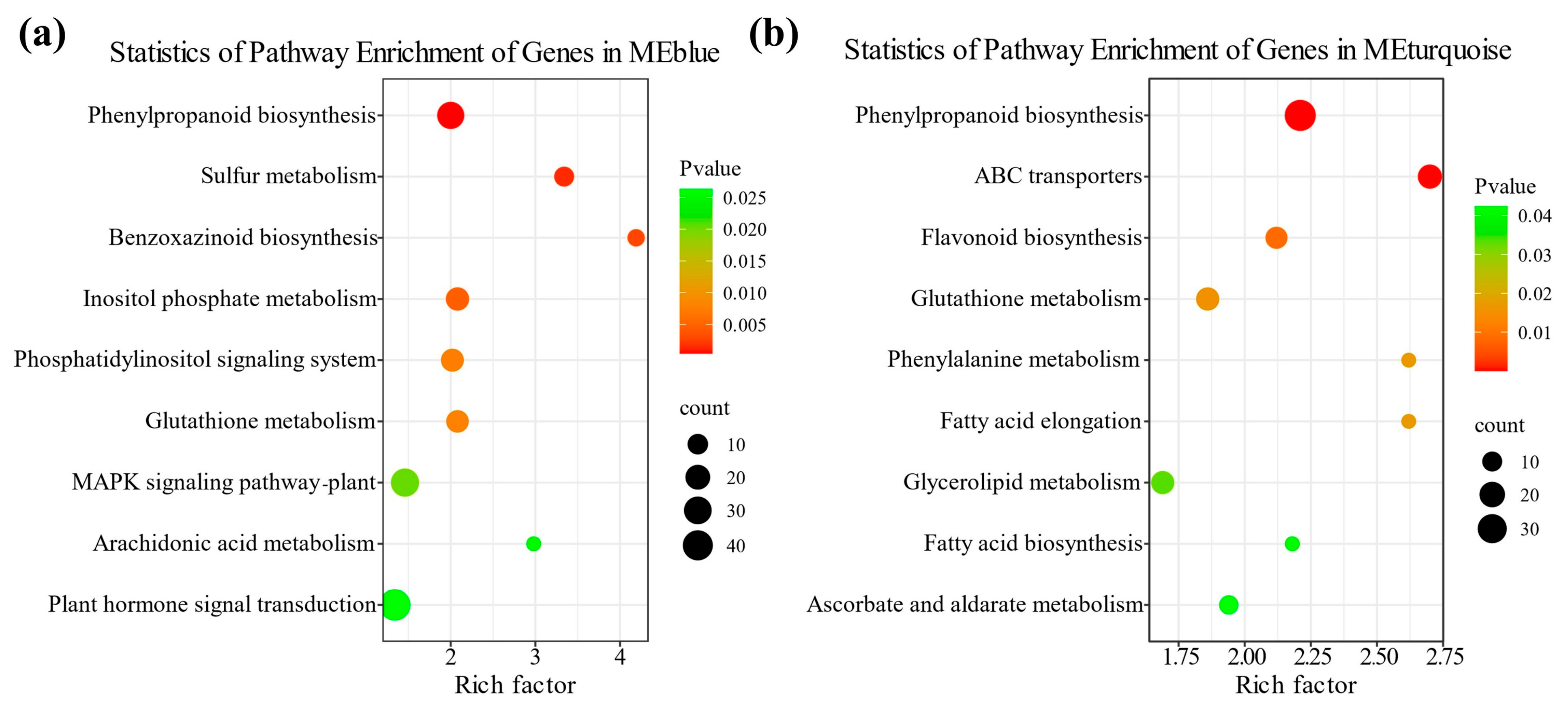
2.5. Weighted Gene Co-Expression Network Analysis of DEGs in Maize Radicles Under TS
2.6. Quantitative Real-Time Polymerase Chain Reaction Analysis of DEGs in Maize Radicles Under TS
2.7. Metabolomics Analysis of Maize Radicles Under TS
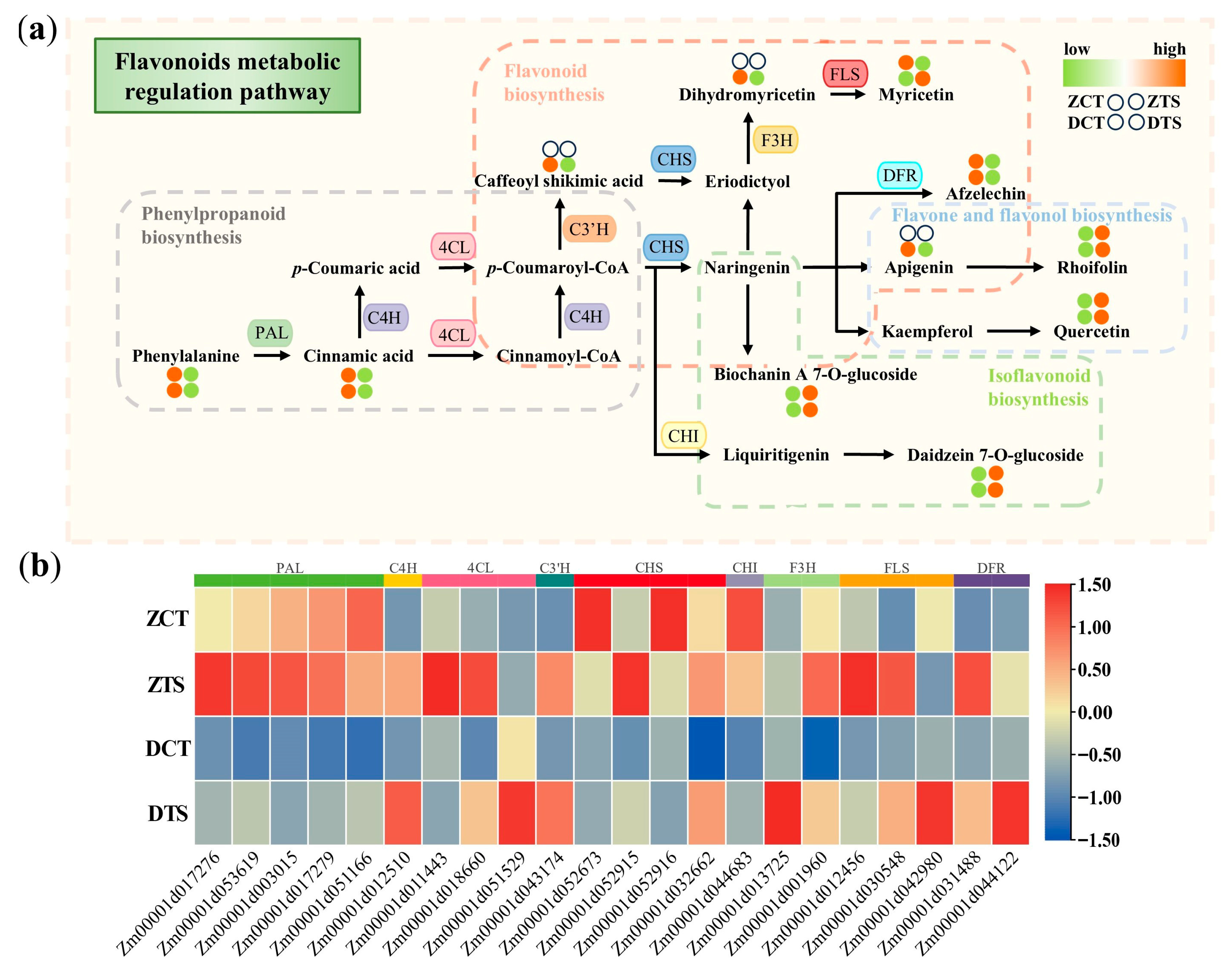
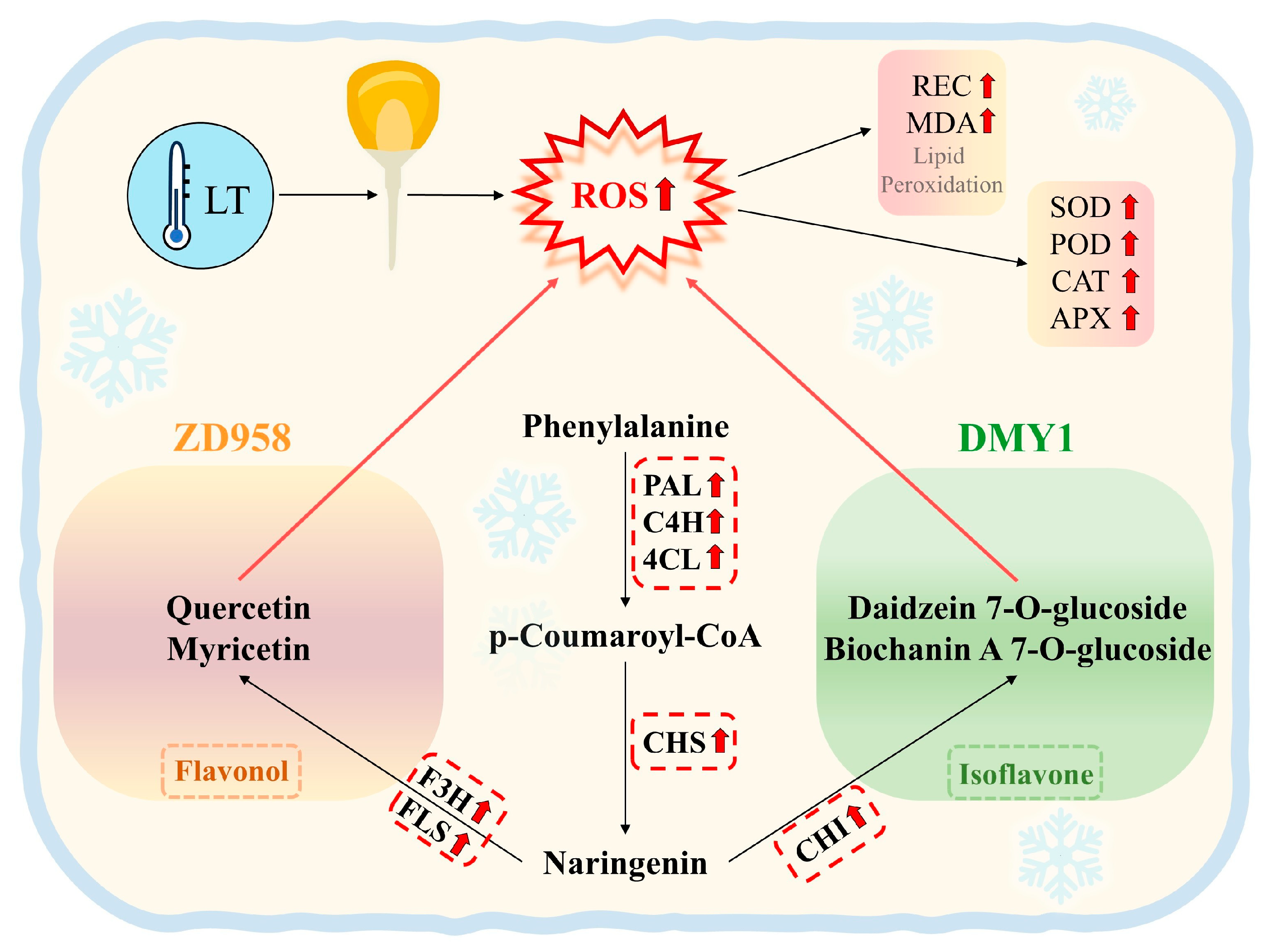
2.8. Analysis of the Regulatory Pathway of Flavonoid Metabolism in Maize Radicles Under TS
3. Discussion
4. Materials and Methods
4.1. Test Varieties and Experimental Design
4.2. Radicle Length, Growth Increment, FW, and DW Measurement
4.3. Physiological and Biochemical Indicator Measurement
4.3.1. Determination of REC and MDA Content in Maize Radicles
4.3.2. Qualitative and Quantitative Analysis of O2− and H2O2
4.3.3. Antioxidant Enzyme Activity Measurement
4.3.4. Determination of TF Content, T-AOC, and 4CL and DFR Activity
4.4. RNA Extraction, Library Construction, and RNA-Seq
4.5. qRT-PCR Analysis
4.6. Metabolomics Analysis
4.7. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| 4CL | 4-coumarate-CoA ligase |
| BP | biological process |
| C4H | trans-cinnamate 4-monooxygenase |
| CAT | catalase |
| CC | cellular component |
| CHS | chalcone synthase |
| CT | control |
| DAB | 3,3′-diaminobenzidine |
| DAM | differentially accumulated metabolites |
| DEG | differentially expressed gene |
| DFR | bifunctional dihydroflavonol 4-reductase |
| DMY1 | Demeiya1 |
| DW | dry weight |
| F3H | naringenin 3-dioxygenase |
| FC | fold change |
| FDR | false discovery rate |
| FLS | flavonol synthase |
| FPKM | fragments per kilobase of exon model per million mapped reads |
| FW | fresh weight |
| GO | Gene Ontology |
| MDA | malondialdehyde |
| MF | molecular function |
| NBT | nitrotetrazolium blue chloride |
| PAL | phenylalanine ammonia-lyase |
| PCA | principal component analysis |
| Phe | phenylalanine |
| POD | peroxidase |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| qRT-PCR | quantitative real-time polymerase chain reaction |
| T-AOC | total antioxidant capacity |
| TF | total flavonoid |
| TS | low-temperature stress |
| SOD | superoxide dismutase |
| REC | relative electrical conductivity |
| ROS | reactive oxygen species |
| WGCNA | weighted gene co-expression network analysis |
| ZD958 | Zhengdan958 |
References
- Xu, Y.; Yang, T.; Zhou, Y.; Yin, S.; Li, P.; Liu, J.; Xu, S.; Yang, Z.; Xu, C. Genome-wide association mapping of starch pasting properties in maize using single-locus and multi-locus models. Front. Plant Sci. 2018, 9, 1311. [Google Scholar] [CrossRef]
- Zhou, Z.; Shi, H.; Fu, Q.; Li, T.; Gan, T.Y.; Liu, S. Assessing spatiotemporal characteristics of drought and its effects on climate-induced yield of maize in Northeast China. J. Hydrol. 2020, 588, 125097. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Z.; Li, J.; Wang, R. Optimum planting density improves resource use efficiency and yield stability of rainfed maize in semiarid climate. Front. Plant Sci. 2021, 12, 752606. [Google Scholar] [CrossRef]
- Wan, J.; Wang, Q.; Zhao, J.; Zhang, X.; Guo, Z.; Hu, D.; Meng, S.; Lin, Y.; Qiu, X.; Mu, L.; et al. Gene expression variation explains maize seed germination heterosis. BMC Plant Biol. 2022, 22, 301. [Google Scholar] [CrossRef] [PubMed]
- Kermode, A.R. Regulatory mechanisms involved in the transition from seed development to germination. Crit. Rev. Plant Sci. 1990, 9, 155–195. [Google Scholar] [CrossRef]
- Qi, X.; Wan, C.; Zhang, X.; Sun, W.; Liu, R.; Wang, Z.; Wang, Z.; Ling, F. Effects of histone methylation modification on low temperature seed germination and growth of maize. Sci. Rep. 2023, 13, 5196. [Google Scholar] [CrossRef]
- Waqas, M.A.; Wang, X.; Zafar, S.A.; Noor, M.A.; Hussain, H.A.; Azher Nawaz, M.; Farooq, M. Thermal stresses in maize: Effects and management strategies. Plants 2021, 10, 293. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, C.; Hou, L.; Wu, X.; Wang, D.; Zhang, L.; Liu, P. Exogenous SA affects rice seed germination under salt stress by regulating Na+/K+ balance and endogenous GAs and ABA homeostasis. Int. J. Mol. Sci. 2022, 23, 3293. [Google Scholar] [CrossRef] [PubMed]
- Balabusta, M.; Szafranska, K.; Posmyk, M.M. Exogenous melatonin improves antioxidant defensein cucumber seeds (Cucumis sativus L.) germinated under chilling stress. Front. Plant Sci. 2016, 7, 12. [Google Scholar] [CrossRef]
- Elder, J.B.; Broome, J.A.; Bushnell, E.A.C. Computational insights into the regeneration of ovothiol and ergothioneine and their selenium analogues by glutathione. ACS Omega 2022, 7, 31813–31821. [Google Scholar] [CrossRef]
- Li, J.; Yu, Q.; Liu, C.; Zhang, N.; Xu, W. Flavonoids as key players in cold tolerance: Molecular insights and applications in horticultural crops. Hortic. Res. 2025, 12, 366. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Wu, Y.; Gao, R.; Zhang, Y.; Zheng, R.; Fang, M.; Li, Y.; Zhang, Y.; Guan, L.; Gao, Y. Integrated metabolomics and transcriptomics reveal the key role of flavonoids in the cold tolerance of chrysanthemum. Int. J. Mol. Sci. 2024, 25, 7589. [Google Scholar] [CrossRef] [PubMed]
- Rychlicka, M.; Rot, A.; Gliszczyńska, A. Biological properties, health benefits and enzymatic modifications of dietary methoxylated derivatives of cinnamic acid. Foods 2021, 10, 1417. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Doseff, A.; Grotewold, E. Flavones: From biosynthesis to health benefits. Plants 2016, 5, 27. [Google Scholar] [CrossRef]
- He, J.; Yao, L.; Pecoraro, L.; Liu, C.; Wang, J.; Huang, L.; Gao, W. Cold stress regulates accumulation of flavonoids and terpenoids in plants by phytohormone, transcription process, functional enzyme, and epigenetics. Crit. Rev. Biotechnol. 2023, 43, 680–697. [Google Scholar] [CrossRef]
- Zhuoma, P.; Tondrob, D.; Qunpei, T.; Fu, J.; Dan, S. Muti-omics revealed the mechanisms of MT-conferred tolerance of Elymus nutans Griseb. to low temperature at XiZang. BMC Plant Biol. 2024, 24, 901. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, L.; Hu, H.; Yang, J.; Cui, J.; Wei, G.; Xu, J. Transcriptome and metabolome changes in Chinese cedar during cold acclimation reveal the roles of flavonoids in needle discoloration and cold resistance. Tree Physiol. 2022, 42, 1858–1875. [Google Scholar] [CrossRef]
- Liu, J.; Wang, T.; Weng, Y.; Liu, B.; Gao, Q.; Ji, W.; Wang, Z.; Wang, Y.; Ma, X. Identification and characterization of regulatory pathways controlling dormancy under lower temperature in alfalfa (Medicago sativa L.). Front. Plant Sci. 2022, 13, 872839. [Google Scholar] [CrossRef]
- Yang, M.; Yang, J.; Su, L.; Sun, K.; Li, D.; Liu, Y.; Wang, H.; Chen, Z.; Guo, T. Metabolic profile analysis and identification of key metabolites during rice seed germination under low-temperature stress. Plant Sci. 2019, 289, 110282. [Google Scholar] [CrossRef]
- Han, L.; Zhong, W.; Qian, J.; Jin, M.; Tian, P.; Zhu, W.; Zhang, H.; Sun, Y.; Feng, J.; Liu, X.; et al. A multi-omics integrative network map of maize. Nat. Genet. 2022, 55, 144–153. [Google Scholar] [CrossRef]
- Qu, X.; Huang, Z.; Baskin, J.M.; Baskin, C.C. Effect of temperature, light and salinity on seed germination and radicle growth of the geographically widespread halophyte shrub Halocnemum strobilaceum. Ann. Bot. 2007, 101, 293–299. [Google Scholar] [CrossRef]
- Li, W.; Li, J.; Zhang, Y.; Luo, W.; Dou, Y.; Yu, S. Effect of reactive oxygen scavenger N,N′-dimethylthiourea (DMTU) on seed germination and radicle elongation of maize. Int. J. Mol. Sci. 2023, 24, 15557. [Google Scholar] [CrossRef]
- Zhao, X.; Wei, Y.; Zhang, J.; Yang, L.; Liu, X.; Zhang, H.; Shao, W.; He, L.; Li, Z.; Zhang, Y.; et al. Membrane lipids’ metabolism and transcriptional regulation in maize roots under cold stress. Front. Plant Sci. 2021, 12, 639132. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Li, W.; Gao, X.; Xu, X.; Zhang, C.; Yu, S.; Dou, Y.; Luo, W.; Yu, L. Transcriptome analysis reveals POD as an important indicator for assessing low-temperature tolerance in maize radicles during germination. Plants 2024, 13, 1362. [Google Scholar] [CrossRef]
- Gu, Y.; He, L.; Zhao, C.; Wang, F.; Yan, B.; Gao, Y.; Li, Z.; Yang, K.; Xu, J. Biochemical and transcriptional regulation of membrane lipid metabolism in maize leaves under low temperature. Front. Plant Sci. 2017, 8, 2053. [Google Scholar] [CrossRef] [PubMed]
- Fenza, M.D.; Hogg, B.; Grant, J.; Barth, S. Transcriptomic response of maize primary roots to low temperatures at seedling emergence. PeerJ 2017, 5, e2839. [Google Scholar] [CrossRef]
- He, F.; Shen, H.; Lin, C.; Fu, H.; Sheteiwy, M.S.; Guan, Y.; Huang, Y.; Hu, J. Transcriptome analysis of chilling-imbibed embryo revealed membrane recovery related genes in maize. Front. Plant Sci. 2016, 7, 1978. [Google Scholar] [CrossRef]
- Saltveit, M.E. Heat shocks increase the chilling tolerance of rice (Oryza sativa) seedling radicles. J. Agric. Food Chem. 2002, 50, 3232–3235. [Google Scholar] [CrossRef] [PubMed]
- Cheshmi, M.; Khajeh-Hosseini, M. Single count of radicle emergence, DNA replication during seed germination and vigour in alfalfa seed lots. Seed Sci. Technol. 2020, 48, 367–380. [Google Scholar] [CrossRef]
- Demir, I.; Kuzucu, C.O.; Ermis, S.; Öktem, G. Radicle emergence as seed vigour test estimates seedling quality of hybrid cucumber (Cucumis sativus L.) cultivars in low temperature and salt stress conditions. Horticulturae 2022, 9, 3. [Google Scholar] [CrossRef]
- Massardo, F.; Corcuera, L.; Alberdi, M. Embryo physiological responses to cold by two cultivars of oat during germination. Crop Sci. 2000, 40, 1694–1701. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J.; Xu, Q.; Wang, D.; Di, H.; Huang, J.; Yang, X.; Wang, Z.; Zhang, L.; Dong, L.; et al. Identification of candidate tolerance genes to low-temperature during maize germination by GWAS and RNA-seq approaches. BMC Plant Biol. 2020, 20, 333. [Google Scholar] [CrossRef]
- Velázquez-Márquez, S.; Conde-Martínez, V.; Trejo, C.; Delgado-Alvarado, A.; Carballo, A.; Suárez, R.; Mascorro, J.O.; Trujillo, A.R. Effects of water deficit on radicle apex elongation and solute accumulation in Zea mays L. Plant Physiol. Biochem. 2015, 96, 29–37. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, Y.; Yao, H.; Wu, J.; Sun, H.; Gong, H. Silicon improves seed germination and alleviates oxidative stress of bud seedlings in tomato under water deficit stress. Plant Physiol. Biochem. 2014, 78, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Lotfi, N.; Soleimani, A.; Vahdati, K.; Çakmakçı, R. Comprehensive biochemical insights into the seed germination of walnut under drought stress. Sci. Hortic. 2019, 250, 329–343. [Google Scholar] [CrossRef]
- Ren, S.; Tan, J.; Zhou, S.; Sun, H.; Li, H.; Li, W.; Li, N.; Wu, J.; Ren, X.; Ci, J.; et al. Germplasm selection and comprehensive evaluation of maize inbred lines at germination and seedling stage for saline–alkali tolerance. Agronomy 2025, 15, 626. [Google Scholar] [CrossRef]
- Wei, X.; Wang, J.; Xu, C.; Zhao, Y.; Pu, X.; Wang, W.; Lu, G. Analysis of germination characteristics and metabolome of Medicago ruthenica in response to saline-alkali stress. Front. Plant Sci. 2025, 16, 1592555. [Google Scholar] [CrossRef]
- Xu, J.; Qiao, X.; Tian, Z.; Zhang, X.; Zou, X.; Cheng, Y.; Lu, G.; Zeng, L.; Fu, G.; Ding, X.; et al. Proteomic analysis of rapeseed root response to waterlogging stress. Plants 2018, 7, 71. [Google Scholar] [CrossRef]
- Shen, C.; Yuan, J.; Qiao, H.; Wang, Z.; Liu, Y.; Ren, X.; Wang, F.; Liu, X.; Zhang, Y.; Chen, X.; et al. Transcriptomic and anatomic profiling reveal the germination process of different wheat varieties in response to waterlogging stress. BMC Genet. 2020, 21, 93. [Google Scholar] [CrossRef]
- Fan, Y.; Cui, C.; Liu, Y.; Wu, K.; Du, Z.; Jiang, X.; Zhao, F.; Zhang, R.; Wang, J.; Mei, H.; et al. Physiological and transcriptional responses of sesame (Sesamum indicum L.) to waterlogging stress. Int. J. Mol. Sci. 2025, 26, 2603. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Wang, S.; Zhang, H.; Liu, Y.; Yang, M. Integrative transcriptomic and metabolomic analyses reveal the flavonoid biosynthesis of Pyrus hopeiensis flowers under cold stress. Hortic. Plant J. 2023, 9, 395–413. [Google Scholar] [CrossRef]
- Jiang, F.; Lv, S.; Zhang, Z.; Chen, Q.; Mai, J.; Wan, X.; Liu, P. Integrated metabolomics and transcriptomics analysis during seed germination of waxy corn under low temperature stress. BMC Plant Biol. 2023, 23, 190. [Google Scholar] [CrossRef]
- Bai, Y.; Dai, Q.; He, Y.; Yan, L.; Niu, J.; Wang, X.; Xie, Y.; Yu, X.; Tang, W.; Li, H.; et al. Exogenous diethyl aminoethyl hexanoate alleviates the damage caused by low-temperature stress in Phaseolus vulgaris L. seedlings through photosynthetic and antioxidant systems. BMC Plant Biol. 2025, 25, 75. [Google Scholar] [CrossRef] [PubMed]
- Meng, A.; Wen, D.; Zhang, C. Maize seed germination under low-temperature stress impacts seedling growth under normal temperature by modulating photosynthesis and antioxidant metabolism. Front. Plant Sci. 2022, 13, 843033. [Google Scholar] [CrossRef]
- Hodges, D.M.; Andrews, C.J.; Johnson, D.A.; Hamilton, R.I. Antioxidant enzyme and compound responses to chilling stress and their combining abilities in differentially sensitive maize hybrids. Crop Sci. 1997, 37, 857–863. [Google Scholar] [CrossRef]
- Ali, M.B.; Hahn, E.J.; Paek, K.Y. Effects of temperature on oxidative stress defense systems, lipid peroxidation and lipoxygenase activity in Phalaenopsis. Plant Physiol. Biochem. 2005, 43, 213–223. [Google Scholar] [CrossRef]
- Shin, S.K.; Cho, H.W.; Song, S.E.; Im, S.S.; Bae, J.H.; Song, D.K. Oxidative stress resulting from the removal of endogenous catalase induces obesity by promoting hyperplasia and hypertrophy of white adipocytes. Redox Biol. 2020, 37, 2213–2317. [Google Scholar] [CrossRef] [PubMed]
- Kesawat, M.S.; Satheesh, N.; Kherawat, B.S.; Kumar, A.; Kim, H.U.; Chung, S.M.; Kumar, M. Regulation of reactive oxygen species during salt stress in plants and their crosstalk with other signaling molecules—Current perspectives and future directions. Plants 2023, 12, 864. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; He, J.; Rusling, J.F. Electrochemical transformations catalyzed by cytochrome P450s and peroxidases. Chem. Soc. Rev. 2023, 52, 5135–5171. [Google Scholar] [CrossRef]
- Li, S. Novel insight into functions of ascorbate peroxidase in higher plants: More than a simple antioxidant enzyme. Redox Biol. 2023, 64, 102789. [Google Scholar] [CrossRef]
- Fu, D.; Qi, J.; Su, L.; Wang, X.; Wang, M.; Chen, B.; Yu, X.; Zhao, X.; Gao, W.; Guo, X.; et al. Chalcone synthase 2 (BpCHS2), a structural gene, was activated by low temperature to promote anthocyanin synthesis in Broussonetia papyrifera to improve its cold tolerance. Plant Physiol. Biochem. 2025, 222, 109656. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Q.; Ma, C.; Zhang, Z.; Cao, H.; Kong, Y.; Yue, C.; Hao, X.; Chen, L.; Ma, J.; et al. Global transcriptome profiles of Camellia sinensis during cold acclimation. BMC Genom. 2013, 14, 415. [Google Scholar] [CrossRef] [PubMed]
- Moliterni, V.M.C.; Paris, R.; Onofri, C.; Orrù, L.; Cattivelli, L.; Pacifico, D.; Avanzato, C.; Ferrarini, A.; Delledonne, M.; Mandolino, G. Early transcriptional changes in Beta vulgaris in response to low temperature. Planta 2015, 242, 187–201. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, S.; Qin, B.; Sun, H.; Yuan, X.; Wang, Q.; Xu, J.; Yin, Z.; Du, Y.; Du, J.; et al. Analysis of the transcriptome and metabolome reveals phenylpropanoid mechanism in common bean (Phaseolus vulgaris) responding to salt stress at sprout stage. Food Energy Secur. 2023, 12, e481. [Google Scholar] [CrossRef]
- Amrine, K.C.H.; Blanco-Ulate, B.; Cantu, D. Discovery of core biotic stress responsive genes in Arabidopsis by weighted gene co-expression network analysis. PLoS ONE 2015, 10, e0118731. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Cao, S.; Zhao, Y.; Wang, R.; Yin, X. Integrated transcriptomics and metabolomics analyses revealed mechanisms of Trichoderma harzianum-induced resistance to downy mildew in grapevine. Physiol. Mol. Plant Pathol. 2025, 137, 102619. [Google Scholar] [CrossRef]
- Xiao, P.; Qu, J.; Wang, Y.; Fang, T.; Xiao, W.; Wang, Y.; Zhang, Y.; Khan, M.; Chen, Q.; Xu, X.; et al. Transcriptome and metabolome atlas reveals contributions of sphingosine and chlorogenic acid to cold tolerance in Citrus. Plant Physiol. 2024, 196, 634–650. [Google Scholar] [CrossRef]
- Jeon, J.; Kim, J.K.; Wu, Q.; Park, S.U. Effects of cold stress on transcripts and metabolites in tartary buckwheat (Fagopyrum tataricum). Environ. Exp. Bot. 2018, 155, 488–496. [Google Scholar] [CrossRef]
- Dunn, W.B.; Broadhurst, D.; Begley, P.; Zelena, E.; Francis-McIntyre, S.; Anderson, N.; Brown, M.; Knowles, J.D.; Halsall, A.; Haselden, J.N.; et al. Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nat. Protoc. 2011, 6, 1060–1083. [Google Scholar] [CrossRef]
- Shi, H.; Chan, Z. The cysteine2/histidine2-type transcription factor ZINC FINGER OF ARABIDOPSIS THALIANA 6-activated C-REPEAT-BINDING FACTOR pathway is essential for melatonin-mediated freezing stress resistance in Arabidopsis. J. Pineal Res. 2014, 57, 185–191. [Google Scholar] [CrossRef]
- Xing, J.; Ye, X.; Huo, K.; Ding, Z.; Tie, W.; Xie, Z.; Li, C.; Meng, F.; Hu, W. Integrated metabolomic and transcriptomic analyses revealed the overlapping response mechanisms of banana to cold and drought stress. Plant Physiol. Biochem. 2025, 222, 109766. [Google Scholar] [CrossRef]
- Peng, Z.; Wang, Y.; Zuo, W.; Gao, Y.; Li, R.; Yu, C.; Liu, Z.; Zheng, Y.; Shen, Y.; Duan, L. Integration of metabolome and transcriptome studies reveals flavonoids, abscisic acid, and nitric oxide comodulating the freezing tolerance in Liriope spicata. Front. Plant Sci. 2022, 12, 764625. [Google Scholar] [CrossRef]
- Wang, L.; Nan, H.; Zhang, M.; Guang, L.; Meng, J.; Liu, M.; Meng, Y.; Chen, W.; Fan, Y.; Huang, H.; et al. GhADT5 enhances alkali stress tolerance in cotton by regulating phenylalanine-derived flavonoid biosynthesis and antioxidant defense. BMC Plant Biol. 2025, 25, 225. [Google Scholar] [CrossRef]
- Kim, J.I.; Hidalgo-Shrestha, C.; Bonawitz, N.D.; Franke, R.B.; Chapple, C. Spatio-temporal control of phenylpropanoid biosynthesis by inducible complementation of a cinnamate 4-hydroxylase mutant. J. Exp. Bot. 2021, 72, 3061–3073. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Liu, X.; Gong, Q.; Cao, J.; Shen, W.; Yin, X.; Grierson, D.; Zhang, B.; Xu, C.; Li, X.; et al. Three AP2/ERF family members modulate flavonoid synthesis by regulating type IV chalcone isomerase in citrus. Plant Biotechnol. J. 2021, 19, 671–688. [Google Scholar] [CrossRef] [PubMed]
- Hacquard, S.; Wang, E.; Slater, H.; Martin, F. Impact of global change on the plant microbiome. New Phytol. 2022, 234, 1907–1909. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Sun, X.; Weiszmann, J.; Weckwerth, W. System-level and granger network analysis of integrated proteomic and metabolomic dynamics identifies key points of grape berry development at the interface of primary and secondary metabolism. Front. Plant Sci. 2017, 8, 1066. [Google Scholar] [CrossRef]
- Rohde, P.; Hincha, D.K.; Heyer, A.G. Heterosis in the freezing tolerance of crosses between two Arabidopsis thaliana accessions (Columbia-0 and C24) that show differences in non-acclimated and acclimated freezing tolerance. Plant J. 2004, 38, 790–799. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Long, Q.; Qiu, S.; Man, J.; Ren, D.; Xu, N.; Luo, R. OsAAI1 increases rice yield and drought tolerance dependent on ABA-mediated regulatory and ROS scavenging pathway. Rice 2023, 16, 35. [Google Scholar] [CrossRef]
- Fridovich, I. Superoxide anion radical (O2−), superoxide dismutases, and related matters. J. Biol. Chem. 1997, 272, 18515–18517. [Google Scholar] [CrossRef]
- Xia, Z.; Wang, M.; Xu, Z. The maize sulfite reductase is involved in cold and oxidative stress responses. Front. Plant Sci. 2018, 9, 1680. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, B.; Xu, Z.; Shi, Z.; Chen, S.; Huang, X.; Chen, J.; Wang, X. Involvement of reactive oxygen species in endosperm cap weakening and embryo elongation growth during lettuce seed germination. J. Exp. Bot. 2014, 65, 3189–3200. [Google Scholar] [CrossRef]
- Neto, A.D.d.A.; Prisco, J.T.; Enéas-Filho, J.; Abreu, C.E.B.d.; Gomes-Filho, E. Effect of salt stress on antioxidative enzymes and lipid peroxidation in leaves and roots of salt-tolerant and salt-sensitive maize genotypes. Environ. Exp. Bot. 2006, 56, 87–94. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases I. occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Maehly, A.C.; Chance, B. The assay of catalases and peroxidases. Methods Biochem. Anal. 1954, 1, 357–424. [Google Scholar] [CrossRef]
- Aebi, H. [13] Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Hu, E.; Cai, Y.; Xie, Z.; Luo, X.; Zhan, L.; Tang, W.; Wang, Q.; Liu, B.; Wang, R.; et al. Using clusterProfiler to characterize multiomics data. Nat. Protoc. 2024, 19, 3292–3320. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xu, J.; Gao, Y.; Wang, C.; Guo, G.; Luo, Y.; Huang, Y.; Hu, W.; Sheteiwy, M.S.; Guan, Y.; et al. The synergistic priming effect of exogenous salicylic acid and H2O2 on chilling tolerance enhancement during maize (Zea mays L.) seed germination. Front. Plant Sci. 2017, 8, 1153. [Google Scholar] [CrossRef] [PubMed]
- Nicot, N.; Hausman, J.F.; Hoffmann, L.; Evers, D. Housekeeping gene selection for real-time RT-PCR normalization in potato during biotic and abiotic stress. J. Exp. Bot. 2005, 56, 2907–2914. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dou, Y.; Luo, W.; Zhang, Y.; Li, W.; Zhang, C.; Lv, Y.; Liu, X.; Yu, S. Integrated Transcriptome–Metabolome Analysis Reveals the Flavonoids Metabolism Mechanism of Maize Radicle in Response to Low Temperature. Plants 2025, 14, 2988. https://doi.org/10.3390/plants14192988
Dou Y, Luo W, Zhang Y, Li W, Zhang C, Lv Y, Liu X, Yu S. Integrated Transcriptome–Metabolome Analysis Reveals the Flavonoids Metabolism Mechanism of Maize Radicle in Response to Low Temperature. Plants. 2025; 14(19):2988. https://doi.org/10.3390/plants14192988
Chicago/Turabian StyleDou, Yi, Wenqi Luo, Yifei Zhang, Wangshu Li, Chunyu Zhang, Yanjie Lv, Xinran Liu, and Song Yu. 2025. "Integrated Transcriptome–Metabolome Analysis Reveals the Flavonoids Metabolism Mechanism of Maize Radicle in Response to Low Temperature" Plants 14, no. 19: 2988. https://doi.org/10.3390/plants14192988
APA StyleDou, Y., Luo, W., Zhang, Y., Li, W., Zhang, C., Lv, Y., Liu, X., & Yu, S. (2025). Integrated Transcriptome–Metabolome Analysis Reveals the Flavonoids Metabolism Mechanism of Maize Radicle in Response to Low Temperature. Plants, 14(19), 2988. https://doi.org/10.3390/plants14192988





